Abstract
Calcific aortic stenosis is the most common indication for surgical valve replacement in the United States. For years this disease has been described as a passive degenerative process during which serum calcium attaches to the valve surface and binds to the leaflet to form nodules. Therefore, surgical treatment of this disease has been the approach towards relieving outflow obstruction in these patients. Recent studies demonstrate an association between atherosclerosis and its risk factors for aortic valve disease. In 2008, there are increasing number of epidemiology and experimental studies to provide evidence that this disease process is not a passive phenomena. There is an active cellular process that develops within the valve leaflet and causes a regulated bone formation to develop. If the atherosclerotic hypothesis is important in the initiation of aortic stenosis than treatments used in slowing the progression of atherosclerosis may be effective in patients with aortic valve disease. This review will review the pathogenesis and the potential for medical therapy in the management of patients with calcific aortic stenosis by examining the lessons provided from the experimental research.
Keywords: Valvular Heart Disease, Lipids, Pathophysiology Atherosclerosis, Experimental Models
Introduction
Calcific aortic stenosis is the most common indication for surgical valve replacement in the United States1. With the decline of acute rheumatic fever, calcific aortic stenosis has become the most common indication for valvular disorder in the US. Landmark epidemiologic studies identified risk factors for the aortic valve which are similar to those of vascular atherosclerosis, such as smoking, male gender, hypertension, elevated cholesterol levels, and renal failure2–4. For years this disease process was thought to be due to build-up of nodules along the valve surface to induce a mechanical stenosis in the valve5. Furthermore, surgical therapy for severe symptomatic aortic stenosis is the only treatment option in 2008 as defined in a landmark study from 19685. This study also defined the classic triad of symptoms which include chest pain, shortness of breath and lightheadedness. Finally, this research demonstrated that the life expectancy of this patient population is reduced significantly, if they do not have surgical valve replacement at the onset of symptoms5. Currently, it is a Class I indication for surgical valve replacement according to the American Heart Association and American College of Cardiology guidelines for valvular heart disease6. Over the past decade, there are a growing number of studies evaluating human disease tissues to define the cellular pathways important in this calcific aortic stenosis. This review will bring together the basic science and clinical science to develop a unified approach towards treating this disease.
Aortic Valve Calcification
The presence of calcification in the aortic valve is responsible for valve stenosis. Recent descriptive studies from patient specimens have demonstrated the hallmark features of aortic valve disease: including early atherosclerosis, cell proliferation and osteoblast expression7–10. To understand aortic valve disease, three interrelated events responsible for the development of valve calcification to consider: 1) classical cardiovascular risk factors, 2) genetic factors and 3) valve biology. The interrelationship of these events results in the final common pathway of this disease: a calcifying osteoblast phenotype. The evidence for these three pathways leading to the development of human aortic valve calcification can be found in the experimental and clinical studies outlined in this review.
Traditional Cardiovascular Atherosclerotic Risk Factors
In the past decade, landmark studies2, 3, has described the risk factors for calcific aortic stenosis as identified by large epidemiologic cohort studies which include lipids, hypertension, male gender, renal failure, and diabetes. Many population science papers have subsequently confirmed these findings2, 11, 12,13. These studies have implicated the traditional risk factors for cardiovascular atherosclerosis important in the development of calcific aortic stenosis. The role of lipids as a risk factor for vascular atherosclerosis has been defined in the literature for years. Atherosclerosis is a complex multifactorial process which produces a lesion composed of lipids14, 15, macrophages16, and proliferating smooth muscle cells17 and apoptosis18 which is regulated by endothelial nitric oxide synthase19–23 which over time causes occlusion of the vessel diameter. The understanding of these clinical risk factors are providing the foundation for the cellular studies and the potential for targeted medical therapies for this disease similar to vascular atherosclerosis.
Surgical pathological studies have demonstrated the presence of LDL and atherosclerosis in calcified valves, suggesting a common cellular mechanism24, 25. Furthermore, patients who have the diagnosis of familial hypercholesterolemia develop aggressive peripheral vascular disease, coronary artery disease, as well as aortic valve lesions which calcify with age. The first descriptions of atherosclerotic aortic valve disease have been in patients with Familial Hypercholesterolemia(FH) who have an early atherosclerotic lesion along the aortic surface of the valve leaflet26–28,29. The discovery of atherosclerosis in the aortic valve in the FH patient population provides the initial proof of principal for the potential treatment of lipids to slow the progression of aortic valve disease.
