Abstract
Background
Differences in late-stage cancer risk between urban and rural residents are a key component of cancer disparities. Using data from the Illinois State Cancer Registry 1998–2002, we investigate the rural-urban gradient in late-stage cancer risk for four major types of cancer: breast, colorectal, lung and prostate.
Methods
Multilevel modeling is used to evaluate the role of population composition and area-based contextual factors in accounting for rural-urban variation. Instead of a simple binary rural-urban classification, we use a finer-grained classification that differentiates the densely populated city of Chicago from its suburbs and from smaller metropolitan areas, large towns and rural settings.
Results
For all four cancers, risk is highest in the most highly urbanized area and decreases as rurality increases, following a J-shaped progression that includes a small upturn in risk in the most isolated rural areas. For some cancers, these geographic disparities are associated with differences in population age and race; for others, the disparities remain after controlling for differences in population composition and ZIP code socioeconomic characteristics and spatial access to health care.
Conclusion
The observed pattern of urban disadvantage emphasizes the need for more extensive urban-based cancer screening and education programs.
Keywords: neoplasms, health disparities, stage at diagnosis, rural
Cancer stage at the time of diagnosis is critically important in affecting the long-term health and well-being of cancer patients. Detection at an early stage increases the likelihood that cancers can be successfully treated, reducing the risk of morbidity and mortality and enhancing long-term prognosis. In the United States, there are wide disparities in late-stage cancer risk among population groups and among geographic regions. Low income, vulnerable populations are more likely to be diagnosed with cancer at a distant or `late' stage. Late-stage risk also varies geographically. Some studies identify a greater risk of late-stage diagnosis among rural residents who face long distances in accessing cancer screening services. However, research on rural-urban disparities has produced mixed and conflicting findings that question whether rural residents are disadvantaged in late-stage risk.
This research investigates the rural-urban gradient in late-stage cancer risk in Illinois for 1998–2002. Using data from the Illinois State Cancer Registry, we examine fine-grained patterns of rural-urban variation for four major types of cancer: breast, colorectal, lung and prostate. The objectives are to determine the extent and direction of rural-urban gradient in late-stage risk for each type of cancer and to analyze the roles of individual demographic characteristics, and ZIP code level contextual variables in accounting for rural-urban variations.
Background
Studies of rural-urban disparities in late-stage cancer risk in the United States present a mixed picture of geographic variation. Conventional wisdom suggests that rural residents have a higher risk of late diagnosis due to numerous barriers to obtaining preventive health services and screening for early detection. These barriers include: poor geographical access to primary care and cancer screening services (1, 2), lack of insurance (3), and lack of knowledge about screening guidelines (4, 5, 6). Concentration of vulnerable population groups, such as the elderly, in rural areas makes these barriers even more significant.
Some empirical investigations find support for the hypothesis of rural disadvantage uncovering higher rates of late-stage cancer among rural residents (7, 8). African-Americans living in rural areas are particularly disadvantaged (9), as are residents of remote and impoverished rural areas (10). Rural areas have also been observed to have lower rates of cancer screening, which results in increased late-stage disease, and a higher proportion of unstaged cancers (11, 12, 10, 13). Evidence of rural disadvantage also comes from research in other countries (14, 15, 16).
An equally diverse body of research suggests little or no rural-urban gradient in late-stage cancer risk. Urban/rural residence had no significant association with cancer stage for breast cancer and melanoma patients in California (17). For colorectal cancer in California, controlling for socioeconomic status eliminated rural-urban disparities in late-stage risk (18). Lung cancer survival was unrelated to urban versus rural residence, but strongly associated with socioeconomic deprivation (19). Similar findings have been reported for colorectal cancer in North Carolina (20) and breast cancer in New Zealand (21). There is also evidence that although rural residents may have been disadvantaged in the past, the rural-urban divide has closed over time (17, 22). Knowledge of and access to cancer screening has increased substantially in many rural areas (23).
Some studies even suggest a reverse pattern of rural-urban disadvantage in which late-stage risk is higher in cities than in rural areas (25). Among breast cancer patients in Florida, living in urban areas was associated with a higher risk of late-stage presentation (24). Intra-urban research shows high rates of late-stage diagnosis in impoverished inner-city communities, rates that may be indicative of clusters of urban disadvantage (26, 27, 28, 29). A recent study of colorectal and lung cancers in the U.S (30) revealed that after controlling for social and demographic factors linked to late presentation, risk is higher in urban than rural areas.
These contrasting findings highlight the need for further research into the rural-urban gradient in late-stage cancer risk. Many previous studies are limited by their reliance on a simple binary classification of urban versus rural that fails to capture the geographic diversity of residential environments in the U.S. Moreover, the definitions of rural and urban differ depending on whether counties or smaller geographic units were used in constructing the rural-urban classification. Past research also suggests that rural-urban disparities may vary among cancer types reflecting differences in availability and accessibility of screening services and health education and socio-demographic differences in affected populations. Cancer stage is also associated with individual and contextual variables such as age, education and economic deprivation, and these confounding factors need to be considered in studies of geographic disparities.
