Abstract
Head and neck squamous cell carcinomas (HNSCC) are one of the leading causes of cancer deaths world wide. Up-regulation of the epidermal growth factor receptor (EGFR) and BCL-2 family anti-apoptosis proteins in these cancers is linked to aggressive tumor growth, metastasis and chemoresistance. Infection of two HNSCC cell lines, SCC25 and CAL27 by an Ad5 mutant (lp11w) defective in coding for the viral anti-apoptosis protein, E1B-19K efficiently induced apoptotic cell death. In cells infected with lp11w there was a dramatic down-regulation of EGFR by apoptosis-dependent and -independent mechanisms. The levels of the anti-apoptotic proteins BCL-2, BCL-xL and MCL- 1 were also down-regulated in lp11w-infected cells compared to uninfected or Ad5 wt infected cells. Infection with lp11w also enhanced sensitivity of the HNSCC cells to the chemotherapeutic drug cisplatin. Our results suggest that adenoviral vectors defective in E1B-19K would be valuable for efficient down-regulation of cell survival proteins and EGFR in epithelial cancers and could be exploited as oncolytic agents to treat HNSCCs.
Keywords: adenovirus; E1B-19K, EGFR; BCL-2, BCL-xL; MCL-1; apoptosis; cisplatin; HNSCC
Introduction
Head and neck cancers are emerging as a major malignant disease in the US accounting for about 2.2% of all cancer deaths in humans (Jemal et al., 2008). Squamous cell carcinoma accounts for more than 90% of all head and neck cancers (Marur and Forastiere, 2008). More than 500,000 new cases of head and neck squamous cell carcinoma (HNSCC) are detected every year world wide (Parkin et al., 2005). The majority of head and neck carcinomas are highly refractory to various chemotherapy and radiotherapy regimens (Baselga et al., 2006; Bonner et al., 2006; Huang, Bock, and Harari, 1999). A hallmark of HNSCCs is that they overexpress the epidermal growth factor receptor (EGFR) (Kalyankrishna and Grandis, 2006). Elevated EGFR-mediated signaling appears to be linked with increased cell proliferation, tumor growth and metastasis as a result of activation of cell cycle regulatory proteins (reviewed by (Siddiquee and Turkson, 2008)). The chemoresistance of HNSCCs has been attributed to overexpression of BCL-2 family anti-apoptosis protein such as BCL-xL, BCL-2 and MCL-1. EGFR-signaling has been linked to overexpression of these anti-apoptosis proteins (reviewed by (Siddiquee and Turkson, 2008)). Agents such as anti-EGFR antibodies and EGFR tyrosine kinase inhibitors are being widely used to sensitize HNSCC cells for anticancer agents (reviewed in (Reuter, Morgan, and Eckardt, 2007)), Strategies such as the use of anti-sense oligonucleotides targeted against BCL-xL and BCL-2 and short peptides or BH3 mimetics corresponding to the BH3 domain of BH3-only pro-apoptotic proteins that engage members of the BCL-2 family anti-apoptotic proteins (Bauer et al., 2005; Sharma et al., 2005) have also been reported to sensitize HNSCC cells for chemotherapeutic agents.
Chemotherapy and radiotherapy constitute the standard modality of treatment for localized HNSCCs. However, relapses after such treatments are frequent and are generally incurable and fatal (Boyle et al., 1993), highlighting the need for alternate modalities of treatment for these ‘difficult’ cancers. The use of oncolytic viral vectors for the treatment of HNSCC appears to be a promising approach since HNSCC are localized in the upper aerodigestive tract and the tumors could be readily injected with the viral vectors. Further, regional metastasis of these tumors to cervical lymph nodes could also be treated with localized administration of the viral vectors which would be expected to be an efficacious mode of treatment either as a primary treatment or as an adjunct. Both replication-defective and replication-competent adenovirus vectors have been used for localized treatment of HNSCC. A replication-defective vector expressing p53 (Ad-p53, Advexin) is in phase III clinical trials (Boyle et al., 1993; Pan et al., 2009). The use of a replication-competent vector ONYX-015, in combination therapy with radiation or treatment with cisplatin and 5-flurouracil for treatment of HNSCC, resulted in enhanced efficacy compared to treatment with the vector alone (Khuri et al., 2000). The use of conditionally replicating adenovirus vectors that exploit transcriptional strategies specific to tumor cells or targeted to HNSCC via EGFR have also been explored (Blackwell et al., 1999; Chia et al., 2004; Toivonen et al., 2009).
We undertook a study to identify potential adenovirus vectors that are more suited to counter the effects of the anti-apoptotic proteins on chemosensitization of HNSCC cells. Since both E1A and E1B regions of adenovirus modulate the cellular apoptotic machinery, we examined the effect of a battery of adenovirus mutants in the E1A and E1B regions. We show that infection with an adenovirus type 5 mutant defective in E1B-19K mediates efficient down-regulation of EGFR and BCL-2 family anti-apoptotic proteins and confers chemosensitivity to HNSCC cells.
Materials and Methods
Cells and chemicals
The human tongue squamous cell carcinoma cell lines SCC25 and CAL27 were purchased from ATCC (Manassas, VA). SCC25 cells were cultured in DMEM-Ham's F-12 media supplemented with hydrocortisone (400 ng/ml). CAL27 cells were cultured in DMEM. The growth media were supplemented with fetal bovine serum (10%), penicillin (10,000 U/mL), streptomycin (10 mg/mL), and amphotericin B (25 µg/mL) that were purchased from Sigma (St. Louis, MO). Cisplatin (cis-Diammineplatinum (II) dichloride) and cytosine arabinoside (AraC, 1β-D-arabinofuranosyl cytosine) were purchased from Sigma (St. Louis, MO). The caspase inhibitor zVAD-fmk was purchased from MBL International (Woburn, MA).
Virus
Ad5 (VR1516) was obtained from American type culture collection and is designated here as Ad5-RM (Reference Material). It corresponds to the adenovirus type 5 reference material (ARM) described elsewhere (Sugarman et al., 2003). Ad5 mutants and recombinants AdΔE1B, Ad-BCL-xL, Ad-BCL-xL-mt1 and Ad-BCL-xL-mt8 (Subramanian et al., 2007), lp11Δ327, lp11w, lp53w (Subramanian, Vijayalingam, and Chinnadurai, 2006), dl312 (Jones and Shenk, 1979; Subramanian, Tarodi, and Chinnadurai, 1995), dl327 (Thimmappaya et al., 1982), dl1101, dl1102 and dl1108 (Jelsma et al., 1988) have been described previously. Ad5-E1AΔ2-11 was constructed by co-transfection of an E1A plasmid (pLendE1AΔ2-11) with pacAd5-9.2-100 (Anderson et al., 2000) into 293 cells. The stocks of different viruses were prepared in 293 cells and banded in CsCl gradients and titrated in A549 or 293 cells.
Cell viability assays
Cell viability was determined by the MTS assay (Promega) as described (Subramanian, Vijayalingam, and Chinnadurai, 2006). SCC25 cells (5×103 cells/well of 96-well plate) and CAL27 cells (1×104 cells/well of 96-well plate) were infected with various viruses at indicated MOI (PFU/cell). The viability assays were performed 48 or 144 hr after infection.
Antibodies, Immunofluorescence and Western blot analyses
Rabbit monoclonal antibody against EGFR (# 04-338), rabbit polyclonal antibody against BAX (# 06-499), BAK (# 06-536) and mouse monoclonal antibody against E1A (M73) were purchased from Millipore (Billerica, MA). Rabbit polyclonal antibodies specific to Ad5 (adenovirus) and caspase-3 antibody were purchased from Abcam (Cambridge, MA) and Cell Signaling Technology (Danvers, MA), respectively. Rabbit polyclonal antibodies specific to MCL-1 were purchased from Stressgen (B.C., Canada). BCL-2 (hamster polyclonal), BIM (rabbit polyclonal) and PARP (mouse monoclonal) antibodies were purchased from BD Pharmingen (San Jose, CA). Rabbit polyclonal antibody to BIK, mouse monoclonal antibody to BCL-xL and goat polyclonal antibody to actin were purchased from Santa Cruz Biotechnology (San Diego, CA). The antibodies used in immunofluorescence analysis, mouse monoclonal EGFR antibody (SC-120) and secondary antibody conjugated with Alexa Fluor 488 were purchased from Santa Cruz Biotechnology (San Diego, CA) and Invitrogen (Carlsbad, CA), respectively. At 24 hr post-infection, cells were lyzed and the proteins were fractionated by NuPAGE 4–12% Bis-Tris gel (Invitrogen) electrophoresis. The separated proteins were electrotransferred onto a nitrocellulose membrane and probed with a primary antibody followed by horseradish peroxidase-conjugated secondary antibodies and analyzed with a chemiluminescence detection system (Roche Applied Science, Indianapolis, IN).