Experimental Models of Valvular Heart Disease
If atherosclerotic risk factors are important in the development of valvular heart disease, than experimental models of atherosclerosis are important in the understanding this disease process. Studies in mice and rabbits have confirmed that experimental hypercholesterolemia causes both atherosclerosis and calcification in the aortic valves10, 30–34. The experimental hypercholesterolemia diet has been used for over one hundred years for to evaluate the mechanisms of vascular disease. Table 1 demonstrates the in vivo rabbit and mouse models of valvular heart disease. The first study to describe early endothelial abnormalities in the aortic valves was in experimental hypercholesterolemia rabbits35–38. This hypothesis has been further developed in the rabbit model to test for multiple markers of the atherosclerotic process within the valve which are critical steps towards the development of valvular calcification process. The initial disease markers described in valve atherosclerosis include cell proliferation and apoptosis30. The early valve lesion of aortic valve sclerosis was also shown in a rabbit model of hypercholesterolemia39. This model was extended using atorvastatin to modify gene markers to define the bone and atherosclerotic pathways and to demonstrate attenuation with a statin10. Atorvastatin attenuated the early gene markers of bone formation and macrophage infiltration along the aortic surface of the experimental hypercholesterolemia aortic valve10. The rabbit model of cholesterol with and without atorvastatin was extended to three and six months duration, to determine the timing of calcification in the valves. Three months of cholesterol induced the early mineralization and eNOS modification in the valve as shown by MicroCT and standard eNOS assays21.
Table 1. Experimental Animal Models of Valvular Heart Disease.
List of Experimental Models of Aortic Valve Disease.
| Author | Year | Model | Diet | Medical Therapy | Experimental Results |
|---|---|---|---|---|---|
| Rabbit | |||||
| Sarphie(38) | 1985 | New Zealand White Rabbit | Cholesterol | None | Endothelial structral changes along aortic surface of the aortic valve |
| Sarphie(37) | 1985 | New Zealand White Rabbit | Cholesterol | None | Anioic Surface Properties of the Endothelium |
| Sarphie(36) | 1986 | New Zealand White Rabbit | Cholesterol | None | Cytochemical Surface Properties |
| Sarphie(35) | 1987 | New Zealand White Rabbit | Cholesterol | None | Interactions of IgG and beta VLDL |
| Rajamannan(30) | 2001 | New Zealand White Rabbit | Cholesterol | None | Apoptosis |
| Rajamannan(10) | 2002 | New Zealand White Rabbit | Cholesterol | Atorvastatin | Cell Proliferation, Extracellular Matrix production, Foam Cell Formation, Cbfal expression, Statin Inhibits Cell Proliferation and Matrix |
| Rajamannan(21) | 2005 | New Zealand White Rabbit | Cholesterol | Atorvastatin | eNOS expression and Regulation in the Aortic Valve, Calcification |
| Rajamannan(40) | 2005 | Watanabe Rabbit/LDLR−/− | Cholesterol | Atorvastatin | Lrp5/Wnt/Cbfa1 regulation in severely calcified aortic valves and regulation of cell proliferation in myofibroblast cell culture |
| Drolet{31) | 2003 | New Zealand White Rabbit | Cholesterol and Vitamin D | None | Hemodynamic Evidence of Early Stenosis |
| Cimini(39) | 2005 | New Zealand White Rabbit | Cholesterol | None | Early Aortic Valve Sclerosis and Atherosclerosis |
| Arishiro(43) | 2007 | Japanese Rabbit | Cholesterol | Olmesartan | ARB1 inhibits endothelial disurption and atherosclerosis |
| Mouse Species | |||||
| Shao(61) | 2005 | LDLR−/− | Cholesterol | None | Valvular Fibrosis via Wnt-Msx2 Regulation |
| Drolet(34) | 2006 | LDLR−/− | Cholesterol | None | Hemodynmic Evidence of Early Stenosis and Metabolic Syndorme |
| Weiss (32) | 2006 | LDLR−/−Apob100/100 | Cholesterol | None | Hemodynamic Evidence of Severe Stenosis, Mineralization and Abnormal Oxidative stress |
| Aikawa(33) | 2007 | ApoE−/− | Cholesterol | None | Multiomodality imaging in Atherosclerotic Aortic Valves- Measuring Osteogenic Activites |
| Tanaka (45) | 2007 | ApoE−/− | Cholesterol | None | Mechanistic study for Aortic valve Disease |
The next experimental proof of principle in the rabbit model, was to demonstrate complex calcification with chronic duration of cholesterol diet to prove the bone differentiation mechanism. 6 months of cholesterol diet treatment induced marked thickening and complex calcification in the aortic valve leaflets with pharmacologic attenuation with atorvastatin by MicroCT analysis40. The effects of statins were also confirmed by testing in vitro for the inhibition of extracellular matrix 41, 42. Finally, the most recent experimental model tested the effect of an angiotensin receptor blocker(ARB) on the inhibition of atherosclerotic pathways in the rabbit model of hypercholesterolemia43. These findings demonstrate experimentally the beneficial effects of HMG CoA Reductase Agents and ARB’s in vivo and in vitro.
