Abstract
Background
The differences in total hip arthroplasty (THA) survivorship may be influenced by individual susceptibility to periprosthetic osteolysis. This may be driven by functional polymorphisms in the genes for cytokines and cytokine receptors involved in the development of osteolysis in THA, thereby having an effect on the individual's phenotype.
Methods
We performed a study on 22 single-nucleotide polymorphisms (SNPs) for 11 cytokines and two cytokine receptor candidate genes for association with severity of acetabular osteolysis and risk to failure in THA. Samples from 205 unrelated Caucasian patients with cementless type THA (ABG 1) were investigated. Distribution of investigated SNP variants between the groups of mild and severe acetabular osteolysis was determined by univariate and multivariate analysis. Time-dependent output variables were analyzed by the Cox hazards model.
Results
Univariate analysis showed: 1) TNF-238*A allele was associated with severe osteolysis (odds ratio, OR = 6.59, p = 0.005, population attributable risk, PAR 5.2%); 2) carriers of the IL6-174*G allele were 2.5 times more prone to develop severe osteolysis than non-carriers (OR = 2.51, p = 0.007, PAR = 31.5%); 3) the carriage of IL2-330*G allele was associated with protection from severe osteolysis (OR = 0.55, p = 0.043). Based on logistic regression, the alleles TNF-238*A and IL6-174*G were independent predictors for the development of severe acetabular osteolysis. Carriers of TNF-238*A had increased cumulative hazard of THA failure according to Cox model (p = 0.024). In contrast, IL2-330*G allele predicted lower cumulative hazard of THA failure (p = 0.019).
Conclusion
Genetic variants of proinflammatory cytokines TNF-alpha and IL-6 confer susceptibility to severe OL. In this way, presence of the minor TNF allele could increase the cumulative risk of THA failure. Conversely, SNP in the IL2 gene may protect carriers from the above THA complications.
Background
Total hip arthroplasty (THA) is a procedure for alleviating end-stage osteoarthritis with significant impact on the quality of life in these patients [1]. However, the life-in-service of joint implants is limited predominantly by aseptic loosening and periprosthetic osteolysis both causally linked to wear debris that are generated continuously by THA [2]. Phagocytosed wear particles provoke macrophages, fibroblasts and other cells to release proinflammatory cytokines and mediators that attract other precursor cells. As a result, chronic inflammatory milieu and a foreign body granuloma develop [3]. This, together with direct inhibition of osteoblast function by wear particles [4], distorts homeostasis in bone in favour of excessive bone resorption, i.e. osteolysis, which can lead to aseptic loosening or periprosthetic fracture.
The concept of cytokines as initiators and perpetuators of particle disease is supported by reports on proinflammatory cytokine (IL-1, IL-6, TNF-α) up-regulation in macrophages and fibroblasts in response to wear particles [5,6]. These pleiotropic cytokines substantially promote the recruitment and maturation of osteoclast precursors at the bone-prosthesis interface [7]. Particle disease/osteolysis might be also facilitated by down-regulation of immunomodulatory cytokines with anti-osteoclastogenic properties (e.g. IL-4, IL-10, IFN-γ) as has been already demonstrated in inflammatory joint conditions [8,9].
However, so called "particle disease" cannot sufficiently explain either the variable degrees of osteolysis found in patients with similar wear rates (and thus exposure to wear particles) or differences in THA survivorship even in the case of the same implant and similar wear rate [10]. For this reason, the concept of individual susceptibility to osteolysis must play a role. Generally, the interaction between the prosthesis and host can be influenced by several less or well known mechanisms including the allergic hypersensitivity, non-allergic and toxic response to a material constituent of the implant [11,12]. Hypothetically, variation in genes for cytokines can alter gene function and/or expression which may affect the individual's resistance/susceptibility to severe osteolysis [13]. In support of this concept, Wilkinson et al. reported increased rate of osteolysis in patients carrying the A allele of the TNF-238 single nucleotide polymorphism (SNP), [14]. Other studies have addressed variation in single genes for other candidate molecules in relationship with aseptic loosening [15-17]. Surprisingly, only one study reported the variation in the axis of RANKL/RANK/OPG (at position RANK+575*T) that is considered the single most influencing regulator of osteoclastogenesis [18]. Interesting results were published by Gordon et al. on the association of periprosthetic osteolysis and polymorphism in genes for Wnt canonical pathway (FRZB 200Trp, FRZB 200Arg: 324Arg haplotype), [19]. Recently, the same team identified the association between carriage of IL1RN +2018*C allele and a decreased risk of osteolysis after THA [20]. Taken together, none of the reported findings has been replicated in independent samples thus still being considered as preliminary. Therefore, researchers in the field are strongly encouraged to perform well-organized replication studies to enable a meta-analysis [21].
This study was conducted to investigate the contribution of genetic variation in proinflammatory/immunomodulatory cytokine genes to the risk of development of severe periprosthetic osteolysis. In addition, this study addressed the question whether there is an association between particular cytokine gene variants and risk of THA failure.
Methods
Subjects
Between February 2004 and June 2007 blood samples were collected by venopuncture from 205 patients with mild and severe acetabular bone defects around THA. The subjects were divided into these two groups according to the size of their acetabular bone defects. This was determined by the classification of Saleh et al. from preoperative radiographs and confirmed intraoperatively [22]. Intraoperatively bone defects were evaluated distinguishing at the acetabular site: no significant bone loss (type I), contained bone loss (type II), moderate uncontained bone loss (type III), severe uncontained bone loss (types IV) and pelvic discontinuity (type V). Briefly, if patients fulfilled the criteria for acetabular bone defects of type I and II they were considered as having mild osteolysis (N = 89) while others with more extensive bone defects (types III to V) were classified as severe osteolysis (N = 116).