FACS analysis
SCC25 cells were mock infected or infected with dl312, Ad5-RM, and lp11w (100 PFU/cell). At 24 hr post infection, cells were trypsinized and stained with recombinant human annexin-V APC (CALTAG laboratories, Invitrogen) and propidium iodide (PI) (Roche Applied Science) in annexin-V binding buffer (BD Pharmingen) according to manufacturer recommendation. Stained cells were analyzed on a FACS calibur flow cytometer (Becton Dickinson, Palo Alto, CA) and the data were analyzed with CellQuest software (Becton Dickinson).
Confocal microscopy
SCC25 and CAL27 cells were plated on glass cover slips, infected with indicated viruses at an MOI of 50 PFU/cell and at 20 hr post infection cells were fixed with 3.7% formaldehyde at room temperature for 10 min and permeabilized with methanol at −20°C for 6 min. The cells were rehydrated with three washes of PBS (phosphate-buffered saline) and blocked with 3% BSA (bovine serum albumin) in PBS. Cells were incubated in mouse monoclonal EGFR antibody at 1:100 dilution and secondary antibody (conjugated with Alexa Flour 488) at 1:300 dilution. Antibodies were diluted in PBS containing 3% BSA. Nuclei were stained with PI at 0.3 µg/ml. The cover slips were mounted onto slides with Vectashield mounting media (Vector Laboratories Inc. Burlingame, CA). Cells were imaged at 60× using an Olympus FV-1000 MPE Confocal microscope and a water immersion 20× (NA = 0.95) plan-achromatic objective lens operated at an optical zoom of 3× and with a 10 µsec dwell time and a resolution of 1024 × 1024. Post image acquisition processing was carried out with Image J software and then cropped in Adobe Photoshop.
Light and Transmission Electron Microscopy
SCC25 cells were mock infected or infected with indicated viruses at an MOI of 100 PFU/cell. At 24 hr post infection, cells were collected by treatment with trypsin and washed with PBS. Cell pellets were fixed in 3.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.25) containing 5% sucrose and 2 mM calcium chloride for 16 hr at 4°C. The cell pellets were then washed 2 × 15 min in 0.1 M sodium cacodylate buffer containing 5% sucrose (this and all subsequent steps up to polymerization are at room temperature) and post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer containing 5% sucrose for 4 hr at room temperature on a rotator. The pellets were then washed 2 × 10 min in distilled water, dehydrated in 2 × 10 min steps through 35%, 50%, 75%, 95% and 100% ethanol, washed 2 × 10 min in propylene oxide and infiltrated with a 1:1 mixture of PolyBed resin (Polysciences, Inc., Warrington, PA) and propylene oxide overnight on a rotator. The pellets were then incubated in fresh PolyBed resin for 6 hr, transferred to BEEM capsules filled with fresh resin, and polymerized overnight at 70°C. For light microscopy 0.5 µm thick sections were cut using glass knives on a Reichert Ultracut E ultramicrotome (Depew, NY), collected and heat-attached to glass slides, stained with toluidine blue and cover-slipped in Permount mounting medium. Light micrographs were taken with a Zeiss research light microscope and an Olympus digital camera. For TEM 50 nm sections were cut with a diamond knife on a Reichert Ultracut E ultramicrotome, collected on 200 mesh copper grids, post-stained with uranyl acetate and lead citrate, and viewed and photographed on a JEOL 100CX electron microscope. EM negatives were digitized using an Epson V700 scanner and copied on to Microsoft PowerPoint.
Viral replication
Real time PCR analysis was used to quantify the relative viral DNA copy number. The PCR reaction was carried out using primers corresponding to the E3 region as described (Ying et al., 2009). The following primers were used-forward primer -5’ TTT ATG AAA TGT GCG AGA TTA CCA T-3’, probe – 5’-CAA ACA GTA TAA GTT GTG GCC CCC-3’ and reverse primer -5’ GCG AGC ACT GTA ATT AGC ATA GC-3’. The probe was modified with the fluorophore 6-FAM at the 5’ end and the quencher TAMRA at the 3’ end. The PCR reactions were carried out using Real-time PCR 7500 (Applied Biosystems).
Results
Oncolytic activity of Ad mutants
In order to identify adenovirus vectors with enhanced oncolytic activity in HNSCC cells, we first compared the oncolytic activity of Ad5-RM and several mutants bearing lesions in the E1A region (Fig. 1). The E1A region codes two protein isoforms of 289 amino acid residues and 243 amino acid residues from two differentially spliced mRNAs of 13S and 12S. We included viral mutants (that express both mRNA species) with deletions in exon 1 known to affect the major functional domains of E1A proteins (Pelka et al., 2008). The specific interest on exon 1 was based on a report that showed enhanced oncolysis of ovarian cancer cells by an exon 1 mutant that induced a unique mode of cell death (Baird et al., 2008). A mutant that lacked most of the E1A coding region (dl312) was included as negative control. In addition to the mutants in the E1A region, we also examined the activity of a mutant in the E1B-19K region (lp11w) and a mutant in the i-leader region (lp53w). These two mutants were included on the basis of our report that they cause enhanced cell lysis and viral spread in a lung cancer cell line (Subramanian, Vijayalingam, and Chinnadurai, 2006).
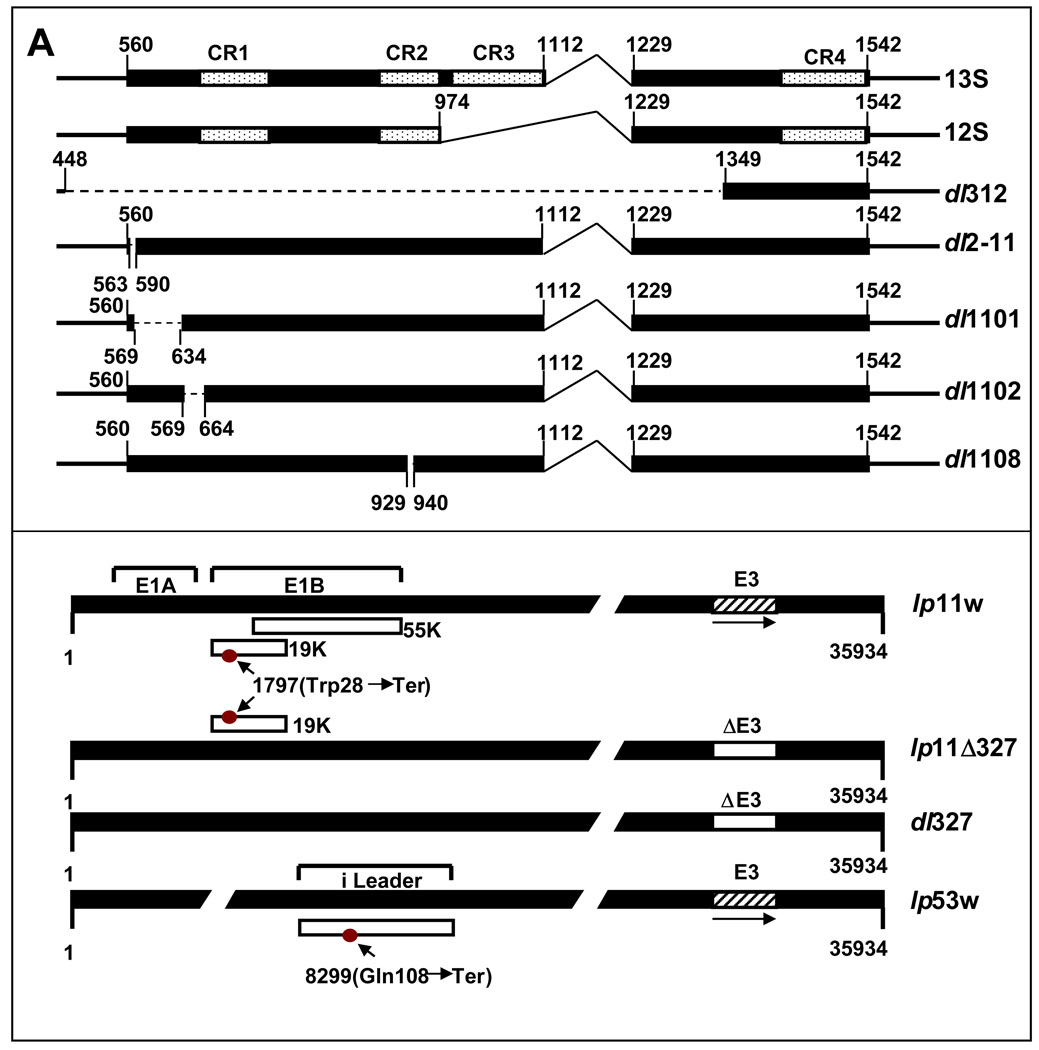
Figure 1. Effect of Ad5 mutants on cell lysis.
