Abstract
Semantic memory refers to knowledge about people, objects, actions, relations, self, and culture acquired through experience. The neural systems that store and retrieve this information have been studied for many years, but a consensus regarding their identity has not been reached. Using strict inclusion criteria, we analyzed 120 functional neuroimaging studies focusing on semantic processing. Reliable areas of activation in these studies were identified using the activation likelihood estimate (ALE) technique. These activations formed a distinct, left-lateralized network comprised of 7 regions: posterior inferior parietal lobe, middle temporal gyrus, fusiform and parahippocampal gyri, dorsomedial prefrontal cortex, inferior frontal gyrus, ventromedial prefrontal cortex, and posterior cingulate gyrus. Secondary analyses showed specific subregions of this network associated with knowledge of actions, manipulable artifacts, abstract concepts, and concrete concepts. The cortical regions involved in semantic processing can be grouped into 3 broad categories: posterior multimodal and heteromodal association cortex, heteromodal prefrontal cortex, and medial limbic regions. The expansion of these regions in the human relative to the nonhuman primate brain may explain uniquely human capacities to use language productively, plan, solve problems, and create cultural and technological artifacts, all of which depend on the fluid and efficient retrieval and manipulation of semantic knowledge.
Keywords: brain mapping, fMRI, meta-analysis, positron emission tomography, semantics
Introduction
The human brain has an enormous capacity to acquire knowledge from experience. The characteristic shapes, colors, textures, movements, sounds, smells, and actions associated with objects in the environment, for example, must all be learned from experience. Much of this knowledge is represented symbolically in language and underlies our understanding of word meanings. These relationships between words and the stores of knowledge they signify are known collectively as the “semantics” of a language (Bréal 1897). In this article, we use the more general term “semantic processing” to refer to the cognitive act of accessing stored knowledge about the world.
For most languages, semantic properties of words are readily distinguished from their structural properties. For example, words can have both spoken (phonological) and written (orthographic) forms, but these surface forms are typically related to word meanings only through the arbitrary conventions of a particular vocabulary. There is nothing, for example, about the letter sequences DOG or CHIEN that inherently links these sequences to a particular concept. Conversely, it is trivial to construct surface forms (e.g., CHOG) that possess all of the phonological and orthographic properties of words in a particular language, but which have no meaning in that language. In this article, we hold to a simple, operational distinction between 1) the processes of analyzing surface form (phonology, orthography), and 2) semantic processes, which concern access to knowledge not directly represented in the surface form.
Semantic processing is a defining feature of human behavior, central not only to language, but also to our capacity to access acquired knowledge in reasoning, planning, and problem solving. Impairments of semantic processing figure in a variety of brain disorders such as Alzheimer disease, semantic dementia, fluent aphasia, schizophrenia, and autism. The neural basis of semantic processing has been studied extensively by analyzing patterns of brain damage in such patients (e.g., Alexander et al. 1989; Hart and Gordon 1990; Chertkow et al. 1997; Tranel et al. 1997; Gainotti 2000; Mummery et al. 2000; Hillis et al. 2001; Damasio et al. 2004; Dronkers et al. 2004). On the whole, this evidence suggests a broadly distributed neural representation, with particular reliance on inferotemporal and posterior inferior parietal regions. Semantic processing has also been addressed in a large number of functional neuroimaging studies conducted on healthy volunteers, using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). The aim of the present study is to conduct a meta-analysis of this functional neuroimaging research, which now includes over 500 published studies. Several excellent reviews on neuroimaging studies of semantic processing have been presented previously, which focused mainly on the evidence for organization of object knowledge by taxonomic categories (Joseph 2001; Martin and Chao 2001; Bookheimer 2002; Thompson-Schill 2003; Damasio et al. 2004; Gerlach 2007). The present analysis differs from these prior efforts in including a larger number and broader range of studies, in adopting specific inclusion and exclusion criteria for identifying semantic processing experiments, and in the construction of probabilistic maps for summarizing the data.
Considered for inclusion were any PET or fMRI studies in which words (either spoken or written) were used as stimulus materials. Thus, the goal of the current study is to identify brain systems that access meaning from words. This approach contrasts with several previous reviews that included and even emphasized studies in which object pictures were used to elicit knowledge retrieval (Joseph 2001; Martin and Chao 2001; Damasio et al. 2004; Gerlach 2007). Our focus on linguistic materials reflects our present concern, which is not how objects are recognized, but rather how conceptual knowledge is organized and accessed. Although the knowledge stores underlying word comprehension may be activated similarly during word and object recognition tasks, there is also evidence that these 2 semantic access routes are not identical. For example, object recognition engages a complex, hierarchical perceptual stream that encodes progressively more abstract representations of object features and their spatial relationships (Marr 1982; Tanaka et al. 1991; Logothetis and Sheinberg 1996; Humphreys and Forde 2001). Certainly not all of these perceptual representations are encoded in language or even available to awareness. At present, it is not clear that comprehension of a word necessarily entails activation of a detailed perceptual representation of the object to which it refers, at least not to the same degree as that evoked by the object itself. In fact, many functional neuroimaging studies suggest different patterns of activation during matched word and picture recognition tasks (Gorno-Tempini et al. 1998; Moore and Price 1999b; Chee et al. 2000; Hasson et al. 2002; Bright et al. 2004; Gates and Yoon 2005; Reinholz and Pollmann 2005). The existence of patients with profound visual object recognition disorders but relatively intact word comprehension also argues against a complete overlap between the knowledge systems underlying word and object recognition (Warrington 1985; Lissauer 1889; Farah 1990; Davidoff and De Bleser 1994). In the interest of maintaining a clear focus on the processing of concepts rather than percepts, we thus elected to include in this analysis only those experiments that used words as stimuli.
Specific exclusion criteria are a critical feature of the present study. The numerous published neuroimaging studies concerning semantic processing address a variety of specific topics and employ a wide range of tasks and task comparisons (here referred to as “contrasts”). The authors of these studies often began from very different theoretical perspectives and varied in their interpretation of task demands. One approach to selecting material for a meta-analysis would have been to include any study considered by the original authors to have addressed semantic processing, as indicated, for example, by the study title or list of keywords. We found during initial attempts to review these reports, however, that markedly different, largely nonoverlapping activation patterns were frequently observed across studies. Furthermore, this discordance was related mainly to variability in task selection and the type of task contrast used. Three problematic features of this literature were of particular interest and were found with some regularity. First, many studies employed contrasting tasks that differed on phonological or orthographic processing demands in addition to semantic processing demands (see examples in Table 1). It is a general principle of functional neuroimaging studies, and one that warrants strong emphasis, that the “activations” measured using these methods are in fact representations of the relative differences in neural activity between 2 or more brain states. Thus, the pattern of activated brain regions observed in a study putatively targeting semantic processes depends not only on the cognitive processes elicited by the semantic task, but also on the processes elicited, or not elicited, by the comparison task. If the comparison task does not make demands on phonological and orthographic processes that approximate those made by the semantic task (as is the case, e.g., with any control task involving unpronounceable or unnameable stimuli), then the resulting activation is as likely to reflect phonological or orthographic processes as semantic processes. Such contrasts were excluded from the present analysis.
Table 1.
Example contrasts in which the semantic and control conditions are not matched on orthographic or phonological processing demands
| Semantic condition | Control condition | ||
| Stimulus, for example | Task | Stimulus, for example | Task |
| horse | Read silently | / / / / / | View silently |
| horse | Read aloud | υβγσφ | Say “OK” |
| horse | Read silently | nbgsj | Read silently |
| horse | Does it have an ascender? | nbgsj | Does it have an ascender? |
| horse | Is it living or nonliving? | nbgsj | Does it have more than 4 letters? |
| horse, zebra | Are they related in meaning? | nbgsj, nbqsj | Are they identical? |
| “horse” | Listen | distorted speech | Listen |
| “horse” | Is it living or nonliving? | tones | Is it high or low in pitch? |
| “horse” | Is it living or nonliving? | distorted speech | Is it a male or female voice? |
| “horse” | Is it living or nonliving? | “ba” | Is it “ba” or “pa”? |
Note: The “living/nonliving” task is intended to represent a variety of similar semantic decision tasks. All examples are from the studies reviewed.
A second, more prevalent problem concerns contrasts in which the conditions differed in task difficulty. Functional neuroimaging measurements are very sensitive to differences in response time, accuracy, and level of effort between tasks (see, e.g., Braver et al. 1997; Jonides et al. 1997; Honey et al. 2000; Adler et al. 2001; Braver et al. 2001; Ullsperger and von Cramon 2001; Gould et al. 2003; Binder et al. 2004; Binder, Medler, et al. 2005; Mitchell 2005; Sabsevitz et al. 2005; Desai et al. 2006; Lehmann et al. 2006; Tregallas et al. 2006). Such differences pose a problem if there are cognitive systems supporting general task performance functions that are modulated by task difficulty. Likely examples of such domain-general systems include a sustained attention network for maintaining arousal, a selective attention system for focusing neural resources on a particular modality or sensory object in the environment (e.g., a visual display), a working memory system for keeping task instructions and task-relevant sensory representations accessible, a response selection mechanism for mapping the contents of working memory to a response, a response inhibition system for preventing premature or prepotent responses from being made in error, and an error-monitoring system for adjusting response criteria and response time deadlines to minimize such errors. These systems, located mainly in frontal, anterior cingulate, and dorsal parietal cortices (Corbetta et al. 1998; Carter et al. 1999; Duncan and Owen 2000; Grosbras et al. 2005; Owen et al. 2005), are necessary for completing any task. If this is the case, and if the level of activation in these systems depends on general task demands, then it follows that activation can never be attributed with certainty to semantic processing when this activation has resulted from a contrast in which general task demands differ. Such contrasts (see examples in Table 2) were therefore excluded from the present analysis.
Table 2.
Examples for which the contrasted conditions are not matched on general task difficulty due to task set, word frequency, ambiguity, or priming effects
| Hard word condition | Easy word condition | ||
| Stimulus, for example | Task | Stimulus, for example | Task |
| horse | Generate associated word | horse | Read |
| — | Generate names of animals | — | Generate months of the year |
| horse | Is it living or nonliving? | horse | Read silently |
| impala | Is it living or nonliving? | horse | Is it living or nonliving? |
| horse | Is it living or nonliving? | horse (repeated) | Is it living or nonliving? |
| horse | Is it living or nonliving? | zebra | Does it contain the letter ‘a'? |
| horse, zebra | Are they related in meaning? | horse, horse | Are they identical? |
| house, zebra | Are they related in meaning? | horse, horse | Are they the same font size? |
| house, zebra | Are they related in meaning? | horse, ZEBRA | Are they the same case? |
| bank, river | Are they related in meaning? | lake, river | Are they related in meaning? |
| house, zebra | Is the second item a word? | horse, zebra | Is the second item a word? |
Note: All examples are from the studies reviewed.
A final issue concerns the interpretation of states in which no overt task is required. Many neuroimaging studies used states described as “passive” or “resting” in which subjects are given either no task, a minimally demanding task such as fixating a point in the visual field, or a nominal task for which compliance is uncertain and unknowable (e.g., “clear your mind,” “focus on the scanner sounds”). Although such conditions can be useful as a low-level baseline, particularly for sensory stimulation studies, their use in semantic studies is problematic. Most people report experiencing vivid and memorable thoughts and mental images during such conscious, attentive states (James 1890; Antrobus et al. 1966; Pope and Singer 1976; Singer 1993; Giambra 1995; Binder et al. 1999; McKiernan et al. 2006). We argued previously that the processes supporting such “task-unrelated thoughts” are essentially semantic, because they depend on activation and manipulation of acquired knowledge about the world (Binder et al. 1999; McKiernan et al. 2006). If such states involve semantic processing, their use as a baseline runs the risk of subtracting away any semantic processing elicited by an overt semantic task. Such a contrast (an active semantic task compared with no task) also violates the stipulations, discussed above, that task conditions be matched on low-level processing demands and on overall difficulty, and would thus be expected to produce “false positive” activation of surface form processing and general executive systems. Such contrasts were therefore excluded from the present analysis. Also excluded were contrasts in which 2 conditions that both involve passive stimulation were compared, such as passively listening to words versus pseudowords. In these cases, the processes underlying generation of task-unrelated thoughts, which we consider to be largely semantic in nature, would predominate in both of the conditions being contrasted, resulting in little or no relative activation of semantic systems.
In summary, the present meta-analysis represents a critical review in which selection criteria are based on specific theoretical distinctions between semantic and surface form processing, and between semantic processes and more general processes required for task execution. Our aim is thus to identify brain regions that contribute specifically to the semantic component of word recognition, that is, the activation of stored conceptual knowledge, apart from the accompanying analysis of surface form or generation of an overt task response.
Materials and Methods
Study Identification
Procedures for identifying candidate studies were designed to be as inclusive as possible. Candidate studies were identified through searches of the PubMed, Medline, and PsychINFO online databases for the years 1980 through 2007. This search was conducted using the following Boolean operation applied to title, abstract, and keyword fields: <“brain mapping” OR “functional magnetic resonance imaging” OR “fMRI” OR “positron emission tomography” OR “PET” OR “neuroimaging”> AND <“semantics” OR “semantic memory” OR “category” OR “conceptual knowledge”>. This step yielded 2832 unique items. Abstracts from these studies were then initially screened to identify those that used either fMRI or PET and included healthy human participants, yielding 790 articles. Abstracts from these articles were then evaluated in more depth to identify experiments that used word stimuli and included either a general or specific semantic contrast. If this information could not be determined from the abstract, the article was also included. This second screening step yielded 431 articles. Online tables of contents and abstracts for selected journals focusing on cognition and neuroimaging, including “Brain and Language,” “Human Brain Mapping,” “Journal of Cognitive Neuroscience,” and “NeuroImage,” and previously published reviews of semantic neuroimaging studies (Cabeza and Nyberg 2000; Joseph 2001; Martin and Chao 2001; Bookheimer 2002; Devlin, Russell, et al. 2002; Price and Friston 2002; Martin and Caramazza 2003; Thompson-Schill 2003; Damasio et al. 2004; Vigneau et al. 2006; Gerlach 2007) were then manually searched back to 1995 for relevant articles, yielding an additional 72 items. Finally, any additional relevant articles known to the authors, cited in the initial set of articles, or encountered during the review process were added to the list, resulting in a total of 520 articles that underwent full review.
The full review process included a complete reading of each article by 1 of the 4 authors, followed by application of the following inclusion criteria:
fMRI or PET study involving healthy adult human participants,
use of spoken or written word stimuli,
use of one or more semantic contrasts (see definition below),
incorporation of controls for sensory and word-form (orthographic and phonological) processes,
incorporation of controls for general executive and response processes,
use of a control task with overall difficulty at least as great as the semantic task,
image data acquired over all or most of the supratentorial brain,
availability of peak activation coordinates from a group activation map.
In an effort to be as inclusive as possible, criteria 6 and 7 were not applied in a rigid manner. For example, studies that did not include adequate documentation of task performance were included if the reviewer judged the tasks to be approximately equal in difficulty. Studies were also included if the control task was more difficult than the semantic task, following the logic that relative activation during the easier semantic task could not in such cases be due to greater demands on general executive processes. Criterion 7 was applied to avoid sampling bias for particular brain regions, but studies were included if nearly all of the cerebrum was imaged. Cases in which adherence to criteria was ambiguous were reviewed by a second author to reach a consensus view.
All studies included after full review by one of the authors were then reviewed by a second author to confirm eligibility. Rare disagreements were resolved by further discussion among the authors.
Types of Semantic Contrasts
Two types of contrasts are relevant to the goal of identifying activation due to semantic processing. The first, which we refer to as a “general semantics” contrast, follows from the operational distinction between word structure and meaning discussed above. A general contrast is one between a condition that elicits high levels of access to word meaning and a condition that elicits lower levels of access to word meaning. The contrast must include controls for processing word structure (phonology and orthography) as well as for general executive and response processes. The 3 most common general contrasts were:
Words versus Pseudowords. Pseudowords are spoken or written stimuli with structural properties similar to real words. The task performed on the words and pseudowords is nominally equivalent (e.g., an orthographic or phonological matching task, reading aloud, or lexical decision), such that the main difference emphasized by the contrast is the additional access to meaning in the word condition.
Semantic Task versus Phonological Task. This contrast involves a comparison between different tasks, one of which focuses attention on semantic aspects of the stimuli (e.g., a semantic decision task) and the other on structural (usually phonological) attributes of the stimuli (e.g., a rhyme decision or phoneme detection task). Typically, all the stimuli are words, thus the contrast emphasizes the additional access to word meaning elicited by the task in the semantic condition. Combinations of contrasts 1) and 2) also occur, in which a semantic task involving words is compared with a phonological task involving pseudowords (e.g., Démonet et al. 1992; Cappa et al. 1998; Binder et al. 1999).
High versus Low Meaningfulness. This contrast is a variation on 1), in which the contrasting stimuli differ in meaningfulness but are not words and pseudowords. Some examples include names of famous versus unknown people, related versus unrelated word pairs, and meaningful versus nonsensical sentences.
The other type of semantic contrast, which we designate “specific,” entails a comparison between hypothetically distinct types of conceptual knowledge. The aim of such studies is not to delineate the entire semantic processing system, but rather to identify putative functional subdivisions within the semantic system. Many such studies, for example, involve comparisons between concrete objects from different categories (e.g., animals vs. tools). Such studies are relevant to our aims because they contribute to the identification of brain regions involved specifically in semantic processing. Because we are not concerned here with particular functional subdivisions within the semantic system, activation data from both sides of the contrast (e.g., activation for animals > tools and for tools > animals) were included. As with the general contrasts, several variations can be distinguished based on whether the contrast pertains to stimulus or task manipulations. For example, in experiments involving a stimulus manipulation, a constant task is used (usually a semantic decision) with 2 (or more) contrasting categories of stimuli. In experiments involving a task manipulation, the attentional focus of the participant is switched to different semantic attributes (e.g., color, action, and size) of the same concepts using changes in task set.
We report here results obtained from separate analyses of the general and specific contrasts as well as a global analysis combining all studies. The specific foci were classified according to type of semantic knowledge represented by each contrast, and separate analyses were conducted for each specific knowledge type for which sufficient data were available.
Data Analysis
For each study reviewed, all reported contrasts that met inclusion criteria were included in the analysis. For each such contrast, the reviewer recorded the number of participants contributing to the activation map, the imaging modality used, the type of contrast, a brief description of the stimuli and tasks used in the contrast, the standard space to which the data were normalized, all reported coordinate locations for activation peaks, the Z values associated with each peak (if available), and the published table from which the coordinates were copied.
All coordinates were converted to a single common stereotaxic space. The studies were evenly divided between those that reported coordinates in the standard space of Talairach and Tournoux (1988) and those that used a variation of MNI space (Evans et al. 1993). We converted all MNI coordinates to the Talairach and Tournoux (1988) system using the “icbm2tal” transform (Lancaster et al. 2007). This transform reduces the bias associated with reference frame and scale in MNI–Talairach conversion.
