Abstract
Sepsis has been associated with tumor necrosis factor α (TNF-α) and nitric oxide (NO) overproduction, insulin resistance, and a profound suppression of muscle protein synthesis. However, lesser suppression of muscle protein synthesis in neonatal pigs occurs in response to endotoxin (LPS) when glucose and amino acids are provided. We hypothesize that the LPS-induced TNF-α and NO overproduction down regulates insulin signaling pathway activation in neonatal pigs in the presence of fed levels of insulin, glucose, and amino acids. In skeletal muscle, inducible NOS activity was increased in response to LPS infusion, but phosphorylation of the insulin receptor, insulin receptor substrate-1 (IRS-1), p42/p44 mitogen-activated protein kinase (MAPK), and protein kinase B (PKB), the association of IRS-1 with phosphatidylinositol 3-kinase (PI 3-kinase), and constitutive NOS activity were not altered. In liver, activation of the insulin receptor, IRS-1, and PI 3-kinase were not affected by LPS, but p42 MAPK phosphorylation was increased. The absence of a down-regulation in the insulin signaling cascade in muscle despite the LPS-induced increase in TNF-α and muscle iNOS, may contribute to the near-maintenance of muscle protein synthesis rates in the presence of glucose and amino acids in LPS-infused neonatal pigs.
Keywords: sepsis, insulin sensitivity, nitric oxide, TNF-α, lipopolysaccharide
The rapid gain in protein mass in skeletal muscle during early postnatal life is sustained by elevated rates of muscle protein synthesis, as a result of enhanced responsiveness to the post-prandial rise in insulin and amino acids in the neonatal period (1). In muscle of neonatal pigs, both the activation of the insulin signaling pathway leading to translation initiation, and translation initiation factor activation by glucose, amino acids, and insulin are enhanced (1) when compared to older pigs (2), leading to a relatively high increase in fractional synthesis rates in response to insulin and nutrients (1, 3) to allow rapid growth. When bacterial endotoxin (lipopolysaccharide, LPS) is infused to neonatal pigs to induce a septic-like state, the reduction in protein synthesis rates in skeletal muscle is less profound than that described in septic mature animals (4) when insulin and amino acids levels similar to those seen in the fed state are present (5), suggesting that neonatal animals maintain their anabolic drive even in the presence of a catabolic insult, such as LPS. The mechanisms that regulate the highly responsive muscle protein synthesis during development for the duration of a catabolic illness such as sepsis have not been completely elucidated.
Sepsis is associated with release of proinflammatory mediators and cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α) and nitric oxide (NO), and they appear to be key regulators of the protein metabolic response to sepsis and endotoxin (6, 7). TNF-α, independent of cortisol, has been associated with the sepsis-induced reduction in muscle protein synthesis (8), and the stimulation of NO formation by inducible nitric oxide synthases [(i)NOS] is promoted by LPS and cytokines, including TNF-α (6, 7, 9). There are three NOS isoenzymes; two of these enzymes are expressed constitutively in vascular endothelial cells (eNOS) and in neurons (nNOS) whereas the expression of a third isoenzyme (iNOS) is inducible in a variety of cells (including macrophages, hepatocytes, and vascular smooth muscle) by cellular products of both gram-negative (endotoxin) and gram-positive bacteria (10). NO induction has been implicated in the pathophysiology of sepsis (9, 10), as well as in the regulation of insulin sensitivity (11).
Whole body insulin resistance for glucose metabolism has been demonstrated in septic patients and in infected animals (8). In adult rats, induction of sepsis causes an inhibition of protein synthesis in skeletal muscle that is resistant to the stimulatory actions of insulin (12, 13). Furthermore, the increase in TNF-α level and NOS activity during sepsis induces insulin resistance in mature individuals (9, 14). Studies using cell culture systems suggest that TNF-α stimulates insulin resistance by inducing serine phosphorylation of insulin receptor substrate-1 (IRS-1), converting IRS-1 into an inhibitor of insulin receptor tyrosine kinase, thereby reducing insulin signal activation downstream in the pathway (15, 16) and inhibiting glucose uptake (17). Furthermore, the TNF-α-induced reduction of insulin-stimulated tyrosine phosphorylation of IRS-1 appears to be mediated by activation of protein kinase B (PKB) by TNF-α (18). Tyrosine phosphorylation of the insulin receptor is crucial for activation of downstream signaling-components leading to the metabolic effects of insulin, including the stimulation of protein synthesis (19). Sepsis-induced insulin resistance can also occur by induction of iNOS activity, probably by impairing insulin-stimulated phosphatidylinositol 3-kinase (PI 3-kinase) and PKB activation (20), resulting in a decline in insulin stimulated-glucose uptake (17).
LPS infusion has been reported to increase the activation of the mitogen-activated protein kinases (MAPK) family [p38 MAPK, p42/p44 MAPK] in monocytes and endothelial cells (21), leading to the stimulation of transcription factors which control cytokine expression (22). In cultured muscle cells, the MAP kinase pathway appears to be involved in the stimulation of protein synthesis by insulin (23). Tyrosine phosphorylation of IRS proteins and adaptor protein Shc by the insulin receptor stimulates the activation of MAP kinase pathway resulting in the activation of transcriptional activity in the nucleus (23). However, insulin-stimulated phosphorylation of MAP kinase is markedly diminished in skeletal muscle of LPS-treated adult rats (24). Thus, the regulation of p42/p44 MAPK activity by LPS may play a role in the sepsis-induced insulin resistance in skeletal muscle in mature animals.