The study included only Czech Caucasian patients who were operated on at a single institution and those with an identical cementless prosthesis (ABG, Howmedica, Inc., Staines, England). The ABG 1 prosthesis was designed in the 1980s as a press-fit hemispherical cup and anatomical stem both with hydroxyapatite coating. All polyethylene liners were ram-extruded from Hostalen GUR 4150 and air-sterilized with 25 kGy gamma irradiation [23]. Our local register was scrutinized for patients who were and were not revised for osteolysis while the latter were chosen from patients with the longest follow-up assuming those are of "resistant genotype" against premature failure and development of severe osteolysis (phenotypically mild osteolysis). All patients were contacted and invited for clinical and radiographic examination and blood sampling. In patients with bilateral THA who were not revised on either side by the day of blood sampling, the data for hips with longer follow-up were included while in the case of revision, the data for hips with shorter follow-up were recorded. The reasons for that were similar as above, i.e. hypothesized risk genotype for severe acetabular osteolysis development. Basic demographic and clinically-relevant data for both study groups are shown in Table 1.
Table 1.
Basic characteristics of the THA patients included in the study stratified according to the severity of osteolysis at the acetabular site.
|
Mild osteolysis (Types I, II) |
Severe osteolysis (Types III-V) |
p Value# | |
| Patients, N | 89 | 116 | |
| Gender (men/women) | 35/54 | 33/83 | p = 0.101 |
| Age at index surgery | 48 (27-58) | 45 (24-68) | p = 0.128 |
| Primary diagnosis: | |||
| Osteoarthritis | 35 | 13 | |
| Dysplastic hip | 23 | 62 | p < 0.001 |
| Other diagnoses | 31 | 41 | |
| BMI (kg/m2) | 28.1 (20.3-35.7) | 27.2 (16.0-42.6) | p = 0.062 |
| Revision (yes) | 44 | 113 | p < 0.001 |
| Age at event* (years) | 55 (34-69) | 52 (29-77) | p = 0.005 |
| Time to event* (years) | 9 (2-13) | 6 (3-12) | p < 0.001 |
| Harris hip score | 78 (14-96) | 65 (28-98) | p < 0.001 |
| Prosthesis stable (yes) | 82 | 86 | p < 0.001 |
| Linear wear rate (mm/year) | 0.22 (0.04-0.92) | 0.34 (0.04-2.52) | p = 0.009 |
Data presented as median and range (minimum to maximum) in parentheses. # p values for comparison between the groups of patients with mild/severe osteolysis were calculated by Chi-squared test or Mann-Whitney U test as appropriate. *The "event" was defined as THA revision for revised patients or the final visit for non-revised patients.
All hips included in the study had stable prosthesis at the first year after index surgery. Interpretation of final radiographs consisted of evaluation of implant stability, occurrence and extent of osteolysis. This was performed according to well-known and validated criteria [22,24,25]. In the revised cases (N = 157), the radiographic findings were supplemented with intraoperative findings. In this line, radiographic stability was re-coded (=changed) to loosening in cases where an implant instability was revealed after a weak levering of special tools for the cup/stem removal. Wear measurement was made using a Universal-type measuring microscope in the revised cases. Briefly, the methodology relies on the determination of nine three-dimensional coordinates on the surface of the prosthetic ball fixed inside the retrieved polyethylene cups in both the post-use and manufactured positions. Based on it, the centre of the prosthetic ball at each position and total wear can be calculated using the special computational algorithm. Briefly, previously reported accuracy of the method used for polyethylene wear measurement ranged from 1 to 4 μm and 1 to 9 mm3 for linear and volumetric wear, respectively; reliability of the method was also previously assessed [26,27].
All blood specimens were collected under the same conditions. Written informed consent was obtained from each subject and the study was approved by the Ethics Committee of Palacky University and Teaching Hospital in Olomouc.
Genotyping of cytokine gene single nucleotide polymorphisms
Twenty-two SNPs distributed in 13 genes encoding cytokines and cytokine receptors (IL-1α, IL-1β, IL-1R, IL-1Ra, IL-4Rα, IL-12, IFN-γ, TGF-β, TNF-α, IL-2, IL-4, IL-6 and IL-10) were investigated (see Additional file 1). Candidate genes were chosen in order to reflect a wide spectrum of cytokines and their receptors which have been shown to play a role in the development of osteolysis. The SNPs were selected for each investigated gene based on their previously reported or anticipated functional relevance for cytokine expression and/or structure (e.g. [28]). The location (function) of investigated SNPs within the genes is listed in Additional file 1. The great majority of chosen SNPs became the most widely studied within particular cytokine genes for their possible association with diseases and they are currently considered as "clinically associated" in public databases http://www.ncbi.nlm.nih.gov/projects/SNP.
The technique for genotyping cytokine SNPs has been described elsewhere [29]. Briefly, DNA was extracted from peripheral blood by the standard salting-out procedure [30] and the DNA was handled according to ethical rules. Genotyping was done by polymerase chain reaction with sequence-specific primers (PCR-SSP) using the Heidelberg kit (Cytokine Typing Tray kit, University of Heidelberg, Heidelberg, Germany). The protocol has been described elsewhere http://www.ihwg.org/components/cytokine/CytokineManualII.rtf. In total, 5000 SNP genotypings were performed. We succeeded in assigning the genotype in more than 98% of genotyped samples/SNPs. The nomenclature for the investigated SNPs was adopted from the manual of the Heidelberg kit (Lot No. CYT11). Despite using the typing kit already validated by us [29] and others [31], a subset of six SNPs in IL1A, IL1B, IL6, and TNF genes was tested for genotyping concordance by "in-house" PCR-based methodologies [32,33].