Probabilistic maps of the resulting sets of coordinates were constructed using the “Activation Likelihood Estimate” (ALE) method (Turkeltaub et al. 2002), implemented in the GingerALE software package (Laird et al. 2005) (available at www.brainmap.org), using an 8-mm FWHM 3D Gaussian point spread function and a spatial grid composed of 2 × 2 × 2 mm voxels. This method treats each reported focus as the center of a Gaussian probability distribution. The 3D Gaussian distributions corresponding to all foci included in a given analysis are summed to create a whole-brain map that represents the overlap of activation peaks at each voxel, referred to as the ALE statistic. Subsequent analysis is restricted to a brain volume mask (Kochunov et al. 2002) distributed with the GingerALE software. To determine the null distribution of the ALE statistic for each analysis, a Monte Carlo simulation with 10 000 iterations was performed, in which each iteration consisted of a set of foci equal in number to the observed data, placed at random locations within the analysis volume and convolved with the same point spread function (Laird et al. 2005). Based on these null distributions, the ALE statistic maps for each analysis are converted to voxelwise probability maps.
The probability of chance formation of suprathreshold clusters was then determined by Monte Carlo simulation using in-house software, with 1000 iterations. Each iteration contained randomly located foci equal in number to the observed data and convolved with the same point spread function. The ALE map was then computed for each iteration, and the number and size of voxel clusters were recorded after thresholding each simulated data set at various voxelwise ALE thresholds. ALE maps from each of the observed data sets were then thresholded at an ALE value that yielded a corrected mapwise α < 0.05 after removing clusters smaller than 1000 μL. For visualization of probability values at each voxel, these corrected ALE maps were applied as masks on the probability maps generated by the GingerALE software. These thresholded probability maps are shown in Figs. 3–6.
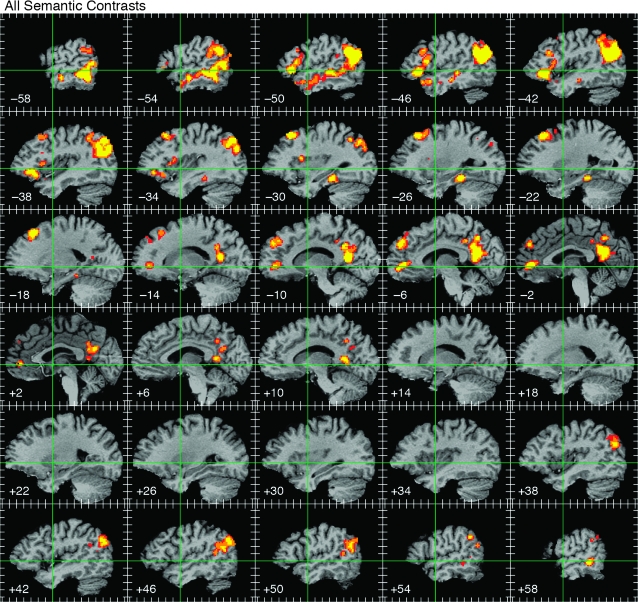
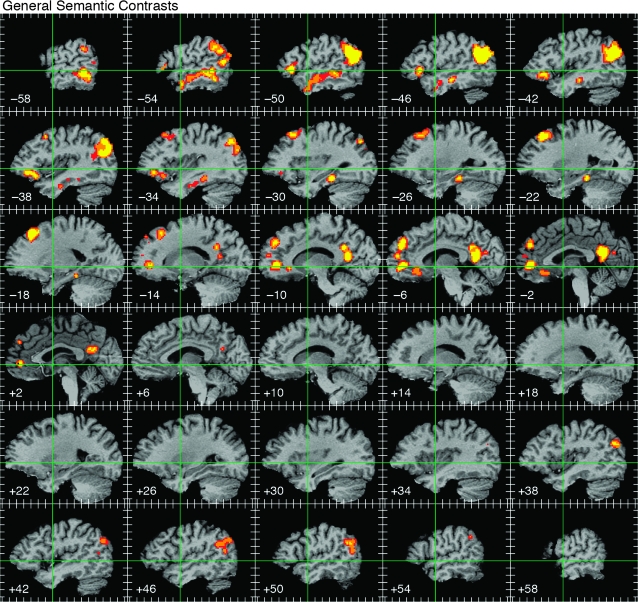
Figure 3.
ALE map of all semantic foci, thresholded at whole-brain corrected P < 0.05. Activations are displayed on serial sagittal sections through the stereotaxic space of Talairach and Tournoux (1988) at 4-mm intervals, with slice locations given at the lower left of each image. Green lines indicate the stereotaxic y and z axes. Tick marks indicate 10-mm intervals. The color scale indicates voxelwise probability values of P < 0.01 (red), P < 0.001 (orange), and P < 0.0001 (yellow).
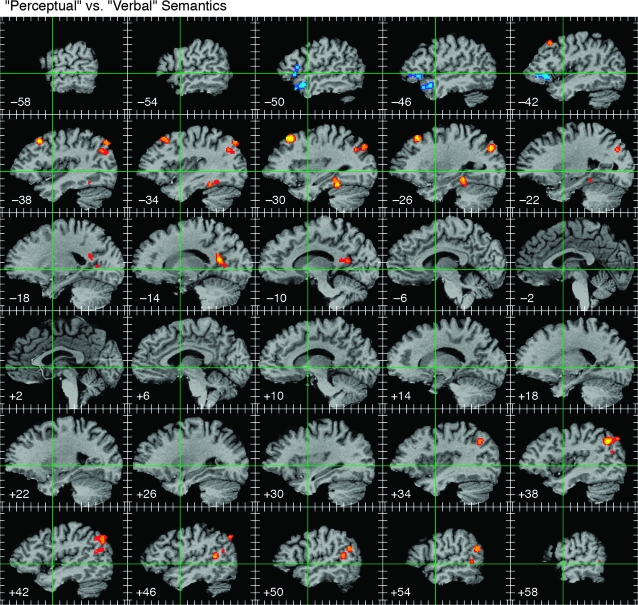
Figure 6.
ALE maps derived from contrasts comparing perceptual (i.e., pertaining to sensory attributes of concrete objects) with verbal (i.e., abstract or encyclopedic) knowledge. The 113 foci representing perceptual knowledge are shown in warm colors, and the 34 foci representing verbal knowledge are shown in cool colors. Formatting and thresholding as in previous figures.
Results
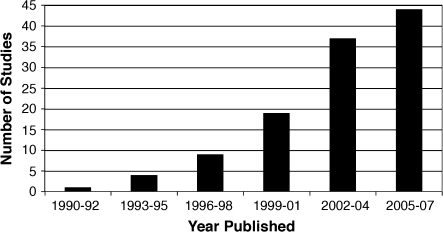
The initial search and screening procedures identified 520 articles, which were subsequently reviewed in detail. A total of 120 articles met inclusion criteria and provided 187 semantic contrasts. Figure 1 shows a breakdown of these studies by year published. A list of the included studies is provided in Appendix 1. Six of the included studies (Paulesu et al. 2000; Giraud and Price 2001; Tyler et al. 2001; Devlin, Russell, et al. 2002; Pilgrim et al. 2002; Liu et al. 2006) met all criteria, but no activation was observed for the semantic contrasts of interest. Among the 400 excluded studies, 10 used techniques other than fMRI or PET, or studied special subject populations. Six were reviews or reanalyses of previously published data. About 20% (82) of the excluded studies did not include any semantic contrasts, 13% (52) did not use word stimuli, 9% (37) used a resting or passive condition as the only control, 31% (127) used active control tasks that were less difficult than the semantic task, and 16% (66) used active control tasks that did not control for word-form (orthographic or phonological) processing. In 17% (69) of the excluded studies, no group activation data were reported for the semantic contrast of interest, or foci were reported only for a priori regions of interest.
Figure 1.
Distribution of the included studies by year published.
Of the 187 contrasts that met all inclusion criteria, 87 were of the general type and 100 of the specific type; 126 used fMRI, and 61 used PET. These studies collectively involved 1642 participants and yielded 1145 activation foci (691 general and 454 specific). (The number of unique individuals involved in these studies could be smaller than 1642. We did not count participants more than once if they were involved in more than one contrast from the same publication. However, the same individual could have been included in more than one publication.)
All Semantic Contrasts
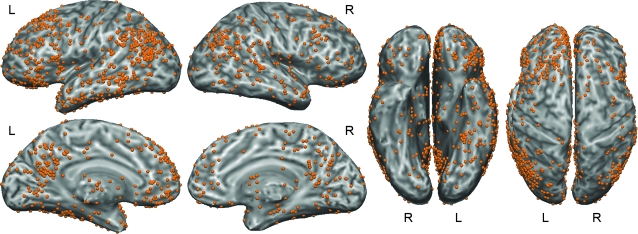
Figure 2 shows activation foci from all of the included studies projected onto an inflated surface model of the cerebral cortex (see Figs. 1 and 2 in the Supplemental Material online for similar images color coded by type of contrast). Of the 1145 published foci, 10 were located in the cerebellum and are therefore not shown. About 68% (771) of the foci were in the left cerebral hemisphere (x < 0) and 32% (362) in the right hemisphere (x > 0), indicating moderate left-hemisphere lateralization. Foci were located throughout the brain, but with strong clustering in some regions, particularly the left inferior parietal lobe. Areas with notably few foci included the precentral and postcentral gyri bilaterally, primary and secondary visual cortices, superior parietal lobules, frontal eye fields, and dorsal anterior cingulate gyri. There was a clear line of demarcation along the lateral bank of the left intraparietal sulcus, separating a large and dense cluster of foci in the inferior parietal lobe from uninvolved cortex in the intraparietal sulcus and superior parietal lobe.
Figure 2.
One thousand one hundred and thirty-five published activation foci from the included studies projected onto an inflated cortical surface.
The thresholded ALE probability map based on all 1145 foci is shown in Figure 3. Activations were lateralized to the left hemisphere and widely distributed in frontal, temporal, parietal, and paralimbic areas. Seven principal regions showed a high likelihood of activation across studies: 1) the angular gyrus (AG) and adjacent supramarginal gyrus (SMG); 2) the lateral temporal lobe, including the entire length of the middle temporal gyrus (MTG) and posterior portions of the inferior temporal gyrus (ITG); 3) a ventromedial region of the temporal lobe centered on the mid-fusiform gyrus and adjacent parahippocampus; 4) dorsomedial prefrontal cortex (DMPFC) in the superior frontal gyrus (SFG) and adjacent middle frontal gyrus (MFG); 5) the inferior frontal gyrus (IFG), especially the pars orbitalis; 6) ventromedial and orbital prefrontal cortex; and 7) the posterior cingulate gyrus and adjacent ventral precuneus. Weaker activations occurred at homologous locations in the right hemisphere, principally the AG, posterior MTG, and posterior cingulate gyrus. See Appendix 2 for details regarding activation of each of these regions in each of the included studies.
General Contrasts
Figure 4 shows the thresholded ALE map for the 691 general semantic foci. This map is very similar to the one derived from all foci, though with generally smaller clusters. Activation in the IFG is more clearly localized to the pars orbitalis. Activation in the left fusiform gyrus extends somewhat farther anteriorly, and there is more extensive involvement of the left ventromedial prefrontal region.
Figure 4.
ALE map of 691 foci resulting from general semantic contrasts (see Methods for details). Formatting and thresholding as in Figure 3.
Specific Contrasts
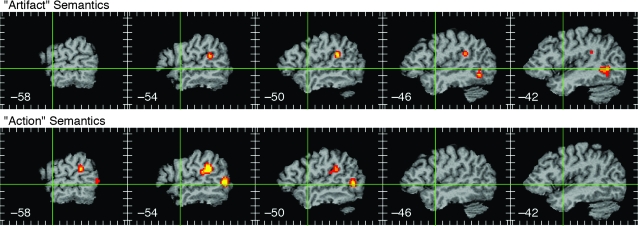
The specific contrasts were further categorized as to the putative type of semantic knowledge examined by each. Many of these types (e.g., auditory, gustatory, olfactory, tactile, visual motion, characteristic object location, emotion, causation, and self knowledge) were examined in only a few studies and thus had too few activation foci to examine separately by meta-analysis. There were 10 studies that examined contrasts between living things (usually animals) and manipulable artifacts (usually tools) (Mummery et al. 1996, 1998; Cappa et al. 1998; Perani et al. 1999; Grossman et al. 2002a; Laine et al. 2002; Kounios et al. 2003; Thioux et al. 2005; Wheatley et al. 2005; Goldberg et al. 2006), providing 41 “living” and 29 “artifact” foci. “Living” foci showed no significant overlap in the ALE analysis. As shown in Figure 5, “artifact” foci showed significant overlap at the left lateral temporal–occipital junction, where posterior MTG and ITG meet anterior occipital cortex (roughly BA 37), and in the ventral left SMG (BA 40) near the junction with the superior temporal gyrus (STG).
Figure 5.
ALE map of 29 foci resulting from contrasts targeting knowledge of manipulable artifacts (top) and ALE map of 40 foci from contrasts targeting knowledge of actions (bottom). Formatting and thresholding as in previous figures.
There were 10 studies that examined action knowledge relative to other types (Martin et al. 1995; Noppeney and Price 2003; Tyler, Stamatakis, et al. 2003; Davis et al. 2004; Boronat et al. 2005; Noppeney et al. 2005; Baumgaertner et al. 2007; Eschen et al. 2007; Ruschmeyer et al. 2007; Tomasino et al. 2007), providing 40 “action” foci. Significant overlap for these foci occurred in the ventral left SMG and posterior left MTG (BA 37). As shown in Figure 5, the SMG focus overlaps the SMG region observed in the “artifact” studies. The posterior MTG cluster associated with action knowledge was slightly dorsal and lateral to the temporal–occipital cluster of “artifact” foci.
There were 17 studies that examined the distinction between perceptually encoded knowledge (i.e., knowledge of concrete objects derived from sensory-motor experience) and verbally encoded knowledge (i.e., knowledge acquired through language) (Paivio 1986). The majority of these studies used contrasts between concrete and abstract concepts (Jessen et al. 2000; Wise et al. 2000; Grossman et al. 2002b; Fiebach and Friederici 2003; Giesbrecht et al. 2004; Noppeney and Price 2004; Whatmough et al. 2004; Binder, Medler, et al. 2005; Binder, Westbury, et al. 2005; Sabsevitz et al. 2005; Wallentin, Østergaard, et al. 2005; Bedny and Thompson-Schill 2006; Fliessbach et al. 2006), whereas a few others examined this distinction using tasks that required explicit knowledge of perceptual versus verbal facts (Fletcher et al. 1995; Noppeney and Price 2003; Lee and Dapretto 2006; Ebisch et al. 2007). Significant overlap for the 113 “perceptual” foci occurred in the AG bilaterally, left mid-fusiform gyrus, left DMPFC, and left posterior cingulate (Fig. 6). Significant overlap for the 34 “verbal” foci occurred in the left IFG (mainly pars orbitalis) and left anterior superior temporal sulcus (STS).
Discussion
PET and fMRI activation studies are based, directly or indirectly, on differences between 2 or more brain activity states. The “activation maps” produced by these methods represent relative changes in brain activity, not absolute activity levels. Interpretation of such activations, therefore, cannot be based solely on the processing demands of one of the task states, but rather requires a joint analysis of the processing demands elicited by each of the task states and the degree to which they differ. The goal of the present meta-analysis was to clarify the brain regions specifically involved in semantic processing, a topic that has been the source of much debate (for a sample of conflicting views, see Wernicke 1874; Head 1926; Petersen et al. 1988; Démonet et al. 1993; Thompson-Schill et al. 1997; Tranel et al. 1997; Hillis et al. 2001; Martin and Caramazza 2003; Patterson et al. 2007). In contrast to previous meta-analyses on this topic, we used explicit criteria to define activation representing semantic processing; these criteria referred to differences between the stimuli and tasks used to generate each activation map. To be considered for inclusion, a contrast had to involve a difference in either the degree to which stored knowledge was accessed (“general” contrast) or the specific type of knowledge accessed (“specific” contrast). These differences in stored knowledge access could be elicited through manipulation of stimulus characteristics (e.g., words vs. pseudowords, meaningful vs. meaningless sentences, famous vs. unfamiliar names, and animals vs. tools), through manipulation of the subject's attention via task instructions (e.g., semantic vs. phonological decisions, color vs. action decisions), or both.
Another central feature of the present study that distinguishes it from previous reviews was the application of strict exclusion criteria. To minimize contamination of the results by nonsemantic processes, studies were excluded if the semantic condition of interest also made greater demands on low-level sensory, orthographic, phonological, syntactic, working memory, attentional, response selection, or motor processes. (Note that studies were excluded only when the semantic condition of interest made greater demands on these processes, not when the comparison condition made greater demands.) The 2 most common reasons for exclusion were inadequate controls for phonological processing due to use of unpronounceable or nonlinguistic stimuli in the comparison condition, and inadequate controls for general task performance processes. The latter type of exclusion was the most common and warrants further discussion, because in our view many prior studies have not clearly distinguished knowledge access processes from more general cognitive processes that are not specific to semantic tasks. The critical point we wish to make is that all consciously executed, goal-directed tasks require at minimum a set of domain-general processes that include maintenance of attention, direction of attention to relevant information (external or internal), maintenance of this relevant information in a short-term memory store, maintenance of the task goal and task procedures in working memory, decision, response selection, and error monitoring. These processes are necessary for all goal-directed cognitive tasks, including semantic tasks; however, our aim here was to identify brain regions engaged specifically in semantic processes. Thus, it was critical to exclude activation contrasts in which the semantic condition of interest engaged these processes to a greater degree than the comparison condition, including all contrasts in which the semantic task was more difficult than the comparison task. In addition to the examples given in the introduction, another illustrative case are the many studies involving either semantic priming or repetition suppression, in which unprimed or new words are compared with primed or repeated words (e.g., Mummery et al. 1999; Wagner et al. 2000; Yasuno et al. 2000; Rossell et al. 2001; Copland et al. 2003; Rossell et al. 2003; Matsumoto et al. 2005; Wible et al. 2006). One underlying hypothesis of these studies is that unprimed or new words require more semantic processing than primed or repeated words that have already been processed, and behavioral data uniformly support this hypothesis by showing longer response times for the unprimed/new items. Though it is likely that unprimed/new items elicit more extensive semantic processing, it is also an inescapable fact, in our view, that they also require greater attentional and executive resources. Exclusion of these studies from the present meta-analysis was therefore necessary to isolate the semantic processes of interest, even though the activation maps from these contrasts probably do reflect, at least in part, semantic processes.