We have demonstrated that endotoxin decreases muscle protein synthesis in neonatal pigs (5) and abrogates the mRNA-binding step in translation initiation, which is insulin regulated, but not the initiator methionyl-tRNA-binding step, which is not regulated by insulin (34). Therefore, we hypothesize that endotoxin also down regulates the insulin signaling pathway activation upstream of translation initiation in the presence of fed levels of insulin, glucose, and amino acids. For comparison, insulin-signaling proteins were also examined in the liver, whose protein synthesis rates rise in response to LPS. Since TNF-α and NO may play a role in modulating muscle insulin sensitivity, we also determined NOS activity in skeletal muscle of LPS-infused neonatal pigs.
EXPERIMENTAL PROCEDURES
Animals
Two crossbred (Landrace × Yorkshire × Hampshire × Duroc) pregnant sows (Agriculture Headquarters, Texas Department of Criminal Justice, Huntsville, Texas) were housed and fed (5084, PMI Feeds, Richmond, IN) in lactation crates in individual environmentally controlled rooms for 1 to 2 wk before farrowing. After farrowing, catheters were inserted into the jugular vein and carotid artery of each piglet, as previously described (5), and pigs were returned to the sow and were allowed to suckle freely until studied. The protocol was approved by the Animal Care and Use Committee of Baylor College of Medicine and was conducted in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals.
Materials
BioMag goat anti-mouse IgG and goat anti-rabbit IgG magnetic beads were obtained from Polysciences, Inc. (Warrington, PA) and the magnetic sample rack from Promega (Madison, WI). Reagents for SDS-PAGE were from Bio Rad Laboratories (Richmond, CA), and the protein assay kit from Pierce (Rockford, IL). Anti-phosphotyrosine (PY) antibodies and anti-IRS-1 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), anti-PI 3-kinase (p85) from Mbl International (Watertown, MA), anti-phospho-Ser473 PKB from Cell Signaling Technology, and phosphospecific p44/42 mitogen-activated (MAP) kinase (Thr202/Tyr204) E10 monoclonal antibodies from New England Biolabs (Beverly, MA). The enhanced chemiluminescence Western blotting detection kit (ECL-Plus) was obtained from Amersham (Arlington Heights, IL). Other chemicals and reagents were from Sigma Chemical Co. (St. Louis, MO).
Experimental design
Piglets (5–6 days of age; 2.2 ± 0.37 kg) from two litters were assigned randomly to control (n=10) and LPS (n=10) treatment groups. Before the study began, the animals were removed from the sow and fasted overnight in a heated room (84°F), with free access to water but no feed. One hour before the LPS infusion was initiated, animals were infused with dextrose at a rate of 800 mg•kg−1•hr−1 and a balanced amino acid mixture (5) at a rate of 1.8 mmol total amino acids•kg−1•hr−1 to simulate a normal fed state. One hour after the initiation of the dextrose/amino acid infusion, the LPS group received a continuous infusion 10 µg •kg−1•hr−1·of E. coli endotoxin (lyophilized E. Coli Serotype 0111-B4, Sigma Chemical Co, St. Louis, MO) that was continued for 8 h while the control group received an equal volume of sterile normal saline solution (0.9% sodium chloride) at the same rate as the LPS infusion. 8 h after the LPS infusion was initiated, pigs were euthanized with an intravenous dose of pentobarbital sodium (50 mg/kg body weight). Longissimus dorsi muscle and liver were rapidly removed, frozen in liquid nitrogen, and stored at −70° C until analysis.
Hormone and substrate determinations and measurement of protein synthesis in pig muscle and liver
The plasma concentrations of TNF-α and IL-1, cortisol, insulin, glucose, and amino acids were determined as previously described (3, 5). The fractional rate of protein synthesis was measured with a flooding dose of [3H] phenylalanine (3), and was calculated as previously described (3, 5).
Preparation of tissue extracts, immunoprecipitation, and Western blot analysis
Muscle and liver samples were homogenized with a Polytron (Brinkmann Instruments, Inc) and solubilized as previously described (2). The homogenate was incubated for 45 min at 4°C with gentle mixing and then centrifuged at 35,000 g for 1 hour at 4°C. The supernatant was collected and an aliquot was assayed for protein concentration using the BCA assay (Pierce, Rockford, Il).
