Abstract
The role of morphogens in bone regeneration has been widely studied, whereas the effect of matrix cues, particularly on stem cell differentiation, are less well understood. In this work, we investigated the effects of arginine-glycine-aspartate (RGD) ligand conformation (linear vs cyclic RGD) on primary human bone marrow stromal cell (hBMSC) and D1 stem cell osteogenic differentiation in three-dimensional (3D) culture and compared their response with that of committed MC3T3-E1 preosteoblasts to determine whether the stage of cell differentiation altered the response to the adhesion ligands. Linear RGD densities that promoted osteogenic differentiation of committed cells (MC3T3-E1 preosteoblasts) did not induce differentiation of hBMSCs or D1 stem cells, although matrices presenting the cyclic form of this adhesion ligand enhanced osteoprogenitor differentiation in 3D culture. This may be due to enhanced integrin–ligand binding. These studies indicate that biomaterial design parameters optimized for differentiated cell types may not directly translate to stem cell populations, because less-committed cells may require more instruction than differentiated cells. It is likely that design of synthetic extracellular matrices tailored to promote stem cell differentiation may enhance bone regeneration by transplanted cells.
Introduction
Bone tissue engineering is a new therapeutic approach that aims to regenerate or repair bone tissue using scaffolds, cells, and inductive factors to actively stimulate tissue formation.1–5 Isolation of sufficient numbers of primary human osteoblasts is difficult because it requires sacrifice of healthy bone tissue, and this has stimulated interest in the use of osteoprogenitor cells as a source for bone regeneration. Because these multipotential precursor cells are self-renewing, maintain their differentiation ability, and can be readily expanded in culture, stem cells have great potential for clinical utility to repair or regenerate tissues.6 Unlike embryonic stem cells, which have the capability to recapitulate all the tissues of the body (e.g., cells derived from all three germ layers), adult stem cells are more limited in their differentiation capability. However, it is likely that adult stem cells will have greater clinical utility in the near future, because the application of these cells for autologous therapies is straightforward. Derived from the peripheral blood, bone marrow, muscle, and even adipose tissue, adult progenitor cells can be expanded in culture and induced down a specific lineage to form mature, differentiated cells that can be used to repair or regenerate tissue.7 There are more than 800 clinical trials currently investigating the use of human adult stem cells for the treatment of a variety of diseases,8 and it is likely that, in many situations, the effective use of stem cells will require a synthetic extracellular matrix (ECM) analog to tightly regulate their fate.
The majority of clinical studies using adult stem cells to regenerate bone have focused on localized delivery via scaffolds to provide site-specific delivery of large quantities of cells.9–11 A variety of synthetic and natural materials have been investigated as synthetic ECMs (sECMs) to deliver the cells. Furthermore, these scaffolds may allow control of the specific microenvironment and strongly influence cell behavior and clinical outcomes.12 Many studies have focused on the delivery of signaling molecules such as growth factors for bone regeneration, whereas the effect of matrix cues, particularly on stem cell differentiation, is less well understood.13–15 However, presentation of appropriate adhesion cues may be critical, because growth factor delivery without optimal scaffold properties has failed in some studies to induce significant bone formation.16
The presentation of adhesion ligands from sECMs provides an attractive approach to regulate osteoprogenitor cell differentiation. Matrix cues that enhance cell differentiation in vitro have been demonstrated to correlate with enhanced bone regeneration in vivo,17 suggesting that in vitro studies with appropriate model systems can provide useful data for design of cell transplantation vehicles. Alginate hydrogels provide a useful model sECM for these types of studies, because alginate is a well-characterized material with good biocompatibility and low toxicity, and its gels have a highly hydrated polymer network similar to native ECMs.18 Alginate gels have previously been used to study cell-matrix interactions in vitro and in vivo, are extensively used as a cell immobilization and transplantation material,16,17,19,20 and can be covalently modified with arginine-glycine-aspartate (RGD) peptide sequences commonly found in ECM proteins (e.g., collagen, fibronectin, vitronectin) using standard carbodiimide chemistry.21
This study addressed the hypothesis that sECMs presenting cyclic RGD sequences to encapsulated cells would enhance osteoprogenitor differentiation better than linear RGD sequences. The native conformation of the RGD sequence found in ECM proteins such as bone sialoprotein (BSP) is a loop conformation,22 and cyclic RGD-presenting peptides have higher binding affinity and selectivity than linear peptides.23,24 To address the hypothesis, alginate hydrogels were modified with the linear RGD peptide sequence (glycine4-arginine-glycine-aspartate-serine-proline (G4RGDSP)) or the cyclic RGD peptide (glycine4-cystine-arginine-glycine-aspartate-serine-proline-cystine (G4CRGDSPC)). The osteoprogenitors employed in these studies were primary human bone marrow stromal cells (hBMSCs), a heterogeneous cell population consisting of cells at various stages of differentiation, and the clonally derived mouse bone marrow stromal D1 cell line.25 BMSCs are useful for clinical applications, but the use of a homogenous cell population may be better suited for examining how ECM cues influence stem cell fate while eliminating effects due to the presence of other progenitor cell types. D1 stem cells are multipotent and have been demonstrated to differentiate toward adipocytes, chondrocytes, and osteoblasts.26–28 D1 cells also express osteocalcin and type I collagen (α1)2α2 messenger RNA (mRNA), as well as high levels of alkaline phosphatase (ALP) and matrix mineralization in vitro.25 We investigated the effects of RGD ligand presentation on primary hBMSC and D1 stem cell osteogenic differentiation in three-dimensional (3D) culture and compared the cell response with that of committed MC3T3-E1 preosteoblasts to determine whether the stage of cell differentiation altered the response to the adhesion ligands.