Hemodynamic studies have evolved to study the noninvasive evidence for the development of aortic stenosis31, in rabbits treated with cholesterol + vitamin D model. These studies demonstrate the presence of early stenosis with an increase in the pulse wave Doppler velocity across the aortic valve leaflet. This was further confirmed in the LDLR−/− mouse model of hypercholesterolemia34. The next hemodynamic study demonstrated severe stenosis and calcification with chronic cholesterol treatment for 24 months of cholesterol.44 The study tested the genetic knock-out mouse which lacks the receptor for the Low-density lipoprotein receptor and expresses only the receptor for the human apoB100 (LDLr−/−apoB100/100) in an aging genetic mouse model which developed mineralization in the valve. The imaging experiments have been taken one step further to measure the development of the osteoblast phenotype in the aortic valve by using multimodality imaging33. The authors hypothesize that the flexion area of the aortic leaflets near the attachment of the aortic root (commissure) encounters the highest mechanical forces, which might induce endothelial cell activation/injury and expression of adhesion molecules such as VCAM-1, intracellular adhesion molecules-1, and E-selectin. Molecular imaging of the earliest stagesof calcification may identify high-risk valves while disease is silent and may enable the monitoring of valvular osteogenic activity during therapeutic interventions such as lipid lowering.
An important mechanistic study, demonstrated in native aortic valves in hypercholesterolemic mice that ten percent of cells are bone marrow derived cells within the atherosclerotic lesion45. These investigators hypothesize that likely both altered lipid metabolism and aging are essential for the development of murine aortic sclerosis, which potentially causes functional stenosis and regurgitation. Their findings suggest that some of the smooth muscle-like and osteoblast-like cells in degenerative valves might derive from bone marrow. It is likely that growth factors expressed in the endothelium with abnormal oxidative stress may play a role, at least in part, in the recruitment and homing of bone-marrow-derived cells to the site of valvular remodeling. Future studies evaluating mechanisms of the myofibroblast differentiation process, stem cell homing experiments, other medical therapies will provide further proof of the mechanisms for valvular heart disease and further understanding of the osteoblast differentiation cascade.
Genetics of Aortic Valve Disease
A growing number of studies are providing further evidence towards the genetic predisposition for aortic valve disease. Two of the studies have correlated genetic lipoprotein abnormalities in patients predisposed to the development of calcific aortic stenosis46, 47. The initial genetic study demonstrated an association of the vitamin D receptor polymorphism with calcific aortic valve stenosis. These investigators found that the B allele of the vitamin D receptor is more common in patients with calcific aortic valve stenosis48. In this study, the investigators found an association between the B allele predisposes the carriers to a decrease in calcium absorption and therefore an increase in bone loss. The discovery of this polymorphism further confirms the potential abnormal bone signaling pathways important in the development of this disease.
The next landmark discovery is the loss of function mutation in the Notch1 receptor in patients with calcific aortic stenosis49. These patients were identified in the Texas Heart Study as having valvular heart disease and accelerated calcification. It is important to note that the kindred of patients also had associated congenital heart abnormalities present in individual family members. Thus, implicating Notch1 in the development of congenital heart abnormalities as well as accelerated valve calcification. Another study demonstrated that the Pvull polymorphisms in the estrogen receptor alpha gene is related to both the presence of aortic stenosis in postmenpausal women and to lipid levels in adolescent females, suggesting that this polymorphism may influence the risk of aortic stenosis by affecting gender and lipid levels50. An interesting study51, demonstrated a familial aggregation for calcific aortic valve disease in the western part of France. These investigators found that the geographic distribution of calcific aortic valve disease is highly heterogeneous, with an average frequency of operated calcific aortic valve disease of 1.13 per 1000 inhabitants but up to 9.38 per 1000 in specific parishes. These genetic and familial studies show that lipids, Vitamin D, Estrogen receptor and Notch1 signaling in addition to a familial aggregation have important implications in the development of aortic stenosis and that an early atherosclerotic lesion secondary to genetic lipid abnormalities are important in the early initiation of this disease. Future genetic testing in the development of calcification in patients without traditional risk factors may play an important role in the treatment of this disease.