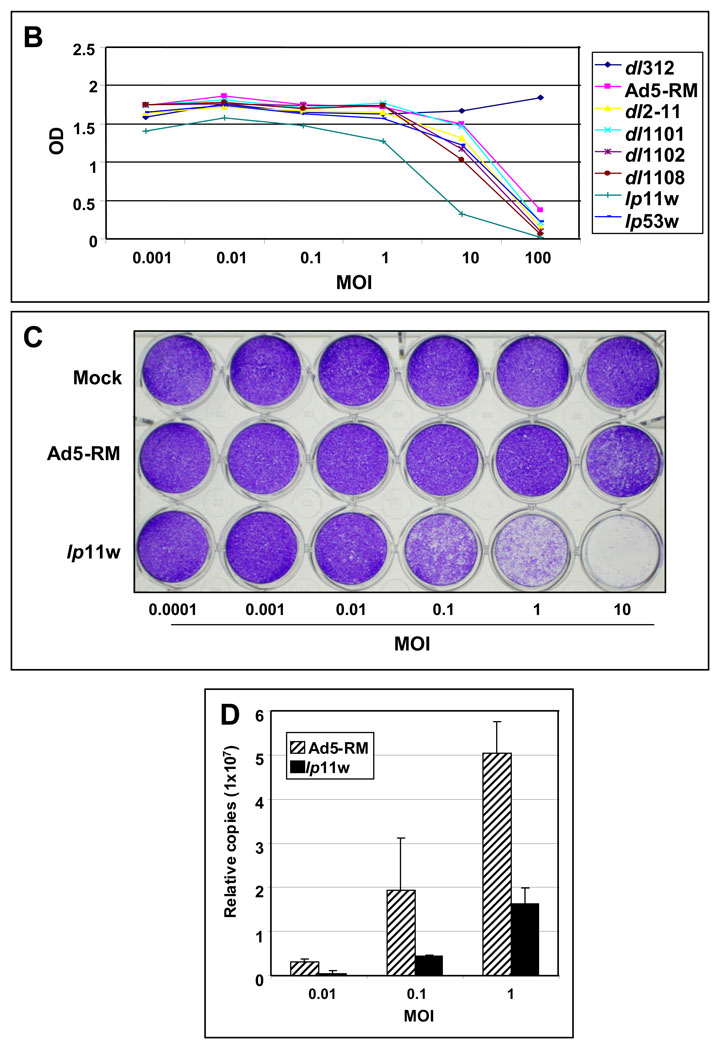
(A). Diagrammatic illustration of Ad5 mutants. The top panel shows the organization of the E1A region and mutants in the E1A region. The bottom panel shows mutants in E1B, E3 and ‘I’-leader regions. The nucleotide positions corresponding to various mutations are indicated. (B). Effect of Ad5 mutants on cell viability. SCC25 cells in 96-well plates were infected with indicated viruses at various MOIs (PFU/cell), and cell viability was determined by the MTS assay 144 hr after infection. (C) Viral spread assay. SCC25 cells in 24-well plates were infected with Ad5-RM or lp11w at various MOIs and stained with crystal violet 144 hr after infection. (D). Replication of Ad5-RM and lp11w. The relative viral genome copy number was determined by real-time PCR analysis 144 hr after infection of SSC25 cells with Ad5-RM or lp11w.
A prototypical HNSCC cell line, SCC25, was infected with various viral mutants (Fig. 1A) at different MOIs and the cell viability was determined by the MTS assay 6 days post infection (Fig. 1B). Mutant dl312 did not cause significant cell death while mutant lp11w caused the most pronounced cell death. The effect of lp11w was also compared with Ad5-RM in a viral spread assay. SCC25 cells in 24-well plates were either mock-infected or infected with Ad5-RM or lp11w at different MOIs and stained with crystal violet after 144 hr post infection (Fig. 1C). At an MOI of 10, lysis was detectable in cells infected with Ad5-RM. A comparable level of lysis was observed in cells infected with lp11w at an MOI of 0.1 suggesting that lp11w was at least 100-fold more efficient in cell lysis and cell to cell spread. In spite of enhanced cell lysis, lp11w replicated at reduced efficiency in SSC25 cells as the cells infected with lp11w contained about one third of the viral DNA copies compared to cells infected with Ad5-RM (Fig. 1D). Based on these assays, we chose lp11w for further studies.
Apoptotic activity of lp11w
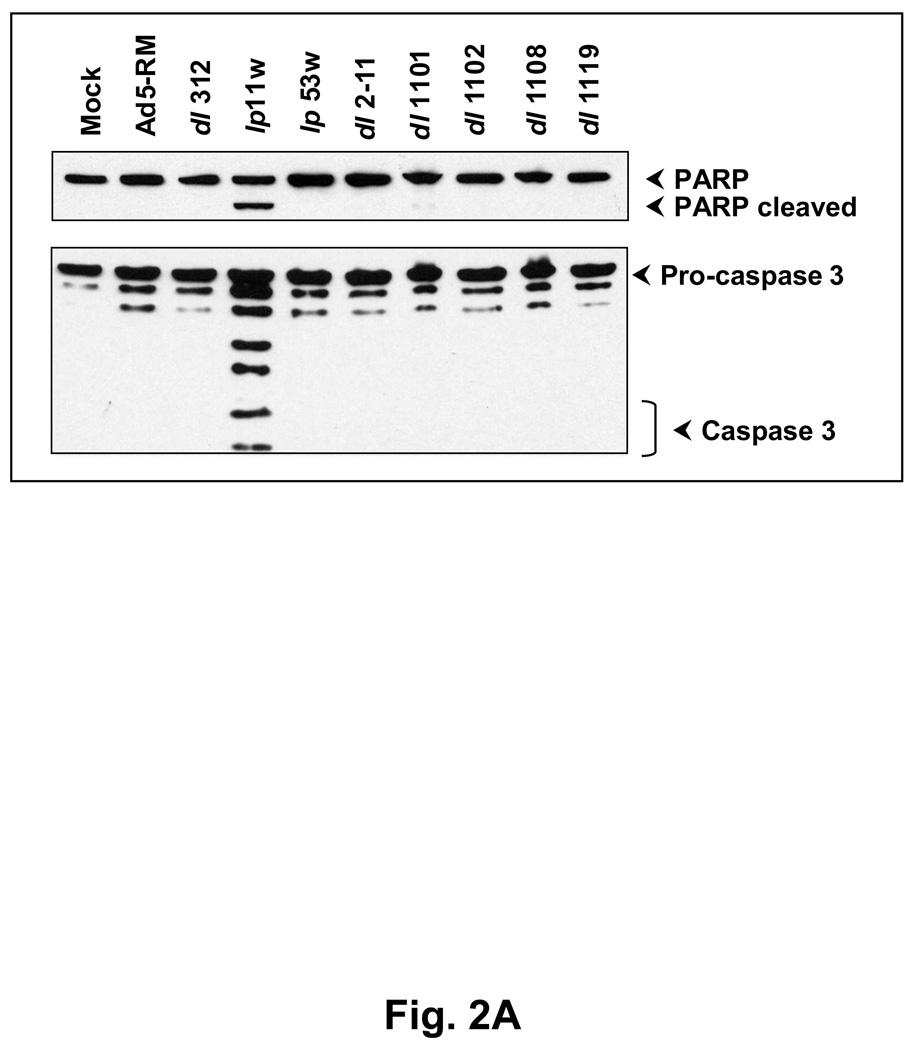
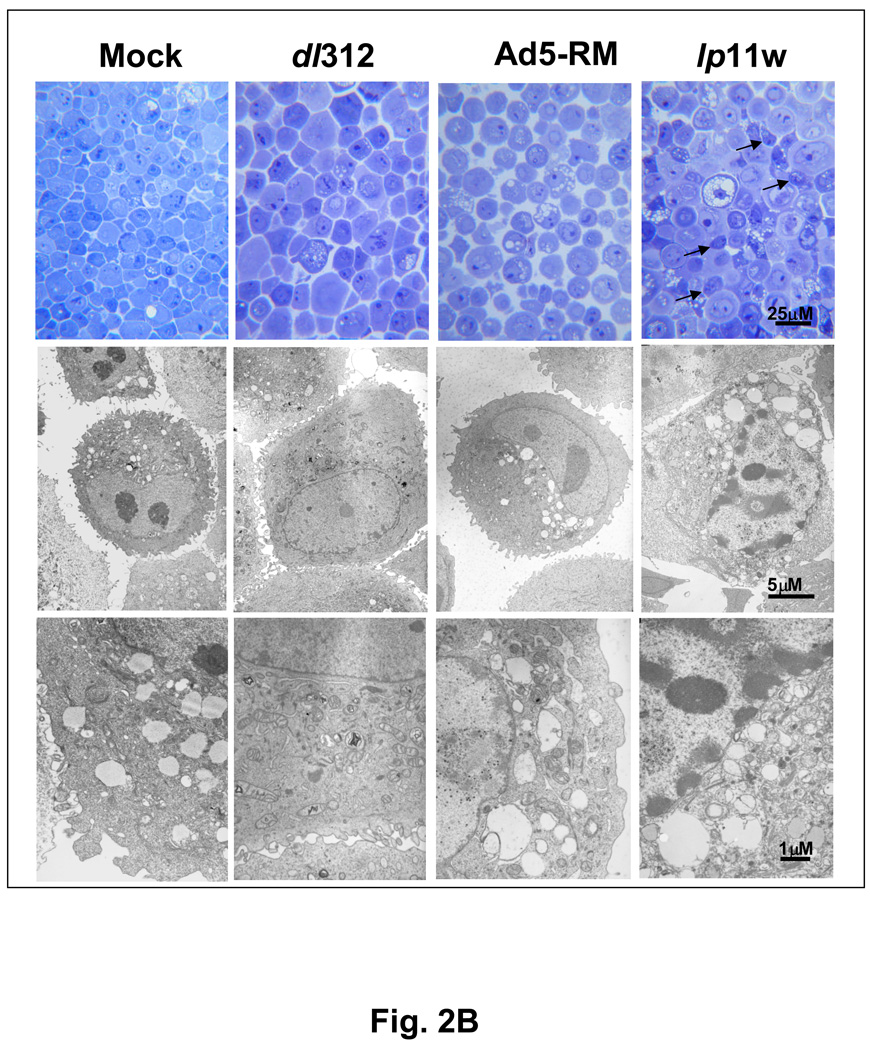
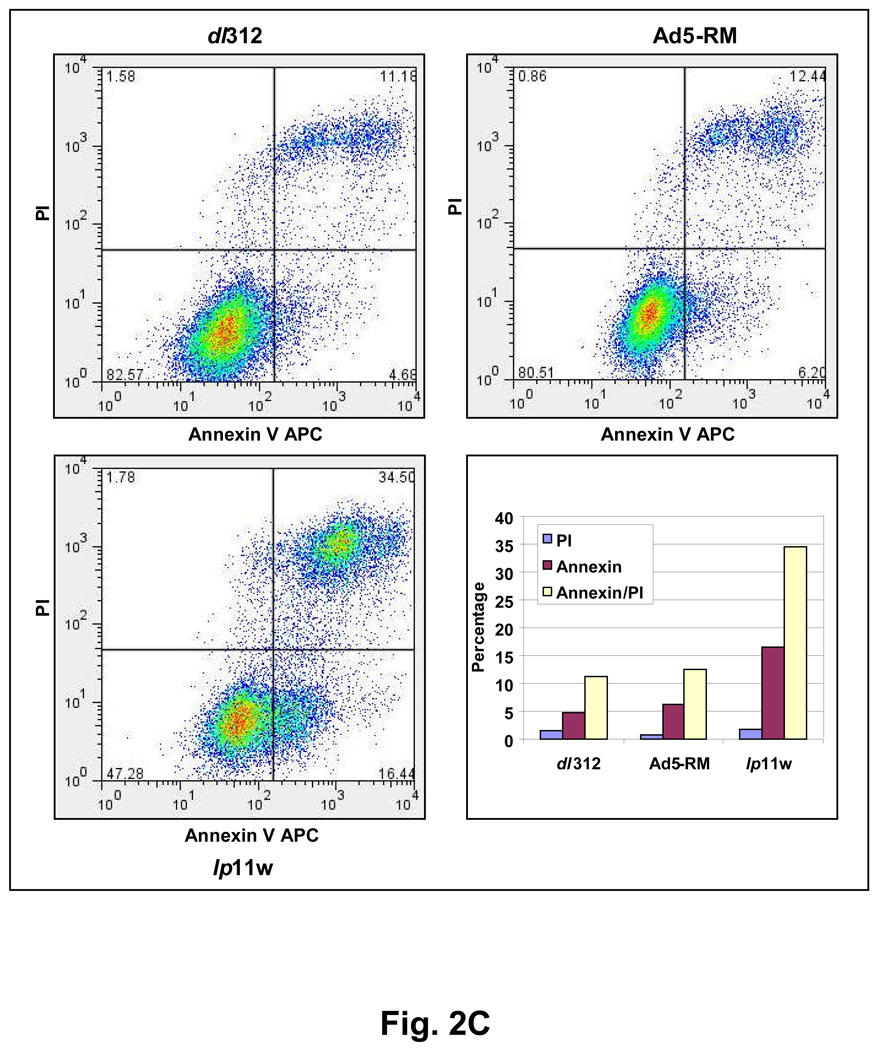
