Abstract
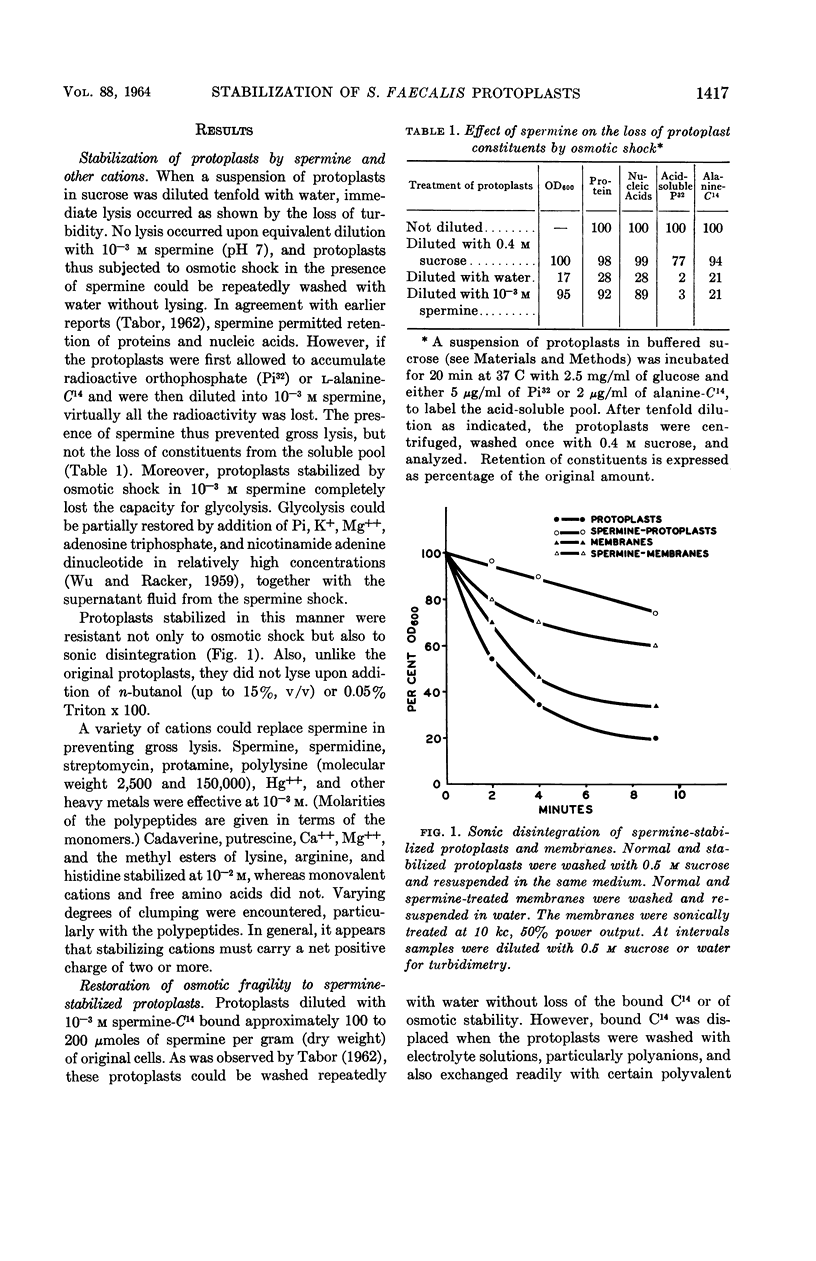
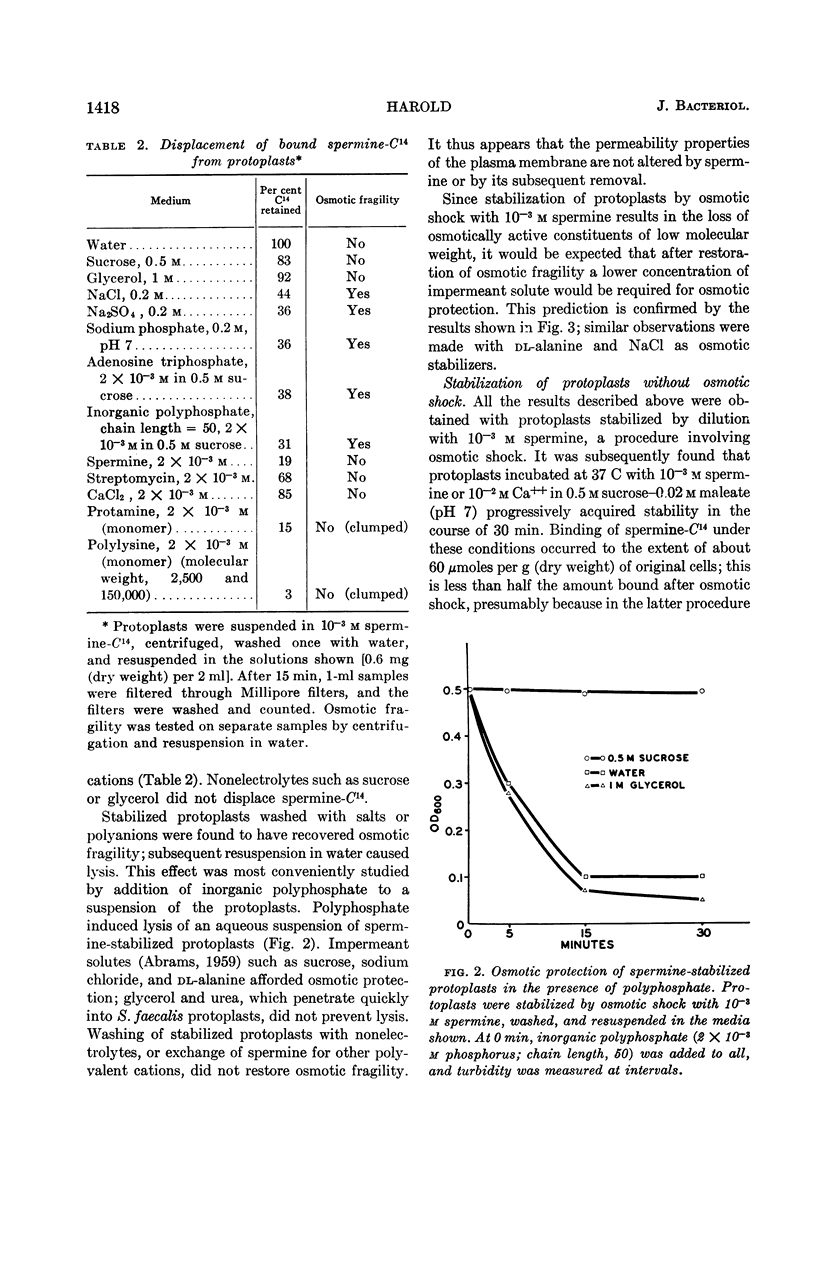
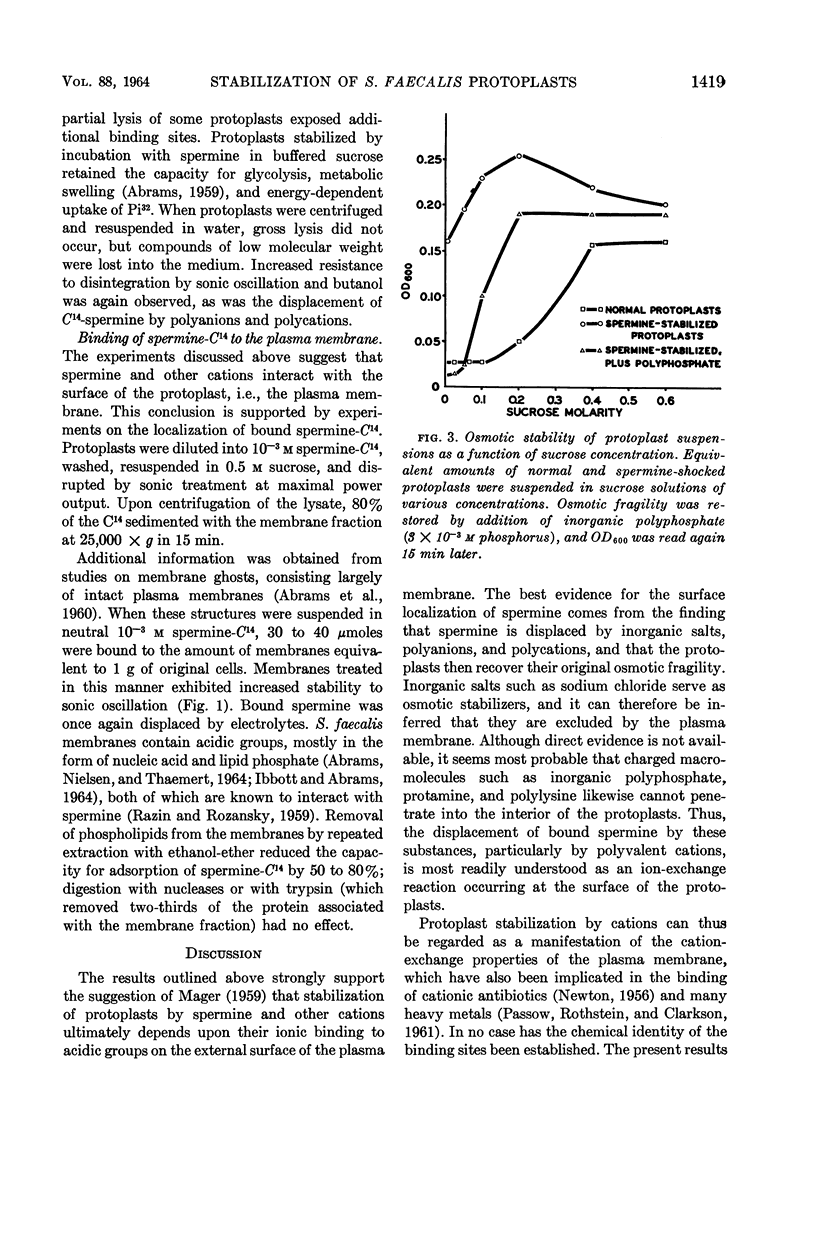
Harold, F. M. (National Jewish Hospital, Denver, Colo.). Stabilization of Streptococcus faecalis protoplasts by spermine. J. Bacteriol. 88:1416–1420. 1964.—Lysis of protoplasts of Streptococcus faecalis subjected to osmotic shock was prevented by the presence of 10−3m spermine and other divalent cations. Protein and nucleic acids were largely retained, but compounds of low molecular weight were discharged into the medium and the capacity for glycolysis was lost. Under these conditions, spermine was bound to the protoplasts. It could not be removed by washing with water or nonelectrolytes, but was displaced by salts, polyanions, and polycations. Removal of the spermine restored the osmotic fragility of the protoplasts, which could once again be protected from lysis by impermeant solutes. Protoplasts were also stabilized, in the absence of osmotic shock, by prolonged incubation with cations in 0.5 m sucrose. By either procedure, the protoplasts became