Materials and Methods
Cell culture
Murine preosteoblast MC3T3-E1 cells, a generous gift from Dr Renny Franceschi (University of Michigan, Ann Arbor MI) were cultured in alpha minimum essential medium (α-MEM) (without ascorbic acid, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS, Invitrogen) and 100 units/mL penicillin-streptomycin (PS, In-vitrogen) at 37°C and 5% carbon dioxide (CO2). A mouse clonally derived BMSC line (D1 stem cells,25 ATCC, Manassas, VA) was cultured in Dulbecco's modified Eagle medium (DMEM; ATCC) supplemented with 10% FBS (ATCC) and 100 units/mL PS.
hBMSCs were isolated from bone marrow aspirate obtained from NDRI (Philadelphia, PA) using Ficoll-Paque (GE Healthcare, Piscataway, NJ) per the manufacturer's instructions. Cells at the low-density interphase were collected, rinsed twice with phosphate buffered saline (PBS), and transferred to tissue culture flasks. hBMSCs were cultured in α-MEM containing 10% FBS and 1% PS. Cells were left unperturbed for 1 week to allow the adherent cell fraction to adhere and proliferate. After 1 week, fresh culture medium was added, and cells were fed every 2 to 3 days thereafter until further passaging. All hBMSCs used in these studies were from the same donor (18-year-old male) and used at passage 5 and below.
For osteogenic characterization of D1 cells, cells were seeded into 12-well tissue culture plates at 5000 cells/cm2 and cultured in serum containing DMEM supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerophosphate for 3 weeks. For osteogenic characterization of hBMSCs, cells were cultured in α-MEM supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 0.1 μM dexamethasone (Sigma, St. Louis, MO). The state of differentiation was analyzed by quantifying ALP activity and osteocalcin secretion and staining for mineral deposition (von Kossa).
For adipogenic characterization of D1 stem cells, cells were cultured in DMEM containing 10% FBS and 1% PS supplemented with 10−7 M dexamethasone. For adipogenic characterization of hBMSCs, cells were cultured in Poietics adipogenic maintenance (containing insulin) and induction (containing insulin, dexamethasone, indomethacin, and 3-isobutyl-1-methyl-xanthine, as described from manufacturer) media for three cycles of induction and maintenance (Lonza Bioscience, Inc., Basel, Switzerland). Cells undergoing adipogenic induction were stained with Oil Red O for lipid vacuoles.
For chondrogenic characterization of D1 stem cells and hBMSCs, cells were pelleted in a 15-mL conical tube and cultured as cell pellets with DMEM containing 10% FBS and 1% PS supplemented with 10 ng/mL transforming growth factorbeta-1 (TGF-β1; R&D Systems, Minneapolis, MN) for 14 days. Cells pellets were harvested, fixed, paraffin embedded, sectioned (5-μm-thick sections), and analyzed for glycosaminoglycans using Safranin O staining.
The DNA content of the samples was determined using the Hoechst 33258 DNA dye assay using calf thymus as a standard. An equal volume (50–100 μL) of Caron's lysis buffer (0.025 M tris hydrochloric acid (HCl), 0.4 M sodium chloride, 0.5% sodium dodecyl sulfate, adjusted to pH 7.4) was added to the cell lysates and sonicated for 4 to 5 seconds before DNA analysis.
Reverse transcription polymerase chain reaction
For reverse transcription polymerase chain reaction (RT-PCR) of D1 cells encapsulated in alginate gels (3D culture), samples were dissolved in 50 mM ethylenediaminetetraacetic acid (EDTA) in PBS for 30 minutes at 4°C to release the encapsulated cells. Upon centrifugation (at 400 g) and aspiration of the EDTA solution, RNA was isolated from samples using a RNeasy mini kit (Qiagen, Valencia, CA). RNA was quantified using the Nanodrop (Nanodrop Technologies, Wilmington, DE) and analyzed using the Bioanalyzer RNA Nano kit (Agilent Technologies, Inc., Santa Clara, CA). One μg of each RNA sample was reverse transcribed to complementary DNA (cDNA) in a 50-μL reaction using the high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA). The PCR reaction performed using the primers for osteocalcin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Invitrogen) and the HotStarTaq Plus PCR kit (Qiagen) using a MJ Tetrad Thermal Cycler (Bio-Rad, Hercules, CA). Cycling conditions were 5 min activation at 95°C, 1 min denaturation at 94°C, 1 min annealing at 60°C, 1 min extension at 72°C for 35 cycles, and a final extension for 10 min at 72°C. Primer sequences (obtained from Yanai et al.29) from 5′ to 3′:
Osteocalcin forward: CAAGTCCCACACAGCAGCTT
Osteocalcin reverse: AAAGCCGAGCTGCCAGAGTT
GAPDH forward: TGAAGGTCGGTGTGAACGGATTTG GC
GAPDH reverse: CATGTAGGCCATGAGGTCCACCAC
Materials preparation
Peptide-modified alginate was prepared using standard carbodiimide chemistry as described previously21 from Ultrapure MVG, molecular weight 270,000 g/mol (Pronova, Oslo, Norway) alginate and linear G4RGDSP or cyclic G4CRGDSPC peptide for certain studies (Peptides International, Inc., Louisville, KY). The overall number or density of RGD peptides covalently conjugated to alginate polymer was varied by altering the concentration of RGD peptide employed in the carbodiimide reaction and the correlation between the number of coupled RGD peptide, and the input concentration has previously determined.21 Although not measured directly, the coupling efficiency of the cyclic peptide would be expected to be similar to that of the linear RGD peptide, because the carbodiimide reaction for peptide conjugation occurs between the carboxy groups on the alginate polymer chain and the terminal amine of the peptides, and the sequence of the two peptides is similar (linear G4RGDSP, cyclic G4CRGDSPC). We have confirmed that the conjugation chemistry maintains the cyclic nature of the cyclic RGD peptide (absence of free thiols) as assessed qualitatively using the 5,5′-dithiobis(2-nitrobenzoate (DTNB) assay. Briefly, DTNB solution was added to tubes containing alginate only, cyclic RGD-modified alginate, cyclic RGD peptide (negative control), and denatured cyclic RGD peptide (exposing free thiols, positive control). No colorimetric change was observed in the cyclic RGD peptide or cyclic RGD peptide–modified alginate condition (data not shown), indicating that the cyclic RGD peptide–modified alginate maintained its cyclic structure.