Study design and statistical analysis
This study investigated eventual associations between severity of acetabular osteolysis in THA (primary outcome) and twenty-two SNPs across eleven genes coding for proinflammatory/immunomodulatory cytokines and also for two cytokine receptor genes, all located across ten chromosomes. Distribution of investigated SNP variants was compared by univariate analysis between the groups of patients with severe (N = 116) and mild (N = 89) osteolysis. Three SNPs emerging from this analysis were tested also for association with secondary outcome of the study, i.e. cumulative hazard of THA failure. The revision of THA was considered as the end point in this analysis although it is known that the decision-making on the timing of revision depends on several factors including those unrelated to the prosthesis failure [34]. Subsequently, a multivariate analysis using logistic regression was performed in order to evaluate interactions of different covariates on the primary outcome. Finally, association of investigated cytokine SNP variants with secondary outcome of the study (cumulative hazard of THA failure) was adjusted for severity of osteolysis.
Comparison of genotypes and carriage rates
Univariate analysis was performed by comparisons of genotype and phenotype frequencies between subgroups of THA patients using χ2 test; the term 'phenotype frequency' (i.e., carriage rate) gives the number of subjects carrying one (or two) copies of a particular allele on one or both (maternal and paternal) chromosomes. Odds ratios (OR) were calculated for carriers of risk allele compared to non-carriers. Population attributable risk (PAR) was calculated according to the protocol described elsewhere [35]. χ2 goodness-of-fit test which compares observed and expected genotype numbers for each investigated SNP was used to test for deviation of genotype distribution from the Hardy-Weinberg (H-W) equilibrium. P value less than 0.05 was considered as significant.
Cumulative estimates and other statistics
Multivariate analyses using logistic regression were performed by forward stepwise (likelihood ratio) method. Cox regression analysis was used to assess cumulative hazard of revision after THA. The time to the event was determined as the time that elapsed since THA implantation to the date of revision or until the final follow-up, whichever occurred first. Homogeneity of the groups was tested using the Chi-squared and Mann-Whitney U tests. All statistics were calculated with the SPSS 15.0 (SPSS Inc, Chicago, IL, USA).
Results
Clinical outcome of patients with THA according to the severity of OL
Patients with severe osteolysis had significantly lower Harris Hip Score at the last follow-up in comparison with those with mild osteolysis (mean 65 versus 78 points, p < 0.001). They were revised in 113/116 (97.4%) cases in comparison with 44/89 (49.4%, p < 0.001) of patients with mild osteolysis. THA was classified as "stable" in 74.1% (86/116) and 92.1% (82/89) in patients with severe and mild osteolysis (p < 0.001), respectively. Furthermore, patients with severe osteolysis were characterised by shorter time to revision/final checking out (median 6 years) when compared with mild osteolysis group (median 9 years; p < 0.001). The reasons for revision were as follows: osteolysis around a stable cup 85/34 (in groups with severe/mild osteolysis), aseptic loosening of the cup 27/7 and periprosthetic fracture 3/1. The most frequent location of acetabular osteolysis was zone II (88%) in revised cases according to DeLee and Charnley classification [36]. In unrevised cases, location of osteolysis was in zone II (42%) followed by zones I (28%) and III (12%).
The distribution of cytokine/cytokine receptor gene SNPs in THA patients
To determine the distribution of the twenty two cytokine/cytokine receptor SNPs, the group of 205 well-characterised THA patients was genotyped using PCR-SSP technique. Of the 22 investigated SNPs, the distribution of genotypes deviated from the Hardy-Weinberg (H-W) equilibrium for one SNP in the group of THA patients as a whole (TGFB1 codon 25: p = 0.01).
However, we did not observe any deviation from H-W equilibrium for this SNP in our sample of Czech healthy subjects investigated by the same methodology [29]. Furthermore, the deviation of TGFB1 codon 25 SNP could occur due to the random fluctuation when H-W testing for multiple markers was applied. We, therefore, used data on this TGFB1 SNP for further analyses with caution. This approach is in compliance with the current recommendations for the reporting of genetic association studies [37].
Association of investigated cytokine/cytokine receptor gene SNPs with severity of acetabular OL in THA
Of 22 analysed cytokine/cytokine receptor SNPs, the proportion of TNF-238*A allele carriers was higher among the patients with severe than mild osteolysis (OR = 6.59, p = 0.005, PAR = 5.2%, Figure 1A). Similarly, carriers of the IL6-174*G allele were overrepresented among patients with severe osteolysis compared to those with mild osteolysis (OR = 2.51, p = 0.007, PAR = 31.5%, Figure 1B). By contrast, the IL2-330*G allele was underrepresented in patients with severe osteolysis in comparison to those with mild ostelysis (OR = 0.55, p = 0.043, Figure 1C) and, thus, appeared to protect against severe osteolysis.
Figure 1.