resistant not only to osmotic lysis but also to sonic oscillation. It is concluded that the stabilization of protoplasts resulted from ionic binding of the cation to acidic sites on the external surface of the plasma membrane. This conferred upon the membrane additional mechanical strength, perhaps by the cross-linking of subunits, but did not alter its permeability to extracellular solutes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P., JOHNSON F. B. Adenosine triphosphatase in isolated bacterial cell membranes. J Biol Chem. 1960 Dec;235:3649–3662. [PubMed] [Google Scholar]
- ABRAMS A., NIELSEN L., THAEMERT J. RAPIDLY SYNTHESIZED RIBONUCLEIC ACID IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FECALIS PROTOPLASTS. Biochim Biophys Acta. 1964 Feb 17;80:325–337. doi: 10.1016/0926-6550(64)90104-5. [DOI] [PubMed] [Google Scholar]
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- FEW A. V., GILBY A. R., SEAMAN G. V. An electrophoretic study on structural components of Micrococcus lysodeikticus. Biochim Biophys Acta. 1960 Feb 12;38:130–136. doi: 10.1016/0006-3002(60)91202-6. [DOI] [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- HAROLD F. M. ACCUMULATION OF INORGANIC POLYPHOSPHATE IN AEROBACTER AEROGENES. I. RELATIONSHIP TO GROWTH AND NUCLEIC ACID SYNTHESIS. J Bacteriol. 1963 Aug;86:216–221. doi: 10.1128/jb.86.2.216-221.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGER J. The stabilizing effect of spermine and related polyamines and bacterial protoplasts. Biochim Biophys Acta. 1959 Dec;36:529–531. doi: 10.1016/0006-3002(59)90195-7. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSOW H., ROTHSTEIN A., CLARKSON T. W. The general pharmacology of the heavy metals. Pharmacol Rev. 1961 Jun;13:185–224. [PubMed] [Google Scholar]
- RAZIN S., ROZANSKY R. Mechanism of the antibacterial action of spermine. Arch Biochem Biophys. 1959 Mar;81(1):36–54. doi: 10.1016/0003-9861(59)90173-0. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D. Quantitative amino acid assimilation in homofermentative metabolism. Arch Biochem Biophys. 1953 Aug;45(2):447–458. doi: 10.1016/s0003-9861(53)80021-4. [DOI] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]