The number of RGD peptides per alginate chain is termed the degree of substitution (DS), with DS 1 defined as one RGD peptide per alginate polymer chain, etc. Alginates were reconstituted in α-MEM containing 1% PS to make a 2% hydrogel solution. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. Alginate hydrogel disks were ionically cross-linked using calcium sulfate slurry in a 25:1 molar ratio before being cast between glass plates. Disks (10 mm in diameter, 1 mm thick) were made using an arch punch (McMaster-Carr, Atlanta, GA) and maintained in α-MEM with 1% PS. Alginate hydrogel beads (∼ 1 mm diameter) were formed using ionic cross-linking of alginate solution in a 0.1-m calcium chloride bath.17
Cell proliferation on alginate
Cells were seeded onto alginate disks at a seeding density of 10,000 cells/cm2 and allowed to adhere for 5h. Disks were then transferred to new plates, and fresh medium was added. At 5 h and days 1, 2, and 4, disks were transferred to 15-mL tubes containing 1 mL of trypsin (Invitrogen) and incubated for 5 min (37°C), and 50 mM EDTA/PBS (pH 7.4) was added to each tube to dissolve the disks. Cell counts from the solution were obtained using a Z2 Coulter Counter (Beckman Coulter, Fullerton, CA). Quadruplicate samples were assayed for each condition. Growth rates were calculated from cell counts comparing cell numbers at day 5 with cell numbers 5 h postseeding.
3D differentiation studies
Cells were encapsulated in 2% peptide-modified alginate hydrogels at a concentration of 20 × 106 cells/mL alginate (D1 cells) or 15×106 cells/mL alginate (primary hBMSCs) using uniform mixing. The cell-alginate solution was formed into disks (as previously described) for D1 cells and beads (approximately 1 mm in diameter)30 for hBMSCs. Alginate beads were cultured in 100-mL spinner flasks, and alginate disks were cultured in a 24-well tissue culture plate (one well per disk) on an orbital shaker (operating at approximately 84rpm) at 37°C and 5% CO2. DMEM was changed every 2 days and supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerophosphate (Sigma) with or without 10 ng/mL BMP-2 for D1 cells or α-MEM (Invitrogen) supplemented with 50 μg/mL ascorbic acid, 10 mm β-glycerophosphate, and 0.1 μM dexamethasone (Sigma) for hBMSCs.
At each time-point, 1 mL of fresh medium (without supplements) was added to each sample, and the plate was placed on an orbital shaker (operating at approximately 84 rpm) overnight at 37°C. Medium was collected and frozen for osteocalcin analysis. Cells were lysed in passive lysis buffer (Promega, Madison, WI), and lysates were frozen for ALP and DNA analysis. Samples were dissolved in 50 mM EDTA in PBS for 30 min at 4°C to release the encapsulated cells. Upon centrifugation (at 400 g) and aspiration of the EDTA solution, the cell pellet was rinsed once with PBS and then lysed with 150 μL passive lysis buffer (Promega). Cell lysates were then frozen and used for ALP and DNA analysis.
Osteogenic assays
ALP activity was quantified using a method based on Lowry et al.31 Cell lysates were collected in passive lysis buffer (Promega), and a portion was assayed with 50 mM p-nitrophenyl phosphate in 100 mM glycine and 1 mM magnesium chloride at 37°C. The absorbance of the p-nitrophenol product from the reaction was read at 405 nm on a microplate reader (BioTek Synergy HT, Winooski, VT). The enzyme activity (expressed in of nmol of p-nitrophenol/min) was calculated based on serially diluted p-nitrophenol standards and normalized to total DNA content.
Osteocalcin synthesis was assayed using a mouse osteocalcin enzyme-linked immunosorbent assay kit, per the manufacturer's instructions (Biomedical Technologies, Inc., Stoughton, MA). The calcium content of cell lysates was assayed by dissolving cultures with 1 M HCl and analyzing them using a calcium kit (Wako Pure Chemical, Osaka, Japan) according to the manufacturer's instructions.
For von Kossa staining, cell layers were fixed in 70% ethanol, and a 5% silver nitrate solution was applied to the cell layer and exposed to light for 15min. Unreacted silver nitrate was removed by addition of 5% sodium thiosulfate. Cell layers were rinsed several times in PBS before obtaining images with an Olympus IX81 inverted microscope (Melville, NY).
Statistical analysis
Statistical significance of data was assessed using one-way analysis of variance with respect to conditions for the respective experiment (varying culture medium supplements or RGD ligand presentation), followed by a post hoc comparison using the Tukey test.
Results
Stem cell characterization
The multipotential differentiation capabilities of primary hBMSCs and the clonally derived D1 cell line toward adipogenic, chondrogenic, and osteogenic lineages have been previously described.32,33 In these studies, the adipogenic, chondrogenic, and osteogenic differentiation of hBMSCs and D1 cells was first assessed to confirm that the cells used in our experiments maintained their multipotential capability. D1 cells cultured in adipogenic induction conditions for 3 weeks stained positively for Oil Red O, indicating the presence of lipid vacuoles (Fig. 1A). Under chondrogenic induction conditions (micromass pellet culture for 2 weeks in medium supplemented with 10 ng/mL TGF-β1), both cell types stained positively with Safranin-O (Fig. 1B), indicating the presence of glycosaminoglycans typical of a cartilage matrix. hBMSCs and D1 cells also stained positively for von Kossa under osteogenic induction conditions (serum containing medium supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerophosphate, with the addition of 0.1 μM dexamethasone for hBMSCs), indicative of matrix mineralization (Fig. 1C). Thus, the hBMSCs and D1 cells used in these studies are both multipotential cell populations capable of forming cartilage, fat, and bone.
FIG. 1.

Multipotential differentiation of primary human bone marrow stromal cells (hBMSCs) (first row) and D1 stem cells (second row). Cells were cultured in adipogenic (A), chondrogenic (B), and osteogenic (C) conditions to assess the capability of these multipotential cells to exhibit markers typical of adipocytes, chondrocytes, and osteoblasts, respectively. For adipogenic conditions, cells were cultured for 21 days and stained with Oil Red O for lipid vacuoles (red). hBMSCs were counterstained with hematoxylin (blue), whereas D1 cells (A, 2nd row) were not. For chondrogenic conditions, cell pellets were cultured for 14 days in medium supplemented with 10 ng/mL transforming growth factor beta-1. Cell pellets were fixed, sectioned, and stained with Safranin-O for detection of glycosaminoglycans. To assess osteogenic differentiation, cells were cultured in complete medium supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerophosphate and with (hBMSC) or without (D1 cells) 0.1 μM dexamethasone and subjected to von Kossa staining to highlight mineral formation (day 21).