Methods
We present a multilevel statistical analysis of rural-urban disparities in late-stage cancer presentation for the four major types of cancer (breast, colorectal, lung and prostate) in Illinois. Illinois is an appropriate study area because it encompasses a diverse range of geographic settings from the densely populated Chicago metropolitan area to low-density, remote rural regions. Data on all cancer cases for the years 1998–2002 were obtained from the Illinois State Cancer Registry (ISCR). The ISCR was certified as a Gold Standard registry by the NAACCR for each and every year from 1998–2002 (per personal communication with Dr. Tiefu Shen, Director of ISCR, on Oct.22, 2008). Cases among Illinois residents that were diagnosed in neighboring states are included, and the completeness of case ascertainment is estimated at 98 percent (31). The data set comprises individual records of cancer incidence in Illinois, and each cancer case is geocoded to the ZIP code of residence. For each cancer patient, variables describing cancer type, age group, sex, race, diagnosis stage and year are included.
ISCR uses a classification scheme consistent with SEER summary stage to measure stage at diagnosis (32). The in situ and localized categories (stage of disease code = 0, 1) were considered early stage, and regional and distant categories (codes 2–7) were considered late stage. Unstaged cases or those with unknown stage were excluded. The percent of cases lacking stage information varied among the four cancer sites, ranging from 5.1% for breast cancer to 13.1% for lung cancer (Table 1).
Table 1.
Cancer Stage by Type in Illinois 1998–2002
| No. of cases | Unstaged | Late-stage | |||
|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | ||
| Breast | 44,070 | 2,226 | 5.1 | 15,454 | 36.9 |
| Colorectal | 36,150 | 3,283 | 9.1 | 20,672 | 62.9 |
| Lung | 43,778 | 5,726 | 13.1 | 30,324 | 79.7 |
| Prostate | 42,291 | 4,260 | 10.1 | 5,920 | 15.6 |
The percentage of patients diagnosed with late-stage disease differs substantially by cancer type. Nearly 80 percent of lung cancer patients present with late-stage disease, compared to only 15.6 percent for prostate cancer. Breast cancer (36.9%) and colorectal cancer (62.9%) have intermediate values. These inequalities by cancer type reflect biological, social and health care factors including differences in awareness and information about early diagnosis, and in the availability of screening procedures for early detection.
Multilevel modeling was used to analyze the rural-urban gradient in late-stage cancer risk and to evaluate the role of individual demographic characteristics (age, sex, race) and ZIP code-level contextual factors in accounting for rural-urban variation. Multilevel modeling is an appropriate method for investigating contextual effects – the effects of the local environment or neighborhood on health outcomes (33). The dependent variable in the multilevel models is a binary variable representing late-stage diagnosis (1=late) for individual cancer patients; thus we model an individual cancer patient's risk of late diagnosis.
Independent variables exist at two levels: individual and ZIP code. The individual-level data come from the ISCR. Due to privacy and confidentiality restrictions, we only have access to a limited set of variables -- age category, race, cancer type, cancer stage at diagnosis and ZIP code of residence – for each cancer patient. Three dummy-coded, individual-level independent variables were included in the models: young age (< 50 years), older age (≥70 years) and race. At the ZIP code level, independent variables representing rural-urban location, spatial access to health care, and socio-economic characteristics of ZIP codes are included (Table 2).
Table 2.
Variable Definitions
| Independent Variable | Definition |
|---|---|
| Individual-level | |
| Age<50 | Age < 50 years |
| Age≥70 | Age ≥ 70 years |
| Black | Black race (1=black, 0=other) |
| ZIP code-level | |
| Chic_sub | Chicago suburb |
| Other_metro | Other metropolitan area |
| Large town | Town with population 10,000−50,000 |
| Rural | Small town and rural area |
| Income | Median household income in logarithm |
| Non_English | % population unable to speak English |
| Access | Spatial access to primary care |
| Time1 | Travel time to nearest mammography or colonoscopy facility |
Travel time is only included in the models for breast and colorectal cancer.
To differentiate various types of urban, suburban and rural locations, we subdivided the state into five zones based on the rural-urban commuting areas classification scheme (RUCA) developed by the Office of Rural Health Policy (34). The RUCA scheme classifies areas on the basis of urbanized population and commuting flow. We rely on the four-tiered RUCA taxonomy which classifies ZIP codes into: 1) urban core areas, 2) suburban areas, 3) large town areas (urbanized population 10,000–49,999) and 4) small town and isolated rural areas. This classification was devised by researchers at the University of Washington based on census tract data aggregated into ZIP code areas (35).