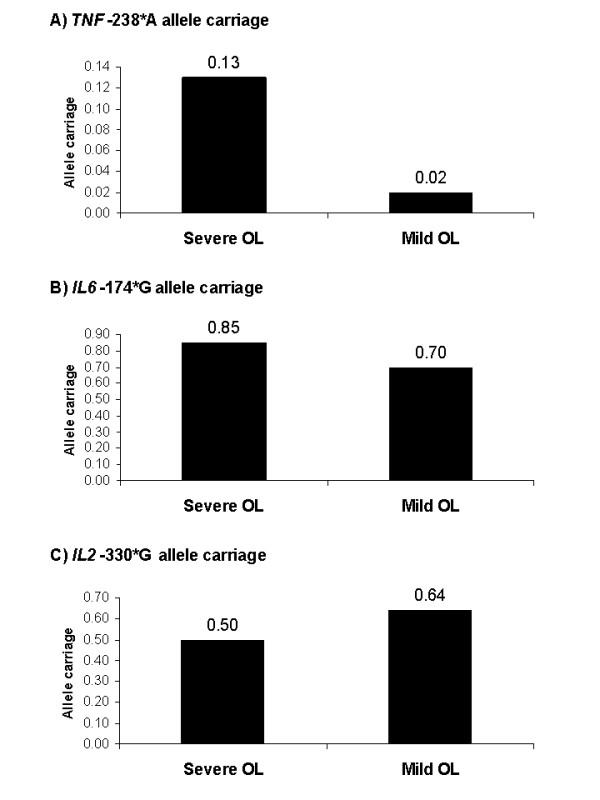
Comparison of proportion of TNF-238*A (A), IL6-174*G (B), and IL2-330*G (C) allele carriers in the groups of patients with severe and mild osteolysis. Comparison severe versus mild osteolysis: TNF-238*A: p = 0.005, OR = 6.59 (95% CI: 1.47-29.64), PAR% = 5.2. IL6-174*G: p = 0.007, OR = 2.51, (95% CI: 1.27-4.98), PAR% = 31.5. IL2-330*G: p = 0.043, OR = 0.55, (95% CI: 0.31-0.98). OR: odds ratio, CI: confidence interval, PAR%: population attributable risk percentage
Multivariate analysis of primary outcome (severity of osteolysis) used regression model including age at index THA, gender, weight/height, primary diagnosis, linear wear rate (LWR) and genetic variants (TNF-238*A, IL6-174*G, IL2-330*G). In this analysis, primary diagnosis (p = 0.002) and LWR (p = 0.004) significantly predicted severe osteolysis. Because LWR covariate strongly limited analysis [it was measured only in revised cases - 157/205 of all THA patients, 76.5%], it was removed from the final regression analysis. In a model without LWR the alleles TNF-238*A (p = 0.045) and IL6-174*G (p = 0.049) appeared to be further predictors for the development of more severe acetabular osteolysis, but the contribution of IL2-330*G allele was not apparent here.
Cumulative risk of THA failure and its relationship to the cytokine/cytokine receptor gene SNPs
Since the rate of THA revision is considered the most critical parameter in assessing THA outcome and at the same time aseptic loosening together with periprosthetic osteolysis is the most frequent cause of THA failure, we were interested in whether cumulative hazard of THA failure (defined as a revision due to aseptic loosening or osteolysis) was associated with cytokine/cytokine receptor SNP variants. In agreement with the observed associations of the TNF-238*A allele with severe osteolysis, TNF-238*A carriers were characterised by increased cumulative hazard of THA failure compared to patients with the TNF-238 GG genotype (p = 0.024, Figure 2A). However, the presence of the IL6-174*G allele in THA patients did not change the cumulative hazard of THA failure. On the other hand, the protective effect of the IL2-330*G allele was further confirmed in terms of lower cumulative hazard of THA failure in IL2-330*G allele carriers compared to non-carriers (p = 0.019, Figure 2B). Nevertheless, neither TNF-238*A nor IL2-330*G alleles predicted changes in the cumulative hazard of THA failure independently from osteolysis as was seen after adjustment of Cox regression analysis for the severity of osteolysis.
Figure 2.
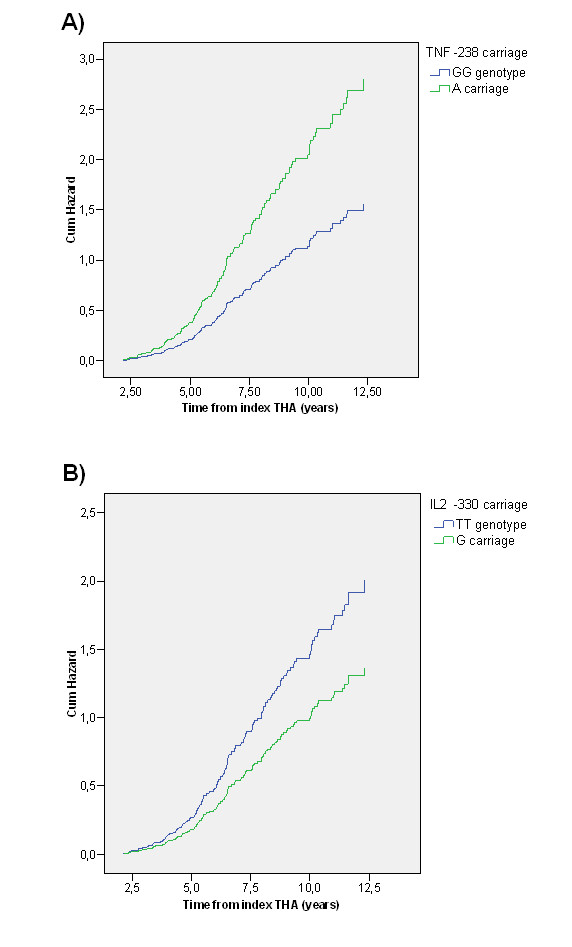
Comparison of cumulative hazard of THA failure between the carriers (green curve) and non-carriers (blue curve) of TNF-238*A (A) and IL2-330*G (B) alleles assessed by Cox regression analysis. TNF-238*A vs. TNF-238 GG homozygotes: p = 0.024. IL2-330*G vs. IL2-330 TT homozygotes: p = 0.019.
Discussion
This comprehensive study investigated the association between extent of acetabular osteolysis in THA and polymorphic variants across a wide spectrum of genes encoding for cytokines/cytokine receptors with inflammatory and/or immunomodulatory properties. In particular, the study suggests that SNPs in genes for proinflammatory cytokines TNF-α and IL-6 may play a role in the pathogenesis of osteolysis. In addition, a SNP in the gene for the Th1 cytokine IL-2 was found to be a factor associated with severity of acetabular osteolysis and risk for premature THA failure.