Since E1B-19K is an anti-apoptosis protein and mutants defective in the 19K locus induce apoptosis in several cultured cell lines (Chinnadurai, 1998; Cuconati and White, 2002), we examined the apoptotic activity of lp11w. We analyzed the activation of caspase-3 and proteolytic processing of poly (ADP-ribose) polymerase (PARP) and other biochemical signatures of apoptosis. Among the mutants examined, only lp11w induced caspase-3 activation (formation of 20 kD and 18 kD subunits) and proteolytic processing of PARP (a caspase target), suggesting efficient apoptosis induction by lp11w (Fig. 2A). Light microscopic and electron microscopic analysis of thin sections of SCC25 cells infected with lp11w revealed condensed chromatin and apoptotic bodies (Fig. 2B). Such features were not readily detectable in cells infected with Ad5-RM. Similarly, the level of apoptosis was also measured by FACS analysis of cells simultaneously stained with annexin V-APC and the non-vital stain propidium iodide (PI). While there was no major difference in non-apoptotic (necrotic) cell death (based on the PI-staining pattern) among cells infected with different viruses, there was increased apoptosis as indicated by annexin V-staining (which detects externalization of membrane phosphatidyl serine during early phase of apoptosis) in cells infected with lp11w compared to mock-infected cells and cells infected with Ad5-RM (Fig. 2C). The cell population in the transition phase (apoptosis to necrosis, positive for both Annexin V and PI staining) also paralled the apoptotic population. These results showed that mutant lp11w was highly apoptogenic in HNCSS cells. The levels of apoptosis induced by lp11w in HNSCC cells were consistently higher than in lung cancer and colon cancer cell lines (not shown).
Figure 2. Apoptotic activity of lp11w.
(A). Effect of Ad5 mutants on caspase activation. SCC25 cells were infected with indicated mutants and proteolytic processing of pro-caspase 3 and PARP were determined by western blot analysis. (B). Ultra-structure of infected cells. Thin sections of Ad-infected cells were analyzed by light microscopy (top panel) and by electron microscopy (bottom two panels). Apoptotic cells in the top panel of lp11w-infected samples are pointed out by arrows. (C). Flow cytometric analysis of annexin V-staining patterns of Ad-infected cells. The quantification of annexin V-positive and propidium iodide-positive cells is shown in the bar diagram.