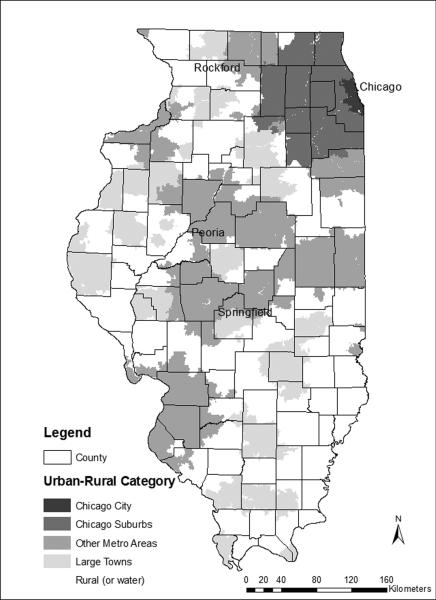
Developed mainly for analyzing rural issues, RUCA codes do not differentiate well within urban metropolitan areas. For example, most ZIP codes in the Chicago metropolitan area, including the city of Chicago and surrounding suburbs, are classified into RUCA category one (urban core) despite their geographic and population differences. Smaller cities such as Peoria and Rockford are also lumped into category one, ignoring the fact that they differ greatly in population density and health care availability from Chicago and its suburbs. To represent better these urban and suburban environments, we modified and expanded the RUCA scheme as follows (Figure 1):
Chicago City. With a population of 2.9 million in 2000, Chicago represents a distinct geographic setting due to its high population density, large poor and immigrant populations, large concentration of hospitals, doctors and other healthcare providers and well-developed public transit system.
Chicago Suburbs. Forming an expanding ring around Chicago, these areas are characterized by moderate-density residential and commercial development, a growing and increasingly diverse population, and strong linkages with the central city. Chicago suburbs were identified as parts of the Chicago metropolitan statistical area located in Illinois and not including the city itself.
Other Metropolitan. This group consists of smaller cities and suburbs in other parts of the state such as the cities of Peoria and Springfield. ZIP codes in RUCA categories 1 and 2 (urban core and suburban) were selected, and those not located in Chicago or its suburbs were placed in the `Other Metropolitan' category.
Large Towns. This group comprises RUCA category 3, towns with populations between 10,000 and 50,000 and their surrounding rural areas with high commuting into the town.
Rural. These areas represent RUCA category 4, small towns (population < 10,000) and isolated rural areas.
Figure 1.
Rural-urban classifications for Illinois ZIP code areas
Four dummy-coded variables describe the five types of rural-urban settings of the ZIP code of residence as described above (see Table 2). In all models, the reference category represents the city of Chicago, so Chicago ZIP codes serve as the basis for comparison. In the models, coefficients for these geographic variables describe the difference in late-stage risk for patients living in a particular type of area relative to the risk for patients living in Chicago, with all other independent variables held constant.
In addition to these rural-urban classifications, several variables at the ZIP code level, obtained from 2000 Census data for ZIP Code Tabulation Areas, were included as area-based socio-economic indicators (36). A large number of socio-economic variables from the 2000 Census were examined by estimating models using different combinations of variables. Most of the socio-economic variables were eliminated because they had high levels of multicollinearity. The final model uses two area-based socio-economic indicators: median household income (in logarithm) and percent of population that does not speak English. These were chosen because they are relatively uncorrelated and represent two distinct dimensions of socio-economic status – economic deprivation/wellbeing and populations disadvantaged by language ability. Although many researchers advocate the use of poverty as an economic indicator (36), we observed that poverty varies much less across rural areas in Illinois than does median income. Thus income is a more sensitive indicator of economic wellbeing, especially in rural areas.
The final two ZIP code-level independent variables describe spatial access to health care from cancer patient's ZIP code of residence: spatial access to primary care and travel time to the nearest cancer screening facility (Table 2). The latter variable is only used in the models for breast and colorectal cancer, because those two types of cancer have clearly identifiable screening services (mammography and colonoscopy). All mammography and colonoscopy facilities in the state were geocoded by address. Travel times from the centroid of each ZIP code to the nearest screening facility were estimated based on real-world road networks accounting for the type of road and adjusting for typically lower travel speeds in densely populated urban areas (37).
Spatial access to primary care was estimated using the two-step floating catchment area method (2SFCA) (37, 38). For each ZIP code, the 2SFCA computes a numerical value that represents the ratio of the local supply of primary care physicians to the local demand (population) for primary care. Supply and demand are measured in a floating window within a fixed range (i.e., 30 minutes) of travel time. A high value for this spatial access measure represents a high ratio of supply to demand – a large number of primary care physicians in the local area compared to population.
The multilevel models specify individual cancer patients nested within ZIP codes – a two-level hierarchical model. A logistic multilevel formulation was used because the dependent variable is binary (late-stage). The specific model formulation used in this research is a two-level `intercepts-as-outcomes model'. Such models assume that the effects of individual-level variables are fixed across ZIP codes and that ZIP code intercepts vary as a function of ZIP code socioeconomic and spatial variables. To evaluate rural-urban variation and assess whether individual and contextual variables account for that variation, we entered independent variables into the multilevel models in blocks. The first models included only the four urban-rural variables; next individual-level variables representing age and race were entered. In the final models, all ZIP-code socioeconomic and spatial variables were included (Table 3). All models were estimated using STATA statistical software.
Table 3.