Osteoblast Phenotype is the Final Common Pathway for Aortic Valve Calcification
The presence of calcification in the aortic valve is responsible for hemodynamic progression of aortic valve stenosis. Recent descriptive studies from patient specimens have demonstrated the critical features of aortic valve calcification: including osteoblast expression, cell proliferation and atherosclerosis7–10. These studies define the biochemical and histological characterization of these valve lesions. Furthermore, these studies have also shown that specific bone cell phenotypes are present in calcifying valve tissue from human specimens52, 53. The vascular biologists have performed the initial studies54,55 which demonstrate the ability of calcifying vascular cells have the multipotential ability to differentiate to calcifying phenotypes. Calcification in the aortic valve is the final common pathway that leads to aortic valve stenosis. This was confirmed in a landmark echocardiographic study56, demonstrating severe aortic stenosis and severe calcification have a worse prognosis than patients with mild calcification and severe aortic stenosis. The data further corroborates the evidence that calcification is the defining feature clinically for prognostic future prognostic implications for this patients population.
Studies have shown that cardiovascular calcification is composed of hydroxyapatite deposited on a bone-like matrix of collagen, osteopontin (OP), and other minor bone matrix proteins 8, 57, 58. This was confirmed histologically with the presence of osteoblast bone formation in calcified aortic valves removed from surgical valve replacement8, 9. In addition, osteopontin expression has been demonstrated in the mineralization zones of heavily calcified aortic valves obtained at autopsy and surgery7, 8. This discovery has been extended in a study which shows by RTPCR analysis, histomorphometry and microCT that an osteoblast-like cellular phenotype is present in calcified aortic valves removed at the time of surgical valve replacement9. The increased gene expression of osteopontin, bone sialoprotein, and Cbfa1 (the osteoblast specific transcription factor for bone formation) were increased in the calcified aortic valves as compared to the control valves from heart transplantation. This is the first to provide the evidence for the gene differentiation pathway in this calcifying tissue and an upregulation of the osteoblast gene program. These discoveries are the foundation for the hypothesis that the cells residing in the aortic valve have the potentiality to differentiate into a bone forming cell which over time mineralizes and expresses an ossification phenotype.
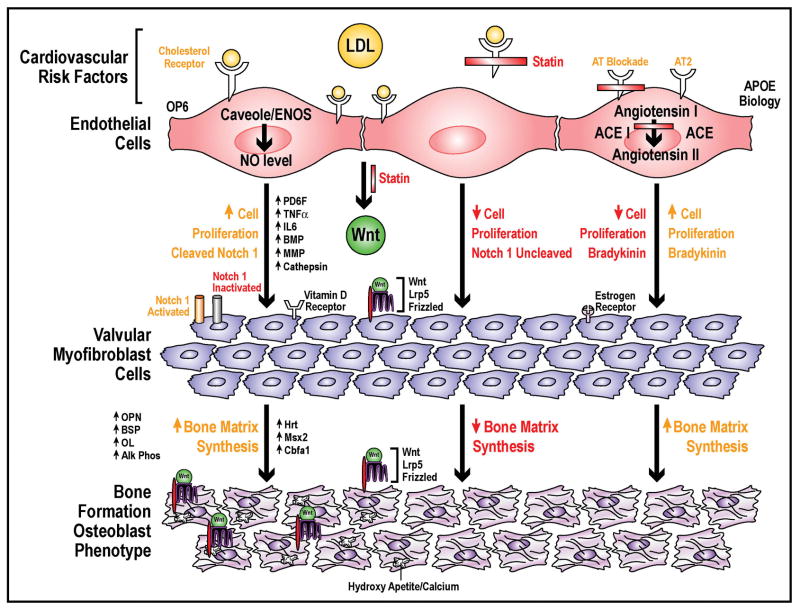
To test the osteoblast hypothesis further, evidence for signaling pathways are important in the development of this disease. The Lrp5 pathway was discovered to regulate bone formation in different diseases of the bone59, 60. There are three studies which have confirmed the regulation of the Lrp5/Wnt pathway in cardiovascular calcification in experimental models of calcification40,52,61. The Lrp5 pathway is one of many signaling pathways important in the development of bone formation. The Lrp5 receptor and other signaling pathways are important in the development of calcific aortic stenosis. Many of these signaling factors are similar to those found in vascular atherosclerosis and bone formation. Matrix MetalloProteinases (MMP)53, 62, Interleukin 163, Transforming growth factor-beta(TGF-beta)64, purine nucleotides42, 65, RANK66, osteoprotegrin(OPG)66, and TNF alpha67, have all been identified as signaling pathways important in the development of this disease process. Evidence, for the angiotensin converting enzyme pathway expressed and colocalize with LDL in calcified aortic valves also plays a role in future potential medical therapy68. Recent studies have shown that an increased expression of elastolytic cathepsins S, K, and V and their inhibitor Cystatin C in stenotic aortic valves69. These signaling studies from ex vivo human calcified aortic valves are the critical links between the experimental and translational implications for the future treatment of valvular heart disease. Figure 1, demonstrates the signaling pathways and cellular events important in the development of this disease. In the presence of lipids, the aortic valve endothelium is activated and abnormal oxidation state develops. The myofibroblast cells then begin to proliferate and synthesize extracellular bone matrix proteins with the upregulation of the various signaling pathways outlined in this review. These proteins overtime mineralize and calcify. ACE inhibitors and statins have the potential to modify this disease process and slow the progression of this disease.