To determine the tyrosine phosphorylation of IR and IRS-1, protein samples from tissue extract preparations were immunoprecipitated with anti-mouse anti-IR and anti-IRS-1 antibody, respectively. To determine the activation of PI 3-kinase in muscle, we measured the association of IRS-1 with p85 subunit of the PI 3-kinase by immunoprecipitating tissue homogenate with mouse anti-IRS-1 antibody, followed by immunoblotting with anti-p85 antibody. The immunoprecipitants were subjected to Western blot analysis as previously described (2). To determine phosphorylation of p44/42 MAP kinase, tissue extracts were separated by SDS-PAGE on a 10% acrylamide gel followed by Western blot analysis using anti-phosphospecific p44/42 MAP kinase (1:2000) that recognizes the proteins only when they are phosphorylated at Thr202/Tyr204 (25). The blots were then quantified by computerized densitometry (Molecular Dynamics Pharmacia, Piscataway, NY) and the phosphorylated forms were normalized by the total protein recovered from the immunoprecipitate.
NOS assay
NO synthase activity was measured as previously described (26). Briefly, skeletal muscle (~ 0.250 g) was homogenized in 1 ml 50 mmol/L HEPES buffer (pH 7.4) containing 1 mmol/L EDTA and protease inhibitors. The homogenate was centrifuged at 600 × g at 4° C for 10 min, and the supernatant was used for NOS assay. For determining iNOS activity, the assay mixture (0.2 ml) contained 0.1 mmol/L (6R)-5,6,7,8-tetrahydro-L-biopterin, 1 mmol/L dithiothreitol, 1 mmol/L MgCl2, 1 mg/L calmodulin, 0.1 mmol/L NADPH, 0.1 mmol/L FAD, 0.1 mmol/L FMN, 0.1 mmol/L L-[U-14C]arginine (150 Bq/nmol), 0.1 mmol/L L-valine (an inhibitor of arginase), 0.1 mmol/L L-citrulline (to prevent the potential recycling of 14C-citrulline into arginine), 2 mmol/L EGTA, and tissue extract (~1 mg protein). For determining total NOS activity, the assay mixture contained all above components, except that 2 mmol/L CaCl2 replaced 2 mmol/L EGTA. Radioactivity blanks containing all above components plus 2 mmol/L NG – methyl-L-arginine (an inhibitor of NOS) were included to improve assay specificity. cNOS was calculated by subtracting iNOS from total NOS activity.
Statistics
Analysis of variance was used to assess the effect of LPS infusion. Probability values of <0.05 were considered statistically significant. Data are presented as mean ± SEM.
RESULTS
Clinical, hormonal, and metabolic markers during experimentally induced sepsis
The data on the metabolic response to LPS in neonatal pigs have been published previously (5). As previously described (5), body temperature and heart rate were significantly higher (P < 0.05) in LPS-treated than in control pigs (Table 1). Cortisol and TNF-α were higher (P < 0.05) in LPS-treated than in control pigs. LPS infusion induced a reduction in plasma glucose and BCAA concentrations in LPS-treated than in control pigs (P< 0.05).
Table 1.
Clinical, hormonal, and metabolic markers in LPS-infused and control neonatal pigs
| Markers | Control | LPS |
|---|---|---|
| A. Clinical: | ||
| Temperature | 100.7 ±0.2 (°F) |
105.0 ± 0.4* (°F) |
| Heart Rate | 167 ±6 (bpm) |
197 ±5* (bpm) |
| B. Hormonal: | ||
| Insulin | 9.5 ±1.5 (µU/mL) |
11.4 ±2.3 (µU/mL) |
| Cortisol | 3 ±0.6 (mg/dL) |
25 ±5* (mg/dL) |
| TNF-α | 28 ±7 (pg/mL) |
879 ±167* (pg/mL) |
| C. Metabolic: | ||
| Glucose | 174 ±12 (mg/dL) |
97 ±5* (mg/dL) |
| BCAA | 802 ±46 (nmol/mL) |
550 ±72* (nmol/mL) |
Values are mean ± SEM (n=10/group) obtained at the end of an 8-hour infusion of LPS for all parameter except TNF-α for which the peak level obtained at 1 hour after LPS infusion is shown. Previously reported data from reference 5.
Significantly different from control value (P< 0.05)
Protein synthesis in skeletal muscle and liver
The data on fractional protein synthesis have been published previously (5) and are included here for comparison. LPS infusion altered protein synthesis rates in skeletal muscle and liver as previously described (5). LPS infusion caused a small (−11%), but significant reduction (P < 0.05) in the fractional rate of protein synthesis in skeletal muscle (controls, 19.5 ± 0.5 %/day; LPS, 17.4 ± 0.6 %/day). Furthermore, LPS infusion increased the fractional rate of liver protein synthesis (P < 0.05; controls, 70.8 ± 2.8 %/day; LPS, 86.2 ± 3.8 %/day).
NOS activity in skeletal muscle
The activity of iNOS was not detectable in skeletal muscle of control neonatal pigs (Table 2). In response to LPS infusion, iNOS activity in skeletal muscle increased sharply (P < 0.001). The activity of constitutive NOS (cNOS), which consists of eNOS and nNOS, was not different between groups. Total NOS activity was higher (P < 0.001) in LPS-treated than in control pigs, because of increased iNOS activity. Because the liver has an exceedingly high activity of arginase that rapidly degrades arginine (27), we could not measure NOS activity using [14C] arginine as the substrate in this tissue.
Table 2.