Multilevel Model Coefficients by Cancer Type, 1998–20021
| Breast | Colorectal | Lung | Prostate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chic_sub | −.181* | −.065* | −.001 | −.092* | −.087* | −.029 | −.007 | −.012 | −.017 | −.189* | −.105* | −.062 |
| Other_metro | −.278* | −.146* | −.110* | −.131* | −.122* | −.056 | −.172* | −.183* | −.252* | −.128* | −.039 | −.012 |
| Large town | −.336* | −.162* | −.167* | −.272* | −.250* | −.198* | −.331* | −.338* | −.431* | −.191* | −.065 | −.063 |
| Rural | −.207* | −.032 | −.063 | −.169* | −.156* | −.085 | −.204* | −.212* | −.323* | −.164* | −.035 | −.038 |
| Age<50 | .547* | .541* | .216* | .216* | .195* | .188* | .129 | .128 | ||||
| Age≥70 | −.279* | −.279* | −.082* | −.083* | −.234* | −.233* | −.174* | −.177* | ||||
| Black | .373* | .370* | .083* | .077* | −.068* | −.085* | .289* | .279* | ||||
| Income | −.134* | −.047 | −.110* | −.087 | ||||||||
| Non_English | .012* | .002 | −.001 | .003 | ||||||||
| Access | −37.6* | 20.0 | −27.2* | −9.9 | ||||||||
| Time | .002 | 0.000 | ||||||||||
For each type of cancer, the first model includes only the four dummy independent variables for the five-category rural-urban classifications; the second model adds three dummy-coded, individual-level variables (age and race); and the third model adds four zip-code-level variables (two socio-economic indicators and two for spatial access to health care). A negative coefficient means that the variable is associated with a reduced risk of late-stage cancer. A positive value means that the variable increases the risk of late-stage disease
p-value < 0.05
Results
The first set of multilevel models that include only rural-urban location variables reveal significant geographic variation in late-stage cancer risk for all four cancers. Table 3 shows the coefficients for the multilevel models; odds ratios, calculated as exp(b), are mentioned in the text and shown in Figure 2 for categorical variables such as age category and geographic zone. According to Table 3, in every case, the risk of late diagnosis is highest among patients living in the city of Chicago. Those living in the other four geographic zones have significantly lower risk, with the exception of lung cancer patients in the Chicago suburbs (not significant).
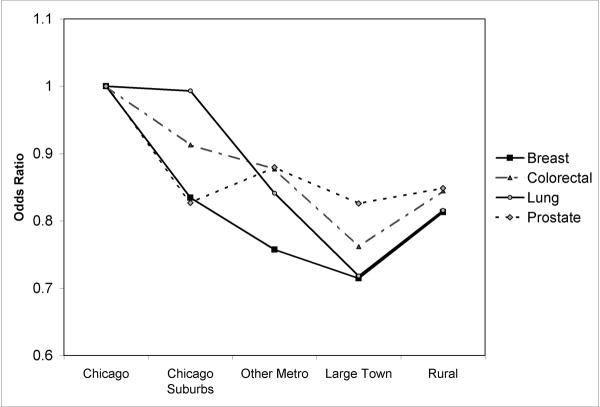
Figure 2.
Rural-urban variation in risk of late-stage diagnosis by cancer type: Odds ratios indicate likelihood of late-stage diagnosis for cancer patients residing in geographic zone compared those living in city of Chicago.
Graphing the odds ratios for each geographic zone and cancer type shows a clear and remarkably consistent rural-urban gradient in late-stage risk (Fig. 2). Risk is highest in Chicago and decreases as we move to less urbanized zones, reaching its nadir in other metropolitan areas and large towns. Risk increases somewhat among patients living in the most rural areas, tracing a reverse J-shaped gradient along the urban-rural continuum. This J-shaped gradient holds for all four cancer types. The gradients are steepest for breast, colon and lung cancers, all of which record the lowest odds ratios among patients living in large towns. These patients are roughly 25 percent less likely to present with late-stage cancer than are their counterparts in the city of Chicago, as indicated by odds ratios (OR) ranging from 0.71 to 0.79. The gradient is less pronounced for prostate cancer, but still patients living outside Chicago are 15–20% less likely to be diagnosed with late-stage disease than those living in the city. Thus, for all cancers, late diagnosis is most concentrated in the highly urbanized city of Chicago and decreases with decreasing urbanization, recording a modest increase in the most isolated rural areas.
To determine if differences in population composition account for these rural-urban variations, individual-level age and race variables were added to the multilevel models for each cancer type (Table 3). In every case, older age is linked to a reduced likelihood of late diagnosis. For breast (OR=.75) and lung (OR=.79) cancer, individuals 70 years and older are 25 percent less likely to be diagnosed with late-stage disease than their younger counterparts. The decrease in risk is less for colorectal and prostate patients, but still statistically significant. In contrast, young age (<50 years) is associated with a higher risk of late diagnosis, and the heightened risk is statistically significant for all but prostate cancer. Racial disparities are also evident. Blacks are more likely than others to be diagnosed with late-stage breast, colorectal and prostate cancer, controlling for age and rural-urban location. The racial disparity is particularly wide for breast (OR=1.45) and prostate cancer (OR=1.34). For lung cancer, black patients are less likely to present with late stage disease.