Figure 1. Cellular, Molecular and Genetic Mechanisms of Calific Aortic Stenosis.
Model Implicating Lipids in the Development of Calcific Aortic Stenosis and the potential for Future Medical Therapies Targeting this disease at the cellular level in the Aortic Valve.
Summary of In vivo, In vitro and Ex vivo Models of Aortic Valve Disease
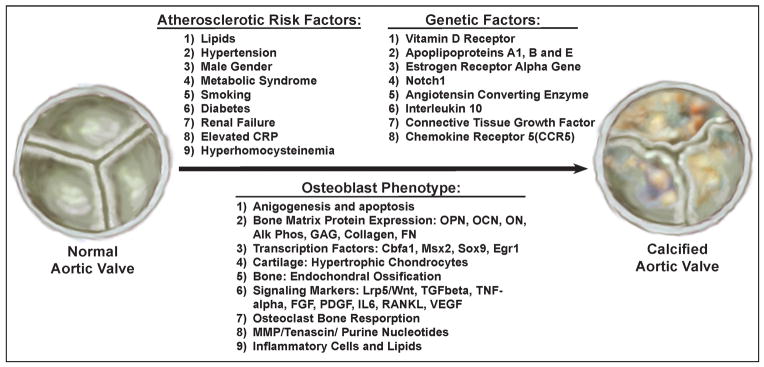
These models have provided the clues for the development of therapeutic approaches for this disease. Understanding these models and genetics will help our future understanding of this complex disease process. First, the initiating events in vascular disease and valvular disease may be similar, but the outcomes and the understanding of treatment of this disease are different because of the different biologic endpoints for vascular disease as compared to valvular heart disease. Figure 2, shows the interrelated events important in the development of aortic valve disease which is important in the understanding of the complexity for developing clinical trials in this field. Future clinical trials will need to include the atherosclerotic hypothesis in addition to the potential of genetics affecting the development of this disease. Finally, understanding the signaling pathways involved in the development of cell proliferation and osteoblast bone formation will allow for the medical therapy for these patients.
Figure 2.
Three Factors Responsible for the Development of Calcific Aortic Stenosis: Cardiovascular Risk Factors, Genetic Factors and Osteoblast Regulatory Pathways.
Discussion
Recent epidemiological studies have revealed that the risk factors for arterial atherosclerosis, male gender, smoking, and elevated serum cholesterol, are similar to the risk factors associated with development of aortic valve stenosis. The risk factors, growing number of models of experimental hypercholesterolemia which produce atherosclerosis in the aortic valve are similar to the early stages of vascular atherosclerotic lesion formation. The interplay of genetics, environmental risk factors and cellular biology play a critical role towards the underlying mechanism of this disease process. In summary, these findings suggest that medical therapies may have a potential role in patients in the early stages of this disease process to prolong the time to severe aortic stenosis and to delay the timing of surgery.
Acknowledgments
Sources of Funding: This work was completed with the support of an American Heart Association Grant-in-Aid (0555714Z) and a grant from the National Institute of Health (5K08HL073927-04, 1R01HL085591-01A1). Contributions: Dr. Rajamannan would like to thank Sheila Macomber for figure preparation and graphic design. Conflict of Interest: Nalini M. Rajamannan is an inventor on a patent for the use of statins in degeneration of aortic valve disease. This patent is owned by the Mayo Clinic and Dr. Rajamannan does not receive any royalties from this patent.