Effect of lp11w on expression of BCL-2 family proteins and EGFR
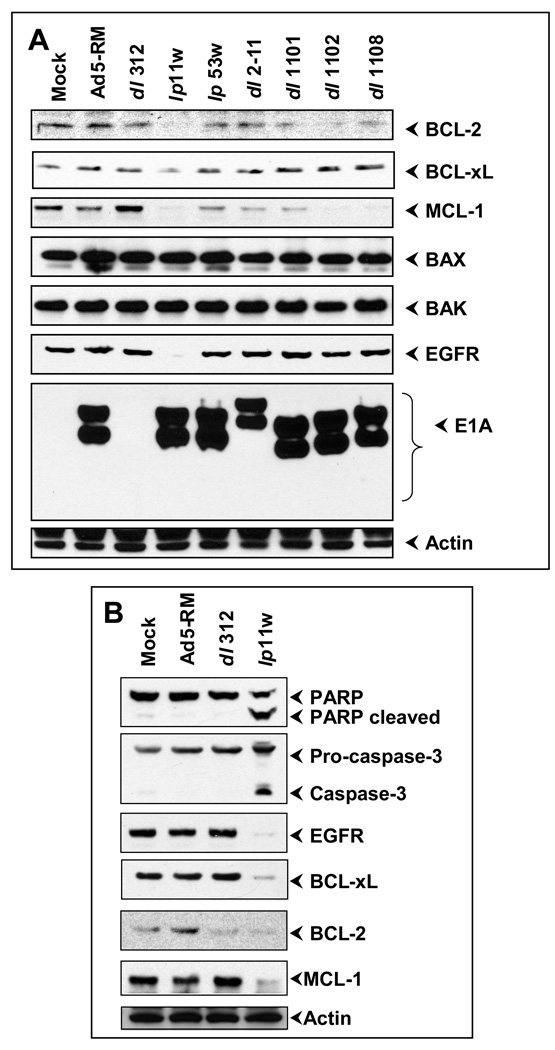
Since overexpression of BCL-2 family anti-apoptosis proteins and EGFR is a hall mark of HNCSS cells, we determined the effect of lp11w on the expression of these proteins. SCC25 cells were infected with various mutants and the levels of various anti-apoptotic and pro-apoptotic proteins and EGFR were examined by western blot analysis (Fig. 3A). There was no significant difference in the levels of the multi-domain (BCL-2 Homology Domain 1–3) pro-apoptotic proteins BAX and BAK and actin. In contrast, the level of MCL-1 was reduced in cells infected with lp11w as well as in cells infected with certain exon 1 mutants such as dl1102 and dl1108. The levels of BCL-xL and BCL-2 were reduced in cells infected with lp11w, albeit the effect on BCL-xL was modest. A striking effect was observed in the expression of EGFR. In cells infected with lp11w, there was no detectable EGFR expression as early as 15 hr post infection. The effect of lp11w on the expression of anti-apoptotic proteins and EGFR was also investigated in another HNSCC cell line, CAL27 (Fig 3B). In these cells also lp11w induced efficient apoptosis as judged by the processing of PARP and activation of caspase-3 (Fig. 3B, top two panels). There was severe loss of BCL-2, MCL-1 and EGFR and modest loss of BCL-xL. These results indicated that in two different HNSCC cell lines, infection with the E1B-19K mutant lp11w resulted in down-regulation of BCL-2 family anti-apoptosis proteins BCL-2, BCL-xL and MCL-1 and EGFR.
Figure 3. Effect of Ad-infection on anti-apoptotic and pro-apoptotic proteins.
(A). Western blot analysis of Ad-infected SCC25 cells. Western-blot analyses were carried out at 15 or 20 hr post infection. (B). Western-blot analysis of Ad-infected CAL27 cells.
Mode of EGFR down-regulation
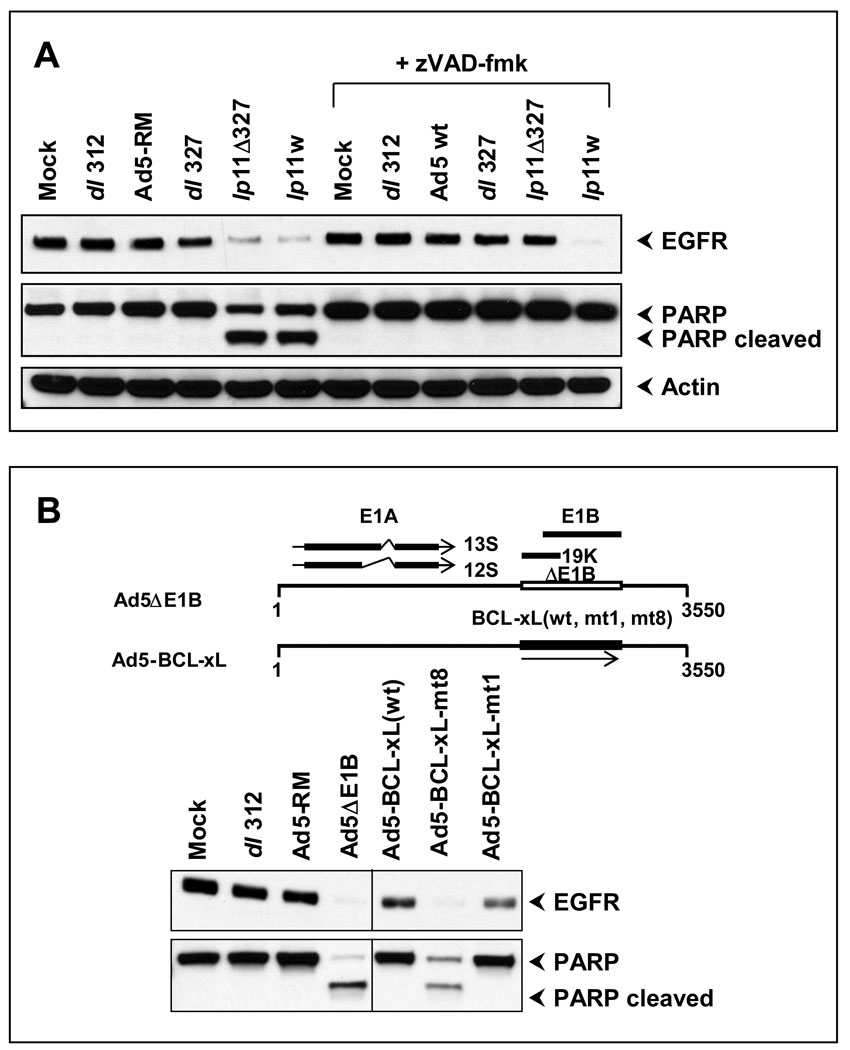
The dramatic effect of lp11w on EGFR down-regulation is of significant interest due to its importance in HNSCC and in other epithelial cancers. We examined the mode of down-regulation of EGFR in lp11w-infected cells. A post-transcriptional mechanism of down-regulation of EGFR that depended on two viral early proteins, RIDα and RIDβ coded by the early region E3 has been identified (Carlin et al., 1989; Tollefson et al., 1991). To determine the role of the E3 region on the observed effect of lp11w, we compared the levels of EGFR between CAL27 cells infected with mutants that contained or lacked the E3 region (dl327) (Thimmappaya et al., 1982). There was no significant difference in the levels of EGFR between cells infected with Ad5-RM or dl327 (ΔE3) (Fig. 4, top panel). In contrast, cells infected with lp11w (+ E3) and cells infected with lp11Δ327 (Subramanian, Vijayalingam, and Chinnadurai, 2006), contained drastically reduced levels of EGFR (Fig. 4). A close comparison of cells infected with lp11Δ327 and cells infected with lp11w revealed that there were higher levels of EGFR down-regulation in cells infected with lp11w than in cells infected with lp11Δ327. These results suggested that the lack of E1B-19K contributed strongly to the down-regulation of EGFR and the presence of E3 contributed additionally. Since reduction of EGFR in cells infected with lp11w and lp11Δ327 accompanied by PARP processing (Fig. 4, middle panel), we concluded that EGFR down-regulation in these cells were predominantly related to the apoptotic activities of these mutants. To substantiate this conclusion, we treated the infected cells with the pan-caspase inhibitor zVAD-fmk. This treatment blocked proteolytic processing of PARP in cells infected with lp11w or lp11Δ327, suggesting inhibition of apoptosis in virus infected cells by zVAD-fmk (Fig. 4, middle panel). The treatment with zVAD-fmk blocked EGFR down-regulation in cells infected with lp11Δ327 but not in cells infected with lp11w. These results suggested that in the absence of apoptosis, the E3 proteins down-regulated EGFR. Similar results were also obtained with SCC25 cells (not shown).
Figure 4. Apoptosis-dependent clearance of EGFR.
(A). CAL27 cells were infected with indicated viruses and incubated with zVAD-fmk (50 µM) or with vehicle and the effects on EGFR and PARP were determined by western blot analysis at 20 hr post-infection. (B). CAL27 cells were infected with indicated mutants and recombinants (top) and the effects on EGFR and PARP were determined by western blot analysis.
The role of apoptosis in down-regulation of EGFR was further substantiated by the use of Ad5 recombinants (that also lacked the sequences the RID-coding region; (Anderson et al., 2000)) that expressed BCL-xL wt or an anti-apoptotic mutant (mt1) or a pro-apoptotic mutant (mt8) (Fig. 4B, top panel) The down-regulation of EGFR was observed only in cells infected with Ad-BCL-xL mt 8 and not in cells infected with Ad-BCL-xL wt or Ad-BCL-xL mt 1 (Figure 4B, bottom panel).