Adding these individual-level, demographic variables reduces the urban-rural gradient in late-stage risk for all cancers with the exception of lung cancer. For prostate cancer, the gradient is almost completely eliminated, suggesting the observed rural-urban disparities are primarily the result of compositional differences in population age and race. For lung and colorectal cancer, the J-shaped gradient in late-stage risk remains, however disparities diminish for colorectal cancer but widen for lung cancer. This means that for lung cancer, the high rates of late-stage diagnosis observed in Chicago city and suburbs are even higher than expected based on the age and racial composition of lung cancer patients. Adjusting for age and race has a different impact on rural-urban differences for breast cancer: Risks converge for rural patients and those in Chicago city, whereas patients living in Chicago suburbs, other metropolitan areas and large towns are significantly less likely to be diagnosed with late-stage disease.
The final models include ZIP code level indicators of socioeconomic conditions and spatial access to health care. These contextual variables achieve statistical significance only in the models for breast and lung cancer (Table 3). In both cases, median income is inversely associated with late diagnosis: residents of higher income areas have a reduced likelihood of late-stage presentation, confirming the strong ties between economic vulnerability and cancer stage found in many research studies (39, 40, 41). Spatial access to primary health care is also statistically significant, and patients living in areas that lack primary health care resources are more likely to present with distance-stage disease. Adding these contextual variables to the models leads to further changes in rural-urban disparities. Disparities are essentially eliminated for prostate and colorectal cancer (except for colorectal patients residing in large towns). Among breast cancer patients, the risk of late-stage diagnosis is less for those living in other metropolitan and large town settings, controlling for patient demographics and area-based indicators of socioeconomic disadvantage and spatial access to health care. In the case of lung cancer, controlling for these factors has the opposite result, revealing wider disparities along the rural-urban continuum. The likelihood of late-stage presentation is highest among residents of Chicago city and suburbs and follows the same J-shaped pattern observed earlier. All else equal, patients living outside the Chicago area are 25–35 percent less likely than their Chicago-area counterparts to present with late-stage lung cancer.
Discussion
A rural-urban gradient in the risk of late-stage cancer is evident for the four major types of cancer in Illinois; however there is little indication of rural disadvantage. Instead we find that the likelihood of late-stage diagnosis is highest among patients living in the most densely populated zone, the city of Chicago. The observed pattern of urban disadvantage provides support for Paquette and Finlayson's (30) finding that for certain cancers, the risk of late-stage presentation is higher among residents of urban areas than among non-urban residents. This study uses a finer-grained rural-urban classification that differentiates the densely populated city of Chicago from its suburbs and from smaller metropolitan areas, large towns and rural settings. For all four cancers, risk decreases as rurality increases, following a J-shaped gradient that includes an upturn in risk in the most isolated rural areas.
For colorectal and prostate cancer, and to a lesser extent breast cancer, rural-urban disparities largely disappear when individual- and ZIP code-level variables are controlled. Thus, the geographic differences observed mainly stem from differences in the age and racial composition of cancer patients and the social and spatial characteristics of the locations in which they live. Concentration of vulnerable populations and economically disadvantaged places in Chicago and its suburbs underpin the high rates of late-stage diagnosis observed in these highly urban areas. Conversely, in the most rural areas, the lower rates of late-stage diagnosis primarily reflect the greater presence of elderly patients who have a lower risk of late-stage diagnosis. Finally, in the case of lung cancer, the rural-urban gradient not only remains after individual and ZIP code characteristics are controlled, but also it becomes more extreme. This suggests that there are unmodelled factors such as cancer awareness or diagnostic differences that vary along the rural-urban continuum leading to systematic disparities in stage at presentation. Lung cancer has the highest percentage of unstaged cases, so staging procedures and accuracy may be important.
These findings also reveal a strong and consistent advantage for patients living in the other metropolitan and large town contexts – an advantage that remains after individual and ZIP code variables are controlled (except for prostate cancer). Other metropolitan areas and large towns are dispersed across the state, ranging in population size from 10,000 to around 300,000. Almost all of these places have primary care physicians, and many have hospital facilities. The lower incidence of late-stage presentation among patients residing in these areas warrants more in-depth investigation, focusing on how the interactions between people and local health care systems affect cancer screening and awareness. For example, residents of these smaller urban places may face fewer space-time constraints in accessing cancer screening services than their counterparts in the most urban and rural settings. Whatever its causes, the lower rate of late-stage presentation in these smaller urban places highlights the scope for improvement elsewhere.