After defining culture conditions for the osteogenic differentiation of D1 cells and hBMSCs, DNA content and ALP and osteocalcin expression were quantified in the same, standard 2D culture system (tissue culture plastic). Osteogenic supplements (50 μg/mL ascorbic acid and 10 mM β-glycerophosphate) enhanced the proliferation (Fig. 2A), ALP activity (Fig. 2B), and osteocalcin synthesis (Fig. 2C) of murine D1 cells. ALP activity of preosteoblasts, osteoblasts, and BMSCs typically increases between 7 and 14 days of culture during active matrix synthesis; then ALP activity decreases as osteocalcin levels increase and matrix calcification occurs.34–36 As shown in Figure 2B, ALP levels for the osteogenic supplement condition peaked at day 7 and then decreased, indicating matrix synthesis earlier than in the medium-only condition. Osteogenic supplements promoted matrix synthesis as indicated by the ALP activity in the medium-only condition not peaking until much later. Serum-containing α-MEM with osteogenic supplements (50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 0.1 μM dexamethasone) also enhanced proliferation (Fig. 3A), ALP activity (Fig. 3B), and osteocalcin synthesis (Fig. 3C) of hBMSCs. ALP activity is similar to reported values of hBMSCs under osteogenic culture conditions.34 These results all together indicate that D1 cells and hBMSCs undergo osteogenic differentiation and express osteogenic markers typical of osteoblasts when cultured under standard 2D conditions (tissue culture plastic).
FIG. 2.
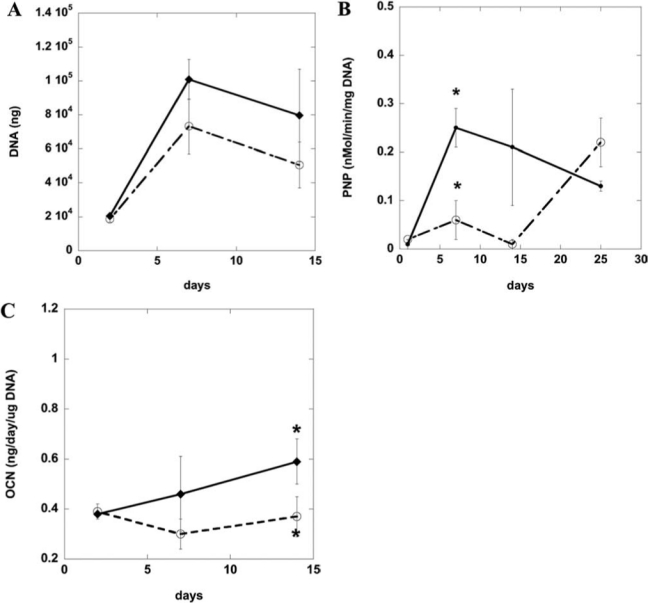
Osteogenic characterization of D1 stem cells on tissue culture plastic. DNA content (A), alkaline phosphatase activity (B), and osteocalcin (C) of D1 cell lysates over time when cultured in tissue culture plastic in Dulbecco's modified Eagle medium alone (open circle) or with osteogenic supplements (solid circle) (50 μg/mL ascorbic acid, 10 mM β-glycerophosphate). *p < 0.05 between noted conditions (medium vs osteogenic supplements) at the same time-point.
FIG. 3.
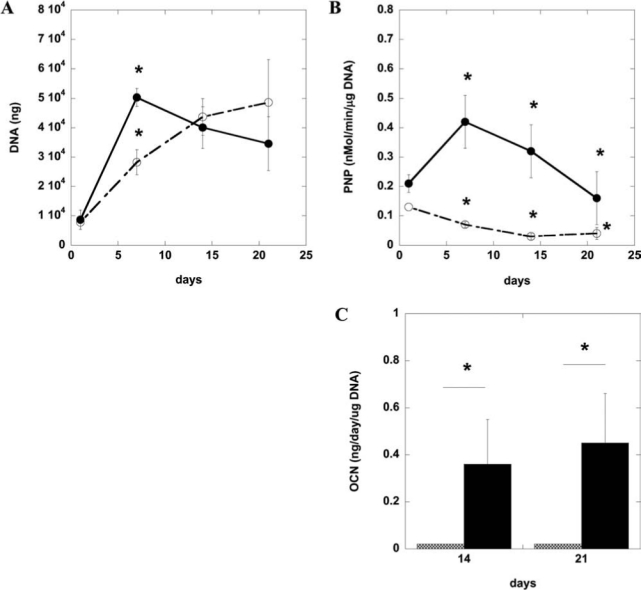
Osteogenic characterization of primary human bone marrow stromal cells (hBMSCs) on tissue culture plastic. DNA content (A), alkaline phosphatase activity (B), and osteocalcin (C) of primary hBMSC lysates over time when cultured in tissue culture plastic in alpha minimum essential medium alone (open circle, hatched bar) or medium with osteogenic supplements (solid circle, filled bar) (50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 0.1μm dexamethasone). Values represent means and standard deviations (n = 4). *p < 0.05 between noted conditions (medium vs osteogenic supplements) at the same time-point.
3D differentiation of hBMSCs and D1 cells
To determine how RGD ligand presentation influenced the differentiation of progenitor cells, D1 cells and hBMSCs were encapsulated in alginate hydrogels modified with linear RGD (degree of substitution [DS] 1, defined as one RGD peptide per alginate polymer chain or overall linear RGD density of 6.25 mg/g alginate) and cultured for 3 weeks under dynamic culture conditions (spinner flask or orbital shaker [84 rpm]) in medium-containing osteogenic supplements. Osteocalcin was the primary marker examined to assess stem cell osteogenic differentiation because it is a late-stage, bone-specific marker and is widely used to assess osteogenic differentiation of stem cells. It has been previously demonstrated that RGD-modified alginate conditions that promoted osteoblast differentiation in vitro (as assessed according to osteocalcin secretion) also correlated with enhanced bone tissue formation.17
Although MC3T3 preosteoblasts readily differentiated toward osteoblasts, as assessed according to osteocalcin secretion (Fig. 4A), D1 stem cells produced minimal osteocalcin protein (Fig. 4B) under these conditions. Doubling the ligand density from DS 1toDS 2 (overall linear RGD density of 12.5 mg/g alginate) did not promote osteocalcin synthesis in D1 cells either (data not shown). Because no osteocalcin protein secretion was detected in D1 cells encapsulated in unmodified alginate and DS 2 linear RGD-modified alginate (Fig. 4B), osteocalcin gene expression of the encapsulated D1 cells was investigated. DS 2 linear RGD–modified alginate slightly up-regulated osteocalcin mRNA of the encapsulated D1 stem cells (Fig. 4C) but was insufficient to drive significant differentiation (e.g., high levels of osteocalcin protein synthesis). Similarly, primary hBMSCs encapsulated in DS 2 linear RGD alginate gels also failed to undergo osteogenic differentiation as assessed according to osteocalcin protein secretion (data not shown); osteocalcin mRNA was not evaluated. All together, these results indicated that matrix cues that successfully induced differentiation of committed preosteoblasts did not drive differentiation of the more-primitive stem cells.