The Semantic System of the Human Brain
The meta-analysis links the following 7 brain regions with semantic processes: 1) posterior inferior parietal lobe (AG and portions of SMG), 2) lateral temporal cortex (MTG and portions of ITG), 3) ventral temporal cortex (mid-fusiform and adjacent parahippocampal gyrus), 4) DMPFC, 5) IFG, 6) ventromedial prefrontal cortex (VMPFC), and 7) posterior cingulate gyrus. One common attribute of these regions is their likely role in high-level integrative processes. All are known to receive extensively processed, multimodal and supramodal input. Recent studies show that even cortical regions formerly considered “unimodal” receive multisensory inputs (Schroeder and Foxe 2004; Cappe and Barone 2005), blurring the traditional distinction between unimodal and heteromodal cortex (Mesulam 1985). A useful qualitative distinction can still be drawn, however, between “modal” cortex, where processing reflects a dominant sensory or motor modality, and “amodal” cortex, where input from multiple modalities is more nearly balanced and highly convergent. For continuity with previous work, we refer to these latter regions as heteromodal, though alternative terms such as supramodal or amodal are perhaps equally valid. The human semantic system thus corresponds in large measure to the network of parietal, temporal, and prefrontal heteromodal association areas, which are greatly expanded in the human relative to the nonhuman primate brain (von Bonin 1962; Geschwind 1965; Brodmann 1994/1909). Evidence supports the subdivision of this network into posterior (temporal/parietal) and frontal components corresponding to storage and retrieval aspects of semantic processing (see discussion below). A second general feature of the semantic system is that it is lateralized to the left hemisphere, though with some bilateral representation (particularly in the AG and posterior cingulate gyrus). The following discussion reviews each of the nodes in this network in greater detail, examining their anatomical characteristics and likely functional roles based on imaging and neuropsychological data.
Angular Gyrus
The most dense concentration of activation foci was in the posterior aspect of the left inferior parietal lobule, a region known historically as the angular gyrus or “pli courbe” (French: “curved gyrus”) (Déjerine 1895). The AG consists of cortex surrounding the parietal extension of the STS; it is formed essentially by the continuation of the superior and middle temporal gyri into the inferior parietal lobe. Its medial boundary is the intraparietal sulcus, which separates it from the superior parietal lobule. The anterior boundary with the SMG is defined by the first intermediate sulcus of Jensen, though this landmark is not always present. Its posterior boundary with the occipital lobe is not well defined. The AG corresponds approximately to BA 39 and in recent cytoarchitectonic studies to PGa and PGp (Caspers et al. 2006). This region is practically nonexistent in lower primates (Brodmann 1994/1909) and is greatly expanded in the human brain relative to its probable homolog in the macaque monkey, area PG/7a (von Bonin and Bailey 1947; Hyvarinen 1982). It is anatomically connected almost entirely with other association regions and receives little or no direct input from primary sensory areas (Mesulam et al. 1977; Hyvarinen 1982; Seltzer and Pandya 1984; Cavada and Goldman-Rakic 1989a, 1989b; Andersen et al. 1990).
Though we use the term angular gyrus, a variety of other labels for activations in this region were encountered in the studies reviewed. Despite its location in the parietal lobe, many refer to it erroneously as the middle temporal gyrus. Others use the terms temporoparietal junction or temporal–parietal–occipital cortex. These concatenated terms strike us as unnecessarily imprecise in this context and should probably be reserved for describing large activations that straddle the boundaries between lobes or extend beyond the parietal lobe. AG activations were also not infrequently mislabeled with BA numbers 40 and 19. (Some of this confusion may be a historical accident stemming from Brodmann's famous illustration, which shows area 39 shrunken to a fraction of its true size relative to surrounding structures [Brodmann 1994/1909]. Other cytoarchitectonic studies have portrayed this region as much more extensive [von Economo and Koskinas 1925; Sarkissov et al. 1955]. Brodmann's intent seems to have been to show both lateral and dorsal brain regions in a single lateral view. This required that the inferior parietal lobule on the lateral surface be reduced in size to accommodate areas on the superior parietal lobule, which are normally not well seen from a lateral view.)
On the other hand, some of the activation foci in this large cluster probably lie outside the AG. Several are just posterior, in what is likely BA 19. Given this evidence from functional imaging, it is possible that at least some cortex in the anterior occipital lobe classically identified as BA 19 may serve a semantic rather than a modal visual associative function. Alternatively, BA 39 may extend farther posteriorly than is typically portrayed. Several other foci in this large cluster were in the SMG (BA 40) just anterior to the AG.
Lesions of the left AG produce a variety of cognitive deficits, including alexia and agraphia (Déjerine 1892; Benson 1979; Cipolotti et al. 1991), anomia (Benson 1979), transcortical sensory aphasia (Damasio 1981; Kertesz et al. 1982; Rapcsak and Rubens 1994), sentence comprehension impairment (Dronkers et al. 2004), acalculia (Gerstmann 1940; Benton 1961; Cipolotti et al. 1991; Dehaene and Cohen 1997), visual-spatial and body schema disorders (Gerstmann 1940; Critchley 1953), ideomotor apraxia (Haaland et al. 2000; Buxbaum et al. 2005; Jax et al. 2006), and dementia (Benson et al. 1982). Perhaps the main conclusion to be drawn from this evidence is that the AG likely plays a role in complex information integration and knowledge retrieval. Given its anatomical location adjoining visual, spatial, auditory, and somatosensory association areas, the AG may be the single best candidate for a high-level, supramodal integration area in the human brain (Geschwind 1965). Several functional imaging studies have shown that the AG is activated in response to semantically anomalous words embedded in sentences, suggesting that it plays a role in integrating individual concepts into a larger whole (Ni et al. 2000; Friederici et al. 2003; Newman et al. 2003). One recent fMRI study found that during auditory sentence comprehension, the AG, alone among the regions activated, showed a late activation relative to baseline that began at the end of the sentence and occurred only when the constituent words could be integrated into a coherent meaning (Humphries et al. 2007). Three studies comparing processing of connected discourse to processing of unrelated sentences or phrases have also shown activation of the AG (Fletcher et al. 1995; Homae et al. 2003; Xu et al. 2005) Considering these various lines of evidence, we propose that the AG occupies a position at the top of a processing hierarchy underlying concept retrieval and conceptual integration. Though it is involved in all aspects of semantic processing, it may play a particular role in behaviors requiring fluent conceptual combination, such as sentence comprehension, discourse, problem solving, and planning.
Lateral and Ventral Temporal Cortex
The meta-analysis identified several regions in the lateral and ventral left temporal lobe, including most of the MTG and portions of the ITG, fusiform gyrus, and parahippocampus. MTG, ITG, and ventral temporal lobe have often been considered modal visual association cortex by analogy with lateral and ventral temporal cortex in the macaque monkey (von Bonin and Bailey 1947; Mesulam 1985); however, the present analysis argues against such an interpretation in the human brain. In fact, many functional imaging studies have demonstrated activation of these regions by auditory stimuli, particularly during language tasks (e.g., Démonet et al. 1992; Binder et al. 1997; Wise et al. 2000; Noppeney et al. 2003; Rissman et al. 2003; von Kriegstein et al. 2003; Xiao et al. 2005; Humphries et al. 2006; Orfanidou et al. 2006; Spitsyna et al. 2006; Baumgaertner et al. 2007). Thus, these regions in the human brain are likely heteromodal cortex involved in supramodal integration and concept retrieval. As in the inferior parietal lobe, the relative expansion of this high-level integrative cortex in the temporal lobe has resulted in modal visual cortex being “pushed” posteriorly and reduced in relative surface area (Orban et al. 2004).
Focal damage to the MTG, though somewhat rare, is strongly associated with language comprehension and semantic deficits (e.g., Hart and Gordon 1990; Hillis and Caramazza 1991; Kertesz et al. 1993; Chertkow et al. 1997; Dronkers et al. 2004). The anterior ventral temporal lobe, including anterior MTG, ITG, and fusiform gyrus, is frequently damaged (usually bilaterally) in herpes simplex encephalitis, often resulting in profound semantic deficits (Warrington and Shallice 1984; Kapur, Barker, et al. 1994; Gitelman et al. 2001; Lambon Ralph et al. 2007; Noppeney et al. 2007). Semantic dementia, the temporal lobe variant of frontotemporal dementia, is characterized by progressive degeneration of the anterior ventrolateral temporal lobes and gradual loss of semantic knowledge (Warrington 1975; Snowden et al. 1989; Hodges et al. 1992, 1995; Mummery et al. 2000; Jefferies and Lambon Ralph 2006; Lambon Ralph et al. 2007; Noppeney et al. 2007). Large lesions of the ventral left temporal lobe have been associated with transcortical sensory aphasia (Damasio 1981; Kertesz et al. 1982; Alexander et al. 1989; Berthier 1999). A striking aspect of many of these temporal lobe injuries is a dissociation in performance across object categories. Patients with anterior temporal damage, for example, occasionally show greater impairment in processing concepts related to living things compared with artifacts (Warrington and Shallice 1984; Warrington and McCarthy 1987; Forde and Humphreys 1999; Gainotti 2000; Lambon Ralph et al. 2007), and the opposite pattern has been reported in patients with posterior temporal and parietal lesions (Warrington and McCarthy 1987, 1994; Hillis and Caramazza 1991; Gainotti 2000). These category-related deficits suggest that the temporal lobe may be a principal site for storage of perceptual information about objects and their attributes. A large number of functional imaging studies provide support for this hypothesis by showing selective activation of the posterior lateral temporal lobe by tool and action concepts (Martin et al. 1995, 1996; Cappa et al. 1998; Chao et al. 1999, 2002; Moore and Price 1999a; Perani et al. 1999; Grossman et al. 2002a; Kable et al. 2002; Phillips et al. 2002; Noppeney et al. 2003; Tyler, Stamatakis, et al. 2003; Davis et al. 2004; Kable et al. 2005; Noppeney et al. 2005; Wallentin, Lund et al. 2005).
Semantic foci in the fusiform and parahippocampal gyri were concentrated in a relatively focal region near the mid-point of these gyri, centered at y = −35 in the Talairach–Tournoux system. The specific role of this region is unknown. It may correspond to the “basal temporal language area” described in electrocortical stimulation mapping studies (Lüders et al. 1991). It is anterior to activation sites observed in functional imaging studies comparing different categories of object pictures (e.g., Perani et al. 1995; Martin et al. 1996; Kanwisher et al. 1997; Epstein and Kanwisher 1998; Chao et al. 1999; Ishai et al. 1999; Moore and Price 1999a; Gorno-Tempini et al. 2000; Okada et al. 2000; Haxby et al. 2001; Chao et al. 2002; Whatmough et al. 2002; Tyler, Bright, et al. 2003; Gerlach et al. 2004; Gerlach 2007). These more posterior activations (typically y < −50) are rarely observed in studies using words, suggesting that they arise from systematic differences between object categories in their constituent visual attributes, which are in turn processed by somewhat different visual perceptual mechanisms (Humphreys and Forde 2001; Hasson et al. 2002). Given its close proximity to these object perception areas, however, several authors have proposed that the mid-fusiform gyrus plays a particular role in retrieving knowledge about the visual attributes of concrete objects (D'Esposito et al. 1997; Chao and Martin 1999; Thompson-Schill, Aguirre, et al. 1999; Wise et al. 2000; Kan et al. 2003; Vandenbulcke et al. 2006; Simmons et al. 2007). This region is also near the hippocampus and massive cortical afferent pathways to the hippocampal formation via parahippocampal and entorhinal cortex (Van Hoesen 1982; Insausti et al. 1987; Suzuki and Amaral 1994). It is thus possible that the parahippocampal component of this cluster acts an interface between lateral semantic memory and medial episodic memory encoding networks (Levy et al. 2004).
The present analysis provides little evidence for involvement of the STG in semantic processing. The STG has long been considered to play a central role in language comprehension (e.g., Wernicke 1874; Geschwind 1971; Bogen and Bogen 1976; Hillis et al. 2001), but anatomical and functional data suggest that it contains mainly modal auditory cortex (von Economo and Koskinas 1925; Galaburda and Sanides 1980; Baylis et al. 1987; Kaas and Hackett 2000; Poremba et al. 2003). Its role in language relates primarily to speech perception and phonological processing rather than to retrieval of word meaning (Henschen 1918–1919; Binder et al. 2000; Wise et al. 2001; Binder 2002; Hickok et al. 2003; Scott and Johnsrude 2003; Indefrey and Levelt 2004; Liebenthal et al. 2005; Buchsbaum and D'Esposito 2008; Graves et al. 2008). Several studies, however, suggest that portions of the left superior temporal sulcus, which includes ventral STG, play a role in processing abstract concepts (see below).
Left DMPFC
We draw particular attention to this region, which has been largely overlooked in reviews on semantic processing despite its consistent activation. It forms a distinctive, diagonally oriented band extending from the posterior–medial aspect of the MFG, across the superior frontal sulcus and dorsal SFG, and onto the medial surface of the SFG. It corresponds roughly to BA 8, extending into BA 9 medially. We use the term “dorsomedial” to distinguish this region from “dorsolateral prefrontal cortex” located lateral and ventral to it in the lateral MFG and inferior frontal sulcus.
Lesions of the left dorsal and medial frontal lobe cause transcortical motor aphasia, a syndrome characterized by sparse speech output but otherwise normal phonological abilities (Luria and Tsvetkova 1968; Freedman et al. 1984; Alexander and Benson 1993). There is typically a striking disparity between cued and uncued speech production in this syndrome. Patients can repeat words and name objects relatively normally, but are unable to generate lists of words within a category or invent nonformulaic responses in conversation. In other words, patients perform well when a simple response is fully specified by the stimulus (a word to be repeated or object to be named) but poorly when a large set of responses is possible (Robinson et al. 1998). This pattern suggests a deficit specifically affecting self-guided, goal-directed retrieval of semantic information. The location of the DMPFC, adjacent to motivation and sustained attention networks in the anterior cingulate gyrus and just anterior to premotor cortex, makes this region a likely candidate for this semantic retrieval role.
The left DMPFC has not been delineated in previous discussions of the prefrontal cortex and semantic retrieval processes. Analysis of medial frontal lesions in transcortical motor aphasia has usually centered on the supplementary motor area (SMA), a region of medial premotor cortex (BA 6) posterior to the DMPFC, perhaps because of the attention drawn to this region in earlier stimulation mapping studies (Penfield and Roberts 1959). Some authors, citing the involvement of SMA and anterior cingulate cortex in motor planning, attention, and motivation processes, dismissed the deficits in patients with left medial frontal lesions as nonlinguistic in nature (Damasio 1981). Others have recognized the linguistic nature of the retrieval deficit while attributing this to SMA damage (Masdeu et al. 1978; Freedman et al. 1984; Goldberg 1985). The DMPFC and SMA have a common arterial supply (the callosomarginal branch of the anterior cerebral artery) and for this reason are usually damaged together in ischemic lesions. Although we agree that focal SMA damage is unlikely to produce a linguistic deficit, we propose that the specific linguistic deficit affecting fluent semantic retrieval in many of these patients is due to DMPFC damage anterior to the SMA.
Left IFG
The left IFG was implicated in several early imaging studies of semantic processing (Petersen et al. 1988; Frith et al. 1991; Kapur, Rose, et al. 1994), and much subsequent discussion has focused on this region (e.g., Démonet et al. 1993; Buckner et al. 1995; Fiez 1997; Thompson-Schill et al. 1997; Gabrieli et al. 1998; Poldrack et al. 1999; Thompson-Schill et al. 1999; Wagner et al. 2000; Roskies et al. 2001; Wagner et al. 2001; Bookheimer 2002; Chee et al. 2002; Gold and Buckner 2002; Devlin et al. 2003; Nyberg et al. 2003; Simmons et al. 2005; Goldberg et al. 2007). Consistent with prior reviews (Fiez 1997; Bookheimer 2002), the meta-analysis shows clear involvement of the anterior–ventral left IFG in semantic processing. This region corresponds to the “pars orbitalis” (BA 47). More posterior and dorsal parts of the IFG were also activated, though less consistently.
Imaging studies have also frequently implicated the left IFG in phonological, working memory, and syntactic processes (e.g., Démonet et al. 1992; Zatorre et al. 1992; Paulesu et al. 1993; Buckner et al. 1995; Fiez 1997; Smith et al. 1998; Fiez et al. 1999; Poldrack et al. 1999; Burton et al. 2000; Embick et al. 2000; Poldrack et al. 2001; Gold and Buckner 2002; Friederici et al. 2003; Nyberg et al. 2003; Davis et al. 2004; Indefrey and Levelt 2004; Fiebach et al. 2005; Owen et al. 2005; Tan et al. 2005; Grodzinsky and Friederici 2006). Many studies have also shown increased BOLD responses in the IFG as task difficulty increases, possibly due to increased working memory or phonological processing demands (e.g., Braver et al. 1997, 2001; Jonides et al. 1997; Honey et al. 2000; Adler et al. 2001; Ullsperger and von Cramon 2001; Gould et al. 2003; Binder, Medler, et al. 2005; Mitchell 2005; Sabsevitz et al. 2005; Desai et al. 2006; Lehmann et al. 2006; Tregallas et al. 2006). Though we attempted to remove contrasts in which semantic processing was confounded with phonological processing or overall difficulty, these screening efforts were likely imperfect due to the absence of appropriate behavioral data in many published studies. It is thus possible that some of the IFG activation foci, particularly those outside the pars orbitalis, are the result of residual phonological or working memory confounds.
As is well known, IFG lesions typically impair phonological, articulatory planning, and syntactic rather than semantic processes (Broca 1861; Mohr 1976; Caramazza et al. 1981; Alexander and Benson 1993), though a few cases of transcortical sensory aphasia have been reported (Otsuki et al. 1998; Maeshima et al. 1999, 2004; Sethi et al. 2007). Strokes affect the posterior aspect of the IFG more commonly than the anterior region; isolated lesions of the pars orbitalis are practically unknown. Devlin et al. (2003) applied transcranial magnetic stimulation (TMS) to the anterior IFG in 8 healthy participants during performance of semantic decision and perceptual (size) decision tasks. TMS slowed participants’ reaction time on the semantic but not on the control task, supporting a role for this region in semantic processing. Accuracy on the semantic task, however, was not affected by TMS. It may be that the anterior–ventral IFG contributes to semantic processing in the healthy brain but is not absolutely necessary for task completion (Price et al. 1999). Damage to this region thus impairs processing “efficiency,” resulting in slowing of responses without actual errors.
Left VMPFC
This group of foci occupy cortex in the cingulate gyrus and medial SFG anterior to the genu of the corpus callosum, the subgenual cingulate gyrus, gyrus rectus, and medial orbital frontal cortex. The involved region of anterior cingulate cortex is anterior and ventral to the more dorsal region of anterior cingulate cortex implicated in many studies of working memory, response conflict, error detection, and executive control functions (e.g., Carter et al. 1999; Duncan and Owen 2000; Barch et al. 2001; van Veen and Carter 2002; Owen et al. 2005). We use the term “rostral cingulate gyrus” to emphasize this distinction. The VMPFC corresponds to portions of BA 10, 11, 24, 25, and 32. This region has been linked with motivation, emotion, and reward processing and probably plays a central role in processing the affective significance of concepts (Damasio 1994; Drevets et al. 1997; Mayberg et al. 1999; Bechara et al. 2000; Phillips et al. 2003). It has also been activated in many general semantic contrasts, however, possibly due to incidental processing of the emotional attributes of words (Kuchinke et al. 2005).
Posterior Cingulate Gyrus
This region, which corresponds to BA 23, BA 31, and the retrosplenial region (BA 26, 29, and 30), was one of the most consistently activated. Activation peaks occurred in both hemispheres but more often on the left. A few foci in this cluster were located in the ventral aspect of the precuneus just dorsal to the posterior cingulate, in the region of the subparietal sulcus separating these gyri, or in the ventral parieto-occipital sulcus, which separates the posterior cingulate gyrus from the occipital lobe.