Our results also emphasize the need to look beyond the binary categories of urban and rural in investigating geographic health disparities. In Illinois, late-stage cancer risk is consistently lowest in the two geographic zones that straddle the urban-rural divide: other metropolitan places (normally classified as `urban') and large towns (normally classified as `rural'). Using traditional urban and rural definitions smoothes away variation along the rural-urban continuum, obscuring the high incidence of late diagnosis that exists in the Chicago region, and the (moderately) higher incidence in isolated rural settings. Variation within these broad geographic zones is also important, although not examined here. Studies show marked geographic variation in late diagnosis within large cities like Chicago (27, 28, 29) and similar spatial inequalities are likely to exist in rural areas. Using spatial clustering methods to identify geographic concentrations of high-rate areas enables detailed spatial targeting of public health interventions (42, 43, 44).
Like many studies, we find a higher risk of late-stage cancer among younger patients and a lower risk among older patients. These are likely to result from differences in frequency of primary care visits and age-related cancer screening protocols. We also observe significant racial disparities in late-stage disease for all types of cancer. The higher likelihood of late presentation among black breast, colorectal and prostate cancer patients, controlling for age and ZIP-code socio-economic characteristics confirms the persistent racial disparities in late-stage cancer reported elsewhere (45, 46). Like disadvantaged populations in other countries (24), the black population in the U.S. is distinctly vulnerable to late diagnosis for these types of cancer.
The causes of these racial disparities are not well understood. Emerging evidence for breast cancer points to biological differences in tumor size, type and nodal involvement (47). At the same time, contextual and cultural factors that affect use of screening services and the quality and effectiveness of those services are also likely to be important (48). Our results indicate that such factors go beyond the socioeconomic and sociocultural characteristics of residential areas and their spatial accessibility to health care. Black patients may face different kinds of constraints and opportunities in accessing health care than do patients of other racial groups, and such differences are not captured in our modeling strategy.
For lung cancer, the racial disparity is reversed: blacks are less than others likely to be diagnosed with late-stage lung cancer, although the decrease in risk is small. This finding is not consistent with national data which show a heightened risk of late diagnosis among black lung cancer patients (49). However, this study controls for age and area-based socioeconomic characteristics which have been found consistently to increase late-stage risk. Understanding how socioeconomic deprivation interacts with and affects racial disparities in lung cancer is an important topic for future investigation.
Conclusion
Rural-urban inequalities in late-stage cancer risk are an important dimension of persistent disparities in cancer morbidity and mortality. Our results indicate that the odds of late-stage presentation are not highest among patients living in rural areas but among those living in the most urbanized setting, the Chicago metropolitan area. Thus, we find a reversal of the commonly held view that risks are highest for rural residents. The concentration of health disadvantage in highly urbanized places emphasizes the need for more extensive urban-based cancer screening and education programs, especially programs targeted to the most vulnerable urban populations and neighborhoods. At the state level, late-stage risk varies systematically along a detailed rural-urban continuum, with both low and high-rate areas cutting across the traditional rural-urban divide. This raises questions about the use of a simple binary rural-urban classification in investigating geographic health disparities. Determining whether the J-shaped trend observed here holds for other types of cancer in other geographic contexts is an important topic for future research investigation. Unraveling the causes of these geographic inequalities also requires attention. Although the limited set of individual and contextual variables considered here accounts for some rural-urban variation, much remains unexplained. Why for example, are risks consistently lower among patients living in large towns in rural areas? Addressing such questions calls for analysis of how people in particular geographic contexts interact with local health care providers, and how providers respond to local population health needs - issues that define a challenging research agenda.
Acknowledgement
Financial support from the National Cancer Institute (NCI), National Institutes of Health (NIH), under Grant 1-R21-CA114501-01, is gratefully acknowledged. Points of view or opinions in this paper are those of the authors and do not necessarily represent the official position or policies of NCI.