FIG. 4.
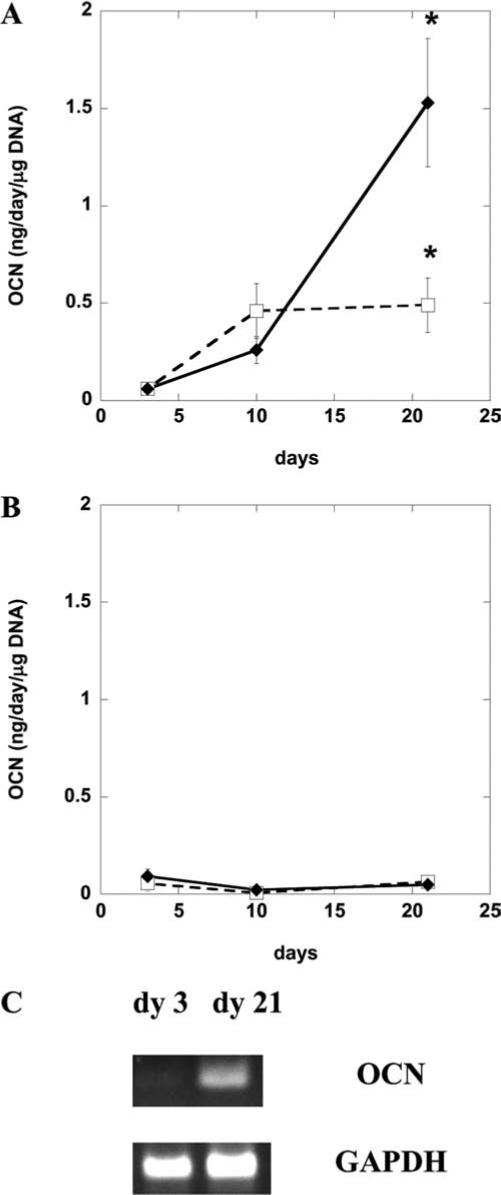
Differentiation of MC3T3 (A) and D1 cells (B) in three-dimensional culture. Cells were encapsulated in linear arginine-glycine-aspartate (RGD)-modified alginate (diamond) (degree of substitution [DS] 1, equivalent to 1 mole of RGD per mole of alginate and total linear RGD density of 6.25 mg RGD/g alginate) or unmodified alginate (open square). (A) MC3T3 osteocalcin secretion (normalized to DNA) assessed at days 3, 10, and 21 of culture. (B) D1 osteocalcin secretion and osteocalcin messenger RNA levels (C) at days 3, 10, and 21 of culture. Cells were cultured at 37°C and 5% carbon dioxide for 21 days, and medium was supplemented with 50 μg/mL ascorbic acid only for osteogenic induction of MC3T3s and 50μg/mL ascorbic acid and 10mM β-glycerophosphate for D1 cells. Values represent means and standard deviations (n = 4). *p < 0.05 between noted conditions (unmodified vs DS 2 linear RGD) at the same time-point.
We hypothesized that the presentation of adhesion ligand sequences with greater affinity may promote the proliferation and differentiation of osteoprogenitors, because the modification of materials with cyclic RGD peptides has been reported to stimulate osteoblast adhesion, proliferation, and bone formation.37,38 Although DS 2 cyclic RGD–modified alginate substrates did not significantly enhance the adhesion, spreading, or proliferation of D1 stem cells in 2D culture, the hBMSC growth rate was almost three times as high on DS 2 cyclic RGD– as on DS 2 linear RGD–presenting substrates in this condition (Fig. 5A). Furthermore, the higher-affinity ligand sequence also increased the cell spreading area of primary hBMSCs 5 h postseeding (data not shown).
FIG. 5.
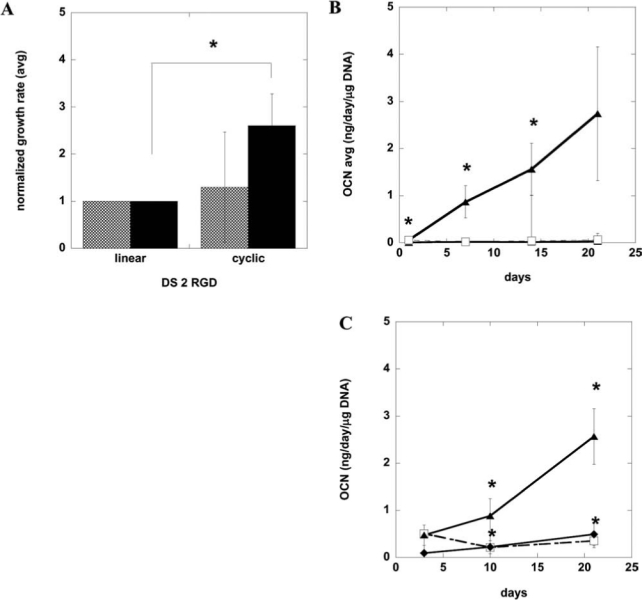
Proliferation and differentiation of human bone marrow stromal cells (hBMSCs) and D1 stem cells in three-dimensional culture using cyclic versus linear arginine-glycine-aspartate (RGD) peptides. (A) Normalized growth rates of D1 cells (cross-hatch) and primary hBMSCs (filled) cultured on DS 2 linear or cyclic RGD–presenting alginate substrates. Growth rates were calculated from cell counts 5 days postseeding and normalized by dividing the growth rate for each cell type on cyclic RGD by the growth rate for the same cell type cultured on gels with the same density of linear RGD. Osteocalcin protein secretion of D1 (B) and primary hBMSCs (C) encapsulated in DS 2 cyclic RGD–modified alginate (triangle), DS 2 linear RGD–modified alginate (diamond), or unmodified alginate (open square). D1 cells were cultured in medium supplemented with 50 μg/mL ascorbic acid and 10 mM β-glycerophosphate for osteogenic induction at 37°C and 5% carbon dioxide for 21 days. Primary hBMSCs were cultured in medium supplemented with 50 μg/mL ascorbic acid, 10 mM β-glycerophosphate, and 0.1μm dexamethasone. Values represent means and standard deviations (n = 3 or 4). *p < 0.05 between noted conditions.