This general region has been linked with episodic and visuospatial memory functions (Valenstein et al. 1987; Rudge and Warrington 1991; Gainotti et al. 1998; Aggleton and Pearce 2001; Vincent et al. 2006; Epstein et al. 2007), emotion processing (Maddock 1999), spatial attention (Mesulam 1990; Small et al. 2003), visual imagery (Hassabis et al. 2007; Johnson et al. 2007; Burgess 2008), and other processes (Vogt et al. 2006). Of these, the association with episodic memory may be most likely. Posterior cingulate and adjacent retrosplenial cortex have strong reciprocal connections with the hippocampal complex via the cingulum bundle (Morris et al. 1999; Kobayashi and Amaral 2003, 2007). A number of patients with focal lesions to this region have presented with amnestic syndromes (Valenstein et al. 1987; Heilman et al. 1990; Rudge and Warrington 1991; Takayama et al. 1991; Katai et al. 1992; Gainotti et al. 1998; McDonald et al. 2001). Retrosplenial and surrounding posterior cingulate cortex are affected early in the course of Alzheimer disease, which typically presents as an episodic memory encoding deficit (Desgranges et al. 2002; Nestor et al. 2003).
If posterior cingulate cortex is involved primarily in encoding episodic memories, why is it consistently activated in contrasts that emphasize semantic processing? The likely answer has to do with the nature of episodic memory, the presumed evolutionary purpose of which is to form a record of past experience for use in guiding future behavior. Not all experiences are equally useful in this regard; thus, the brain has evolved a strategy of preferentially recording highly meaningful experiences, that is, experiences that evoke associations and concepts. Familiar examples of this phenomenon include the enhanced learning of words encoded during semantic relative to perceptual tasks, imageable relative to abstract words, and emotional relative to neutral words (Paivio 1968; Craik 1972 #762; Bock 1986). In each case, the enhanced retrieval of conceptual information (semantic retrieval) leads to enhanced episodic encoding. Several related theories of this phenomenon have been proposed (Cohen and Eichenbaum 1993; McClelland et al. 1995; O'Reilly and Rudy 2001), all of which postulate that episodic memory encoding involves the formation of large-scale representations through interactions between neocortex and the hippocampal system. The role of the neocortex is to compute ongoing perceptual, semantic, affective, and motor representations during the episode, while the hippocampal system binds these spatiotemporal cortical events into a unique event configuration. The important point is that the amount of episodic encoding that occurs is highly correlated with the degree of semantic processing evoked by the episode. We propose that the posterior cingulate gyrus, by virtue of its strong connections with the hippocampus, acts as an interface between the semantic retrieval and episodic encoding systems, similar to the role postulated above for the parahippocampal gyrus.
Homologues of the Human Semantic System in the Macaque Monkey Brain
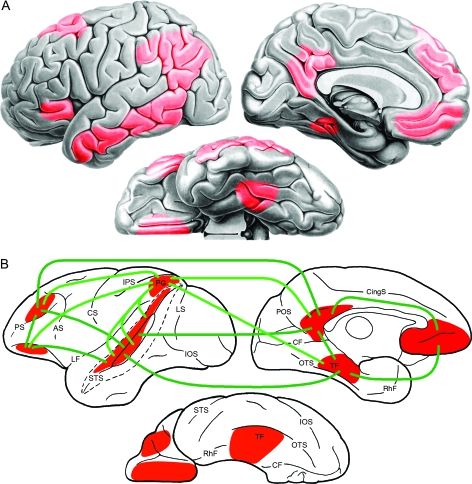
The posterior inferior parietal lobe of the macaque monkey, variously designated 7a (Vogt and Vogt 1919) or PG (von Bonin and Bailey 1947), and more recently subdivided into 2 subregions, PG and Opt (Pandya and Seltzer 1982; Gregoriou et al. 2006), is a likely homologue of the human AG with similar heteromodal functional characteristics (Hyvarinen 1982). Its principal connections are with visual and “polysensory” regions in the upper bank and fundus of the STS (areas TPO, STP, MST, and IPa), the parahippocampal gyrus (areas TF and TH), dorsolateral prefrontal cortex (mainly area 46), rostrolateral orbitofrontal cortex (area 11), and posterior cingulate gyrus (Jones and Powell 1970; Mesulam et al. 1977; Leichnitz 1980; Petrides and Pandya 1984; Seltzer and Pandya 1984, 1994; Selemon and Goldman-Rakic 1988; Cavada and Goldman-Rakic 1989a, 1989b; Andersen et al. 1990). Notably, the same STS, parahippocampal, prefrontal, and posterior cingulate regions with which PG/7a is connected are themselves all strongly interconnected (Jones and Powell 1970; Seltzer and Pandya 1976, 1978, 1989, 1994; Baleydier and Mauguiere 1980; Vogt and Pandya 1987; Selemon and Goldman-Rakic 1988; Morris et al. 1999; Blatt et al. 2003; Kobayashi and Amaral 2003, 2007; Padberg et al. 2003; Parvizi et al. 2006). These 6 regions thus form a distinct, large-scale cortical network that is strikingly similar in location and function to the human semantic system (Fig. 7). The other chief component of the human system, VMPFC, is roughly homologous to the medial orbitofrontal (BA 10, 14, 25, 32) region of the macaque. Although this region has no connection with PG/7a, it is strongly connected to middle and anterior STS, posterior cingulate and retrosplenial cortex, parahippocampus, and hippocampus (Seltzer and Pandya 1989; Barbas 1993; Cavada 2000; Blatt et al. 2003; Kobayashi and Amaral 2003; Saleem et al. 2007). Thus, the macaque brain contains a well-defined network of polysensory, heteromodal, and paralimbic areas that are several processing stages removed from primary sensory and motor regions and likely to be involved in computation of complex, nonperceptual information. We propose that this network is a nonhuman primate homologue of the human semantic system, responsible for storage of abstract knowledge about conspecifics, food sources, objects, actions, and emotions. Anatomical differences between the human and macaque systems are consistent with the known expansion of prefrontal, parietal, and temporal heteromodal cortex in the human brain, which has enabled in humans further abstraction of knowledge from perceptual events, ultimately culminating in the development of formal symbol systems to represent and communicate this knowledge.
Figure 7.
Summary diagrams comparing (A) the large-scale semantic network of the human brain and (B) a probable homologous network in the macaque monkey brain, comprised of posterior inferior parietal cortex (PG/7a), STS, parahippocampal cortex (TF, TH), dorsolateral prefrontal cortex, posterior cingulate and retrosplenial cortex, lateral orbital frontal cortex, and VMPFC. Green lines indicate the principal cortical connections of these regions in the monkey, based on studies using anterograde and retrograde tracer techniques (Jones and Powell 1970; Seltzer and Pandya 1976, 1978, 1984, 1989, 1994; Mesulam et al. 1977; Baleydier and Mauguiere 1980; Leichnitz 1980; Petrides and Pandya 1984; Vogt and Pandya 1987; Selemon and Goldman-Rakic 1988; Cavada and Goldman-Rakic 1989a, 1989b; Andersen et al. 1990; Barbas 1993; Morris et al. 1999; Cavada 2000; Blatt et al. 2003; Kobayashi and Amaral 2003, 2007; Padberg et al. 2003; Parvizi et al. 2006; Saleem et al. 2007). All connections indicated are monosynaptic and reciprocal.
The macaque parietal/frontal/STS network illustrated in Figure 7 has often been interpreted as playing a central role in visuospatial processing and spatial allocation of attention (Mesulam 1981; Hyvarinen 1982; Seltzer and Pandya 1984; Selemon and Goldman-Rakic 1988). This view is supported by a large number of studies showing cells in the posterior inferior parietal lobe of the macaque that respond to oculomotor, limb movement, and spatial attention tasks (Mountcastle et al. 1975; Hyvarinen 1982; Andersen et al. 1997). This model is clearly at odds, however, with our proposal that these regions are involved in long-term storage and retrieval of object and action knowledge. In our view, the characterization of this network as visuospatial/attentional does not account for the prominent connections of these frontoparietal areas with polysensory and paralimbic areas. We believe these models can be reconciled by a consideration of known subdivisions of the macaque posterior parietal lobe. Research over the past 20 years has clarified the connectivity and functional properties of several areas immediately anteromedial to PG/7a in the macaque intraparietal sulcus (LIP, VIP, and MIP), which appear to play a greater role in visuospatial and attention processes than PG/7a (Andersen et al. 1990, 1997; Rushworth et al. 1997; Chafee and Goldman-Rakic 1998; Duhamel et al. 1998). Unlike PG/7a, these IPS regions have little or no connectivity with the temporal lobe or paralimbic regions (Seltzer and Pandya 1984; Cavada and Goldman-Rakic 1989a; Suzuki and Amaral 1994). They are connected strongly to the frontal eye fields and premotor cortex, whereas PG/7a is connected to more anterior and dorsal prefrontal regions (area 46) (Cavada and Goldman-Rakic 1989b; Andersen et al. 1990). Numerous functional imaging studies have also clearly linked the IPS and frontal eye fields in humans with visuospatial and attention functions (Corbetta et al. 1998; Grefkes and Fink 2005; Grosbras et al. 2005). Thus, we propose that the posterior parietal spatial attention system in both the human and macaque is confined mainly to cortex in the IPS and superior parietal lobule, and that there is a distinct functional and anatomical boundary between this IPS system and adjacent inferior parietal cortex involved in semantic knowledge representation. This boundary line appears to correspond in both species to the superior margin of the lateral (posterior) bank of the IPS (see Fig. 2).
Evidence for Distinct Semantic Subsystems
In addition to brain networks supporting semantic processing in general, particular regions may be relatively specialized for processing specific object categories, attributes, or types of knowledge. Prior reviews on this topic have included studies that used object pictures as stimuli, whereas the present meta-analysis was confined to studies using words. The number of such studies that examined specific types of semantic knowledge was relatively small, and activation peaks from these studies showed little overlap. The clearest pattern emerged from the 10 studies examining action knowledge (Fig. 5). Two distinct activation clusters were observed in left SMG and posterior MTG. Lesions in these areas have been associated with impairments of action knowledge and ideomotor apraxia in many neuropsychological studies (Tranel et al. 1997, 2003; Haaland et al. 2000; Buxbaum et al. 2005; Jax et al. 2006). The SMG focus lies just posterior to somatosensory association cortex; thus, it seems likely that this region stores abstract somatosensory (e.g., proprioceptive) knowledge acquired during learning and performance of complex motor sequences. The likely homologue of this region in the macaque monkey is area PF in the anterior inferior parietal lobe, a region known to contain mirror neurons responsive to both action observation and performance (Rizzolatti and Craighero 2004). Activation of this region in humans by words, which merely refer conceptually to actions, lends support to the idea that the information stored there is semantic in nature, coding complex actions performed on objects for a specific purpose (Rothi et al. 1991; Buxbaum 2001; Buxbaum et al. 2005, 2006; Fogassi et al. 2005). The posterior MTG focus is just anterior to visual motion processing areas in the MT complex (Tootell et al. 1995), suggesting that this region stores knowledge about the visual attributes of actions. As previously suggested (Martin et al. 2000), this specialization of posterior MTG for processing action knowledge may explain the frequently observed preferential activation of this region by pictures of tools (Martin et al. 1996; Mummery et al. 1996; Cappa et al. 1998; Chao et al. 1999, 2002; Moore and Price 1999a; Perani et al. 1999; Devlin, Moore, et al. 2002; Phillips et al. 2002; Damasio et al. 2004). Indeed, the studies examining knowledge of manipulable artifacts (relative to living things) produced areas of overlap at very similar sites in the SMG and near the junction of posterior MTG, ITG, and lateral occipital lobe. In contrast, the studies examining knowledge of living things (usually animals) relative to other categories produced no significant areas of overlap, consistent with several prior reviews (Devlin, Russell, et al. 2002; Gerlach 2007).
Given the presence of mirror neurons in premotor cortex (Rizzolatti and Craighero 2004) and prior imaging evidence that the inferior frontal region responds to pictures of manipulable objects (Martin et al. 1996; Binkofski et al. 1999; Perani et al. 1999; Chao and Martin 2000; Gerlach et al. 2002; Kellenbach et al. 2003; Buxbaum et al. 2006), it is somewhat surprising that frontal cortex did not show consistent activation in studies of action or artifact words. Several of the included action and artifact studies did report activation in this general region (Martin et al. 1995; Kounios et al. 2003; Tyler, Statamatakis, et al. 2003; Wheatley et al. 2005; Ruschmeyer et al. 2007), yet these foci did not cluster sufficiently to produce an activation in the ALE analysis. Evidence suggests that inferior frontal and inferior parietal cortices play somewhat different roles in action processing, with the parietal system more closely associated with knowledge of specific object-related actions (Buxbaum 2001; Buxbaum et al. 2005; Creem-Regehr and Lee 2005; Fogassi et al. 2005). Consistent with this distinction, patients with inferior parietal lesions may have impaired recognition of object-related pantomimes performed by others (“representational ideomotor apraxia”), whereas patients with inferior frontal lesions apparently do not (Varney and Damasio 1987; Rothi et al. 1991; Buxbaum et al. 2005). Thus, it is possible that action and artifact words, which in any case do not seem to activate motor representations as readily as pictures do (Rumiati and Humphreys 1998), engage inferior frontal systems only weakly compared with inferior parietal areas concerned with object-related action knowledge.
Some theorists have emphasized a distinction between perceptual (“image-based”) and verbal representations in semantic memory (Paivio 1986), exemplified by the contrast between concrete and abstract words. A number of functional imaging studies have examined this distinction (see Binder 2007 for a review). Thirteen studies included in the present meta-analysis showed areas of stronger activation for concrete compared with abstract words, with overlap in bilateral AG, left mid-fusiform gyrus, left DMPFC, and left posterior cingulate cortex (Fig. 6). This pattern is very similar to the network observed in the general semantic meta-analysis (Fig. 4). Eight studies showed areas of stronger activation for verbal compared with perceptual knowledge. These included comparisons between abstract and concrete words, abstract and concrete stories, and encyclopedic (i.e., verbal factual) versus perceptual knowledge. Abstract concepts are generally more difficult to process than concrete concepts, and several other studies had to be excluded from the meta-analysis because of this confound. Areas associated with verbal semantic processing included the left IFG (mainly pars orbitalis) and left anterior STS. These dissociations support a distinction between perceptually based knowledge, stored in heteromodal association areas such as the AG and ventral temporal lobe, and verbally encoded knowledge, which places greater demands on left anterior perisylvian regions. This dissociation is supported by a range of neuropsychological studies showing relative impairment of abstract word processing in patients with perisylvian lesions (Goodglass et al. 1969; Coltheart et al. 1980; Roeltgen et al. 1983; Katz and Goodglass 1990; Franklin et al. 1995) and relative impairment of concrete word processing in patients with extrasylvian (mainly ventral temporal lobe) lesions (Warrington 1975, 1981; Warrington and Shallice 1984; Breedin et al. 1995; Marshall et al. 1998).
Semantic Processing and Autobiographical Memory Retrieval
The semantic system identified here is virtually identical to the large-scale network identified in recent studies of autobiographical memory retrieval (Maguire 2001; Svoboda et al. 2006). (In their comprehensive review of the autobiographical memory literature, Svoboda et al. [2006] refer to the AG as “temporoparietal junction,” though all of the foci they report in this region are in the inferior parietal lobe.) There are cogent theoretical and empirical reasons to distinguish general semantic from autobiographical memory (Tulving 1972; De Renzi et al. 1987; Yasuda et al. 1997), yet this nearly complete overlap observed in functional imaging studies is striking. Autobiographical memory refers to knowledge about one's own past, including both remembered events, known as episodic autobiographical memory (e.g., “I remember playing tennis last weekend”), and static facts about the self, known as semantic autobiographical memory (e.g., “I like to play tennis”). Most autobiographical memory begins as detailed knowledge about recently experienced events (i.e., episodic memories). With time, these specific memories lose their perceptual detail and come to more closely resemble semantic knowledge (Johnson et al. 1988; Levine et al. 2002; Addis et al. 2004).
There are several reasons to expect overlap between the general semantic and autobiographical memory retrieval systems. First, semantic autobiographical memories, though they differ from general semantic knowledge in referring to the self, are essentially learned facts and therefore might be supported by the same system that stores and retrieves other learned facts. Second, several theorists have proposed that retrieval of general concepts, such as particular temporal and spatial locations, people, objects, and emotions, is an early processing stage in the retrieval of autobiographical memories and serves to cue the retrieval of these more personal memories (Barsalou 1998; Conway and Pleydell-Pearce 2000). Finally, it is worth emphasizing the perhaps obvious point that autobiographical memories are necessarily composed of concepts and that there could be no retrieval of an autobiographical memory without retrieval of concepts. To recall, for example, that “I played tennis last weekend” logically entails retrieval of the concepts “tennis,” “play,” and “weekend.” Thus, the essential distinction between general semantic retrieval and autobiographical memory retrieval lies in the self-referential aspect of the latter, which may be a relatively minor component of the overall process, at least from a neural standpoint.
A few neuroimaging studies have directly compared autobiographical and general semantic retrieval and reported greater activation in the semantic network during the autobiographical condition, particularly in the medial prefrontal cortex, AG, and posterior cingulate region (Graham et al. 2003; Maguire and Frith 2003; Addis et al. 2004; Levine et al. 2004). Rather than indicating a specialization for autobiographical memory processing in these regions, we believe these data reflect the fact that semantic autobiographical memories are typically more perceptually vivid, detailed, contextualized, and emotionally meaningful than general semantic memories. Thus, we propose that semantic autobiographical and general semantic memory processing are supported by largely identical neural networks, but that retrieval of richer and more detailed memories (such as autobiographical memories) engages this network to a greater degree than retrieval of less detailed knowledge.
Semantic Processing and the “Default Network”
As illustrated in Figure 8, the semantic system identified here is also strikingly similar to the human brain “default network” thought to be active during the conscious resting state (Binder et al. 1999; Raichle et al. 2001). This network was originally defined as a set of brain areas that consistently show “task-induced deactivation” in functional imaging studies (Shulman et al. 1997; Binder et al. 1999; Mazoyer et al. 2001; Raichle et al. 2001; McKiernan et al. 2003). Task-induced deactivation refers to decreases in blood flow or BOLD signal during effortful tasks compared with more passive states such as resting, fixation, and passive sensory stimulation.
Figure 8.
Comparison of the left-hemisphere general semantic network indicated in the present ALE meta-analysis (top) and the “default network” (bottom). The latter map represents brain areas that showed task-induced deactivation during performance of a tone discrimination task, that is, higher BOLD signal during a conscious resting baseline compared with the tone task (see Binder et al. 2008 for details). In both types of studies, effects are observed in the AG, posterior cingulate gyrus, DMPFC, VMPFC, ventral temporal lobe, anterior MTG, and ventral IFG. Although effects are stronger in the left hemisphere for both kinds of studies, task-induced deactivation is typically more symmetrical in posterior cingulate and medial prefrontal regions (Shulman et al. 1997; Binder et al. 1999; Mazoyer et al. 2001; Raichle et al. 2001; McKiernan et al. 2003).