References
- 1.Lindroos M, Kupari M, Heikkila J, Tilvis R. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. Journal of the American College of Cardiology. 1993;21(5):1220–1225. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 2.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. Journal of the American College of Cardiology. 1997;29(3):630–634. doi: 10.1016/s0735-1097(96)00563-3. [DOI] [PubMed] [Google Scholar]
- 3.Otto CM, Lind BK, Kitzman DW, Gersh BJ, Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. [comment] New England Journal of Medicine. 1999;341(3):142–147. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 4.Aronow WS, Ahn C, Kronzon I, Goldman ME. Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. American Journal of Cardiology. 2001;88(6):693–695. doi: 10.1016/s0002-9149(01)01821-5. [DOI] [PubMed] [Google Scholar]
- 5.Ross J, Jr, Braunwald E. Aortic stenosis. Circulation. 1968;38(1 Suppl):61–67. doi: 10.1161/01.cir.38.1s5.v-61. [DOI] [PubMed] [Google Scholar]
- 6.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114(5):e84–231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. [comment] Circulation. 1995;92(8):2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 8.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103(11):1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 9.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107(17):2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105(22):2260–2265. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palta S, Pai AM, Gill KS, Pai RG. New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation. 2000;101(21):2497–2502. doi: 10.1161/01.cir.101.21.2497. [DOI] [PubMed] [Google Scholar]
- 12.Aronow WS, Ahn C, Kronzon I. Association of mitral annular calcium with symptomatic peripheral arterial disease in older persons. Am J Cardiol. 2001;88(3):333–334. doi: 10.1016/s0002-9149(01)01657-5. [DOI] [PubMed] [Google Scholar]
- 13.Briand M, Lemieux I, Dumesnil JG, Mathieu P, Cartier A, Despres JP, Arsenault M, Couet J, Pibarot P. Metabolic syndrome negatively influences disease progression and prognosis in aortic stenosis. J Am Coll Cardiol. 2006;47(11):2229–2236. doi: 10.1016/j.jacc.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 14.Desai MY, Rodriguez A, Wasserman BA, Gerstenblith G, Agarwal S, Kennedy M, Bluemke DA, Lima JA. Association of cholesterol subfractions and carotid lipid core measured by MRI. Arterioscler Thromb Vasc Biol. 2005;25(6):e110–111. doi: 10.1161/01.ATV.0000166599.78182.6c. [DOI] [PubMed] [Google Scholar]
- 15.Subbaiah PV, Gesquiere LR, Wang K. Regulation of the selective uptake of cholesteryl esters from high density lipoproteins by sphingomyelin. J Lipid Res. 2005;46(12):2699–2705. doi: 10.1194/jlr.M500263-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Kim WJ, Chereshnev I, Gazdoiu M, Fallon JT, Rollins BJ, Taubman MB. MCP-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem Biophys Res Commun. 2003;310(3):936–942. doi: 10.1016/j.bbrc.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 17.Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27(Kip1), p21(Cip1), and p16(Ink4) on vascular smooth muscle cell proliferation. Circulation. 2000;101(17):2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- 18.Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24(24):3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 19.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. Journal of Biological Chemistry. 1998;273(37):24266–24271. doi: 10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 20.Venema RC, Sayegh HS, Kent JD, Harrison DG. Identification, characterization, and comparison of the calmodulin-binding domains of the endothelial and inducible nitric oxide synthases. Journal of Biological Chemistry. 1996;271(11):6435–6440. doi: 10.1074/jbc.271.11.6435. [DOI] [PubMed] [Google Scholar]
- 21.Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005;91(6):806–810. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charest A, Pepin A, Shetty R, Cote C, Voisine P, Dagenais F, Pibarot P, Mathieu P. Distribution of SPARC During Neovascularization of Degenerative Aortic Stenosis. Heart. 2006 doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ngo DT, Heresztyn T, Mishra K, Marwick TH, Horowitz JD. Aortic stenosis is associated with elevated plasma levels of asymmetric dimethylarginine (ADMA) Nitric Oxide. 2007;16(2):197–201. doi: 10.1016/j.niox.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien KD, Reichenbach DD, Marcovina SM, Kuusisto J, Alpers CE, Otto CM. Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of ‘degenerative’ valvular aortic stenosis. Arteriosclerosis, Thrombosis & Vascular Biology. 1996;16(4):523–532. doi: 10.1161/01.atv.16.4.523. [DOI] [PubMed] [Google Scholar]