To determine whether a higher-affinity ligand sequence would promote differentiation of stem cells in 3D culture, D1 cells and hBMSCs were encapsulated in cyclic RGD–presenting alginate matrices in the presence of osteogenic supplements. DS 2 cyclic RGD–modified alginate significantly up-regulated the osteocalcin synthesis in D1 stem cells (Fig. 5B) and primary hBMSCs (Fig. 5C), whereas an alginate gel presenting the same molar ratio of the linear RGD ligand did not up-regulate osteocalcin secretion (Fig. 4B [D1 cells], hBMSC osteocalcin protein data not shown). Encapsulated D1 cells and hBMSCs were viable throughout the culture period, as assessed according to the quantitative tetrazolium compound assay (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS), inner salt; Cell-Titer 96 Aqueous Nonradioactive Cell Proliferation Assay, Promega, Madison, WI) or 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) bromide staining, respectively. Linear and cyclic RGD–modified alginates enhanced cell viability over unmodified alginate gels for both cell types (data not shown).
Discussion
It is widely reported that hBMSCs undergo differentiation toward multiple lineages,32,39 and the multipotential capability of the primary hBMSCs used in these studies and a clonally derived murine bone marrow cell line (D1 cells) was confirmed by culturing these cells under conditions that induced differentiation toward bone, fat, and cartilage, because the cells stained positive for markers typical of osteoblasts, adipocytes, and chondrocytes. Supplements that induced osteogenic differentiation of both cell types included ascorbic acid and β-glycerophosphate, which are essential for matrix synthesis.40 Although dexamethasone enhanced osteogenic differentiation and matrix mineralization of hBMSCs, it inhibited differentiation and matrix mineralization of murine D1 cells (data not shown). Dexamethasone has also been reported to decrease mineralization and osteocalcin mRNA in MC3T3-E1 preosteoblasts.41 Differences in the species of origin and stage of differentiation influence cell response to signaling molecules42 and may have contributed to the observed differences in hBMSC and D1 cell response to dexamethasone and BMP-2.
Alkaline phosphatase and osteocalcin secretion from hBMSCs and D1 stem cells under osteogenic induction conditions in tissue culture were similar in magnitude to those reported for MC3T3 preosteoblasts in standard 2D culture35,36 but not in 3D culture in alginate hydrogels that presented linear peptides. Osteocalcin secretion was negligible in osteoprogenitors maintained in 3D culture, whereas osteocalcin synthesis in MC3T3 preostoblasts remained high in similar culture conditions. RGD-modified alginate (DS 2 linear) up-regulated osteocalcin mRNA levels in D1 stem cells but were insufficient to induce significant osteocalcin secretion.
Progenitor cells encapsulated within gels presenting a higher-affinity RGD sequence (cyclic RGD instead of linear RGD peptide) promoted differentiation of D1 stem cells and primary hBMSCs. Peak osteocalcin values for both cell types were approximately 2 ng/day per μg of DNA, similar to those reported for MC3T3 preosteoblasts and primary murine osteoblasts.35,36 Furthermore, osteogenic differentiation of stem cells within DS 2 cyclic RGD alginate did not require growth factors, such as BMP-2. However, it is possible that additional supplementation with BMP-2 or other growth factors may improve differentiation of progenitor cells, especially primary hBMSCs, under these conditions. It is likely that the observed difference in cell response to the different peptide sequences is due to higher integrin binding affinity to the cyclic RGD sequence, preferential engagement of specific integrin receptors, or both.43,44 Greater bond formation has been demonstrated to enhance osteogenic differentiation of preosteoblasts encapsulated in RGD-presenting alginate gels and, similarly, to drive differentiation of osteoprogenitors.45 Cyclic RGD peptides are also highly selective toward the αv integrin, which regulates matrix responsiveness by activating the osteoblast-specific transcription factor Core binding factor alpha-1/runt related transcription factor 2 during osteogenesis.46 It is not expected that the differences in cell response between linear and cyclic RGD peptide-modified alginate is due to differences in coupling efficiency of the peptide to the alginate polymer. Peptide conjugation of the linear and cyclic RGD peptide to alginate polymer was prepared using carbodiimide chemistry, in which the reaction occurs between the carboxy groups on the alginate polymer chain and the terminal amine of the peptides. Because the sequence of the two peptides is similar (linear G4RGDSP, cyclic G4CRGDSPC), it is not anticipated that the coupling efficiencies of the two peptides are significantly different. We have also confirmed that the conjugation chemistry maintains the cyclic nature of the cyclic RGD peptide (absence of free thiols) as assessed according to the DTNB assay (data not shown).