There is now a general consensus that task-induced deactivation occurs because certain types of neural processes active during passive states are “interrupted” when subjects are engaged in effortful tasks (Binder et al. 1999; Raichle 2006), though the precise nature of these default neural processes remains a topic of debate. Evidence suggests that high levels of ongoing “spontaneous” neural activity are necessary for maximizing responsiveness of the cortex to changes in input (Ho and Destexhe 2000; Salinas and Sejnowski 2001; Chance et al. 2002), and this ongoing activity seems to account for much of the high resting metabolic needs of the cortex (Attwell and Laughlin 2001). The mere fact that there are high levels of neural activity in the resting state does not explain, however, why this activity decreases in particular brain regions during active task states. Such an explanation would seem to depend on an account of resting state activity in terms of the cognitive and affective processes in which subjects preferentially engage during passive states, such as episodic memory encoding and retrieval, monitoring and evaluating the internal and external environment, visual imagery, emotion processing, and working memory (Andreasen et al. 1995; Shulman et al. 1997; Mazoyer et al. 2001; Raichle et al. 2001; Simpson et al. 2001; Stark and Squire 2001).
Binder et al. (1999) proposed that semantic processing constitutes a large component of the cognitive activity occurring during passive states. This proposal was based first on phenomenological considerations. The everyday experience of spontaneous thoughts, mental narratives, and imagery that James referred to as the “stream of consciousness” (James 1890) is ubiquitous and undeniable. Participants in controlled laboratory conditions reliably report such “task-unrelated thinking,” the content of which includes concepts, emotions, and images (Antrobus et al. 1966; Antrobus 1968; Horowitz 1975; Pope and Singer 1976; Teasdale et al. 1993, 1995; Giambra 1995; McKiernan et al. 2003). Performance of effortful perceptual and short-term memory tasks reliably suppresses task-unrelated thoughts, suggesting a direct competition between exogenous and endogenous signals for attentional and executive resources. “Interrupting the stream of consciousness” thus provides a straightforward explanation for task-induced deactivation.
The second consideration emphasized by Binder et al. was the potential adaptive advantage of systems that allow “ongoing” retrieval of conceptual knowledge. In contrast to the sensory, spatial attention, and motor processes engaged when an external stimulus requires a response, ongoing conceptual processes operate primarily on internal stores of knowledge built from past experience. They are not random or “spontaneous” but rather play a profoundly important role in human behavior. Their purpose is to “make sense” of prior experience, solve problems that require computation over long periods of time, and create effective plans governing behavior in the future. This uniquely human capability to perform high-level computations “off-line” is surely the principal explanation for our ability to adapt, create culture, and invent technology.
The final evidence offered by Binder et al. was an fMRI study in which participants were scanned while resting, while performing a perceptual task with no semantic content, and while making semantic decisions about words. Relative to the resting state, the perceptual task produced deactivation in the usual default network (AG, posterior cingulate gyrus, VMPFC, and DMPFC). Critically, however, the semantic decision task did not deactivate these regions. Finally, the same regions were activated when contrasting the semantic decision task with a phonological task that controlled for sensory and executive processes. These results show not only that the semantic network (as defined by the semantic–phonological task contrast) is virtually identical to the default state network, but also that task-induced deactivation in these regions can be modulated by task content. Deactivation occurs when the task or stimuli make little or no demands on semantic processing. When the task itself engages the semantic system, however, deactivation does not occur. This pattern indicates that the default network itself is engaged in semantic processing, both during passive states and when presented with a semantic task. This pattern has since been replicated in a number of studies comparing word tasks, pseudoword tasks, and passive or resting conditions. Pseudowords reliably deactivate the default network relative to the resting state, whereas words do not (Baumgaertner et al. 2002; Henson et al. 2002; Mechelli et al. 2003; Rissman et al. 2003; Ischebeck et al. 2004; Binder, Medler, et al. 2005; Xiao et al. 2005; Humphries et al. 2007). This result is fully consistent with the model just proposed, because pseudowords have little meaning and thus do not engage the semantic system.
Conceptual versus Perceptual Processing
The observation that semantic processing engages a network of heteromodal association areas distinct from modal sensory and motor systems provides support for the traditional distinction between conceptual and perceptual processes (Fodor 1983). Under this view, conceptual processes operate on “internal” sources of information, such as semantic and episodic memory, which can be retrieved and manipulated at any time, independent of ongoing external events. In contrast, perceptual processes operate on “external” information derived from immediate, ongoing sensory and motor processes that coordinate interactions with the external environment. Many tasks, of course, require simultaneous processing of both internal and external information, including, for example, any behavior involving recognition of a familiar stimulus such as a word or picture. In such instances, external sensory information is given meaning by activation of associated internal information (Neisser 1967; Aurell 1979).
Functional imaging evidence for such a distinction is illustrated dramatically in Figure 9, which shows areas identified with semantic processing in the present meta-analysis in red. Yellow areas in the figure were activated during a task in which subjects read pseudowords aloud, thereby engaging ventral visual form perception, dorsal visual and spatial attention, motor articulation, and auditory perceptual systems bilaterally (Binder, Medler, et al. 2005). Apart from a few areas of overlap in the anterior ventral visual stream, STS, and IFG, these large-scale networks are largely distinct and complementary, together covering much of the cortical surface. Similar imaging evidence supporting a general distinction between large-scale “intrinsic” and “extrinsic” networks has been reported recently by other researchers (Fox et al. 2005; Fransson 2005; Golland et al. 2007).
Figure 9.
Composite map of complementary human brain networks for processing internal and external sources of information. Red areas indicate the general semantic network identified in the current meta-analysis. Yellow indicates areas activated in 24 healthy adults during oral reading of visually presented pseudowords (pronounceable but meaningless letter strings) compared with a resting baseline (Binder, Medler, et al. 2005). The latter task activates unimodal visual and auditory areas bilaterally, dorsal attention systems in the IPS and FEF bilaterally, and bilateral motor, premotor, dorsal anterior cingulate, and dorsolateral prefrontal systems involved in response production. Overlap between these 2 major networks is minimal.
Although these observations confirm a general distinction between conceptual and perceptual systems in the brain, other evidence suggests that this distinction is not absolute (Gallese and Lakoff 2005). For example, several studies have shown involvement of primary motor and premotor cortex in the comprehension of action verbs (Hauk et al. 2004; Pulvermuller et al. 2005; Tettamanti et al. 2005). Similarly, there is evidence that high-level visual cortex participates in the processing of object nouns (Martin et al. 1995; James and Gauthier 2003; Kan et al. 2003; Simmons et al. 2007). Word-related activation of these motor and sensory areas is likely to be somewhat subtle compared with activation of heteromodal conceptual regions and to depend to a greater extent on the specific sensorimotor attributes of the word concept. Furthermore, there are as yet few published studies that have focused on such specific attributes. Thus, the present meta-analysis may underrepresent the involvement of sensory and motor systems in comprehension of word stimuli. Defining the extent of this involvement is an important topic for future research.
Comparison with Previous Large-Scale Reviews of Semantic Processing
The current results differ somewhat from conclusions reached in previous reviews. Cabeza and Nyberg (2000) reviewed functional imaging studies published prior to 1999. From an initial sample of 275 studies in a range of cognitive domains, they found 31 involving semantic memory retrieval tasks. Activation foci from these studies clustered mainly in the left IFG, with a few additional foci in the left MTG. The authors provided a brief description of the task contrasts used in each study and drew attention to the importance of control tasks, but made no attempt to exclude studies with word-form or task difficulty confounds. Of these 31 studies, only 4 passed our criteria for inclusion, with the remainder excluded mainly for phonological or task difficulty confounds.
Vigneau et al. (2006) collected 65 studies published prior to 2005 that concerned semantic processing. The authors did not report how the studies were identified but indicated that they excluded contrasts that used visual fixation or resting controls. Both object and word studies were included. No attempt was made to identify phonological or task difficulty confounds. Only activation peaks in the lateral temporal, lateral frontal, and inferior parietal lobes were included in the analysis. Foci were found throughout these regions, including the STG, MTG, IFG, and premotor cortex. The findings of Vigneau et al. thus differ from the current results in showing numerous foci in the STG (due to inclusion of studies comparing speech with non–speech sounds) and in the posterior IFG and premotor cortex (likely due to phonological and working memory confounds), and in the a priori exclusion of data from ventral temporal, dorsomedial prefrontal, posterior cingulate, and ventromedial frontal areas. Of the 65 studies selected by Vigneau et al., only 17 passed our criteria for inclusion; most were excluded because of task confounds or use of object stimuli. Another 53 studies that passed our criteria and were published prior to 2005 were not identified by Vigneau et al.
Future meta-analyses of cognitive imaging studies would benefit from the establishment of a more formal ontological system for defining the cognitive processes represented by an “activation.” The aim of such meta-analyses is to identify the neural correlates of a specific cognitive process or related set of processes, but this enterprise cannot logically succeed without an objective means of defining the cognitive components represented by an activation. The “objects” in such an ontology would correspond to particular experimental conditions (i.e., cognitive processing states), specified by sets of operationally-defined stimulus and task attributes. Contrasts between experimental conditions would constitute “relations” defining the cognitive processes that differ between conditions. The system would rest on a set of agreed-upon axioms concerning stimuli, tasks, and their attributes (e.g., “words are familiar symbols,” “familiar symbols have associations,” “associations are stored in semantic memory,” etc.). The interpretive power of such an ontology would be invaluable not only for retrospective meta-analysis but also for designing and interpreting future studies within a common theoretical framework.
Conclusions
The neural systems specialized for storage and retrieval of semantic knowledge are widespread and occupy a large proportion of the cortex in the human brain. The areas implicated in these processes can be grouped into 3 broad categories: posterior heteromodal association cortex (AG, MTG, and fusiform gyrus), specific subregions of heteromodal prefrontal cortex (dorsal, ventromedial, and inferior prefrontal cortex), and medial paralimbic regions with strong connections to the hippocampal formation (parahippocampus and posterior cingulate gyrus). The widespread involvement of heteromodal cortex is notable in that these regions are greatly expanded in the human relative to the nonhuman primate brain. This evolutionary expansion of neural systems supporting conceptual processing probably accounts for uniquely human capacities to use language productively, plan the future, solve problems, and create cultural and technological artifacts, all of which depend on the fluid and efficient retrieval and manipulation of conceptual knowledge.
Funding
National Institutes of Health (grants R01 NS33576, R01 NS35929, R01 DC006287, R03 DC008416, T32 MH019992, and F32 HD056767).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Supplementary Material
Acknowledgments
The authors thank B. Douglas Ward for invaluable technical assistance. Conflict of Interest: None declared.
Appendix 1: List of Included Studies
Assaf M, Calhoun VD, Kuzu CH, Kraut MA, Rivkin PR, Hart J, et al. Neural correlates of the object-recall process in semantic memory. Psychiatry Research: Neuroimaging 2006; 147: 115–126.
Baumgaertner A, Buccino G, Lange R, McNamara A, Binkofski F. Polymodal conceptual processing of human biological actions in the left inferior frontal lobe. European Journal of Neuroscience 2007; 25: 881–889.
Baumgaertner A, Weiller C, Buchel C. Event-related fMRI reveals cortical sites involved in contextual sentence integration. Neuroimage 2002; 16: 736–745.
Bedny M, Thompson-Schill SL. Neuroanatomically separable effects of imageability and grammatical class during single-word comprehension. Brain and Language 2006; 98: 127–139.
Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. Journal of Cognitive Neuroscience 1999; 11: 80–93.
Binder JR, McKiernan KA, Parsons M, Westbury CF, Possing ET, Kaufman JN, et al. Neural correlates of lexical access during visual word recognition. Journal of Cognitive Neuroscience 2003; 15: 372–393.
Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage 2005a; 27: 677–693.
Binder JR, Westbury CF, Possing ET, McKiernan KA, Medler DA. Distinct brain systems for processing concrete and abstract concepts. Journal of Cognitive Neuroscience 2005b; 17: 905–917.
Blaxton TA, Bookheimer SY, Zeffiro TA, Figlozzi CM, Gaillard WD, Theodore WH. Functional mapping of human memory using PET: comparisons of conceptual and perceptual tasks. Canadian Journal of Experimental Psychology 1996; 50: 42–56.
Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, et al. Specialization of phonological and semantic processing in Chinese word reading. Brain Research 2006; 1071: 197–207.
Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, et al. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Cogn Brain Research 2005; 23: 361–373.
Cai C, Kochiyama T, Osaka K, Wu J. Lexical/semantic processing in dorsal left inferior frontal gyrus. Neuroreport 2007; 18: 1147–1151.
Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F. The effects of semantic category and knowledge type on lexical-semantic access: A PET study. Neuroimage 1998; 8: 350–359.
Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, et al. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. Journal of Cognitive Neuroscience 2004; 16: 167–177.
Clark D, Wagner AD. Assembling and encoding word representations: fMRI subsequent memory effects implicate a role for phonological control. Neuropsychologia 2003; 41: 304–317.
Collette F, Majerus S, Van der Linden M, Dabe P, Degueldre C, Delfiore G, et al. Contribution of lexico-semantic processes to verbal short-term memory tasks: A PET activation study. Memory 2001; 9: 249–259.
Copland DA, de Zubicaray GI, McMahon K, Eastburn M. Neural correlates of semantic priming for ambiguous words: An event-related fMRI study. Brain Research 2007; 1131: 163–172.
Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychological Science 1999; 10: 26–34.
Crosson B, Radonovich K, Sadek JR, Gokcay D, Bauer RM, Fischler IS, et al. Left-hemisphere processing of emotional connotation during word generation. Neuroreport 1999; 10: 2449–2455.
D'Arcy RCN, Ryner L, Richter W, Service E, Connolly JF. The fan effect in fMRI: Left hemisphere specialization in verbal working memory. Neuroreport 2004; 15: 1851–1855.
Dapretto M, Bookheimer SY. Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron 1999; 24: 427–432.
Daselaar SM, Veltman DJ, Rombouts ARB, Raaijmakers JGW, Lazeron RHC, Jonker C. Medial temporal lobe activity during semantic classification using a flexible fMRI design. Behavioural Brain Research 2002; 136: 399–404.
Davis MH, Meunier F, Marslen-Wilson WD. Neural responses to morphological, syntactic, and semantic properties of single words: An fMRI study. Brain and Language 2004; 89: 439–449.
Démonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, et al. The anatomy of phonological and semantic processing in normal subjects. Brain 1992; 115: 1753–1768.
Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. Journal of Cognitive Neuroscience 2003; 15: 71–84.
Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Jalal Fadili M, et al. Is there an anatomical basis for category-specificity? Semantic memory studies with PET and fMRI. Neuropsychologia 2002; 40: 54–75.
Ebisch SJH, Babiloni C, Del Gratta C, Ferretti A, Perrucci MG, Caulo M, et al. Human neural systems for conceptual knowledge of proper object use: A functional magnetic resonance imaging study. Cerebral Cortex 2007; 17: 2744–2751.
Eschen A, Freeman J, Dietrich T, Martin M, Ellis J, Martin E, et al. Motor brain regions are involved in the encoding of delayed intentions: A fMRI study. International Journal of Psychophysiology 2007; 64: 259–268.
Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia 2003; 42: 62–70.
Fiebach CJ, Friederici AD, Muller K, von Cramon DY. FMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience 2002; 14: 11–23.
Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 1995; 57: 109–128.
Fliessbach K, Wesi S, Klaver P, Elger CE, Weber B. The effect of word concreteness on recognition memory. Neuroimage 2006; 32: 1413–1421.
Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imageability on word processing in human cortex. Cerebral Cortex 2004; 14: 521–529.
Giraud AL, Price CJ. The constraints functional neuroimaging places on classical models of auditory word processing. Journal of Cognitive Neuroscience 2001; 13: 754–765.
Gitelman DR, Nobre AC, Sonty S, Parrish TB, Mesulam MM. Language network specializations: An analysis with parallel task designs and functional magnetic resonance imaging. Neuroimage 2005; 26: 975–985.
Goldberg RF, Perfetti CA, Schneider W. Distinct and common cortical activations for multimodal semantic categories. Cognitive, Affective and Behavioral Neuroscience 2006a; 6: 214–222.
Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. Journal of Neuroscience 2006b; 26: 4917–4921.
Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, et al. The neural systems sustaining face and proper-name processing. Brain 1998; 121: 2103–2118.
Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, et al. A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 2000; 14: 353–360.
Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, et al. The neural basis for category-specific knowledge: an fMRI study. Neuroimage 2002a; 15: 936–948.
Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, et al. Neural representation of verb meaning: An fMRI study. Human Brain Mapping 2002b; 15: 124–134.
Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: A PET study. Journal of Cognitive Neuroscience 1999; 11: 383–398.
Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proceedings of the National Academy of Sciences USA 1999; 96: 5884–5889.
Henson RNA. Repetition effects for words and nonwords as indexed by event-related fMRI: a preliminary study. Scandinavian Journal of Psychology 2001; 42: 179–186.
Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus nonwords and initial versus repeated face presentations. Neuroimage 2002; 15: 83–97.
Herbster AN, Mintun MA, Nebes RD, Becker JT. Regional cerebral blood flow during word and nonword reading. Human Brain Mapping 1997; 5: 84–92.
Homae F, Hashimoto R, Nakajima K, Miyashita Y, Sakai KL. From perception to sentence comprehension: the convergence of auditory and visual information of language in the left inferior frontal cortex. Neuroimage 2002; 16: 883–900.
Homae F, Yahata N, Sakai KL. Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage 2003; 20: 578–586.
Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience 2006; 18: 665–679.
Ischebeck A, Indefrey P, Usui N, Nose I, Hellwig F, Taira M. Reading in a regular orthography: An fMRI study investigating the role of visual familiarity. Journal of Cognitive Neuroscience 2004; 16: 727–741.
Jessen F, Heun R, Erb M, Granath DO, Klose U, Papassotiropoulos A, et al. The concreteness effect: Evidence for dual-coding and context availability. Brain and Language 2000; 74: 103–112.
Joubert SA, Beauregard M, Walter N, Bourgouin P, Beaudoin G, Leroux J-M, et al. Neural correlates of lexical and sublexical processes in reading. Brain and Language 2004; 89: 9–20.
Kellenbach ML, Brett M, Patterson K. Large, colourful or noisy? Attribute- and modality-specific activations during retrieval of perceptual attribute knowledge. Cognitive, Affective, and Behavioral Neuroscience 2001; 1: 207–221.
Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience 2002; 14: 785–794.
Kosslyn SM, Alpert NM, Thompson WL. Identifying objects at different levels of hierarchy: A positron emission tomography study. Human Brain Mapping 1995; 3: 107–132.
Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: An event-related functional MRI study. Neuroimage 2002; 17: 1761–1772.
Kounios J, Koenig P, Glosser G, DeVita C, Dennis K, Moore P, et al. Category-specific medial temporal lobe activation and the consolidation of semantic memory. Cogn Brain Research 2003; 17: 484–494.
Kuchinke L, Jacobs AM, Grubich C, Vo MLH, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage 2005; 28: 1022–1032.
Kuperberg GR, Lakshmanan BM, Caplan DN, Holcomb PJ. Making sense of discourse: An fMRI study of causal inferencing across sentences. Neuroimage 2006; 33: 343–361.