In this study, carriage of the TNF-238*A allele was associated with severe acetabular osteolysis and risk for premature failure in contrast to the TNF-238 "GG" genotype. Individuals carrying the TNF-238*A were six times more prone to develop severe osteolysis than patients with the GG genotype. Accordingly, the striking risk for severe acetabular osteolysis associated with TNF-238*A carriage could be detected here at statistically significant level though this variant is relatively rare (see power calculations in Additional file 1). Our study, therefore, confirms that TNF-238 SNP plays a role in the development of osteolysis as suggested by Wilkinson et al. [14]. Concerning another SNP in TNF gene promoter, our findings are concordant to those of Wilkinson et al. and Kolundzic et al. [17], who found no relationship between the carriage of the TNF-308*A allele and risk for osteolysis development or premature prosthetic failure.
To properly evaluate the contribution of SNPs in the TNF gene to osteolysis development and prognosis of THA, it should be acknowledged that the minor TNF-238*A allele occurs rarely in the normal population, limiting its contribution to the overall "population" risk of severe acetabular osteolysis (PAR value, 5.2%). This SNP may, however, serve as a marker for other causative polymorphism(s) in the TNF locus or neighbouring polymorphic major histocompatibility complex (MHC) genes. This interpretation would be also in accordance with equivocal data from in vitro/ex vivo analyses of the functional influence of TNF-238 and also TNF-308 polymorphisms on TNF gene transcription and subsequent cytokine expression, processes which seem to be highly cell or stimulus specific [38,39].
A SNP in the IL6 gene promoter was another polymorphism implicated in the pathogenesis of OL in this study. IL-6 is a multifunctional cytokine also involved in the regulation of bone metabolism; therefore its participation in the processes occurring at the bone-prosthesis interface is plausible [6]. Here, IL6-174*G allele carriage was associated with the increased risk of severe osteolysis according to both the univariate and multivariate analyses. However, this SNP in gene for IL-6 did not influence the risk for cumulative hazard of THA failure. Association of IL6-174*G with severe osteolysis could not currently be supported by straightforward "mechanistic" explanation because its functional role on IL-6 expression is controversial. The allele IL6-174*G was originally associated with higher induced expression of IL-6 in vitro [40], however this was not subsequently confirmed [41]. Importantly, Terry et al. revealed that effect of IL6 genetic variation on IL-6 expression is tissue-specific and dependent on other environmental factors [41,42]. This observation may explain the opposite roles of IL6-174 SNP observed in different clinical conditions [40,43,44]. Our results, therefore, allow us to speculate that complex local response around THA provides adequate conditions/stimuli promoting "penetrance" of the IL6-174 variant in terms of its effect on IL-6 expression. Concerning previous findings on IL6 gene in THA outcome, Malik et al. [16] like us, reported no association of IL6-174 SNP with the risk of premature failure of cemented THA. The association of the IL6 SNP (positions -597 and -572) with the earlier prosthetic failure has been reported by Kolundzic et al. [17]. However, their study had a small number of patients and included both hips in the same patient into the analysis.
Based on our data the IL2-330*G genetic variant may function protectively against severe osteolysis which could result in significantly lower cumulative hazard of THA failure. Interestingly, Campos et al. [45] found that polymorphism in the IL2-330*G was not associated with premature failure of dental implants. Due to biological and mechanical similarities between THA and dental implants, both studies suggest a protective role of IL2 SNP on the prosthetic-bone interface. IL-2 regulates both survival and death of regulatory T cells which might affect the osteoclast life cycle [46]. However, the functional relevance of the investigated IL2 SNP is not clear [47,48] and thus the role of IL-2, and therefore also Th1 lymphocytes in the pathogenesis of periprosthetic osteolysis has yet to be elucidated [11]. Whatever the mechanism behind possible IL-2 participation in pathological processes around THA, if proven in other centres/populations, the protective effects of the IL2-330*G would have clear implications because the G allele is carried by more than 50% of Europeans [29,49].
While interpreting the results of the current study, one should consider the theory of multifactorial susceptibility to complex disease and current issues of genetic association studies (GAS). Any individual genome contains many functional variants and many of these can influence the development of osteolysis in conjunction with other biological, mechanical and material factors. Furthermore, one may also conceivably argue that other genetic "elements" than those identified in this study may be involved in the complex process of particular disease via phenomenon of linkage disequilibrium [50]. Future investigations should be directed at detailed analyses of specified candidate regions on chromosomes 4q, 6p and 7q in order to search for further genetic markers of osteolysis developing after THA. Finally, based on current experience showing that the great proportion of GAS has not been replicated in independent samples, further validation through replication studies is strictly recommended [51]. In this regard, it should be emphasised that our study replicated the findings of Wilkinson et al. for TNF-238*A [14].
Limitations and strengths of our study
We are aware that relative heterogeneity of the primary diagnoses leading to index THA may be linked to the THA outcome including osteolysis. Nevertheless, genetic variants of cytokine genes (namely TNF-238*A and IL6-174*G) appeared to be independent predictors of severe osteolysis after adjustment for primary diagnosis and other relevant factors in the regression analysis. Moreover, the vast majority of dysplastic hips (over 98%) were of Hartofilakidis type I which influence the THA survival only insignificantly, and the remaining ones were of type II [52]. In addition, selection bias might have an influence on the reported findings because not all patients with the ABG 1 prosthesis could be included in the study (205 of 506). In non-revised cases the severity of bone defects was determined primarily from radiography which could have led to underestimation of the true bone defects. Despite the rigorously created design of this association study, false positive findings cannot be excluded. On the other hand, only patients with identical prosthesis were included which eliminated the influence of inter-prosthetic differences on osteolysis development and prosthetic failure. In addition, surgery was performed at the single institution by a limited number of experienced surgeons which should minimize the role for surgery-related differences on failure of the implant.