Effect of LPS infusion on NOS activity in skeletal muscle of neonatal pigs
| Treatment | Inducible NOS (iNOS) activity |
Constitutive NOS (cNOS) activity |
Total NOS activity |
|---|---|---|---|
| (pmol/30min/mg protein) | |||
| Control | n.d. | 265.5 ± 18.4 | 265.5 ± 18.4 |
| LPS | 395.5 ± 30.2* | 246.6 ± 16.2 | 642.1 ± 31.9* |
Values are means ± SEM.
Significant different from control pigs (P < 0.001).
n.d.: none detected.
Insulin receptor, IRS-1, PI 3-Kinase, PKB, and p42/p44 MAPK activation in skeletal muscle
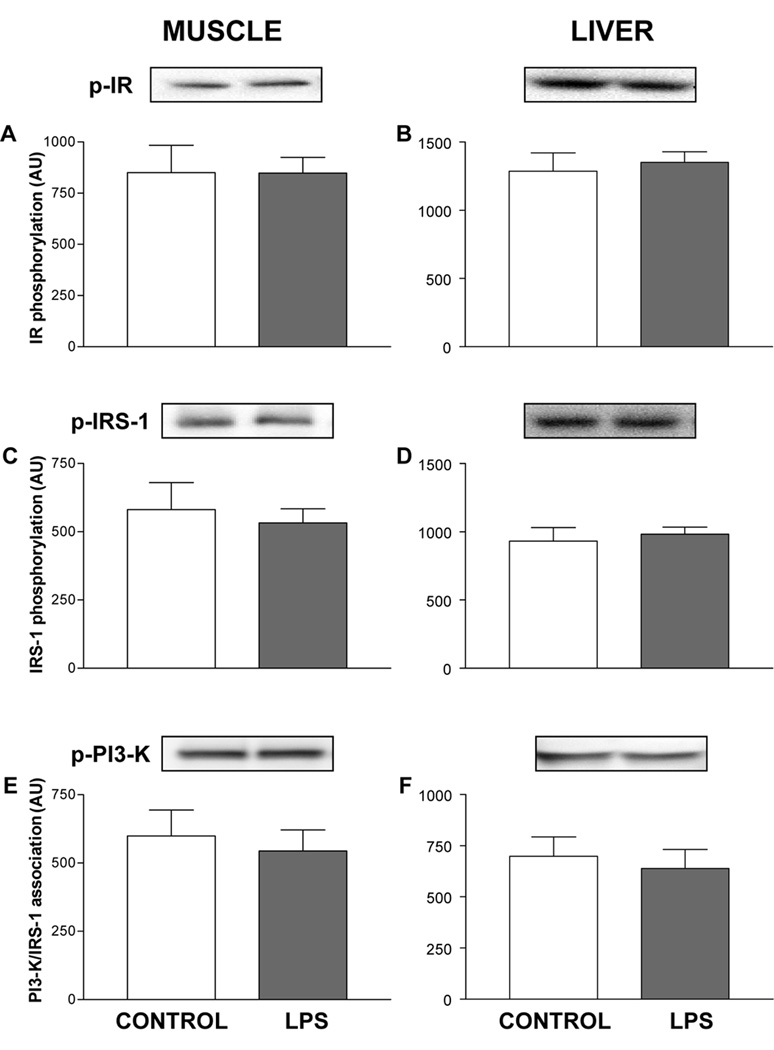
In skeletal muscle, LPS treatment did not alter insulin receptor phosphorylation (Fig. 1A), a fundamental step required for activation of downstream insulin signaling-components (19). Infusing LPS for 8 hours did not alter IRS-1 tyrosine phosphorylation (Fig. 1C), the association of IRS-1 with PI 3-kinase (Fig. 1E), PKB phosphorylation (Fig. 2A), and the phosphorylation of p42 or p44 MAPK in muscle of neonatal pigs (Fig. 2C and 2E).
Figure 1.
Activation of early steps in the insulin-signaling pathway in skeletal muscle and liver of control and LPS-infused neonatal pigs. Phosphorylated proteins, as shown in the blot, were corrected by the total protein recovered from the immunoprecipitate, as shown in the figure. Results are means ± SEM (n=10/group).
Figure 2.