References
- 1.Liff JM, Chow WH, Greenberg RS. Rural-urban differences in stage at diagnosis. possible relationship to cancer screening. Cancer. 1991;67(5):1454–1459. doi: 10.1002/1097-0142(19910301)67:5<1454::aid-cncr2820670533>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, McLafferty S, Escamilla V, Luo L. Late-stage breast cancer diagnosis and healthcare access in Illinois. Professional Geographer. 2008;60:54–69. doi: 10.1080/00330120701724087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carr WP, Maldonado G, Leonard PR, Halberg JU, Church TR, Mandel JH, Dowd B, Mandel JS. Mammogram utilization among farm women. J Rural Health. 1996;12(4 Suppl):278–290. doi: 10.1111/j.1748-0361.1996.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 4.Bryant H, Mah Z. Breast cancer screening attitudes and behaviors of rural and urban women. Prev Med. 1992;21(4):405–418. doi: 10.1016/0091-7435(92)90050-r. [DOI] [PubMed] [Google Scholar]
- 5.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21:182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 6.Elnicki DM, Morris DK, Shockcor WT. Patient-perceived barriers to preventive health care among indigent, rural Appalachian patients. Arch Intern Med. 1995;155:421–424. [PubMed] [Google Scholar]
- 7.Fazio L, Cotterchio M, Manno M, McLaughlin J, Gallinger S. Association between colonic screening, subject characteristics, and stage of colorectal cancer. The American Journal of Gastroenterology. 2005;100(11):2531–9. doi: 10.1111/j.1572-0241.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 8.Jemal A, Ward E, Wu X, Martin HJ, McLaughlin CC, Thun MJ. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiology, Biomarkers and Prevention. 2005;14(3):590–5. doi: 10.1158/1055-9965.EPI-04-0522. [DOI] [PubMed] [Google Scholar]
- 9.Amey CH, Miller MK, Albrecht SL. The role of race and residence in determining stage at diagnosis of breast cancer. Journal of Rural Health. 1997;13:99–108. doi: 10.1111/j.1748-0361.1997.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 10.Lengerich EJ, Rubio A, Brown PK, Knight EA, Wyatt SW. Results of coordinated investigations of a national colorectal cancer education campaign in Appalachia. Preventing Chronic Disease. 2005;3(2):A32. [PMC free article] [PubMed] [Google Scholar]
- 11.Coughlin SS, Thompson TD, Hall HI, Logan P, Uhler RJ. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer. 2002;94:2801–2812. doi: 10.1002/cncr.10577. [DOI] [PubMed] [Google Scholar]
- 12.Higginbotham JC, Moulder J, Currier M. Rural v. urban aspects of cancer: first-year data from the Mississippi Central Cancer Registry. Family and Community Health. 2001;24(2):1–9. doi: 10.1097/00003727-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Schootman M, Kinman E, Farria D. Rural-urban differences in ductal carcinoma in situ as a proxy for mammography use over time. J Rural Health. 2003;19(4):470–476. doi: 10.1111/j.1748-0361.2003.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell NC, Elliot AM, Sharp L, et al. Rural and urban differences in stage at diagnosis of colorectal and lung cancers. Br J Cancer. 2001;84:910–914. doi: 10.1054/bjoc.2000.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pozet A, Westeel V, Berion P, Danzon A, Debieuvre D, Breton JL, Monnier A, Lahourcade J, Dalphin JC, Mercier M. Rurality and survival differences in lung cancer: a large population-based multivariate analysis. Lung Cancer. 2008;59(3):291–300. doi: 10.1016/j.lungcan.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman M, de Pinho H, Cooper D, Sayed R, Dent DM, Gudgeon A, van Zyl J, Rosenberg L, Shapiro S. Breast cancer incidence and determinants of cancer stage in the Western Cape. South African Medical Journal. 2000;90(12):1212–6. [PubMed] [Google Scholar]
- 17.Blair SL, Sadler GR, Bristol R, Summers C, Tahar Z, Saltzstein SL. Early cancer detection among rural and urban Californians. BMC Public Health. 2006;6:194. doi: 10.1186/1471-2458-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988–2000. Cancer. 2006;107(5 Suppl):1189–95. doi: 10.1002/cncr.22016. [DOI] [PubMed] [Google Scholar]
- 19.Shugarman LR, Sorbero ME, Tian H, Jain AK, Ashwood JS. An exploration of urban and rural differences in lung cancer survival among medicare beneficiaries. American Journal of Public Health. 2008;98(7):1280–7. doi: 10.2105/AJPH.2006.099416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinney AY, Harrell J, Slattery M, Martin C, Sandler RS. Rural-urban differences in colon cancer risk in blacks and whites: the North Carolina Colon Cancer Study. The Journal of Rural Health. 2006;22(2):124–30. doi: 10.1111/j.1748-0361.2006.00020.x. [DOI] [PubMed] [Google Scholar]
- 21.Bennett H, Marshall R, Campbell I, Lawrenson R. Women with breast cancer in Aotearoa New Zealand: the effect of urban versus rural residence on stage at diagnosis and survival. New Zealand Medical Journal. 2007;120(1266):U2831. [PubMed] [Google Scholar]
- 22.McElroy JA, Remington PL, Gangnon RE, Hariharan L, Andersen LD. Identifying geographic disparities in the early detection of breast cancer using a geographic information system. Prev Chronic Dis. 2006;3(1):A10. 2006. [PMC free article] [PubMed] [Google Scholar]
- 23.Lisovicz N, Johnson RE, Higginbotham J, Downey JA, Hardy CM, Fouad MN, Hinton AW, Partridge EE. The Deep South Network for cancer control. Cancer. 2006;107(S8):1971–1979. doi: 10.1002/cncr.22151. [DOI] [PubMed] [Google Scholar]