- 25.Olsson M, Thyberg J, Nilsson J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol. 1999;19(5):1218–1222. doi: 10.1161/01.atv.19.5.1218. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JL, Brown MS. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci U S A. 1973;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprecher DL, Schaefer EJ, Kent KM, Gregg RE, Zech LA, Hoeg JM, McManus B, Roberts WC, Brewer HB., Jr Cardiovascular features of homozygous familial hypercholesterolemia: analysis of 16 patients. American Journal of Cardiology. 1984;54(1):20–30. doi: 10.1016/0002-9149(84)90298-4. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi A, Miyatake K, Yutani C, Beppu S, Tsushima M, Yamamura T, Yamamoto A. Characteristic cardiovascular manifestation in homozygous and heterozygous familial hypercholesterolemia. 1999 Mar;1999:410–418. doi: 10.1016/s0002-8703(99)70485-0. [DOI] [PubMed] [Google Scholar]
- 29.Rajamannan NM, Edwards WD, Spelsberg TC. Hypercholesterolemic aortic-valve disease. New England Journal of Medicine. 2003;349(7):717–718. doi: 10.1056/NEJMc031360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajamannan NM, Sangiorgi G, Springett M, Arnold K, Mohacsi T, Spagnoli LG, Edwards WD, Tajik AJ, Schwartz RS. Experimental hypercholesterolemia induces apoptosis in the aortic valve. Journal of Heart Valve Disease. 2001;10(3):371–374. [PubMed] [Google Scholar]
- 31.Drolet MC, Arsenault M, Couet J. Experimental aortic valve stenosis in rabbits. Journal of the American College of Cardiology. 2003;41(7):1211–1217. doi: 10.1016/s0735-1097(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 32.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation. 2006;114(19):2065–2069. doi: 10.1161/CIRCULATIONAHA.106.634139. [DOI] [PubMed] [Google Scholar]
- 33.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 34.Drolet MC, Roussel E, Deshaies Y, Couet J, Arsenault M. A high fat/high carbohydrate diet induces aortic valve disease in C57BL/6J mice. J Am Coll Cardiol. 2006;47(4):850–855. doi: 10.1016/j.jacc.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Sarphie TG. Interactions of IgG and beta-VLDL with aortic valve endothelium from hypercholesterolemic rabbits. Atherosclerosis. 1987 Dec;:199–212. doi: 10.1016/0021-9150(87)90199-7. [DOI] [PubMed] [Google Scholar]
- 36.Sarphie TG. A cytochemical study of the surface properties of aortic and mitral valve endothelium from hypercholesterolemic rabbits. Exp Mol Pathol. 1986 Jun;:281–296. doi: 10.1016/0014-4800(86)90042-0. [DOI] [PubMed] [Google Scholar]
- 37.Sarphie TG. Anionic surface properties of aortic and mitral valve endothelium from New Zealand white rabbits. Am J Anat. 1985 Oct;:145–160. doi: 10.1002/aja.1001740205. [DOI] [PubMed] [Google Scholar]
- 38.Sarphie TG. Surface responses of aortic valve endothelia from diet-induced, hypercholesterolemic rabbits. Atherosclerosis. 1985 Mar;:283–299. doi: 10.1016/0021-9150(85)90122-4. [DOI] [PubMed] [Google Scholar]
- 39.Cimini M, Boughner DR, Ronald JA, Aldington L, Rogers KA. Development of aortic valve sclerosis in a rabbit model of atherosclerosis: an immunohistochemical and histological study. The Journal of heart valve disease. 2005;14(3):365–375. [PubMed] [Google Scholar]
- 40.Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005;112(9 Suppl):I229–234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114(1 Suppl):I547–552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 42.Osman L, Chester AH, Amrani M, Yacoub MH, Smolenski RT. A novel role of extracellular nucleotides in valve calcification: a potential target for atorvastatin. Circulation. 2006;114(1 Suppl):I566–572. doi: 10.1161/CIRCULATIONAHA.105.001214. [DOI] [PubMed] [Google Scholar]
- 43.Arishiro K, Hoshiga M, Negoro N, Jin D, Takai S, Miyazaki M, Ishihara T, Hanafusa T. Angiotensin receptor-1 blocker inhibits atherosclerotic changes and endothelial disruption of the aortic valve in hypercholesterolemic rabbits. J Am Coll Cardiol. 2007;49(13):1482–1489. doi: 10.1016/j.jacc.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 44.Ortlepp JR, Pillich M, Schmitz F, Mevissen V, Koos R, Weiss S, Stork L, Dronskowski R, Langebartels G, Autschbach R, Brandenburg V, Woodruff S, Kaden JJ, Hoffmann R. Lower serum calcium levels are associated with greater calcium hydroxyapatite deposition in native aortic valves of male patients with severe calcific aortic stenosis. The Journal of heart valve disease. 2006;15(4):502–508. [PubMed] [Google Scholar]
- 45.Tanaka K, Sata M, Fukuda D, Suematsu Y, Motomura N, Takamoto S, Hirata Y, Nagai R. Age-associated aortic stenosis in apolipoprotein E-deficient mice. J Am Coll Cardiol. 2005;46(1):134–141. doi: 10.1016/j.jacc.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 46.Avakian SD, Annicchino-Bizzacchi JM, Grinberg M, Ramires JA, Mansura AP. Apolipoproteins AI, B, and E polymorphisms in severe aortic valve stenosis. Clin Genet. 2001;60(5):381–384. doi: 10.1034/j.1399-0004.2001.600511.x. [DOI] [PubMed] [Google Scholar]