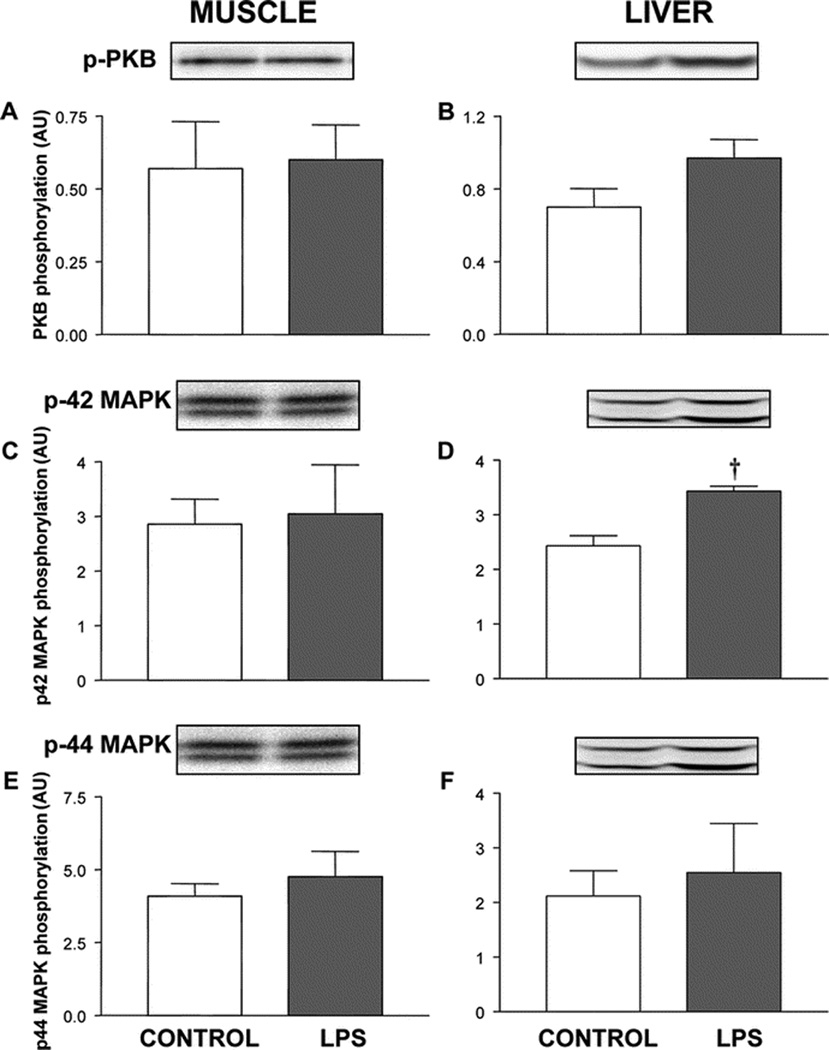
Activation of protein kinase B (PKB) and mitogen-activated protein kinases (MAPK) p42 and p44 in skeletal muscle and liver of control and LPS-infused pigs. Phosphorylated proteins, as shown in the blot, were corrected by the total protein recovered from the immunoprecipitate, as shown in the figure. Results are means ± SEM (n=10/group). † Significantly different from control value (P < 0.05).
Insulin receptor, IRS-1, PI 3-Kinase, PKB, and p42/p44 MAPK activation in liver
In the liver, LPS infusion did not alter phosphorylation of the insulin receptor or IRS-1 (Figure 1B and 1D) or the association of IRS-1 with PI 3-kinase (Fig 1F), but tended to increase serine phosphorylation of PKB (P=0.06; Fig. 2B). LPS infusion increased the phosphorylation of p42 MAPK (P < 0.05), but not p44 MAPK, in liver (Fig 2D and 2F).
DISCUSSION
Insulin signaling in experimentally induced neonatal sepsis
Profound suppression of muscle protein synthesis and reduced activation of the insulin signaling proteins in skeletal muscle, such as insulin receptor and IRS-1, has been reported in mature septic animals (24, 28). However, in LPS-infused neonatal pigs, the response of muscle protein synthesis to insulin is maintained despite depression of the translational process (29), an event that occurs downstream of the insulin signaling pathway and can respond to amino acid stimulation alone. In the current study, when neonatal pigs were maintained on insulin, glucose, and amino acid concentrations similar to those seen in the fed state (5), LPS did not have an effect on the activation of components of the insulin signaling pathway despite similar circulating levels of insulin and reduced glucose and amino acid concentrations. Since LPS only modestly decreased protein synthesis rates in muscle when compared to adult models of sepsis (8), the results suggest that insulin resistance for protein metabolism may not occur in acute endotoxemia in neonatal pigs.
Endotoxin and TNF-α has been implicated in the development of insulin resistance during sepsis (16, 30). In humans, systemic infusion of TNF-α reduces whole-body glucose disposal (31) due to effects on both hepatic and peripheral insulin sensitivity, including skeletal muscle (8), and in rats, high circulating levels of TNF-α have been shown to induce insulin resistance for protein metabolism by decreasing translation initiation (8). In vitro, TNF-α inhibits the activation of the insulin receptor by stimulating phosphorylation of serine residues of IRS-1 (15), which negatively regulates insulin receptor tyrosine phosphorylation, and blunts the insulin-stimulated tyrosine phosphorylation of IRS-1 (18). Since in our study, the increase in TNF-α in response to endotoxin did not alter insulin receptor and IRS-1 tyrosine phosphorylation, we hypothesize that the elevated insulin receptor kinase activity in skeletal muscle of neonatal pigs may overcome potential inhibitory effects of serine-phosphorylated IRS-1 on the insulin receptor. It is also possible that IRS-1 serine phosphorylation will be unaffected by TNF-α or LPS stimulation in the neonatal pig. Moreover, in contrast to findings in adult rats (30), the LPS-induced increase in TNF-α was not associated with altered activation of components of the insulin signaling pathway, suggesting that the response of the insulin signaling cascade to TNF-α in muscle may be affected by development (2).