Also from the genetic epidemiology view, our study meets the recently adopted criteria for GAS [21]. Apart from emphasis on its preliminary character, this study had adequate study power (more than 80% in 19 of 22 investigated SNPs; see Additional file 1) and adhered to quality control methods in genotyping/analytical methodologies [29]; overall genotyping "failure" rate was very low (less than two percent). Importantly, the parameter of PAR was included to estimate the specific contribution of investigated genetic markers among other complex individual and environmental factors promoting development of severe acetabular osteolysis and premature THA failure.
Conclusion
This case-control study associated gene polymorphisms in two proinflammatory cytokines TNF-α and IL-6 with extent of acetabular osteolysis. In addition, the risk for THA failure was increased in carriers of TNF-238*A allele. In contrast, a polymorphism of the gene for regulatory Th1 cytokine IL-2 emerged as a possible factor protecting against premature failure of THA and was negatively associated with osteolysis severity in univariate analysis. Collectively, the data expand the current paradigm of osteolysis as a predominantly inflammatory process and may implicate T-cells and their modulatory cytokines in the particle disease. If this single-centre data are replicated in other centres/population, new avenues will be opened up for the use of genetic testing in pre-surgical stages and also for investigation of immune response modulation in THA.
List of abbreviations used in the study
GAS: Genetic Association Study; IFN: Interferon; IL: Interleukins; M-CSF: Macrophage colony stimulating factor; OR: Odds ratio; PAR: Population attributable risk; RANKL: Receptor activator of NF-κβ ligand; SNPs: Single nucleotide polymorphisms; TGF-β: Transforming growth factor beta; THA: Total hip arthroplasty; TNF-α: Tumour necrosis factor alpha.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All three authors equally contributed to this paper; the order of the authors is alphabetical. JG recruited THA patients into the study and collected clinical details, MP provided healthy population control. FM carried out the genotyping and performed statistical analyses with help of MP's and JG's ideas. Conceptualization and design of the study was joint work of JG (clinical problem of individual predisposition to osteolysis) and MP (immunogenetic approach to its solution and proposal for data analyses/presentation). All three authors contributed to drafting of the paper; the author responsible for the MS integrity is MP.
Authors' information
All three authors are with the Faculty of Medicine and Dentistry, Palacky University, Czech Republic and in parallel with the Faculty Hospital Olomouc, Czech Republic. Associated Professor Jiri Gallo, MD, PhD is the chief of Department of Orthopaedics; Assistant Professor Frantisek Mrazek, MD, PhD is at the Department of Immunology and leads the DNA section of the Tissue Typing laboratory, Faculty Hospital; Professor Martin Petrek, MD, PhD, is the acting head of the Department of Clinical Chemistry & Immunogenetics, the Principal Investigator at the Laboratory of Immunogenomics & Proteomics and the Director of the Tissue Typing Laboratory.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
List of investigated cytokine/cytokine receptor SNPs. The data provided represent the list of investigated cytokine/cytokine receptor SNPs with their gene location, designation in Cytokine CTS-PCR-SSP Tray Kit (University of Heidelberg), NCBI reference SNP cluster report (refSNP), and function/location of each SNP within the particular gene.
Acknowledgments
Acknowledgements
This study was supported by Internal Grant Agency of Ministry of Health, Czech Republic (IGA MZ CR NR 9490 to Frantisek Mrazek).
Drs. V. Havranek, A. Arakelyan and Z. Kubistova assisted in the following partial works related to this report: measurements of polyethylene wear in retrieved THAs (VH), contribution to data analyses (AA) and partial genotyping of population control (ZK). Prof. S.B. Goodman has critically assessed the manuscript. Parts of this work were presented at the 20th European Histocompatibility & Immunogenetics Conference, June 6-9 2006, Oslo, Norway, 17th Annual Meeting of EORS, April 24-26 2008, Madrid, Spain and 9th EFORT Congress, May 29-June 1 2008, Nice, France, where poster by Jiri Gallo et al. was awarded the Jacques-Duparc award.
Contributor Information
Jiri Gallo, Email: jiri.gallo@volny.cz.
Frantisek Mrazek, Email: Frantisek.Mrazek@fnol.cz.
Martin Petrek, Email: martin.petrek@fnol.cz.