Kuperberg GR, McGuire PK, Bullmore ET, Brammer MJ, Rabe-Hesketh S, Wright I, et al. Common and distinct neural substrates for pragmatic, semantic, and syntactic processing of spoken sentences: an fMRI study. Journal of Cognitive Neuroscience 2000; 12: 321–341.
Laine M, Rinne JO, Hiltunen J, Kaasinen V, Sipila H. Different brain activation patterns during production of animals versus artefacts: a PET activation study on category-specific processing. Cogn Brain Research 2002; 13: 95–99.
Lee AC, Graham KS, Simons JS, Hodges JR, Owen AM, Patterson K. Regional brain activations differ for semantic features but not categories. Neuroreport 2002a; 13: 1497–1501.
Lee ACH, Robbins TW, Graham KS, Owen AM. “Pray or prey?” Dissociation of semantic memory retrieval from epiesodic memory processes using positron emission tomography and a novel homophone task. Neuroimage 2002b; 16: 724–735.
Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. Neuroimage 2006; 29: 536–544.
Liu C, Hue C, Chen C, Chuang K, Liang K, Wang Y, et al. Dissociated roles of the middle frontal gyri in the processing of Chinese characters. Neuroreport 2006; 17: 1397–1401.
Luo J, Niki K. Role of medial temporal lobe in extensive retrieval of task-related knowledge. Hippocampus 2002; 12: 487–494.
Majerus S, Van der Linden M, Collette F, Laureys S, Poncelet M, Degueldre C, et al. Modulation of brain activity during phonological familiarization. Brain and Language 2005; 92: 320–331.
Maril A, Wagner AD, Schacter DL. On the tip of the tongue: An event-related fMRI study of semantic retrieval failure and cognitive conflict. Neuron 2001; 31: 653–660.
Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science 1995; 270: 102–105.
Mashal N, Faust M, Hendler T, Jung-Beeman M. An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain and Language 2007; 100: 115–126.
Mechelli A, Josephs O, Lambon Ralph MA, McClelland JL, Price CJ. Dissociating stimulus-driven semantic and phonological effect during reading and naming. Human Brain Mapping 2007; 28: 205–217.
Meyer M, Alter K, Friederici AD, Lohmann G, von Cramon DY. FMRI reveals brain regions mediating slow prosodic modulations in spoken sentences. Human Brain Mapping 2002; 17: 73–88.
Miceli G, Turriziani P, Caltagirone C, Capasso R, Tomaiuolo F, Caramazza A. The neural correlates of grammatical gender: An fMRI investigation. Journal of Cognitive Neuroscience 2002; 14: 618–628.
Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: divisible by what? Journal of Cognitive Neuroscience 1998; 10: 766–777.
Mummery CJ, Patterson K, Hodges JR, Wise RJS. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proceedings of the Royal Society of London B 1996; 263: 989–995.
Nakic M, Smith BW, Busis S, Vythilingam M, Blair RJR. The impact of affect and frequency on lexical decision: The role of the amygdala and inferior frontal cortex. Neuroimage 2006; 31: 1752–1761.
Nichelli P, Grafman J, Pietrini P, Clark K, Lee KY, Miletich R. Where the brain appreciates the moral of a story. Neuroreport 1995; 6: 2309–2313.
Noppeney U, Friston KJ, Price CJ. Effects of visual deprivation on the organization of the semantic system. Brain 2003; 126: 1620–1627.
Noppeney U, Josephs O, Kiebel S, Friston KJ, Price CJ. Action selectivity in parietal and temporal cortex. Cogn Brain Research 2005; 25: 641–649.
Noppeney U, Price CJ. Retrieval of visual, auditory, and abstract semantics. Neuroimage 2002; 15: 917–926.
Noppeney U, Price CJ. Functional imaging of the semantic system: Retrieval of sensory-experienced and verbally learned knowledge. Brain and Language 2003; 84: 120–133.
Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage 2004; 22: 164–170.
Obleser J, Wise RJS, Dresner MA, Scott SK. Functional integration across brain regions improves speech perception under adverse listening conditions. Journal of Neuroscience 2007; 27: 2283–2289.
Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. Journal of Cognitive Neuroscience 2006; 18: 1237–1252.
Otten LJ, Rugg MD. Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cerebral Cortex 2001; 11: 1150–1160.
Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, et al. A cultural effect on brain function. Nature Neuroscience 2000; 3: 91–96.
Perani D, Dehaene S, Grassi F, Cohen L, Cappa SF, Dupoux E, et al. Brain processing of native and foreign languages. Neuroreport 1996; 7: 2439–2444.
Perani D, Schnur T, Tettamanti M, Gorno-Tempini M, Cappa SF, Fazio F. Word and picture matching: A PET study of semantic category effects. Neuropsychologia 1999; 37: 293–306.
Pilgrim LK, Fadili J, Fletcher P, Tyler LK. Overcoming confounds of stimulus blocking: An event-related fMRI design of semantic processing. Neuroimage 2002; 16: 713–723.
Price CJ, Moore CJ, Humphreys GW, Wise RJS. Segregating semantic from phonological processes during reading. Journal of Cognitive Neuroscience 1997; 9: 727–733.
Price CJ, Wise RSJ, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex 1996; 6: 62–70.
Prince SE, Tsukiura T, Cabeza R. Distinguishing the neural correlates of episodic memory encoding and semantic memory retrieval. Psychological Science 2007; 18: 144–151.
Rajah MN, McIntosh AM. Overlap in the functional neural systems involved in semantic and episodic memory retrieval. Journal of Cognitive Neuroscience 2005; 17: 470–482.
Raposo A, Moss HE, Stamatakis EA, Tyler LK. Repetition suppression and semantic enhancement: An investigation of the neural correlates of priming. Neuropsychologia 2006; 44: 2284–2295.
Rissman J, Eliassen JC, Blumstein SE. An event-related fMRI investigation of implicit semantic priming. Journal of Cognitive Neuroscience 2003; 15: 1160–1175.
Robertson DA, Gernsbacher MA, Guidotti SJ, Robertson RRW, Irwin W, Mock BJ, et al. Functional neuroanatomy of the cognitive process of mapping during discourse comprehension. Psychological Science 2000; 11: 255–260.
Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, et al. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cognitive, Affective and Behavioral Neuroscience 2006; 6: 201–213.
Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. Journal of Cognitive Neuroscience 2001; 13: 829–843.
Ruschmeyer S-A, Brass M, Friederici AD. Comprehending prehending: Neural correlates of processing verbs with motor stems. Journal of Cognitive Neuroscience 2007; 19: 855–865.
Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage 2005; 27: 188–200.
Satpute AB, Fenker DB, Waldmann MR, Tabibnia G, Holyoak KJ, Lieberman MD. An fMRI study of causal judgments. European Journal of Neuroscience 2005; 22: 1233–1238.
Scott SK, Leff AP, Wise RJS. Going beyond the information given: a neural system supporting semantic interpretation. Neuroimage 2003; 19: 870–876.
Stringaris AK, Medford NC, Giampietro V, Brammer MJ, David AS. Deriving meaning: Distinct neural mechanisms for metaphoric, literal, and non-meaningful sentences. Brain and Language 2007; 100: 150–162.
Stringaris AK, Medford NC, Giora R, Giampietro VC, Brammer MJ, David AS. How metaphors influence semantic relatedness judgments: The role of the right frontal cortex. Neuroimage 2006; 33: 784–793.
Sugiura M, Sassa Y, Watanabe J, Akitsuki Y, Maeda Y, Matsue Y, et al. Cortical mechanisms of person representation: recognition of famous and personally familiar names. Neuroimage 2006; 31: 853–860.
Thioux M, Pesenti M, Costes N, De Volder A, Seron X. Task-independent semantic activation for numbers and animals. Cogn Brain Research 2005; 24: 284–290.
Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, et al. Neural correlates of verb argument structure processing. Journal of Cognitive Neuroscience 2007; 19: 1753–1767.
Tomasino B, Werner C, Weiss PH, Fink GR. Stimulus properties matter more than perspective: An fMRI study of mental imagery and silent reading of action phrases. Neuroimage 2007; 36: T128–141.
Tyler LK, Russell R, Fadili J, Moss HE. The neural representation of nouns and verbs: PET studies. Brain 2001; 124: 1619–1634.
Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss HE. Objects and their actions: evidence for a neurally distributed semantic system. Neuroimage 2003; 18: 542–557.
Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. Journal of Cognitive Neuroscience 2002; 14: 550–560.
von Kriegstein K, Eger E, Kleinschmidt A, Giraud AL. Modulation of neural responses to speech by directing attention to voices or verbal content. Cogn Brain Research 2003; 17: 48–55.
Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides semantic retrieval. Neuron 2001; 31: 329–338.
Wallentin M, Østergaarda S, Lund TE, Østergaard L, Roepstorff A. Concrete spatial language: See what I mean? Brain and Language 2005; 92: 221–233.
Whatmough C, Verret L, Fung D, Chertkow H. Common and contrasting areas of activation for abstract and concrete concepts: An H2 15O PET study. Journal of Cognitive Neuroscience 2004; 16: 1211–1226.
Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. Journal of Cognitive Neuroscience 2005; 17: 1871–1885.
Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C, et al. Noun imageability and the temporal lobes. Neuropsychologia 2000; 38: 985–994.
Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, et al. Temporally graded activation of neocortical regions in response to memories of different ages. Journal of Cognitive Neuroscience 2007; 19: 1–12.
Xiao Z, Zhang JX, Wang X, Wu R, Hu X, Weng X, et al. Differential activity in left inferior frontal gyrus for pseudowords and real words: an event-related fMRI study on auditory lexical decision. Human Brain Mapping 2005; 25: 212–221.
Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage 2005; 25: 1002–1015.
Appendix 2. Individual Study Details.
| No. | 1st Author | N | Method | Type | Contrast | Activation in brain regions of interest | ||||||
| IPL | MTG | FG/PH | DMPFC | IFG | VMPFC | PC | ||||||
| 1 | Assaf | 18 | fMRI | Gen | Rel–Unrel(SD) | + | + | + | + | + | + | |
| 2 | Baumgaertner | 19 | fMRI | Spec | Action–Motion(SD) | + | + | + | + | |||
| 3 | Baumgaertner | 9 | fMRI | Gen | W–N(LD) | + | + | + | ||||
| 4 | Bedny | 13 | fMRI | Spec | Conc–Abst(SD) | + | + | |||||
| 5 | Binder | 30 | fMRI | Gen | W(SD)–N(PD) | + | + | + | + | |||
| 6 | Binder | 24 | fMRI | Gen | W–N(LD) | + | + | + | + | + | + | + |
| 7 | Binder | 24 | fMRI | Gen | W–N(Read) | + | + | + | + | + | + | + |
| Spec | Conc–Abst(Read) | + | + | + | + | + | + | |||||
| 8 | Binder | 24 | fMRI | Gen | W–N(LD) | + | + | + | + | + | + | + |
| Spec | Conc–Abst(LD) | + | + | + | ||||||||
| 9 | Blaxton | 14 | PET | Gen | W(SD)–N(PD) | + | + | + | + | + | + | |
| 10 | Booth | 13 | fMRI | Gen | W(SD)–W(PD) | + | + | |||||
| 11 | Boronat | 15 | fMRI | Spec | Action–Function(SD) | + | ||||||
| 12 | Cai | 15 | fMRI | Gen | W–N(PD) | + | ||||||
| 13 | Cappa | 13 | PET | Gen | W(SD)–N(PD) | + | + | + | ||||
| Spec | Tool–Animal(SD) | + | + | |||||||||
| 14 | Cato | 26 | fMRI | Spec | Emotion–Neutral(cWG) | + | + | + | + | + | ||
| 15 | Clark | 20 | fMRI | Gen | W–N(PD) | + | + | + | + | + | ||
| 16 | Collette | 12 | PET | Gen | W–N(Rep) | + | ||||||
| 17 | Copland | 14 | fMRI | Gen | Rel–Unrel(LD) | + | ||||||
| 18 | Craik | 8 | PET | Gen | W(SD)–W(PD) | + | + | + | + | + | ||
| 19 | Crosson | 17 | fMRI | Spec | Emotion–Neutral(cWG) | + | ||||||
| 20 | D'Arcy | 10 | fMRI | Gen | Rel–Unrel(SD) | |||||||
| 21 | Dapretto | 8 | fMRI | Gen | SemSP–SynSP(SD) | + | ||||||
| 22 | Daselaar | 6 | fMRI | Gen | W(SD)–W(PD) | + | + | + | + | |||
| 23 | Davis | 12 | fMRI | Spec | Action–Other(SD) | + | ||||||
| 24 | Démonet | 9 | PET | Gen | W(SD)–N(PD) | + | + | + | + | |||
| 25 | Devlin | 12 | fMRI | Gen | W(SD)–W(PD) | + | + | + | + | + | ||
| 26 | Devlin | 26 | Both | Spec | Artifact–Natural(SD) | |||||||
| 27 | Ebisch | 17 | fMRI | Spec | Function–Size(SD) | + | + | + | + | |||
| 28 | Eschen | 10 | fMRI | Spec | Action–Abst(Mem) | |||||||
| 29 | Fiebach | 12 | fMRI | Spec | Conc–Abst(LD) | |||||||
| 30 | Fiebach | 11 | fMRI | Gen | W–N(LD) | + | + | + | ||||
| 31 | Fletcher | 6 | PET | Spec | Story–UnrelSent(SD) | + | + | + | + | |||
| Spec | ThM–Story(SD) | + | + | + | ||||||||
| 32 | Fliessbach | 21 | fMRI | Spec | Conc–Abst(Rec) | + | ||||||
| 33 | Giesbrecht | 10 | fMRI | Spec | Conc–Abst(SD) | + | + | + | ||||
| 34 | Giraud | 12 | PET | Gen | W–N(Rep) | |||||||
| 35 | Gitelman | 14 | fMRI | Gen | W(SD)–W(PD) | + | + | + | + | |||
| 36 | Goldberg | 13 | fMRI | Spec | Natural–Other(SD) | + | + | + | ||||
| 37 | Goldberg | 14 | fMRI | Spec | Color/Taste/ etc.–Other(SD) | + | + | + | + | |||
| 38 | Gorno-Tempini | 6 | PET | Spec | Person–Object(Match) | + | ||||||
| 39 | Gourovitch | 18 | PET | Gen | W(SWG)–W(PWG) | + | + | + | + | |||
| 40 | Grossman | 16 | fMRI | Spec | Tool–Animal(SD) | + | + | + | ||||
| 41 | Grossman | 16 | fMRI | Spec | Motion–Abst(SD) | + | + | |||||
| 42 | Hagoort | 11 | PET | Gen | W–N(Read) | + | + | |||||
| 43 | Henke | 12 | PET | Gen | W(SD)–W(PD) | + | + | |||||
| 44 | Henson | 6 | fMRI | Gen | W–N(OD) | + | + | + | ||||
| 45 | Henson | 12 | fMRI | Gen | W–N(LD) | + | + | + | + | |||
| 46 | Herbster | 10 | PET | Gen | W–N(cRead) | + | ||||||
| 47 | Homae | 9 | fMRI | Gen | W/N(LD)–N(Match) | + | + | + | + | + | ||
| Gen | Sent(SD)–W/N(LD) | + | + | + | + | |||||||
| 48 | Homae | 10 | fMRI | Spec | Story(SD)–UnrelSent(LD) | + | + | + | ||||
| 49 | Humphries | 21 | fMRI | Gen | HighM–LowM(SD) | + | + | + | + | + | ||
| Gen | HighM–N(SD) | + | + | + | + | |||||||
| Gen | LowM–N(SD) | + | + | + | ||||||||
| 50 | Ischebeck | 10 | fMRI | Gen | W–N(cRead) | + | + | + | + | |||
| 7 | Gen | W–N(LD) | + | + | + | + | ||||||
| 51 | Jessen | 14 | fMRI | Spec | Conc–Abst(Mem) | + | + | + | + | |||
| 52 | Joubert | 10 | fMRI | Gen | W–N(cRead) | + | + | |||||
| 53 | Kellenbach | 10 | PET | Spec | Auditory–Visual(SD) | + | + | + | + | |||
| Spec | Size–Color(SD) | + | ||||||||||
| 54 | Kelley | 21 | fMRI | Spec | Self–Other(SD) | + | + | |||||
| 55 | Kosslyn | 12 | PET | Spec | Category–Other(SD) | + | + | |||||
| 56 | Kotz | 13 | fMRI | Gen | W–N(LD) | + | + | + | ||||
| Gen | Rel–Unrel(LD) | + | ||||||||||
| Spec | Category–Association(LD) | + | + | |||||||||
| 57 | Kounios | 16 | fMRI | Spec | Animal–Tool(SD) | + | + | |||||
| Spec | Animal–Abst(SD) | + | ||||||||||
| Spec | Tool–Abst(SD) | + | ||||||||||
| 58 | Kuchinke | 20 | fMRI | Gen | W–N(LD) | + | + | + | + | + | ||
| Spec | Emotion–Other(LD) | + | + | + | + | + | + | |||||
| 59 | Kuperberg | 15 | fMRI | Gen | Rel–Unrel(SD) | + | ||||||
| 60 | Kuperberg | 9 | fMRI | Gen | HighM–LowM(SD) | + | + | + | + | + | ||
| 61 | Laine | 7 | PET | Spec | Animal–Artifact(SWG) | + | + | |||||
| 62 | Lee, A | 10 | PET | Spec | Perceptual–Verbal(SWG) | + | + | |||||
| 63 | Lee, A | 7 | PET | Gen | W–N(Mem) | + | + | + | ||||
| 64 | Lee, S | 12 | fMRI | Spec | Figurative–Literal(SD) | + | + | + | ||||
| 65 | Liu | 12 | fMRI | Gen | W(SD)–W(PD) | |||||||
| 66 | Luo | 12 | fMRI | Gen | Rel–Unrel(SD) | + | + | + | + | + | ||
| 67 | Majerus | 12 | PET | Gen | W–N(Rep) | + | + | |||||
| 68 | Maril | 14 | fMRI | Gen | Fam–Unfam(SD) | + | ||||||
| 69 | Martin | 12 | PET | Spec | Action–Color(SWG) | + | + | + | + | + | + | |
| 70 | Mashal | 15 | fMRI | Gen | Rel–Unrel(SD) | + | + | + | ||||
| Spec | Figurative–Unrel(SD) | + | + | + | + | |||||||
| Spec | Figurative–Literal(SD) | + | + | |||||||||
| 71 | Mechelli | 20 | fMRI | Gen | Rel–Unrel(Read) | + | + | + | + | |||
| 72 | Meyer | 14 | fMRI | Gen | W–N(GJ) | + | ||||||
| 73 | Miceli | 9 | fMRI | Gen | W(SD)–W(PD) | + | + | + | + | |||
| 74 | Mummery | 10 | PET | Gen | W(SD)–W(PD) | + | + | + | + | + | + | + |
| Spec | Location–Color(SD) | + | + | + | + | |||||||
| Spec | Natural–Artifact(SD) | + | ||||||||||
| 75 | Mummery | 6 | PET | Gen | W(SWG)–W(PWG) | + | + | |||||
| Spec | Natural–Artifact(SWG) | + | + | |||||||||
| 76 | Nakic | 13 | fMRI | Spec | Emotion–Other(LD) | + | + | + | + | |||
| 77 | Nichelli | 9 | PET | Gen | Story(SD)–Story(OD) | + | + | + | ||||
| 78 | Noppeney | 23 | fMRI | Spec | Action–Other(SD) | + | ||||||
| Spec | Visual–Other(SD) | + | ||||||||||
| 79 | Noppeney | 27 | fMRI | Spec | Action–Other(SD) | |||||||
| 80 | Noppeney | 12 | PET | Spec | Conc–Abst(SD) | |||||||
| 81 | Noppeney | 9 | PET | Spec | Verbal–Perceptual(SD) | + | + | |||||
| 82 | Noppeney | 15 | fMRI | Spec | Abst–Conc(SD) | + | + | |||||
| 83 | Obleser | 16 | fMRI | Gen | HighM–LowM(Mem) | + | + | + | + | |||
| 84 | Orfanidou | 13 | fMRI | Gen | W–N(LD) | + | + | + | + | + | + | + |
| 85 | Otten | 16 | fMRI | Gen | W(SD)–W(PD) | + | + | |||||
| 86 | Paulesu | 12 | PET | Gen | W–N(Read) | |||||||
| 87 | Perani | 9 | PET | Gen | HighM–LowM(Mem) | + | + | |||||
| 88 | Perani | 8 | PET | Spec | Animal–Artifact(Match) | + | + | |||||
| 89 | Pilgrim | 15 | fMRI | Spec | Natural–Artifact(SD) | |||||||
| 90 | Price | 6 | PET | Gen | W(SD)–W(PD) | + | + | + | ||||
| 91 | Price | 6 | PET | Gen | W(OD)–N(OD) | + | ||||||
| 92 | Prince | 12 | fMRI | Gen | Rel–Unrel(SD) | + | + | + | ||||
| 93 | Rajah | 11 | PET | Gen | W(SD)–W(Rec) | + | + | + | + | + | ||
| 94 | Raposo | 15 | fMRI | Gen | Rel–Unrel(SD) | + | + | + | + | + | ||
| 95 | Rissman | 15 | fMRI | Gen | W–N(LD) | + | + | + | + | |||
| 96 | Robertson | 8 | fMRI | Spec | Story–UnrelSent(cRead) | + | ||||||
| 97 | Rogers | 12 | PET | Spec | Specific–General(SD) | + | ||||||
| 98 | Roskies | 20 | PET | Gen | W(SD)–W(PD) | + | + | + | + | + | + | + |
| 99 | Ruschmeyer | 20 | fMRI | Spec | Action–Abst(LD) | + | + | |||||
| 100 | Sabsevitz | 28 | fMRI | Spec | Conc–Abst(SD) | + | + | + | + | + | + | + |
| 101 | Satpute | 11 | fMRI | Spec | Causation–Association(SD) | + | ||||||
| 102 | Scott | 9 | PET | Gen | W(SD)–W(PD) | + | + | + | + | |||
| 103 | Stringaris | 11 | fMRI | Gen | HighM–LowM(SD) | + | + | + | ||||
| Spec | Figurative–Literal(SD) | + | + | + | + | |||||||
| 104 | Stringaris | 12 | fMRI | Spec | Figurative–Literal(SD) | + | + | + | + | |||
| 105 | Sugiura | 16 | fMRI | Gen | Fam–Unfam(SD) | + | + | + | + | |||
| Spec | Self–Other(SD) | + | + | + | ||||||||
| 106 | Thioux | 6 | PET | Spec | Animal–Number(SD) | + | + | + | ||||
| 107 | Thompson | 14 | fMRI | Spec | Verb2–Verb1(LD) | + | ||||||
| 108 | Tomasino | 15 | fMRI | Spec | Action–Other(SD) | + | ||||||
| 109 | Tyler | 9 | PET | Spec | Conc–Abst(LD) | |||||||
| 110 | Tyler | 12 | fMRI | Spec | Action–Animal(SD) | + | + | |||||
| Spec | Action–Tool(SD) | |||||||||||
| 111 | Vandenberghe | 10 | PET | Gen | LowM–HighM(Match) | + | ||||||
| 112 | von Kriegstein | 14 | fMRI | Gen | Sent(VTarg)–Sent(NVTarg) | + | ||||||
| 113 | Wagner | 13 | fMRI | Gen | LowA–HighA(SD) | + | ||||||
| 114 | Wallentin | 18 | fMRI | Spec | Conc–Abst(SD) | + | + | + | + | + | ||
| 115 | Whatmough | 15 | PET | Spec | Conc–Abst(SD) | + | + | |||||