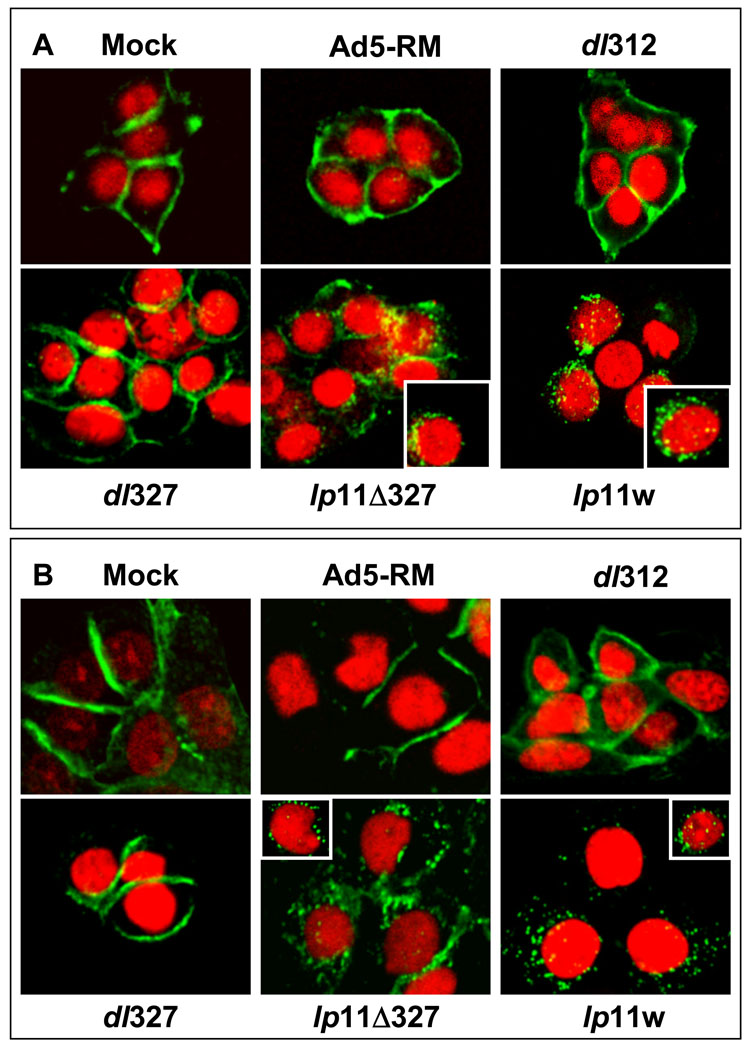
The effect of lp11w on down-regulation of EGFR was also analyzed by indirect immunofluorescence analysis (Fig. 5). CAL27 and SCC25 cells were infected with dl312, dl327, lp11Δ327 or lp11w, stained with the EGFR antibody and also with propidium iodide. In cells infected with dl312 or dl327, EGFR was localized on the plasma membrane. In cells infected with lp11Δ327 or lp11w, EGFR was localized in punctate cytosolic locations, suggesting clearing of EGFR from the cell surface, internalization and degradation. Taken together these results suggested that down-regulation of EGFR in lp11w-infected cells was predominantly the result of apoptosis whereas non-apoptotic down-regulation was mediated by E3 proteins. However, under non-apoptotic conditions, EGFR down-regulation was primarily mediated by the E3 region.
Figure 5. Immunofluorescence analysis of EGFR expression in Ad-infected cells.
CAL27 (A) or SCC25(B) cells were infected with indicated viruses and indirect immunofluorescence analysis and confocal microscopy were carried out cells fixed at 20 hr post-infection.
Chemosensitization of HNSCC cells
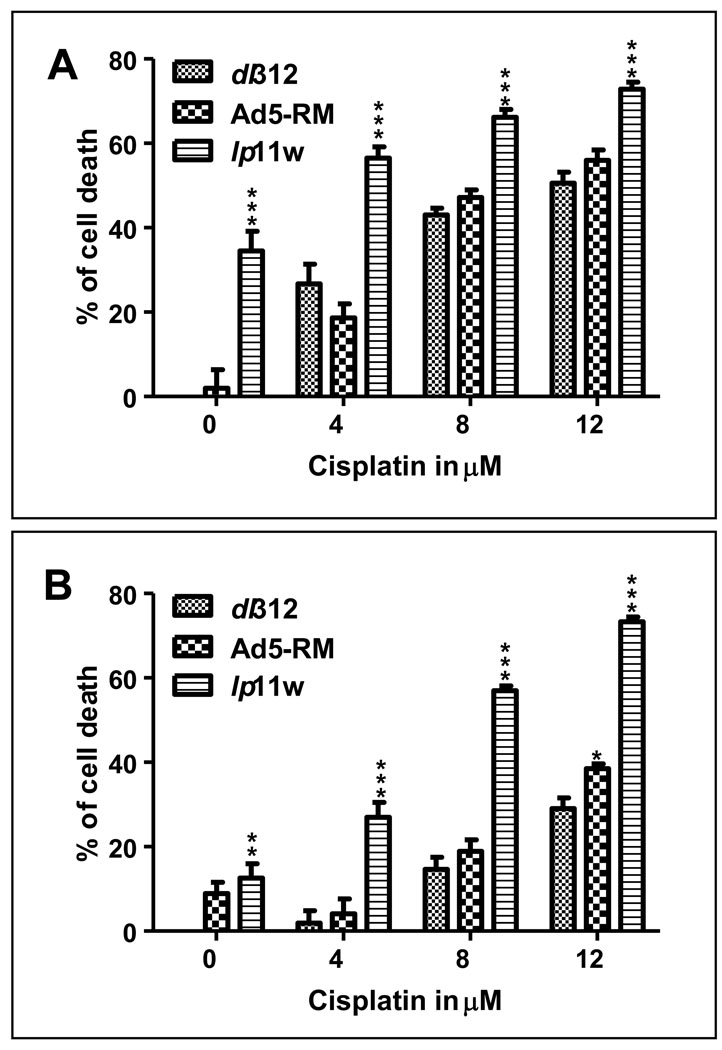
We then examined whether the effects of lp11w on the expression of EGFR and anti-apoptosis proteins would be reflected in conferring chemosensitivity to infected cells. Since cisplatin is the frontline anticancer drug for HNSCCs, we investigated whether lp11w could sensitize CAL27 (a cisplatin resistant cell line) and SCC25 cells for cisplatin toxicity. Both cell lines were infected with dl312 or Ad5-RM or lp11w and treated with cisplatin at indicated concentrations and cell viability was determined by the MTS assay (Fig. 6). CAL27 cells were sensitive to lp11w infection even in the absence of cisplatin resulting in about three-fold enhanced cell death compared to cells infected with Ad5-RM. Treatment with cisplatin resulted in an increase in the overall toxicity in Ad-infected CAL27 cells; infection with lp11w resulted in 2–3-fold enhancement in cell death compared to cells infected with dl312 or Ad5-RM at a lower cisplatin concentration (4 µM) than at higher concentrations (8 and 12 µM). Infection of SCC25 cells with lp11w and treatment with cisplatin resulted in two to three-fold increase in cell death over the range of cisplatin concentrations used (4 to 12 µM). These results suggest that lp11w could be an attractive oncolytic agent for HNSCC either alone or in combination with cisplatin.
Figure 6. Effect of Ad-infection on cisplatin toxicity.
CAL27 (A) and SCC25 (B) cells were infected with dl312 or Ad5-RM or lp11w at an MOI of 10, treated with indicated concentrations of cisplatin at 24 hr post infection and cell viability was determined at 24 hr after drug treatment by the MTS assay. The experiments were repeated at least three times independently and the data are means ± SD. Statistical differences were assessed using two-way ANOVA followed by a Bonferroni post hoc test, where appropriate, using GraphPad Prism 5 software. P-value was determined by comparing dl 312 vs Ad5-RM, dl 312 vs lp11w and Ad5-RM vs lp11w and also with all the cisplatin concentrations. Statistical significance was accepted at a value of *P<0.05, **P<0.01 and ***P<0.001.
Discussion
We have shown that an adenovirus type 5 mutant defective in coding for E1B-19K (lp11w) induced efficient apoptotic cell death in HNSCC cells that were normally refractory to apoptosis-inducing anti-cancer agents as a result of up-regulation of BCL-2 family anti-apoptosis proteins and EGFR. The apoptotic cell death induced by lp11w was accompanied by enhanced viral spread. Previously, other strategies such as overexpression of an adenovirus death protein (ADP) (Doronin et al., 2000; Tollefson et al., 1996) and use of an E1A mutant with a deletion in the CR2 region (Baird et al., 2008) have been reported to enhance the oncolytic potential of Ad vectors. In our study, a CR2 deleted mutant dl1108 induced only modest increment in lysis of HNSCC cells compared to wt, but significantly less than lp11w (Fig. 1B). Although we did not compare lp11w with Ad vectors that overexpress ADP for lysis of HNSCC cells, we have previously shown that mutations in the 19K locus readily compensated for the requirement of ADP in lysis of infected lung cancer cells (Subramanian, Vijayalingam, and Chinnadurai, 2006). It would be interesting to determine the effect of 19K mutant vectors engineered to overexpress ADP in oncolysis of HNSCC cells.