References
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370:1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- Tuan RS, Lee FY, Y TK, Wilkinson JM, Smith RL. What are the local and systemic biologic reactions and mediators to wear debris, and what host factors determine or modulate the biologic response to wear particles? J Am Acad Orthop Surg. 2008;16:S42–48. doi: 10.5435/00124635-200800001-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konttinen YT, Zhao D, Beklen A, Ma G, Takagi M, Kivela-Rajamaki M, Ashammakhi N, Santavirta S. The microenvironment around total hip replacement prostheses. Clin Orthop Relat Res. 2005:28–38. doi: 10.1097/01.blo.0000150451.50452.da. [DOI] [PubMed] [Google Scholar]
- Vermes C, Chandrasekaran R, Jacobs JJ, Galante JO, Roebuck KA, Glant TT. The effects of particulate wear debris, cytokines, and growth factors on the functions of MG-63 osteoblasts. J Bone Joint Surg Am. 2001;83-A:201–211. doi: 10.2106/00004623-200102000-00007. [DOI] [PubMed] [Google Scholar]
- Koulouvaris P, Ly K, Ivashkiv LB, Bostrom MP, Nestor BJ, Sculco TP, Purdue PE. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J Orthop Res. 2008;26:106–116. doi: 10.1002/jor.20486. [DOI] [PubMed] [Google Scholar]
- Shanbhag AS, Kaufman AM, Hayata K, Rubash HE. Assessing osteolysis with use of high-throughput protein chips. J Bone Joint Surg Am. 2007;89:1081–1089. doi: 10.2106/JBJS.F.00330. [DOI] [PubMed] [Google Scholar]
- Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. J Orthop Res. 2005;23:511–519. doi: 10.1016/j.orthres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Tunyogi-Csapo M, Kis-Toth K, Radacs M, Farkas B, Jacobs JJ, Finnegan A, Mikecz K, Glant TT. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: Fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–2408. doi: 10.1002/art.23653. [DOI] [PubMed] [Google Scholar]
- Ramage SC, Urban NH, Jiranek WA, Maiti A, Beckman MJ. Expression of RANKL in osteolytic membranes: association with fibroblastic cell markers. J Bone Joint Surg Am. 2007;89:841–848. doi: 10.2106/JBJS.F.00655. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Hamer AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res. 2005;23:520–525. doi: 10.1016/j.orthres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granchi D, Cenni E, Trisolino G, Giunti A, Baldini N. Sensitivity to implant materials in patients undergoing total hip replacement. J Biomed Mater Res B Appl Biomater. 2006;77:257–264. doi: 10.1002/jbm.b.30445. [DOI] [PubMed] [Google Scholar]
- Ollier WE. Cytokine genes and disease susceptibility. Cytokine. 2004;28:174–178. doi: 10.1016/j.cyto.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Wilson AG, Stockley I, Scott IR, Macdonald DA, Hamer AJ, Duff GW, Eastell R. Variation in the TNF gene promoter and risk of osteolysis after total hip arthroplasty. J Bone Miner Res. 2003;18:1995–2001. doi: 10.1359/jbmr.2003.18.11.1995. [DOI] [PubMed] [Google Scholar]
- Malik MH, Bayat A, Jury F, Kay PR, Ollier WE. Genetic susceptibility to total hip arthroplasty failure--positive association with mannose-binding lectin. J Arthroplasty. 2007;22:265–270. doi: 10.1016/j.arth.2006.02.163. [DOI] [PubMed] [Google Scholar]
- Malik MH, Jury F, Bayat A, Ollier WE, Kay PR. Genetic susceptibility to total hip arthroplasty failure: a preliminary study on the influence of matrix metalloproteinase 1, interleukin 6 polymorphisms and vitamin D receptor. Ann Rheum Dis. 2007;66:1116–1120. doi: 10.1136/ard.2006.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolundzic R, Orlic D, Trkulja V, Pavelic K, Troselj KG. Single nucleotide polymorphisms in the interleukin-6 gene promoter, tumor necrosis factor-alpha gene promoter, and transforming growth factor-beta1 gene signal sequence as predictors of time to onset of aseptic loosening after total hip arthroplasty: preliminary study. J Orthop Sci. 2006;11:592–600. doi: 10.1007/s00776-006-1069-y. [DOI] [PubMed] [Google Scholar]
- Malik MH, Bayat A, Jury F, Ollier WE, Kay PR. Genetic susceptibility to hip arthroplasty failure--association with the RANK/OPG pathway. Int Orthop. 2006;30:177–181. doi: 10.1007/s00264-006-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Southam L, Loughlin J, Wilson AG, Stockley I, Hamer AJ, Eastell R, Wilkinson JM. Variation in the secreted frizzled-related protein-3 gene and risk of osteolysis and heterotopic ossification after total hip arthroplasty. J Orthop Res. 2007;25:1665–1670. doi: 10.1002/jor.20446. [DOI] [PubMed] [Google Scholar]
- Gordon A, Kiss-Toth E, Stockley I, Eastell R, Wilkinson JM. Polymorphisms in the interleukin-1 receptor antagonist and interleukin-6 genes affect risk of osteolysis in patients with total hip arthroplasty. Arthritis Rheum. 2008;58:3157–3165. doi: 10.1002/art.23863. [DOI] [PubMed] [Google Scholar]
- Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, Thomas G, Hirschhorn JN, Abecasis G, Altshuler D, Bailey-Wilson JE, et al. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]
- Saleh KJ, Holtzman J, Gafni A, Saleh L, Davis A, Resig S, Gross AE. Reliability and intraoperative validity of preoperative assessment of standardized plain radiographs in predicting bone loss at revision hip surgery. J Bone Joint Surg Am. 2001;83-A:1040–1046. doi: 10.2106/00004623-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Gallo J, Langova K, Havranek V, Cechova I. Poor survival of ABG I hip prosthesis in younger patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2008;152:163–168. doi: 10.5507/bp.2008.027. [DOI] [PubMed] [Google Scholar]
- Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990:107–128. [PubMed] [Google Scholar]
- Udomkiat P, Wan Z, Dorr LD. Comparison of preoperative radiographs and intraoperative findings of fixation of hemispheric porous-coated sockets. J Bone Joint Surg Am. 2001;83-A:1865–1870. doi: 10.2106/00004623-200112000-00015. [DOI] [PubMed] [Google Scholar]