| 116 | Wheatley | 18 | fMRI | Spec | Animal–Artifact(cRead) | + | + | + | + | + | + | |
| 117 | Wise | 18 | PET | Spec | Conc–Abst(SD) | + | ||||||
| 118 | Woodard | 15 | fMRI | Gen | Fam–Unfam(SD) | + | + | + | + | + | + | + |
| 119 | Xiao | 14 | fMRI | Gen | W–N(LD) | + | + | + | ||||
| 120 | Xu | 22 | fMRI | Spec | Story–UnrelSent(Mem) | + | + | + | + | + | ||
Note: The Contrast column provides a code indicating the contrast used, with tasks indicated in parentheses after stimuli. The terms Action, Animal, Artifact, Association, Auditory, Category, Causation, Color, Emotion, Figurative, Function, General, Literal, Location, Motion, Natural, Number, Perceptual, Self, Size, Specific, Taste, Tool, Verbal, and Visual refer to the object category or knowledge domain emphasized by the stimulus or task. “Other” indicates a miscellaneous combination of categories or domains. Abbreviations used for stimuli: Abst = abstract words, Conc = concrete words, Fam = familiar concepts, Unfam = unfamiliar concepts, N = nonwords (i.e., pseudowords), W = words, HighM = sentences or words with high meaningfulness, LowM = sentences or words with low meaningfulness, HighA = highly associated word pairs, LowA = weakly associated word pairs, Rel = semantically related words, Unrel = semantically unrelated words, Sent = sentences, Story = connected narrative or discourse, UnrelSent = unrelated sentences, SemSP = semantically distinct sentence pairs, SynSP = syntactically distinct sentence pairs, ThM = theory-of-mind narrative, Verb1 = single-argument verb, Verb2 = 2-argument verb. Abbreviations for tasks: GJ = grammaticality judgment, LD = lexical decision, Match = identity matching, Mem = memorization, OD = orthographic decision, PD = phonological decision, cRead = covert reading, Read = oral reading, Rep = oral repetition, Rec = recognition, SD = semantic decision, PWG = phonological word generation, SWG = semantic word generation, cSWG = covert semantic word generation, VTarg = detect a target word or phrase, NVTarg = detect a target nonverbal feature. Examples: W(SD)–N(PD) indicates a semantic decision task on words contrasted with a phonological decision on pseudowords; Conc–Abst(LD) indicates a contrast between concrete and abstract words presented during a lexical decision task. Specific contrasts were almost always performed in both directions (e.g., Animal–Tool and Tool–Animal); for simplicity such complementary contrasts are collapsed into a single row in the table.
Other abbreviations: N = number of subjects in study; Gen = general contrast; Spec = specific contrast; IPL = inferior parietal lobe (angular and supramarginal gyri); MTG = middle temporal gyrus; FG/PH = fusiform and parahippocampal gyri; DMPFC = dorsomedial prefrontal cortex; IFG = inferior frontal gyrus; VMPFC = ventromedial prefrontal cortex; PC = posterior cingulate gyrus.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: insights from animal research. Phil Trans Roy Soc Lond B. 2001;356:1467–1482. doi: 10.1098/rstb.2001.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander MP, Benson DF. The aphasias and related disturbances. In: Joynt RJ, editor. Clinical Neurology. Philadelphia (PA): J.B. Lipincott; 1993. pp. 1–58. [Google Scholar]
- Alexander MP, Hiltbrunner B, Fischer RS. Distributed anatomy of transcortical sensory aphasia. Arch Neurol. 1989;46:885–892. doi: 10.1001/archneur.1989.00520440075023. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Asanuma C, Essick G, Siegel RM. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J Comp Neurol. 1990;296:65–113. doi: 10.1002/cne.902960106. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annual Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Antrobus JS. Information theory and stimulus-independent thought. Br J Psychol. 1968;59:423–430. [Google Scholar]
- Antrobus JS, Singer JL, Greenberg S. Studies in the stream of consciousness: experimental enhancement and suppression of spontaneous cognitive processes. Perceptual Motor Skills. 1966;23:399–417. [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- Aurell CG. Perception: a model comprising two modes of consciousness. Percept Motor Skills. 1979;49:431–444. doi: 10.2466/pms.1979.49.2.431. [DOI] [PubMed] [Google Scholar]
- Baleydier C, Mauguiere F. The duality of the cingulate gyrus in the monkey. Neuroanatomical study and functional hypothesis. Brain. 1980;103:525–554. doi: 10.1093/brain/103.3.525. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–848. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. The content and organization of autobiographical memories. In: Neisser U, Winograd E, editors. Remembering reconsidered: ecological and traditional approaches to the study of memory. Cambridge: Cambridge University Press; 1998. pp. 193–243. [Google Scholar]
- Baumgaertner A, Buccino G, Lange R, McNamara A, Binkofski F. Polymodal conceptual processing of human biological actions in the left inferior frontal lobe. Eur J Neurosci. 2007;25:881–889. doi: 10.1111/j.1460-9568.2007.05346.x. [DOI] [PubMed] [Google Scholar]
- Baumgaertner A, Weiller C, Buchel C. Event-related fMRI reveals cortical sites involved in contextual sentence integration. Neuroimage. 2002;16:736–745. doi: 10.1006/nimg.2002.1134. [DOI] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. Functional subdivisions of the temporal lobe neocortex. J Neurosci. 1987;7:330–342. doi: 10.1523/JNEUROSCI.07-02-00330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bedny M, Thompson-Schill SL. Neuroanatomically separable effects of imageability and grammatical class during single-word comprehension. Brain Lang. 2006;98:127–139. doi: 10.1016/j.bandl.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Benson DF. Aphasia, alexia and agraphia. New York: Churchill Livingstone; 1979. [Google Scholar]
- Benson DF, Cummings JL, Tsai SY. Angular gyrus syndrome simulating Alzheimer's disease. Arch Neurol. 1982;38:616–620. doi: 10.1001/archneur.1982.00510220014003. [DOI] [PubMed] [Google Scholar]
- Benton AL. The fiction of the “Gerstmann syndrome”. J Neurol Neurosurg Psychiatry. 1961;24:176–181. doi: 10.1136/jnnp.24.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier ML. Transcortical aphasias. Hove: Psychology Press; 1999. [Google Scholar]
- Binder JR. Wernicke aphasia: a disorder of central language processing. In: D'Esposito ME, editor. Neurological foundations of cognitive neuroscience. Cambridge (MA): MIT Press; 2002. pp. 175–238. [Google Scholar]
- Binder JR. Effects of word imageability on semantic access: neuroimaging studies. In: Hart J, Kraut MA, editors. Neural basis of semantic memory. Cambridge, UK: Cambridge University Press; 2007. pp. 149–181. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. Conceptual processing during the conscious resting state: a functional MRI study. J Cogn Neurosci. 1999;11:80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional MRI. J Neurosci. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Liebenthal E, Possing ET, Medler DA, Ward BD. Neural correlates of sensory and decision processes in auditory object identification. Nature Neurosci. 2004;7:295–301. doi: 10.1038/nn1198. [DOI] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E. Some neurophysiological constraints on models of word naming. Neuroimage. 2005;27:677–693. doi: 10.1016/j.neuroimage.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Sabsevitz DS. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49:1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Westbury CF, Possing ET, McKiernan KA, Medler DA. Distinct brain systems for processing concrete and abstract concepts. J Cogn Neurosci. 2005;17:905–917. doi: 10.1162/0898929054021102. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI study. Eur J Neurosci. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Pandya DN, Rosene DL. Parcellation of cortical afferents to three distinct sectors in the parahippocampal gyrus of the rhesus monkey: an anatomical and neurophysiological study. J Comp Neurol. 2003;466:161–179. doi: 10.1002/cne.10866. [DOI] [PubMed] [Google Scholar]
- Bock M. The influence of emotional meaning on the recall of words processed for form or self-reference. Psychol Res. 1986;48:107–112. [Google Scholar]
- Bogen JE, Bogen GM. Wernicke's region—where is it? Ann NY Acad Sci. 1976;290:834–843. doi: 10.1111/j.1749-6632.1976.tb25546.x. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA. Distinctions between manipulation and function knowledge of objects: evidence from functional magnetic resonance imaging. Cogn Brain Res. 2005;23:361–373. doi: 10.1016/j.cogbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Bréal M. Essai de sémantique (science des significations) Paris: Librairie Hachette; 1897. [Google Scholar]
- Breedin SD, Saffran EM, Coslett HB. Reversal of a concreteness effect in a patient with semantic dementia. Cogn Neuropsychol. 1995;11:617–660. [Google Scholar]
- Bright P, Moss HE, Tyler LK. Unitary vs. multiple semantics: PET studies of word and picture processing. Brain Lang. 2004;89:417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarques sur le siège de la faculté du langage articulé; suivies d'une observation d'aphemie. Bull Soc Anat Paris. 1861;6:330–357. [Google Scholar]
- Brodmann K. Localisation in the cerebral cortex. London: Smith-Gordon; 1994/1909. [Google Scholar]
- Buchsbaum BR, D'Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20:762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurosci. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial cognition and the brain. Ann NY Acad Sci. 2008;1124:77–97. doi: 10.1196/annals.1440.002. [DOI] [PubMed] [Google Scholar]
- Burton MW, Small S, Blumstein SE. The role of segmentation in phonological processing: an fMRI investigation. J Cogn Neurosci. 2000;12:679–690. doi: 10.1162/089892900562309. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: a call to action. Neurocase. 2001;7:445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Cogn Brain Res. 2005;25:226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JA. Neural substrates of knowledge of hand postures for object grasping and functional onject use: evidence from fMRI. Brain Res. 2006;1117:175–185. doi: 10.1016/j.brainres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Perani D, Schnur T, Tettamanti M, Fazio F. The effects of semantic category and knowledge type on lexical–semantic access: a PET study. Neuroimage. 1998;8:350–359. doi: 10.1006/nimg.1998.0368. [DOI] [PubMed] [Google Scholar]
- Cappe C, Barone P. Heteromodal connections supporting multisensory integration at low levels of cortical processing in the monkey. Eur J Neurosci. 2005;22:2886–2902. doi: 10.1111/j.1460-9568.2005.04462.x. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Berndt RS, Basili AG, Koller JJ. Syntactic processing deficits in aphasia. Cortex. 1981;17:333–348. doi: 10.1016/s0010-9452(81)80021-4. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. Neuroimage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cavada C. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: i. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in the rhesus monkey: iI. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287:422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chafee M, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79:2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nature Neurosci. 1999;2:913–919. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Cortical regions associated with perceiving, naming, and knowing about colors. J Cogn Neurosci. 1999;11:25–35. doi: 10.1162/089892999563229. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A. Representation of manipulable man-made objects in the dorsal stream. Neuroimage. 2000;12:478–484. doi: 10.1006/nimg.2000.0635. [DOI] [PubMed] [Google Scholar]
- Chao LL, Weisberg J, Martin A. Experience-dependent modulation of category-related cortical activity. Cereb Cortex. 2002;12:545–551. doi: 10.1093/cercor/12.5.545. [DOI] [PubMed] [Google Scholar]
- Chee MW, Hon NHH, Caplan D, Lee HL, Goh J. Frequency of concrete words modulates prefrontal activation during semantic judgments. Neuroimage. 2002;16:259–268. doi: 10.1006/nimg.2002.1061. [DOI] [PubMed] [Google Scholar]
- Chee MW, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, Chee M. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures. Neuroimage. 2000;12:392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Chertkow H, Bub D, Deaudon C, Whitehead V. On the status of object concepts in aphasia. Brain Lang. 1997;58:203–232. doi: 10.1006/brln.1997.1771. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Butterworth B, Denes G. A specific deficit for numbers in a case of dense acalculia. Brain. 1991;114:2619–2637. doi: 10.1093/brain/114.6.2619. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge (MA): MIT Press; 1993. [Google Scholar]
- Coltheart M, Patterson K, Marshall J. Deep dyslexia. London: Routledge & Kegan Paul; 1980. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychol Rev. 2000;107:261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Copland DA, de Zubicaray GI, McMahon K, Wilson SJ, Eastburn M, Chenery HJ. Brain activity during automatic semantic priming revealed by event-related functional magnetic resonance imaging. Neuroimage. 2003;20:302–310. doi: 10.1016/s1053-8119(03)00279-9. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo T, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Lockhart RS. Levels of processing: a framework for memory research. J Verb Learn Verb Behav. 1992;11:671–684. [Google Scholar]
- Creem-Regehr S, Lee J. Neural representation of graspable objects: are tools special? Cogn Brain Res. 2005;22:457–469. doi: 10.1016/j.cogbrainres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Critchley M. The parietal lobes. New York: Hafner; 1953. [Google Scholar]
- D'Esposito M, Detre JA, Aguirre GK, Stallcup M, Alsop DC, Tippet LJ, Farah MJ. A functional MRI study of mental image generation. Neuropsychologia. 1997;35:725–730. doi: 10.1016/s0028-3932(96)00121-2. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descarte's error: emotion, reason, and the human brain. New York: Putnam; 1994. [Google Scholar]
- Damasio H. Cerebral localization of the aphasias. In: Sarno MT, editor. Acquired aphasia. Orlando: Academic Press; 1981. pp. 27–50. [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Davidoff J, De Bleser R. Impaired picture recognition with preserved object naming and reading. Brain Cogn. 1994;24:1–23. doi: 10.1006/brcg.1994.1001. [DOI] [PubMed] [Google Scholar]
- Davis MH, Meunier F, Marslen-Wilson WD. Neural responses to morphological, syntactic, and semantic properties of single words: an fMRI study. Brain Lang. 2004;89:439–449. doi: 10.1016/S0093-934X(03)00471-1. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Liotti M, Nichelli P. Semantic amnesia with preservation of autobiographical memory: a case report. Cortex. 1987;23:575–597. doi: 10.1016/s0010-9452(87)80050-3. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L. Cerebral pathways for calculation: double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex. 1997;33:219–250. doi: 10.1016/s0010-9452(08)70002-9. [DOI] [PubMed] [Google Scholar]
- Déjerine J. Anatomie des centres nerveux. Paris: Rueff; 1895. [Google Scholar]
- Déjerine J. Contribution à l'étude anatomo-pathologique et clinique des différentes variétés de cécité verbale. Comptes Rend Séances Soc Biol. 1892;44:61–90. [Google Scholar]
- Démonet J-F, Chollet F, Ramsay S, Cardebat D, Nespoulous J-L, Wise R, Rascol A, Frackowiak R. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115:1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- Démonet JF, Wise R, Frackowiak RSJ. Language functions explored in normal subjects by positron emission tomography: a critical review. Hum Brain Mapp. 1993;1:39–47. [Google Scholar]
- Desai R, Conant LL, Waldron E, Binder JR. FMRI of past tense processing: the effects of phonological complexity and task difficulty. J Cogn Neurosci. 2006;18:278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgranges B, Baron JC, Lalevee C, Giffard B, Viader F, de La Sayette V, Eustache F. The neural substrates of episodic memory impairment in Alzheimer's disease as revealed by FDG-PET: relationship to degree of deterioration. Brain. 2002;125:1116–1124. doi: 10.1093/brain/awf097. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MFS. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Moore CJ, Mummery CJ, Gorno-Tempini ML, Phillips JA, Noppeney U, Frackowiak RSJ, Price CJ. Anatomic constraints on cognitive theories of category specificity. Neuroimage. 2002;15:675–685. doi: 10.1006/nimg.2001.1002. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Jalal Fadili M, Tyler LK. Is there an anatomical basis for category-specificity? Semantic memory studies with PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. J Neurophysiol. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Ebisch SJH, Babiloni C, Del Gratta C, Ferretti A, Perrucci MG, Caulo M, Sitskoorn MM, Romani GL. Human neural systems for conceptual knowledge of proper object use: a functional magnetic resonance imaging study. Cereb Cortex. 2007;17:2744–2751. doi: 10.1093/cercor/bhm001. [DOI] [PubMed] [Google Scholar]
- Embick D, Marantz A, Miyashita Y, O'Neil W, Sakai KL. A syntactic specialization for Broca's area. Proc Natl Acad Sci. 2000;97:6150–6154. doi: 10.1073/pnas.100098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 2007;27:6141–6149. doi: 10.1523/JNEUROSCI.0799-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschen A, Freeman J, Dietrich T, Martin M, Ellis J, Martin E, Kliegel M. Motor brain regions are involved in the encoding of delayed intentions: a fMRI study. Int J Psychophysiol. 2007;64:259–268. doi: 10.1016/j.ijpsycho.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Evans AC, Collins DL, Mills SR, Brown ED, Kelly RL, Peters TM. 3D statistical neuroanatomical models from 305 MRI volumes. IEEE nuclear science symposium and medical imaging conference. 1993:1813–1817. [Google Scholar]
- Farah MJ. Visual agnosia. Disorders of object recognition and what they tell us about normal vision. Cambridge: MIT Press; 1990. [Google Scholar]