- 47.Novaro GM, Sachar R, Pearce GL, Sprecher DL, Griffin BP. Association between apolipoprotein E alleles and calcific valvular heart disease. Circulation. 2003;108(15):1804–1808. doi: 10.1161/01.CIR.0000097560.96431.3E. [DOI] [PubMed] [Google Scholar]
- 48.Ortlepp JR, Hoffmann R, Ohme F, Lauscher J, Bleckmann F, Hanrath P. The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart. 2001;85(6):635–638. doi: 10.1136/heart.85.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437(7056):270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 50.Nordstrom P, Glader CA, Dahlen G, Birgander LS, Lorentzon R, Waldenstrom A, Lorentzon M. Oestrogen receptor alpha gene polymorphism is related to aortic valve sclerosis in postmenopausal women. J Intern Med. 2003;254(2):140–146. doi: 10.1046/j.1365-2796.2003.01179.x. [DOI] [PubMed] [Google Scholar]
- 51.Probst V, Le Scouarnec S, Legendre A, Jousseaume V, Jaafar P, Nguyen JM, Chaventre A, Le Marec H, Schott JJ. Familial aggregation of calcific aortic valve stenosis in the western part of France. Circulation. 2006;113(6):856–860. doi: 10.1161/CIRCULATIONAHA.105.569467. [DOI] [PubMed] [Google Scholar]
- 52.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47(8):1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jian B, Jones PL, Li Q, Mohler ER, 3rd, Schoen FJ, Levy RJ. Matrix metalloproteinase-2 is associated with tenascin-C in calcific aortic stenosis. Am J Pathol. 2001;159(1):321–327. doi: 10.1016/S0002-9440(10)61698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Demer LL. Cholesterol in vascular and valvular calcification. Circulation. 2001;104(16):1881–1883. [PubMed] [Google Scholar]
- 55.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108(20):2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
- 56.Rosenhek R, Binder T, Porenta G, et al. Predictors of outcome in severe asymptomatic aortic stenosis. NEJM. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 57.Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arteriosclerosis, Thrombosis & Vascular Biology. 1997;17(3):547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 58.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92(8):2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 59.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML Osteoporosis-Pseudoglioma Syndrome Collaborative G. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 60.Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 61.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115(5):1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaden JJ, Vocke DC, Fischer CS, Grobholz R, Brueckmann M, Vahl CF, Hagl S, Haase KK, Dempfle CE, Borggrefe M. Expression and activity of matrix metalloproteinase-2 in calcific aortic stenosis. Z Kardiol. 2004;93(2):124–130. doi: 10.1007/s00392-004-1021-0. [DOI] [PubMed] [Google Scholar]
- 63.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis. 2003;170(2):205–211. doi: 10.1016/s0021-9150(03)00284-3. [DOI] [PubMed] [Google Scholar]
- 64.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75(2):457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465–456. [DOI] [PubMed] [Google Scholar]
- 65.Osman L, Amrani M, Isley C, Yacoub MH, Smolenski RT. Stimulatory effects of atorvastatin on extracellular nucleotide degradation in human endothelial cells. Nucleosides Nucleotides Nucleic Acids. 2006;25(9–11):1125–1128. doi: 10.1080/15257770600894196. [DOI] [PubMed] [Google Scholar]
- 66.Kaden JJ, Bickelhaupt S, Grobholz R, Haase KK, Sarikoc A, Kilic R, Brueckmann M, Lang S, Zahn I, Vahl C, Hagl S, Dempfle CE, Borggrefe M. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulate aortic valve calcification. J Mol Cell Cardiol. 2004;36(1):57–66. doi: 10.1016/j.yjmcc.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 67.Kaden JJ, Kilic R, Sarikoc A, Hagl S, Lang S, Hoffmann U, Brueckmann M, Borggrefe M. Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med. 2005;16(5):869–872. [PubMed] [Google Scholar]
- 68.O’Brien KD, Shavelle DM, Caulfield MT, McDonald TO, Olin-Lewis K, Otto CM, Probstfield JL. Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation. 2002;106(17):2224–2230. doi: 10.1161/01.cir.0000035655.45453.d2. [DOI] [PubMed] [Google Scholar]
- 69.Helske S, Syvaranta S, Lindstedt KA, Lappalainen J, Oorni K, Mayranpaa MI, Lommi J, Turto H, Werkkala K, Kupari M, Kovanen PT. Increased expression of elastolytic cathepsins S, K, and V and their inhibitor cystatin C in stenotic aortic valves. Arterioscler Thromb Vasc Biol. 2006;26(8):1791–1798. doi: 10.1161/01.ATV.0000228824.01604.63. [DOI] [PubMed] [Google Scholar]