- Gallo J, Havranek V, Zapletalova J, Mandat D. Measurement of acetabular polyethylene wear of total hip replacement, using a universal measuring microscope. Characteristics of measurements. Acta Chir Orthop Traumatol Cech. 2006;73:28–33. In Czech. [PubMed] [Google Scholar]
- Gallo J, Havranek V, Cechova I, Zapletalova J. Wear measurement of retrieved polyethylene ABG 1 cups by universal-type measuring microscope and X-ray methods. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:321–326. doi: 10.5507/bp.2006.049. [DOI] [PubMed] [Google Scholar]
- Javor J, Bucova M, Ferencik S, Grosse-Wilde H, Buc M. Single nucleotide polymorphisms of cytokine genes in the healthy Slovak population. Int J Immunogenet. 2007;34:273–280. doi: 10.1111/j.1744-313X.2007.00693.x. [DOI] [PubMed] [Google Scholar]
- Kubistova Z, Mrazek F, Tudos Z, Kriegova E, Ambruzova Z, Mytilineos J, Petrek M. Distribution of 22 cytokine gene polymorphisms in the healthy Czech population. Int J Immunogenet. 2006;33:261–267. doi: 10.1111/j.1744-313X.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JA, (Ed) Proceedings of the 13th International Histocompatibility Workshop and Conference. Vol. 1. Seattle: IHWG Press; 2007. Immunobiology of the human MHC. [Google Scholar]
- Hutyrova B, Pantelidis P, Drabek J, Zurkova M, Kolek V, Lenhart K, Welsh KI, Du Bois RM, Petrek M. Interleukin-1 gene cluster polymorphisms in sarcoidosis and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;165:148–151. doi: 10.1164/ajrccm.165.2.2106004. [DOI] [PubMed] [Google Scholar]
- Mrazek F, Gallo J, Arakelyan A, Kubistova Z, Petrek M. Single nucleotide polymorphisms in genes for cytokines interleukin (IL)-2, IL-6 and TNFalpha influence severity of osteolysis after total hip arthroplasty. Tissue Antigens. 2008;71:331–332. [Google Scholar]
- Wroblewski BM, Siney PD, Fleming PA. Charnley low-friction arthroplasty: survival patterns to 38 years. J Bone Joint Surg Br. 2007;89:1015–1018. doi: 10.1302/0301-620X.89B8.18387. [DOI] [PubMed] [Google Scholar]
- Kurreeman FA, Padyukov L, Marques RB, Schrodi SJ, Seddighzadeh M, Stoeken-Rijsbergen G, Helm-van Mil AH van der, Allaart CF, Verduyn W, Houwing-Duistermaat J, et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med. 2007;4:e278. doi: 10.1371/journal.pmed.0040278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976:20–32. [PubMed] [Google Scholar]
- Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE Statement. Hum Genet. 2009;125:131–151. doi: 10.1007/s00439-008-0592-7. [DOI] [PubMed] [Google Scholar]
- Bayley JP, de Rooij H, Elsen PJ van den, Huizinga TW, Verweij CL. Functional analysis of linker-scan mutants spanning the -376, -308, -244, and -238 polymorphic sites of the TNF-alpha promoter. Cytokine. 2001;14:316–323. doi: 10.1006/cyto.2001.0902. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ghosh B, Sharma SK. Association of TNF polymorphisms with sarcoidosis, its prognosis and tumour necrosis factor (TNF)-alpha levels in Asian Indians. Clin Exp Immunol. 2008;151:251–259. doi: 10.1111/j.1365-2249.2007.03564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, Humphries S, Woo P. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest. 1998;102:1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275:18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Schluter B, Raufhake C, Erren M, Schotte H, Kipp F, Rust S, Van Aken H, Assmann G, Berendes E. Effect of the interleukin-6 promoter polymorphism (-174 G/C) on the incidence and outcome of sepsis. Crit Care Med. 2002;30:32–37. doi: 10.1097/00003246-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Michalek J, Svetlikova P, Fedora M, Klimovic M, Klapacova L, Bartosova D, Hrstkova H, Hubacek JA. Interleukin-6 gene variants and the risk of sepsis development in children. Hum Immunol. 2007;68:756–760. doi: 10.1016/j.humimm.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Campos MI, Godoy dos Santos MC, Trevilatto PC, Scarel-Caminaga RM, Bezerra FJ, Line SR. Interleukin-2 and interleukin-6 gene promoter polymorphisms, and early failure of dental implants. Implant Dent. 2005;14:391–396. doi: 10.1097/01.id.0000188470.54417.98. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum. 2007;56:4104–4112. doi: 10.1002/art.23138. [DOI] [PubMed] [Google Scholar]
- Matesanz F, Fedetz M, Leyva L, Delgado C, Fernandez O, Alcina A. Effects of the multiple sclerosis associated -330 promoter polymorphism in IL2 allelic expression. J Neuroimmunol. 2004;148:212–217. doi: 10.1016/j.jneuroim.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hoffmann SC, Stanley EM, Darrin Cox E, Craighead N, DiMercurio BS, Koziol DE, Harlan DM, Kirk AD, Blair PJ. Association of cytokine polymorphic inheritance and in vitro cytokine production in anti-CD3/CD28-stimulated peripheral blood lymphocytes. Transplantation. 2001;72:1444–1450. doi: 10.1097/00007890-200110270-00019. [DOI] [PubMed] [Google Scholar]
- Scola L, Candore G, Colonna-Romano G, Crivello A, Forte GI, Paolisso G, Franceschi C, Lio D, Caruso C. Study of the association with -330T/G IL-2 in a population of centenarians from centre and south Italy. Biogerontology. 2005;6:425–429. doi: 10.1007/s10522-005-4909-9. [DOI] [PubMed] [Google Scholar]
- Dupuis J, O'Donnell CJ. Interpreting results of large-scale genetic association studies: separating gold from fool's gold. JAMA. 2007;297:529–531. doi: 10.1001/jama.297.5.529. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougle A, Hemmady MV, Hodgkinson JP. Severity of hip dysplasia and loosening of the socket in cemented total hip replacement. A long-term follow-up. J Bone Joint Surg Br. 2005;87:16–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of investigated cytokine/cytokine receptor SNPs. The data provided represent the list of investigated cytokine/cytokine receptor SNPs with their gene location, designation in Cytokine CTS-PCR-SSP Tray Kit (University of Heidelberg), NCBI reference SNP cluster report (refSNP), and function/location of each SNP within the particular gene.