NOS activity in experimentally induced neonatal sepsis
The induction of iNOS activity in a variety of tissues during sepsis has been proposed to be part of an adaptive response of the host defense mechanism (9). Furthermore, induction of iNOS activity either by LPS treatment (11) or by obesity (20) has been linked to the impairment of glucose transport in skeletal muscle that contributes to the whole body insulin resistance for glucose metabolism. In our study, LPS infusion increased inducible NOS activity, similar to previous reports (32). Although constitutive NOS (nNOS + eNOS) activity has also been shown to increase in adult rats with sepsis (33), we found that constitutive NOS activity was unaltered in skeletal muscle of LPS-infused piglets. This suggests that the LPS-induced NOS activation in skeletal muscle of neonatal pigs is derived exclusively from iNOS, whereas in adult animals, LPS increases both constitutive NOS and iNOS activities. It has been postulated that iNOS impairs glucose transport by affecting insulin receptor signaling to PI 3- kinase (20) and PKB activation in skeletal muscle of mice (20). In our study, despite the LPS-induced increase on iNOS activity in neonatal skeletal muscle, the activation of early steps of the insulin signaling pathway was not altered by LPS treatment, suggesting that neonatal pigs may be unique in the ability to maintain insulin responsiveness during LPS-induced NO overproduction.
Role of insulin signaling in the regulation of muscle protein synthesis in experimentally induced neonatal sepsis
In adults, sepsis reduces skeletal muscle protein synthesis and is associated with reductions in the activation of translational mechanisms downstream of mTOR (34). In response to insulin and IGF-I treatment, PKB activation increases phosphorylation of mTOR on Ser2448 (35). In our study in neonatal pigs, LPS did not affect PKB phosphorylation on Ser473 despite a 50% reduction in mTOR phosphorylation (34). This finding suggests that in skeletal muscle of LPS-treated neonatal pigs, mTOR phosphorylation at Ser2448 can be modulated through a mechanism distinct from PKB. Since the activation of the insulin signaling pathway in neonatal pigs links with the activation of translation initiation leading to the stimulation of protein synthesis in muscle (1, 2), the lack of correlation between the insulin signaling pathway, the translational process and protein synthesis rates during acute endotoxemia in the neonatal pig may be unique, and may contribute to the relative resistance of muscle protein synthesis to the catabolic effect of sepsis. In this regard, it is possible that insulin will increase global rates of muscle protein synthesis in neonatal sepsis by stimulating protein synthesis in other sub cellular organelles such as the mitochondria, which may require a process separate from mTOR.
MAP kinase pathways are involved in the stimulation of cytokine production during sepsis (22). However, in skeletal muscle of adult rats, LPS treatment reduces p42/p44 MAPK phosphorylation (24). We have shown previously that p42/p44 MAPK phosphorylation in neonatal muscle is augmented in response to LPS in the fasting state, and that insulin attenuates this response (29). In the current study, the lack of effect of LPS infusion on p42 and p44 MAPK phosphorylation in muscle may be consistent with raised insulin levels in response to dextrose and amino acids infused, and may play a role in limiting the sepsis-induced insulin resistance in skeletal muscle in neonatal pigs.
Effect of LPS on insulin signaling in the liver of neonatal pigs
Sepsis and LPS in neonatal pigs stimulates liver protein synthesis (5), and in both healthy and LPS-infused neonatal pigs, liver protein synthesis is unaffected by insulin, in contrast to skeletal muscle, in which insulin increases and LPS decreases protein synthesis (29). In our study, LPS did not affect the activation of early steps of the insulin signaling pathway in the liver of neonatal pigs, similar to reports in adult rats (28). In our study, PKB phosphorylation tended to increase in response to LPS, without an associated increase in PI 3-kinase activation during sepsis, similar to previous reports (29). It is generally accepted that insulin-induced activation of PKB is strictly dependent on the activity of PI 3-kinase (36). However, PI 3-kinase independent activation of PKB has also been reported (37, 38), and we have shown that PKB phosphorylation in the liver also tends to increase in response to insulin in LPS-infused neonatal pigs (29). This suggests that, in liver of septic neonatal pigs, there is an unknown PI 3-kinase independent mechanism that stimulates PKB activation, leading to the stimulation of liver protein synthesis.
In the liver, LPS infusion induced an increase in p42 MAPK phosphorylation, but not p44 MAPK in liver, similar to reports in adult rats challenged by cecal ligation and puncture (39). Since activation of P42/44 MAPK has been associated with enhanced cytokine expression, the increase in protein synthesis in the liver relative to the increase in p42 phosphorylation may reflect acute phase reactant synthesis (22). Previously, we found that LPS increased P42/44 phosphorylation in liver during fasting, and insulin appeared to attenuate this response (29), in contrast to reports in adult rats, where insulin appeared to amplify this response (39). Since there is a lack of effect of LPS infusion on p42 and p44 MAPK phosphorylation in muscle, in contrast to an increase in p42 MAPK phosphorylation in liver, we speculate that the LPS-induced stimulation of components of MAP kinase pathway is dependent on the organ studied (28) and may be related to an increase in cytokine production by the liver.