Cyclic peptides, developed using cyclization of RGD peptides by disulfide bonds, thioether, or rigid aromatic rings, are more resistant to proteolysis47 and may also promote a more stable cell-ligand bond. Enhanced integrin–adhesion ligand binding may then promote matrix-mediated osteogenic differentiation of hBMSCs via the extracellular signal-regulated kinase and focal adhesion kinase signaling pathways.48 In addition to RGD ligands, it is possible that other adhesion ligand sequences (e.g., the synergy proline-histidine-serine-arginine-asparagine (PHSRN) peptide) would enhance osteoprogenitor cell differentiation in the model sECM used in these studies. Because the PHSRN sequence may confer integrin binding specificity49, presentation of the PHRSN with RGD ligands may enhance α5 receptor binding and therefore stem cell differentiation.50–52
Linear RGD densities that promoted osteogenic differentiation of committed cells (MC3T3-E1 preosteoblasts) did not induce differentiation of hBMSCs or D1 stem cells. However, matrices presenting the higher-affinity cyclic form of this adhesion ligand enhanced osteoprogenitor differentiation in three dimensions. These studies indicate that the presentation of appropriate matrix cues are critical for proliferation and differentiation of progenitor cell populations and that bio-material design parameters optimized for differentiated cell types may not directly translate to stem cell populations. The results of this study suggest that less-committed cells may require more instruction than preosteoblasts, and this is consistent with the previous findings that primary rat calvarial osteoblasts transplanted within RGD-modified alginate matrix promoted bone regeneration in vivo, whereas hBMSCs transplanted within the same matrix did not unless provided with additional cues.16,17 In future studies, it would be interesting to further investigate whether the presentation of cyclic RGD peptides similarly enhance the differentiation of hBMSCs toward chondrocytes, adipocytes, or other differentiated cell types. It is likely that design of sECMs tailored to promote stem cell differentiation may enhance tissue regeneration by transplanted cells and may even reduce the need for exogenous growth factors.
Acknowledgments
This work was supported by the U.S. Army Research Laboratory and the U.S. Army Research Office Grant DAAD190310168 and the National Institutes of Health (R37 DE013033).
References
- 1.Holland T.A. Mikos A.G. Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Eng Biotechnol. 2006;102:161. doi: 10.1007/b137205. [DOI] [PubMed] [Google Scholar]
- 2.Hutmacher D.W. Schantz J.T. Lam C.X. Tan K.C. Lim T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J Tissue Eng Regen Med. 2007;1:245. doi: 10.1002/term.24. [DOI] [PubMed] [Google Scholar]
- 3.Betz V.M. Betz O.B. Harris M.B. Vrahas M.S. Evans C.H. Bone tissue engineering and repair by gene therapy. Front Biosci. 2008;13:833. doi: 10.2741/2724. [DOI] [PubMed] [Google Scholar]
- 4.Stevens B. Yang Y. Mohandas A. Stucker B. Nguyen K.T. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater. 2008;85:573. doi: 10.1002/jbm.b.30962. [DOI] [PubMed] [Google Scholar]
- 5.Sokolsky-Papkov M. Agashi K. Olaye A. Shakesheff K. Domb A.J. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59:187. doi: 10.1016/j.addr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Tsutsumi S. Shimazu A. Miyazaki K. Pan H. Koike C. Yoshida E. Takagishi K. Kato Y. Retention of multi-lineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288:413. doi: 10.1006/bbrc.2001.5777. [DOI] [PubMed] [Google Scholar]
- 7.Caplan A.I. Bruder S.P. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259. doi: 10.1016/s1471-4914(01)02016-0. [DOI] [PubMed] [Google Scholar]
- 8.Results of 2007 “stem cells” query in database. Clinicaltrials.gov. Clinicaltrials.gov
- 9.Sittinger M. Hutmacher D.W. Risbud M.V. Current strategies for cell delivery in cartilage and bone regeneration. Curr Opin Biotechnol. 2004;15:411. doi: 10.1016/j.copbio.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald K.K. Cheung C.Y. Anseth K.S. Cellular delivery of TGFbeta1 promotes osteoinductive signalling for bone regeneration. J Tissue Eng Regen Med. 2007;1:314. doi: 10.1002/term.31. [DOI] [PubMed] [Google Scholar]
- 11.Torigoe I. Sotome S. Tsuchiya A. Yoshii T. Takahashi M. Kawabata S. Shinomiya K. Novel cell seeding system into a porous scaffold using a modified low-pressure method to enhance cell seeding efficiency and bone formation. Cell Transplant. 2007;16:729. doi: 10.3727/000000007783465109. [DOI] [PubMed] [Google Scholar]
- 12.Fox J.M. Chamberlain G. Ashton B.A. Middleton J. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 13.Saito N. Okada T. Horiuchi H. Murakami N. Takahashi J. Nawata M. Ota H. Nozaki K. Takaoka K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat Biotechnol. 2001;19:332. doi: 10.1038/86715. [DOI] [PubMed] [Google Scholar]
- 14.Karageorgiou V. Meinel L. Hofmann S. Malhotra A. Volloch V. Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res A. 2004;71:528. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 15.Schmoekel H. Schense J.C. Weber F.E. Gratz K.W. Gnagi D. Muller R. Hubbell J.A. Bone healing in the rat and dog with nonglycosylated BMP-2 demonstrating low solubility in fibrin matrices. J Orthop Res. 2004;22:376. doi: 10.1016/S0736-0266(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 16.Simmons C.A. Alsberg E. Hsiong S. Kim W.J. Mooney D.J. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Alsberg E. Anderson K.W. Albeiruti A. Franceschi R.T. Mooney D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 18.Lee K.Y. Mooney D.J. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 19.Smidsrod O. Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 20.Atala A. Kim W. Paige K.T. Vacanti C.A. Retik A.B. Endoscopic treatment of vesicoureteral reflux with a chondrocyte-alginate suspension. J Urol. 1994;152:641. doi: 10.1016/s0022-5347(17)32671-x. [DOI] [PubMed] [Google Scholar]
- 21.Rowley J.A. Madlambayan G. Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Bio-materials. 1999;20:45. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro H.S. Chen J. Wrana J.L. Zhang Q. Blum M. Sodek J. Characterization of porcine bone sialoprotein: primary structure and cellular expression. Matrix. 1993;13:431. doi: 10.1016/s0934-8832(11)80109-5. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai H. Tajima M. Ueno Y. Giga-Hama Y. Ohba M. Effect of cyclic RGD peptide on cell adhesion and tumor metastasis. Biochem Biophys Res Commun. 1991;177:74. doi: 10.1016/0006-291x(91)91950-h. [DOI] [PubMed] [Google Scholar]