We have observed a dramatic effect of lp11w on the expression of EGFR in HNSCC cells. This is highly significant considering the role of EGFR activation in pathogenesis of epithelial cancers, including HNSCC (Rowinsky, 2004). Overexpression of EGFR has been linked to higher proliferation, tumor growth, metastasis and chemoresistance of cancer cells (Herbst, Fukuoka, and Baselga, 2004; Rowinsky, 2004). Our results strongly suggested that EGFR down-regulation in lp11w infected HNSCC cells was mediated by apoptosis as well as by an E3-depedent non-apoptotic mechanism. Previously, a transcriptional repression mechanism of down-regulation of EGFR mediated by the E1A-12S product has been identified (Flinterman et al., 2003; Flinterman et al., 2007). The transcriptional mechanism was linked to recruitment of a cellular protein p400 by E1A in HNSCC cells (Flinterman et al., 2003; Flinterman et al., 2007). However, we observed that cells infected with an E1A mutant (dl1102) defective in the recruitment of p400 contained lower levels of EGFR rather than expected higher levels (Fig. 3A). The reason for the discrepancy is not known. However, the studies by Flinterman et al. utilized viral mutants that expressed E1A 12S mRNA while our studies used viruses that expressed both 12S and 13S mRNAs (viral mutants expressing only the 12S mRNA might be unsuited as an oncolytic agent due to replication defects). The transcriptional repression activity of E1A is generally more pronounced in cells expressing the 12S mRNA rather than genomic E1A. The post-translational mechanism that targets EGFR for degradation by the RID proteins coded by the E3 region (Carlin et al., 1989; Tollefson et al., 1991) also appear to contribute modestly for down-regulation of EGFR (in an apoptosis-independent fashion) by lp11w. Our results suggest that lp11w that incorporates both apoptosis-dependent (i.e, mediated by E1B-19K mutant) and the apoptosis-independent (i.e, E3-mediated) down-regulation of EGFR in a single vector would be a more potent vector for down-regulation of EGFR in epithelial cancer cells. It should be noted that the widely used Ad5 19K mutants are based on a parental strain (dl309) that lacks the E3-B region that includes the RID-coding region. In addition to lp11w, one of our Ad2 mutant dl250 (Subramanian et al., 1984) that contains the entire E3 region would also be expected to exhibit enhanced clearance of EGFR.
It is possible that EGFR down-regulation via the apoptosis-dependent mechanism may provide an apoptosis amplification loop and enhance the overall apoptotic response. It was shown that EGFR was degraded in cells exposed to certain apoptotic stimuli as a result of caspase activation (Bae et al., 2001; He et al., 2003; Zhuang, Ouedraogo, and Kochevar, 2003). In vitro studies have shown that EGFR was cleaved by caspase-3 in the cytoplasmic domain resulting in a10 kD polypeptide (He, Huang, and Chignell, 2006). With other members of the EGFR family, HER-2 (Strohecker et al., 2008) and HER-4/ERBB-4 (Naresh et al., 2006) caspases were shown to release the intracellular cytoplasmic domain that behaved as BH3-only proteins in promoting apoptosis. It is possible that the cytoplasmic domain of EGFR may also function as a BH3-only protein after caspase cleavage. It should be noted that all members of the EGFR family contain a conserved BH3 domain. These conjectural evidences suggest that EGFR down-regulation during lp11w induced apoptosis may result in apoptosis amplification as a result of caspase cleavage of EGFR and generation of an intracellular cytoplasmic BH3-containing domain. It is possible that the enhanced apoptotic response observed in HNSCC cells might be result of such amplification. It is of interest that we have also observed that infection of lp11w in breast cancer cells resulted in efficient down-regulation of HER-2 (results not shown).
We have observed down-regulation of BCL-2 family anti-apoptosis proteins in cells infected with lp11w. EGFR-signaling via STAT3 activation has been linked to up-regulation of BCL-xL (Song and Grandis, 2000) and BCL-2 (Shen and Kramer, 2004) in HNSCC cells. Thus, it is possible that down-regulation of these anti-apoptosis proteins in lp11w-infected cells may be linked to down-regulation of EGFR. BCL-xL and BCL-2 have been shown to be direct targets for caspases (Cheng et al., 1997; Clem et al., 1998). Caspases activated in cells infected with lp11w might also directly target BCL-xL and BCL-2. EGFR-signaling has also been reported to up-regulate MCL-1 in other cancer cells (Leu, Chang, and Hu, 2000). Although down-regulation of MCL-1 observed in cells infected with lp11w may be related to EGFR down-regulation, other potential mechanisms are possible. In addition to cells infected with lp11w, down-regulation of MCL-1 was observed in cells infected with certain E1A mutants (Fig. 3A). A previous study has shown expression of E1A induced a DNA damage-like response resulting in proteasomal degradation of MCL-1 (Cuconati et al., 2003). Thus, MCL-1 down-regulation in lp11w-infected cells might be the result of multiple E1A effects.
We have shown that infection with lp11w increased the overall cytotoxic effect of cisplatin in HNSCC cells compared to cells infected with Ad5-RM, suggesting the apoptotic effect of lp11w is the contributing factor for the enhanced drug sensitivity. Several previous studies have explored the use of Ad mutants with deletions in E1B-19K as oncolytic agents (Liu et al., 2004; Sauthoff et al., 2000; Yoon et al., 2006). These studies employed E1B-19K-deleted viruses that also contained additional deletions in other early genes such as E1B-55K and E3-B region. Other studies have exploited Ads with only 19K ablation as oncolytic agents and demonstrated increased efficacy in combination with other therapeutic agents for treatment of cancers (Leitner et al., 2009; Liu et al., 2004). Thus, the apoptogenic mutants of Ad5 or Ad2 may be attractive oncolytic agents for HNSCCs. Since these mutants exhibit enhanced intercellular viral spread, they can be used at a relatively low viral dose, minimizing potential side effects of virotherapy in localized treatments of HNSCCs.
Acknowledgments
This work was supported by research grants CA-33616 and CA-84941 from the National Cancer Institute. We thank W.S.M. Wold and Karoly Toth for their comments on the manuscript, Ann Tollefson for reagents and discussions and B. Ying for help with real-time PCR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7(12):1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- Bae SS, Choi JH, Oh YS, Perry DK, Ryu SH, Suh PG. Proteolytic cleavage of epidermal growth factor receptor by caspases. FEBS Lett. 2001;491(1–2):16–20. doi: 10.1016/s0014-5793(01)02167-6. [DOI] [PubMed] [Google Scholar]
- Baird SK, Aerts JL, Eddaoudi A, Lockley M, Lemoine NR, McNeish IA. Oncolytic adenoviral mutants induce a novel mode of programmed cell death in ovarian cancer. Oncogene. 2008;27(22):3081–3090. doi: 10.1038/sj.onc.1210977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Schoffski P, Rojo F, Dumez H, Ramos FJ, Macarulla T, Cajal R, Kisker O, Van Oosterom A, Tabernero J. A phase I pharmacokinetic (PK) and molecular pharmacodynamic (PD) study of the combination of two anti-EGFR therapies, the monoclonal antibody (MAb) cetuximab (C) and the tyrosine kinase inhibitor (TKI) gefitinib (G), in patients (pts) with advanced colorectal (CRC), head and neck (HNC) and non-small cell lung cancer (NSCLC) Journal of Clinical Oncology. 2006;24(18):122s–122s. [Google Scholar]
- Bauer JA, Trask DK, Kumar B, Los G, Castro J, Lee JS, Chen J, Wang S, Bradford CR, Carey TE. Reversal of cisplatin resistance with a BH3 mimetic, (−)-gossypol, in head and neck cancer cells: role of wild-type p53 and Bcl-xL. Mol Cancer Ther. 2005;4(7):1096–1104. doi: 10.1158/1535-7163.MCT-05-0081. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Miller CR, Douglas JT, Li H, Reynolds PN, Carroll WR, Peters GE, Strong TV, Curiel DT. Retargeting to EGFR enhances adenovirus infection efficiency of squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1999;125(8):856–863. doi: 10.1001/archotol.125.8.856. [DOI] [PubMed] [Google Scholar]
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- Boyle JO, Hakim J, Koch W, van der Riet P, Hruban RH, Roa RA, Correo R, Eby YJ, Ruppert JM, Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993;53(19):4477–4480. [PubMed] [Google Scholar]