Perspective
In the present study, the activation of the early insulin signaling components in skeletal muscle is maintained in LPS-infused neonatal pigs, despite increased TNF-α levels and iNOS activity, which have been shown to promote insulin resistance. Maintaining the high activity of the insulin signaling pathway in the face of a septic-like insult may attenuate the catabolic effect of sepsis in the neonate. Further study of the effects of development during sepsis on the interaction between cytokines, nitric oxide, and insulin signaling activation is needed to understand the attenuated decrease in muscle protein synthesis in neonatal when compared to mature animals.
ACKNOWLEDGMENTS
We thank W. Liu for laboratory assistance and F. Biggs for care of animals. The contents of this publication do not necessarily reflect the views or policies of the US Department of Agriculture, nor does mention of trade names, commercial products or organization imply endorsement by the US Government.
Financial Support: Supported, in part, by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01-AR-44474 (Davis) and the United States Department of Agriculture/Agricultural Research Service Cooperative Agreement no. 58-6250-6-001 (Davis), the National Institute of Arthritis and Musculoskeletal and Skin Diseases K08-AR-51563 (Orellana), and National Research Initiative Competitive Grant (2008-35206-18764) from the USDA Cooperative State Research, Education, and Extension Service (Wu and Davis).
Abbreviations
- IRS-1
insulin receptor substrate-1
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- PI 3-kinase
phosphatidylinositol 3-kinase
- PKB
protein kinase B
Footnotes
Pediatric Research Articles Ahead of Print contains articles in unedited manuscript form that have been peer-reviewed and accepted for publication. As a service to our readers, we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting and review of the resulting proof before it is published in its final definitive form. Please note that during the production process errors may be discovered, which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E40–E53. doi: 10.1152/ajpendo.00563.2002. [DOI] [PubMed] [Google Scholar]
- 2.Suryawan A, Escobar J, Frank JW, Nguyen HV, Davis TA. Developmental regulation of the activation of signaling components leading to translation initiation in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2006;291:E849–E859. doi: 10.1152/ajpendo.00069.2006. [DOI] [PubMed] [Google Scholar]
- 3.Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR. Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab. 2000;279:E1226–E1234. doi: 10.1152/ajpendo.2000.279.6.E1226. [DOI] [PubMed] [Google Scholar]
- 4.Lang CH, Frost RA, Jefferson LS, Kimball SR, Vary TC. Endotoxin-induced decrease in muscle protein synthesis is associated with changes in eIF2B, eIF4E, and IGF-I. Am J Physiol Endocrinol Metab. 2000;278:E1133–E1143. doi: 10.1152/ajpendo.2000.278.6.E1133. [DOI] [PubMed] [Google Scholar]
- 5.Orellana RA, O'Connor PM, Nguyen HV, Bush JA, Suryawan A, Thivierge MC, Fiorotto ML, Davis TA. Endotoxemia reduces skeletal muscle protein synthesis in neonates. Am J Physiol Endocrinol Metab. 2002;283:E909–E916. doi: 10.1152/ajpendo.00220.2002. [DOI] [PubMed] [Google Scholar]
- 6.Myers MJ, Farrell DE, Palmer DC, Post LO. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int Immunopharmacol. 2003;3:571–579. doi: 10.1016/S1567-5769(03)00048-1. [DOI] [PubMed] [Google Scholar]
- 7.Beasley D, Eldridge M. Interleukin-1 beta and tumor necrosis factor-alpha synergistically induce NO synthase in rat vascular smooth muscle cells. Am J Physiol. 1994;266:R1197–R1203. doi: 10.1152/ajpregu.1994.266.4.R1197. [DOI] [PubMed] [Google Scholar]
- 8.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab. 2007;293:E453–E459. doi: 10.1152/ajpendo.00204.2007. [DOI] [PubMed] [Google Scholar]
- 9.Titheradge MA. Nitric oxide in septic shock. Biochim Biophys Acta. 1999;1411:437–455. doi: 10.1016/s0005-2728(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 10.Parratt JR. Nitric oxide in sepsis and endotoxaemia. J Antimicrob Chemother. 1998;41:31–39. doi: 10.1093/jac/41.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 11.Kapur S, Bedard S, Marcotte B, Cote CH, Marette A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- 12.Jurasinski C, Gray K, Vary TC. Modulation of skeletal muscle protein synthesis by amino acids and insulin during sepsis. Metabolism. 1995;44:1130–1138. doi: 10.1016/0026-0495(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 13.Vary TC, Jefferson LS, Kimball SR. Insulin fails to stimulate muscle protein synthesis in sepsis despite unimpaired signaling to 4E-BP1 and S6K1. Am J Physiol Endocrinol Metab. 2001;281:E1045–E1053. doi: 10.1152/ajpendo.2001.281.5.E1045. [DOI] [PubMed] [Google Scholar]