- 24.Haynes R, Pearce J, Barnett R. Cancer survival in New Zealand: Ethnic, social and geographical inequalities. Social Science and Medicine. 2008;67(6):928–37. doi: 10.1016/j.socscimed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 25.MacKinnon JA, Duncan RC, Huang Y, Lee DJ, Fleming LE, Voti L, Rudolph M, Wilkinson JD. Detecting an association between socioeconomic status and late stage breast cancer using spatial analysis and area-based measures. Cancer Epidemiology, Biomarkers and Prevention. 2007;16(4):756–62. doi: 10.1158/1055-9965.EPI-06-0392. [DOI] [PubMed] [Google Scholar]
- 26.Abe T, Martin IB, Roche LM. Clusters of census tracts with high proportions of men with distant-stage prostate cancer incidence in New Jersey. American Journal of Preventive Medicine. 2006;30(2, supp. 1):S60–S66. doi: 10.1016/j.amepre.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Barrett RE, Cho YI, Weaver KE, Ryu K, Campbell RT, Dolecek TA, Warnecke RB. Neighborhood change and distant metastasis at diagnosis of breast cancer. Annals of Epidemiology. 2008;18(1):43–7. doi: 10.1016/j.annepidem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Barry J, Breen N. The importance of place of residence in predicting late-stage diagnosis of breast or cervical cancer. Health and Place. 2005;11:15–29. doi: 10.1016/j.healthplace.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status and breast cancer treatment and survival. Journal of the National Cancer Institute. 2002;94:490–6. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 30.Paquette I, Finlayson SR. Rural versus urban colorectal and lung cancer patients: differences in stage at presentation. Journal of The American College of Surgeons. 2007;205(5):636–41. doi: 10.1016/j.jamcollsurg.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 31.Lehnerr M, Havener L. Assessment of Interstate Exchange of Cancer Data: Illinois, 1986–1998. Illinois State Department of Public Health; Springfield, Illinois: 2002. [Google Scholar]
- 32.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. National Cancer Institute; Bethesda, MD: 2001. NIH Pub. No. 01-4969. [Google Scholar]
- 33.Duncan C, Jones K, Moon G. Health-related behavior in context: a multilevel modeling approach. Social Science and Medicine. 1996;42(6):817–30. doi: 10.1016/0277-9536(95)00181-6. [DOI] [PubMed] [Google Scholar]
- 34.Hart LG, Larsen EH, Lishner DM. Rural definitions for health policy and research. American Journal of Public Health. 2005;95(7):1149–1155. doi: 10.2105/AJPH.2004.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rural Health Research Center, University of Washington, http://depts.washington.edu/uwruca/, accessed October 29, 2008.
- 36.Krieger N, Chen J, Waterman P, Soobader M, Subramanian S, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) Journal of Epidemiology and Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo W, Wang F. Measures of spatial accessibility to healthcare in a GIS environment: Synthesis and a case study in Chicago region. Environment and Planning B: Planning and Design. 2003;30:865–884. doi: 10.1068/b29120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang F. Quantitative methods and applications in GIS. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- 39.Liu L, Cozen W, Bernstein L, Ross RK, Deapen D. Changing relationship between socioeconomic status and prostate cancer incidence. Journal of National Cancer Institute. 2001;93:705–9. doi: 10.1093/jnci/93.9.705. [DOI] [PubMed] [Google Scholar]
- 40.Merkin S, Stevenson L, Powe N. Geographic socioeconomic status, race, and advanced-stage breast cancer in New York City. American Journal of Public Health. 2002;92:64–70. doi: 10.2105/ajph.92.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes and Control. 2003;14:761–6. doi: 10.1023/a:1026321923883. [DOI] [PubMed] [Google Scholar]
- 42.Pollack LA, Gotway CA, Bates JH, et al. Use of the spatial scan statistic to identify geographic variations in late stage colorectal cancer in California (United States) Cancer Causes Control. 2006;17:449–457. doi: 10.1007/s10552-005-0505-1. [DOI] [PubMed] [Google Scholar]
- 43.Rushton G, Peleg I, Banerjee A, Smith G, West M. Analyzing geographic patterns of disease incidence: rates of late-stage colorectal cancer in Iowa. Journal of Medical Systems. 2004;28(3):223–236. doi: 10.1023/b:joms.0000032841.39701.36. [DOI] [PubMed] [Google Scholar]
- 44.Sheehan J, DeChello L. A space-time analysis of the proportion of late stage breast cancer in Massachusetts, 1988 to 1997. International Journal of Health Geographics. 2005;4:15. doi: 10.1186/1476-072X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kauh J, Brawley O, Berger M. Racial disparities in colorectal cancer. Current Problems in Cancer Research. 2007;31(3):122–131. doi: 10.1016/j.currproblcancer.2007.01.002. 2007. [DOI] [PubMed] [Google Scholar]
- 46.Martin IK, Newman LA. Disparities in breast cancer. Current Problems in Cancer. 2007;31:134–156. doi: 10.1016/j.currproblcancer.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 47.McBride R, Hershman D, Tsai W, Jacobsen J, Grann V, Neugut D. Within-stage racial disparities in tumor size and number of positive nodes in women with breast cancer. Cancer. 2007 September 15;110(6):1202–1208. doi: 10.1002/cncr.22884. [DOI] [PubMed] [Google Scholar]
- 48.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates: separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97(11):2853–60. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 49.Berger M, Lund M, Brawley O. Racial disparities in lung cancer. Current Problems in Cancer. 2007;31(3):202–209. doi: 10.1016/j.currproblcancer.2007.02.002. [DOI] [PubMed] [Google Scholar]