- Carlin CR, Tollefson AE, Brady HA, Hoffman BL, Wold WS. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278(5345):1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- Chia MC, Shi W, Li JH, Sanchez O, Strathdee CA, Huang D, Busson P, Klamut HJ, Liu FF. A conditionally replicating adenovirus for nasopharyngeal carcinoma gene therapy. Mol Ther. 2004;9(6):804–817. doi: 10.1016/j.ymthe.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Control of apoptosis by human adenovirus genes. Seminars in Virology. 1998;8(5):399–408. [Google Scholar]
- Clem RJ, Cheng EH, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95(2):554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A, Mukherjee C, Perez D, White E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 2003;17(23):2922–2932. doi: 10.1101/gad.1156903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuconati A, White E. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16(19):2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Doronin K, Toth K, Kuppuswamy M, Ward P, Tollefson AE, Wold WS. Tumor-specific, replication-competent adenovirus vectors overexpressing the adenovirus death protein. J Virol. 2000;74(13):6147–6155. doi: 10.1128/jvi.74.13.6147-6155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinterman M, Gaken J, Farzaneh F, Tavassoli M. E1A-mediated suppression of EGFR expression and induction of apoptosis in head and neck squamous carcinoma cell lines. Oncogene. 2003;22(13):1965–1977. doi: 10.1038/sj.onc.1206190. [DOI] [PubMed] [Google Scholar]
- Flinterman MB, Mymryk JS, Klanrit P, Yousef AF, Lowe SW, Caldas C, Gaken J, Farzaneh F, Tavassoli M. p400 function is required for the adenovirus E1A-mediated suppression of EGFR and tumour cell killing. Oncogene. 2007;26(48):6863–6874. doi: 10.1038/sj.onc.1210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Huang JL, Chignell CF. Cleavage of epidermal growth factor receptor by caspase during apoptosis is independent of its internalization. Oncogene. 2006;25(10):1521–1531. doi: 10.1038/sj.onc.1209184. [DOI] [PubMed] [Google Scholar]
- He YY, Huang JL, Gentry JB, Chignell CF. Epidermal growth factor receptor down-regulation induced by UVA in human keratinocytes does not require the receptor kinase activity. J Biol Chem. 2003;278(43):42457–42465. doi: 10.1074/jbc.M303376200. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Fukuoka M, Baselga J. Gefitinib--a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4(12):956–965. doi: 10.1038/nrc1506. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59(8):1935–1940. [PubMed] [Google Scholar]
- Jelsma TN, Howe JA, Evelegh CM, Cunniff NF, Skiadopoulos MH, Floroff MR, Denman JE, Bayley ST. Use of deletion and point mutants spanning the coding region of the adenovirus 5 E1A gene to define a domain that is essential for transcriptional activation. Virology. 1988;163(2):494–502. doi: 10.1016/0042-6822(88)90290-5. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24(17):2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C, Randlev B, Gillenwater AM, Bruso P, Kaye SB, Hong WK, Kirn DH. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- Leitner S, Sweeney K, Oberg D, Davies D, Miranda E, Lemoine NR, Hallden G. Oncolytic Adenoviral Mutants with E1B19K Gene Deletions Enhance Gemcitabine-induced Apoptosis in Pancreatic Carcinoma Cells and Anti-Tumor Efficacy In vivo. Clin Cancer Res. 2009;15(5):1730–1740. doi: 10.1158/1078-0432.CCR-08-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu CM, Chang C, Hu C. Epidermal growth factor (EGF) suppresses staurosporine-induced apoptosis by inducing mcl-1 via the mitogen-activated protein kinase pathway. Oncogene. 2000;19(13):1665–1675. doi: 10.1038/sj.onc.1203452. [DOI] [PubMed] [Google Scholar]
- Liu TC, Hallden G, Wang Y, Brooks G, Francis J, Lemoine N, Kirn D. An E1B-19 kDa gene deletion mutant adenovirus demonstrates tumor necrosis factor-enhanced cancer selectivity and enhanced oncolytic potency. Mol Ther. 2004;9(6):786–803. doi: 10.1016/j.ymthe.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83(4):489–501. doi: 10.4065/83.4.489. [DOI] [PubMed] [Google Scholar]
- Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66(12):6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- Pan JJ, Zhang SW, Chen CB, Xiao SW, Sun Y, Liu CQ, Su X, Li DM, Xu G, Xu B, Lu YY. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;27(5):799–804. doi: 10.1200/JCO.2008.18.9670. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pelka P, Ablack JN, Fonseca GJ, Yousef AF, Mymryk JS. Intrinsic structural disorder in adenovirus E1A: a viral molecular hub linking multiple diverse processes. J Virol. 2008;82(15):7252–7263. doi: 10.1128/JVI.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter CW, Morgan MA, Eckardt A. Targeting EGF-receptor-signalling in squamous cell carcinomas of the head and neck. Br J Cancer. 2007;96(3):408–416. doi: 10.1038/sj.bjc.6603566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK. The erbB family: targets for therapeutic development against cancer and therapeutic strategies using monoclonal antibodies and tyrosine kinase inhibitors. Annu Rev Med. 2004;55:433–457. doi: 10.1146/annurev.med.55.091902.104433. [DOI] [PubMed] [Google Scholar]
- Sauthoff H, Heitner S, Rom WN, Hay JG. Deletion of the adenoviral E1b-19kD gene enhances tumor cell killing of a replicating adenoviral vector. Hum Gene Ther. 2000;11(3):379–388. doi: 10.1089/10430340050015851. [DOI] [PubMed] [Google Scholar]
- Sharma H, Sen S, Lo Muzio L, Mariggio A, Singh N. Antisense-mediated downregulation of anti-apoptotic proteins induces apoptosis and sensitizes head and neck squamous cell carcinoma cells to chemotherapy. Cancer Biol Ther. 2005;4(7):720–727. doi: 10.4161/cbt.4.7.1783. [DOI] [PubMed] [Google Scholar]
- Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol. 2004;165(4):1315–1329. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiquee KAZ, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Research. 2008;18(2):254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19(21):2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- Strohecker AM, Yehiely F, Chen F, Cryns VL. Caspase cleavage of HER-2 releases a Bad-like cell death effector. J Biol Chem. 2008;283(26):18269–18282. doi: 10.1074/jbc.M802156200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T, Kuppuswamy M, Gysbers J, Mak S, Chinnadurai G. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J Biol Chem. 1984;259(19):11777–11783. [PubMed] [Google Scholar]
- Subramanian T, Tarodi B, Chinnadurai G. p53-independent apoptotic and necrotic cell deaths induced by adenovirus infection: suppression by E1B 19K and Bcl-2 proteins. Cell Growth Differ. 1995;6(2):131–137. [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S, Chinnadurai G. Genetic identification of adenovirus type 5 genes that influence viral spread. J Virol. 2006;80(4):2000–2012. doi: 10.1128/JVI.80.4.2000-2012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81(19):10486–10495. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B, Hutchins BM, Mcallister DL, Lu F, Thomas KB. The complete nucleic acid sequence of the adenovirus type 5 reference material (ARM) genome. Bioprocessing Journal. 2003 Sept/Oct;:27–33. [Google Scholar]
- Thimmappaya B, Weinberger C, Schneider RJ, Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Toivonen R, Suominen E, Grenman R, Savontaus M. Retargeting improves the efficacy of a telomerase-dependent oncolytic adenovirus for head and neck cancer. Oncol Rep. 2009;21(1):165–171. [PubMed] [Google Scholar]
- Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WS. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70(4):2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson AE, Stewart AR, Yei SP, Saha SK, Wold WS. The 10,400- and 14,500-dalton proteins encoded by region E3 of adenovirus form a complex and function together to down-regulate the epidermal growth factor receptor. J Virol. 1991;65(6):3095–3105. doi: 10.1128/jvi.65.6.3095-3105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying B, Toth K, Spencer JF, Meyer J, Tollefson AE, Patra D, Dhar D, Shashkova EV, Kuppuswamy M, Doronin K, Thomas MA, Zumstein LA, Wold WS, Lichtenstein DL. INGN 007, an oncolytic adenovirus vector, replicates in Syrian hamsters but not mice: comparison of biodistribution studies. Cancer Gene Ther. 2009 doi: 10.1038/cgt.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon AR, Kim JH, Lee YS, Kim H, Yoo JY, Sohn JH, Park BW, Yun CO. Markedly enhanced cytolysis by E1B-19kD-deleted oncolytic adenovirus in combination with cisplatin. Hum Gene Ther. 2006;17(4):379–390. doi: 10.1089/hum.2006.17.379. [DOI] [PubMed] [Google Scholar]
- Zhuang S, Ouedraogo GD, Kochevar IE. Downregulation of epidermal growth factor receptor signaling by singlet oxygen through activation of caspase-3 and protein phosphatases. Oncogene. 2003;22(28):4413–4424. doi: 10.1038/sj.onc.1206604. [DOI] [PubMed] [Google Scholar]