- 24.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin alphavbeta3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 25.Diduch D.R. Coe M.R. Joyner C. Owen M.E. Balian G. Two cell lines from bone marrow that differ in terms of collagen synthesis, osteogenic characteristics, and matrix mineralization. J Bone Joint Surg Am. 1993;75:92. doi: 10.2106/00004623-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Li X. Cui Q. Kao C. Wang G.J. Balian G. Lovastatin inhibits adipogenic and stimulates osteogenic differentiation by suppressing PPARgamma2 and increasing Cbfa1/Runx2 expression in bone marrow mesenchymal cell cultures. Bone. 2003;33:652. doi: 10.1016/s8756-3282(03)00239-4. [DOI] [PubMed] [Google Scholar]
- 27.Li X. Jin L. Cui Q. Wang G.J. Balian G. Steroid effects on osteogenesis through mesenchymal cell gene expression. Osteoporos Int. 2005;16:101. doi: 10.1007/s00198-004-1649-7. [DOI] [PubMed] [Google Scholar]
- 28.Devine M.J. Mierisch C.M. Jang E. Anderson P.C. Balian G. Transplanted bone marrow cells localize to fracture callus in a mouse model. J Orthop Res. 2002;20:1232. doi: 10.1016/S0736-0266(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 29.Yanai T. Katagiri T. Akiyama S. Imada M. Yamashita T. Chiba H. Takahashi N. Suda T. Expression of mouse osteocalcin transcripts, OG1 and OG2, is differently regulated in bone tissues and osteoblast cultures. J Bone Miner Metab. 2001;19:345. doi: 10.1007/s007740170003. [DOI] [PubMed] [Google Scholar]
- 30.Guo J.F. Jourdian G.W. MacCallum D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 31.Lowry O.H. Roberts N.R. Wu M.L. Hixon W.S. Crawford E.J. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954;207:19. [PubMed] [Google Scholar]
- 32.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 33.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 34.Cheng S.L. Yang J.W. Rifas L. Zhang S.F. Avioli L.V. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology. 1994;134:277. doi: 10.1210/endo.134.1.8275945. [DOI] [PubMed] [Google Scholar]
- 35.Asahina I. Sampath T.K. Hauschka P.V. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- 36.Carpenter T.O. Moltz K.C. Ellis B. Andreoli M. McCarthy T.L. Centrella M. Bryan D. Gundberg C.M. Osteocalcin production in primary osteoblast cultures derived from normal and Hyp mice. Endocrinology. 1998;139:35. doi: 10.1210/endo.139.1.5677. [DOI] [PubMed] [Google Scholar]
- 37.Kantlehner M. Schaffner P. Finsinger D. Meyer J. Jonczyk A. Diefenbach B. Nies B. Holzemann G. Goodman S.L. Kessler H. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1:107. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Lieb E. Hacker M. Tessmar J. Kunz-Schughart L.A. Fiedler J. Dahmen C. Hersel U. Kessler H. Schulz M.B. Gopferich A. Mediating specific cell adhesion to low-adhesive diblock copolymers by instant modification with cyclic RGD peptides. Biomaterials. 2005;26:2333. doi: 10.1016/j.biomaterials.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Krebsbach P.H. Kuznetsov S.A. Bianco P. Robey P.G. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10:165. doi: 10.1177/10454411990100020401. [DOI] [PubMed] [Google Scholar]
- 40.Xiao G. Cui Y. Ducy P. Karsenty G. Franceschi R.T. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 41.Luppen C.A. Smith E. Spevak L. Boskey A.L. Frenkel B. Bone morphogenetic protein-2 restores mineralization in glucocorticoid-inhibited MC3T3-E1 osteoblast cultures. J Bone Miner Res. 2003;18:1186. doi: 10.1359/jbmr.2003.18.7.1186. [DOI] [PubMed] [Google Scholar]
- 42.Lian J.B. Shalhoub V. Aslam F. Frenkel B. Green J. Hamrah M. Stein G.S. Stein J.L. Species-specific glucocorticoid and 1,25-dihydroxyvitamin D responsiveness in mouse MC3T3-E1 osteoblasts: dexamethasone inhibits osteoblast differentiation and vitamin D down-regulates osteocalcin gene expression. Endocrinology. 1997;138:2117. doi: 10.1210/endo.138.5.5117. [DOI] [PubMed] [Google Scholar]
- 43.Pfaff M. Tangemann K. Muller B. Gurrath M. Muller G. Kessler H. Timpl R. Engel J. Selective recognition of cyclic RGD peptides of NMR defined conformation by alpha IIb beta 3, alpha V beta 3, and alpha 5 beta 1 integrins. J Biol Chem. 1994;269:20233. [PubMed] [Google Scholar]
- 44.Pierschbacher M.D. Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984;81:5985. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong H.J. Boontheekul T. Mooney D.J. Quantifying the relation between adhesion ligand-receptor bond formation and cell phenotype. Proc Natl Acad Sci U S A. 2006;103:18534. doi: 10.1073/pnas.0605960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider G.B. Zaharias R. Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80:1540. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 47.Bogdanowich-Knipp S.J. Chakrabarti S. Williams T.D. Dillman R.K. Siahaan T.J. Solution stability of linear vs. cyclic RGD peptides. J Pept Res. 1999;53:530. doi: 10.1034/j.1399-3011.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 48.Salasznyk R.M. Klees R.F. Williams W.A. Boskey A. Plopper G.E. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aota S. Nomizu M. Yamada K.M. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756. [PubMed] [Google Scholar]
- 50.Pistone M. Sanguineti C. Federici A. Sanguineti F. Defilippi P. Santolini F. Querze G. Marchisio P.C. Manduca P. Integrin synthesis and utilization in cultured human osteoblasts. Cell Biol Int. 1996;20:471. doi: 10.1006/cbir.1996.0062. [DOI] [PubMed] [Google Scholar]
- 51.Bennett J.H. Carter D.H. Alavi A.L. Beresford J.N. Walsh S. Patterns of integrin expression in a human mandibular explant model of osteoblast differentiation. Arch Oral Biol. 2001;46:229. doi: 10.1016/s0003-9969(00)00114-x. [DOI] [PubMed] [Google Scholar]
- 52.Petrie T.A. Capadona J.R. Reyes C.D. Garcia A.J. Integrin specificity and enhanced cellular activities associated with surfaces presenting a recombinant fibronectin fragment compared to RGD supports. Biomaterials. 2006;27:5459. doi: 10.1016/j.biomaterials.2006.06.027. [DOI] [PubMed] [Google Scholar]