- 14.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 15.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 16.Peraldi P, Spiegelman B. TNF-alpha and insulin resistance: summary and future prospects. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 17.Del Aguila LF, Claffey KP, Kirwan JP. TNF-alpha impairs insulin signaling and insulin stimulation of glucose uptake in C2C12 muscle cells. Am J Physiol. 1999;276:E849–E855. doi: 10.1152/ajpendo.1999.276.5.E849. [DOI] [PubMed] [Google Scholar]
- 18.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez R, Myers MG, Jr, White MF, Rhoads RE. Stimulation of protein synthesis, eukaryotic translation initiation factor 4E phosphorylation, and PHAS-I phosphorylation by insulin requires insulin receptor substrate 1 and phosphatidylinositol 3-kinase. Mol Cell Biol. 1996;16:2857–2864. doi: 10.1128/mcb.16.6.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 21.van den Blink B, Branger J, Weijer S, Deventer SH, van der Poll T, Peppelenbosch MP. Human endotoxemia activates p38 MAP kinase and p42/44 MAP kinase, but not c-Jun N-terminal kinase. Mol Med. 2001;7:755–760. [PMC free article] [PubMed] [Google Scholar]
- 22.Downey JS, Han J. Cellular activation mechanisms in septic shock. Front Biosci. 1998;3:d468–d476. doi: 10.2741/a293. [DOI] [PubMed] [Google Scholar]
- 23.Combettes-Souverain M, Issad T. Molecular basis of insulin action. Diabetes Metab. 1998;24:477–489. [PubMed] [Google Scholar]
- 24.Fan J, Li YH, Wojnar MM, Lang CH. Endotoxin-induced alterations in insulin insulin-stimulatedphosphorylation of insulin receptor, IRS-1, and MAP kinase in skeletal muscle. Shock. 1996;6:164–170. [PubMed] [Google Scholar]
- 25.Pham PT, Heydrick SJ, Fox HL, Kimball SR, Jefferson LS, Jr, Lynch CJ. Assessment of cell-signaling pathways in the regulation of mammalian target of rapamycin (mTOR) by amino acids in rat adipocytes. J Cell Biochem. 2000;79:427–441. doi: 10.1002/1097-4644(20001201)79:3<427::aid-jcb80>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 26.Meininger CJ, Wu G. Regulation of endothelial cell proliferation by nitric oxide. Methods Enzymol. 2002;352:280–295. doi: 10.1016/s0076-6879(02)52026-7. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes AL, Carvalheira JB, Carvalho CR, Brenelli SL, Saad MJ. Tissue-specific regulation of early steps in insulin action in septic rats. Life Sci. 2001;69:2103–2112. doi: 10.1016/s0024-3205(01)01288-7. [DOI] [PubMed] [Google Scholar]
- 29.Orellana RA, Kimball SR, Suryawan A, Escobar J, Nguyen HV, Jefferson LS, Davis TA. Insulin stimulates muscle protein synthesis in neonates during endotoxemia despite repression of translation initiation. Am J Physiol Endocrinol Metab. 2007;292:E629–E636. doi: 10.1152/ajpendo.00214.2006. [DOI] [PubMed] [Google Scholar]
- 30.McCowen KC, Ling PR, Ciccarone A, Mao Y, Chow JC, Bistrian BR, Smith RJ. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Crit Care Med. 2001;29:839–846. doi: 10.1097/00003246-200104000-00032. [DOI] [PubMed] [Google Scholar]
- 31.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 32.Soeters PB, Hallemeesch MM, Bruins MJ, van Eijk HM, Deutz NE. Quantitative in vivo assessment of arginine utilization and nitric oxide production in endotoxemia. Am J Surg. 2002;183:480–488. doi: 10.1016/s0002-9610(02)00847-4. [DOI] [PubMed] [Google Scholar]
- 33.Gocan NC, Scott JA, Tyml K. Nitric oxide produced via neuronal NOS may impair vasodilatation in septic rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;278:H1480–H1489. doi: 10.1152/ajpheart.2000.278.5.H1480. [DOI] [PubMed] [Google Scholar]
- 34.Kimball SR, Orellana RA, O'Connor PM, Suryawan A, Bush JA, Nguyen HV, Thivierge MC, Jefferson LS, Davis TA. Endotoxin induces differential regulation of mTOR-dependent signaling in skeletal muscle and liver of neonatal pigs. Am J Physiol Endocrinol Metab. 2003;285:E637–E644. doi: 10.1152/ajpendo.00340.2002. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds TH, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem. 2002;277:17657–17662. doi: 10.1074/jbc.M201142200. [DOI] [PubMed] [Google Scholar]
- 36.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi H, Matsuzaki H, Tanaka M, Ono Y, Tokunaga C, Kuroda S, Kikkawa U. Activation of RAC-protein kinase by heat shock and hyperosmolarity stress through a pathway independent of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:7639–7643. doi: 10.1073/pnas.93.15.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sable CL, Filippa N, Hemmings B, Van OE. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 1997;409:253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 39.Maitra SR, Chen E, Rosa D, Valane PD, El Maghrabi MR, Brathwaite CE. Modulations of signal transduction pathways during sepsis and the effects of insulin and mifepristone. Acad Emerg Med. 2003;10:1–8. doi: 10.1111/j.1553-2712.2003.tb01968.x. [DOI] [PubMed] [Google Scholar]