- Fiebach CJ, Friederici AD. Processing concrete words: fMRI evidence against a specific right-hemisphere involvement. Neuropsychologia. 2003;42:62–70. doi: 10.1016/s0028-3932(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G. Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp. 2005;24:79–91. doi: 10.1002/hbm.20070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics and the role of the left inferior prefrontal cortex. Hum Brain Mapp. 1997;5:79–83. [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fliessbach K, Wesi S, Klaver P, Elger CE, Weber B. The effect of word concreteness on recognition memory. Neuroimage. 2006;32:1413–1421. doi: 10.1016/j.neuroimage.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Fodor JA. The modularity of mind: an essay on faculty psychology. Cambridge (MA): MIT Press; 1983. [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;29:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Forde EME, Humphreys GW. Category-specific recognition impairments: a review of important case studies and influential theories. Aphasiol. 1999;13:169–193. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin S, Howard D, Patterson K. Abstract word anomia. Cogn Neuropsychol. 1995;12:549–566. [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M, Alexander MP, Naeser MA. Anatomic basis of transcortical motor aphasia. Neurology. 1984;40:409–417. doi: 10.1212/wnl.34.4.409. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rüschemeyer S-A, Hahne A, Fiebach CJ. The role of left inferior frontal gyrus and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–177. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. A PET study of word finding. Neuropsychologia. 1991;29:1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. Proc Natl Acad Sci USA. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. What the locus of brain lesion tells us about the nature of the cognitive defect underlying category-specific disorders: a review. Cortex. 2000;36:539–559. doi: 10.1016/s0010-9452(08)70537-9. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Almonti S, Di Betta AM, Silveri MC. Retrograde amnesia in a patient with retrosplenial tumour. Neurocase. 1998;4:519–526. [Google Scholar]
- Galaburda A, Sanides F. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol. 1980;190:597–610. doi: 10.1002/cne.901900312. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G. The brain's concepts: the role of the sensory-motor system in conceptual knowledge. Cogn Neuropsychol. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Gates L, Yoon MG. Distinct and shared cortical regions of the human brain activated by pictorial depictions versus verbal descriptions: an fMRI study. Neuroimage. 2005;24:473–486. doi: 10.1016/j.neuroimage.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Gerlach C. A review of functional imaging studies on category specificity. J Cogn Neurosci. 2007;19:296–314. doi: 10.1162/jocn.2007.19.2.296. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Paulson O. When action turns into words. Activation of motor-based knowledge during categorization of manipulable objects. J Cogn Neurosci. 2002;14:1230–1239. doi: 10.1162/089892902760807221. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Paulson OB. Structural similarity and category-specificity: a refined account. Neuropsychologia. 2004;42:1543–1553. doi: 10.1016/j.neuropsychologia.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Gerstmann J. Syndrome of finger agnosia, disorientation for right and left, agraphia and acalculia. Arch Neurol Psychiat. 1940;44:398–408. [Google Scholar]
- Geschwind N. Aphasia. New Engl J Med. 1971;284:654–656. doi: 10.1056/NEJM197103252841206. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. 585–644. [DOI] [PubMed] [Google Scholar]
- Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Conscious Cogn. 1995;4:1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Giesbrecht B, Camblin CC, Swaab TY. Separable effects of semantic priming and imageability on word processing in human cortex. Cereb Cortex. 2004;14:521–529. doi: 10.1093/cercor/bhh014. [DOI] [PubMed] [Google Scholar]
- Giraud AL, Price CJ. The constraints functional neuroimaging places on classical models of auditory word processing. J Cogn Neurosci. 2001;13:754–765. doi: 10.1162/08989290152541421. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Ashburner J, Friston KJ, Tyler LK, Price CJ. Voxel-based morphometry of herpes simplex encephalitis. Neuroimage. 2001;13:623–631. doi: 10.1006/nimg.2000.0734. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Goldberg G. Supplementary motor area structure and function: review and hypothesis. Behav Brain Sci. 1985;8:567–616. [Google Scholar]
- Goldberg RF, Perfetti CA, Fiez JA, Schneider W. Selective retrieval of abstract semantic knowledge in left prefrontal cortex. J Neurosci. 2007;27:3790–3798. doi: 10.1523/JNEUROSCI.2381-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RF, Perfetti CA, Schneider W. Perceptual knowledge retrieval activates sensory brain regions. J Neurosci. 2006;26:4917–4921. doi: 10.1523/JNEUROSCI.5389-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golland Y, Bentin S, Gelbard H, Benjamini Y, Heller R, Nir Y, Hasson U, Malach R. Extrinsic and intrinsic systems in the posterior cortex of the human brain revealed during natural sensory stimulation. Cereb Cortex. 2007;17:766–777. doi: 10.1093/cercor/bhk030. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Hyde MR, Blumstein S. Frequency, picturability and availability of nouns in aphasia. Cortex. 1969;5:104–119. doi: 10.1016/s0010-9452(69)80022-5. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Cipolotti L, Price CJ. Category differences in brain activation studies: where do they come from? Proc Roy Soc London B. 2000;267:1253–1258. doi: 10.1098/rspb.2000.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorno-Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain. 1998;121:2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Ffytche DH, Howard RJ. FMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage. 2003;20:1006–1019. doi: 10.1016/S1053-8119(03)00365-3. [DOI] [PubMed] [Google Scholar]
- Graham KS, Lee AC, Brett M, Patterson K. The neural basis of autobiographical and semantic memory: new evidence from three PET studies. Cogn Affect Behav Neurosci. 2003;3:234–254. doi: 10.3758/cabn.3.3.234. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, Gupta P. The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. J Cogn Neurosci. 2008;20:1698–1710. doi: 10.1162/jocn.2008.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C, Fink GR. The functional organization of the intraparietal sulcus in humans and monkeys. J Anat. 2005;207:3–17. doi: 10.1111/j.1469-7580.2005.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Borra E, Matelli M, Luppino G. Architectonic organization of the inferior parietal convexity of the macaque monkey. J Comp Neurol. 2006;496:422–451. doi: 10.1002/cne.20933. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y, Friederici AD. Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol. 2006;16:240–246. doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Grosbras M-H, Laird AR, Paus T. Cortical regions involved in eye movements, shifts of attention, and gaze perception. Hum Brain Mapp. 2005;25:140–154. doi: 10.1002/hbm.20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. The neural basis for category-specific knowledge: an fMRI study. Neuroimage. 2002a;15:936–948. doi: 10.1006/nimg.2001.1028. [DOI] [PubMed] [Google Scholar]
- Grossman M, Koenig P, DeVita C, Glosser G, Alsop D, Detre J, Gee J. Neural representation of verb meaning: an fMRI study. Hum Brain Mapp. 2002b;15:124–134. doi: 10.1002/hbm.10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Hart J, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomic correlation. Ann Neurol. 1990;27:226–231. doi: 10.1002/ana.410270303. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson U, Levy I, Behrmann M, Hendler T, Malach R. Eccentricity bias as an organizing principle for human high-order object areas. Neuron. 2002;34:490–497. doi: 10.1016/s0896-6273(02)00662-1. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–2430. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Head H. Aphasia and kindred disorders of speech. New York: MacMillan; 1926. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson RT, Day A, Valenstein E, Hammond E, Duara R. Frontal hypermetabolism and thalamic hypometabolism in a patient with abnormal orienting and retrosplenial amnesia. Neuropsychologia. 1990;28:161–169. doi: 10.1016/0028-3932(90)90098-9. [DOI] [PubMed] [Google Scholar]
- Henschen SE. On the hearing sphere. Acta Otolaryngol (Stockh) 1918–1919;1:423–486. [Google Scholar]
- Henson RNA, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: application to words versus nonwords and initial versus repeated face presentations. Neuroimage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: speech, music, and working memory in area Spt. J Cogn Neurosci. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Caramazza A. Category-specific naming and comprehension impairment: a double dissociation. Brain. 1991;114:2081–2094. doi: 10.1093/brain/114.5.2081. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Tuffiash E, Beauchamp NJ, Jacobs MA, Barker PB, Selnes OA. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Ann Neurol. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Ho N, Destexhe A. Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol. 2000;84:1488–1496. doi: 10.1152/jn.2000.84.3.1488. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Graham N, Patterson K. Charting the progression in semantic dementia: implications for the organisation of semantic memory. Memory. 1995;3:463–495. doi: 10.1080/09658219508253161. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Homae F, Yahata N, Sakai KL. Selective enhancement of functional connectivity in the left prefrontal cortex during sentence processing. Neuroimage. 2003;20:578–586. doi: 10.1016/s1053-8119(03)00272-6. [DOI] [PubMed] [Google Scholar]
- Honey GD, Bullmore ET, Sharma T. Prolonged reaction time to a verbal working memory task predicts increased power of posterior parietal cortical activation. Neuroimage. 2000;12:495–503. doi: 10.1006/nimg.2000.0624. [DOI] [PubMed] [Google Scholar]
- Horowitz M. Intrusive and repetitive thought after experimental stress. Arch Gen Psychiatry. 1975;32:1457–1463. doi: 10.1001/archpsyc.1975.01760290125015. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM. Hierarchies, similarity and interactivity in object recognition: ‘category specific’ neuropsychological deficits. Behav Brain Sci. 2001;24:453–509. [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. Neuroimage. 2007;36:924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J. Posterior parietal lobe of the primate brain. Physiol Rev. 1982;62:1060–1129. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: iI. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Ischebeck A, Indefrey P, Usui N, Nose I, Hellwig F, Taira M. Reading in a regular orthography: an fMRI study investigating the role of visual familiarity. J Cogn Neurosci. 2004;16:727–741. doi: 10.1162/089892904970708. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proc Natl Acad Sci. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TW, Gauthier I. Auditory and action semantic features activate sensory-specific perceptual brain regions. Curr Biol. 2003;13:1792–1796. doi: 10.1016/j.cub.2003.09.039. [DOI] [PubMed] [Google Scholar]
- James W. Principles of psychology. Vol. 1. New York: Dover Publications; 1890. The stream of consciousness; pp. 224–290. [Google Scholar]
- Jax SA, Buxbaum LJ, Moll AD. Deficits in movement planning and intrinsic coordinate control in ideomotor apraxia. J Cogn Neurosci. 2006;18:2063–2076. doi: 10.1162/jocn.2006.18.12.2063. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jessen F, Heun R, Erb M, Granath DO, Klose U, Papassotiropoulos A, Grodd W. The concreteness effect: evidence for dual-coding and context availability. Brain Lang. 2000;74:103–112. doi: 10.1006/brln.2000.2340. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. J Exp Psychol Gen. 1988;117:371–376. [PubMed] [Google Scholar]
- Johnson MR, Mitchell KJ, Raye CL, D'Esposito M, Johnson MK. A brief thought can modulate activity in extrastriate visual areas: top-down effects of refreshing just-seen visual stimuli. Neuroimage. 2007;37:290–299. doi: 10.1016/j.neuroimage.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TSP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Jonides J, Schumacher EH, Smith EE, Lauber E, Awh E, Minoshima S, Koeppe RA. The task-load of verbal working memory affects regional brain activation as measured by PET. J Cogn Neurosci. 1997;9:462–475. doi: 10.1162/jocn.1997.9.4.462. [DOI] [PubMed] [Google Scholar]
- Joseph JE. Functional neuroimaging studies of category specificity in object recognition: a critical review and meta-analysis. Cogn Affect Behav Neurosci. 2001;1:119–136. doi: 10.3758/cabn.1.2.119. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci. 2000;97:11793–11799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Kan IP, Wilson A, Thompson-Schill SL, Chatterjee A. Conceptual representations of action in the lateral temporal cortex. J Cogn Neurosci. 2005;17:1855–1870. doi: 10.1162/089892905775008625. [DOI] [PubMed] [Google Scholar]
- Kable JW, Lease-Spellmeyer J, Chatterjee A. Neural substrates for action event knowledge. J Cogn Neurosci. 2002;14:795–805. doi: 10.1162/08989290260138681. [DOI] [PubMed] [Google Scholar]
- Kan IP, Barsalou LW, Solomon KO, Minor JK, Thompson-Schill SL. Role of mental imagery in a property verification task: fMRI evidence for perceptual representations of conceptual knowledge. Cogn Neuropsychol. 2003;20:525–540. doi: 10.1080/02643290244000257. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur N, Barker S, Burrows EH, Ellison D, Brice J, Illis LS, Scholey K, Colbourn C, Wilson B, Loates M. Herpes simplex encephalitis—long-term magnetic resonance imaging and neuropsychological profile. J Neurol Neurosurg Psychiatry. 1994;57:1334–1342. doi: 10.1136/jnnp.57.11.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Rose R, Liddle PF, Zipursky RB, Brown GM, Stuss D, Houle S, Tulving E. The role of the left prefrontal cortex in verbal processing: semantic processing or willed action? Neuroreport. 1994;5:2193–2196. doi: 10.1097/00001756-199410270-00051. [DOI] [PubMed] [Google Scholar]
- Katai S, Maruyama T, Hashimoto T, Yanagisawa N. A case of cerebral infarction presenting as retrosplenial amnesia. Clin Neurol. 1992;32:1281–1287. [PubMed] [Google Scholar]
- Katz RB, Goodglass H. Deep dysphasia: analysis of a rare form of repetition disorder. Brain Lang. 1990;39:153–185. doi: 10.1016/0093-934x(90)90009-6. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K. Actions speak louder than functions: the importance of manipulability and action in tool representation. J Cogn Neurosci. 2003;15:30–46. doi: 10.1162/089892903321107800. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Lau WK, Polk M. The structural determinants of recovery in Wernicke's aphasia. Brain Lang. 1993;44:153–164. doi: 10.1006/brln.1993.1010. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Sheppard A, MacKenzie R. Localization in transcortical sensory aphasia. Arch Neurol. 1982;39:475–478. doi: 10.1001/archneur.1982.00510200017002. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex. II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 2007;502:810–833. doi: 10.1002/cne.21346. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–927. [PubMed] [Google Scholar]
- Kounios J, Koenig P, Glosser G, DeVita C, Dennis K, Moore P, Grossman M. Category-specific medial temporal lobe activation and the consolidation of semantic memory. Cogn Brain Res. 2003;17:484–494. doi: 10.1016/s0926-6410(03)00164-2. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo MLH, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Laine M, Rinne JO, Hiltunen J, Kaasinen V, Sipila H. Different brain activation patterns during production of animals versus artefacts: a PET activation study on category-specific processing. Cogn Brain Res. 2002;13:95–99. doi: 10.1016/s0926-6410(01)00095-7. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–1137. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans AC, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Dapretto M. Metaphorical vs. literal word meanings: fMRI evidence against a selective role of the right hemisphere. Neuroimage. 2006;29:536–544. doi: 10.1016/j.neuroimage.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Lehmann C, Vannini P, Wahlund L, Almkvist O, Dierks T. Increased sensitivity in mapping task demand in visuospatial processing using reaction-time-dependent hemodynamic response predictors in rapid event-related fMRI. Neuroimage. 2006;31:505–512. doi: 10.1016/j.neuroimage.2005.12.064. [DOI] [PubMed] [Google Scholar]
- Leichnitz GR. An intrahemispheric columnar projection between two cortical multisensory convergence areas (inferior parietal lobule and prefrontal cortex): an anterograde study in macaque using HRP gel. Neurosci Lett. 1980;18:119–124. doi: 10.1016/0304-3940(80)90313-4. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand DJ, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16:1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Levy DA, Bayley PJ, Squire LR. The anatomy of semantic knowledge: medial vs. lateral temporal lobe. Proc Natl Acad Sci USA. 2004;101:6710–6715. doi: 10.1073/pnas.0401679101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonetic perception. Cereb Cortex. 2005;15:1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- Lissauer H. Ein fall von Seelenblindheit nebst einem Beitrage zur Theorie derselben. Arch Psychiatr Nervenkr. 1889;21:2–50. [Google Scholar]
- Liu C, Hue C, Chen C, Chuang K, Liang K, Wang Y, Wu C, Chen J. Dissociated roles of the middle frontal gyri in the processing of Chinese characters. Neuroreport. 2006;17:1397–1401. doi: 10.1097/01.wnr.0000233090.00463.35. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Sheinberg DL. Visual object recognition. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- Lüders H, Lesser RP, Hahn J, Dinner DS, Morris HH, Wyllie E, Godoy J. Basal temporal language area. Brain. 1991;114:743–754. doi: 10.1093/brain/114.2.743. [DOI] [PubMed] [Google Scholar]
- Luria AR, Tsvetkova LS. The mechanism of “dynamic aphasia”. Found Lang. 1968;4:296–307. [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Kuwata T, Masuo O, Yamaga H, Okita R, Ozaki F, Moriwaki H, Roger P. Transcortical sensory aphasia due to a left frontal subcortical hemorrhage. Brain Inj. 1999;13:927–933. doi: 10.1080/026990599121124. [DOI] [PubMed] [Google Scholar]
- Maeshima S, Osawa A, Nakayama Y, Miki J. Transcortical sensory aphasia following infarction in the left frontal lobe. Eur Neurol. 2004;52:125–128. doi: 10.1159/000080273. [DOI] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Phil Trans Royal Soc Lond B. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. J Neurosci. 2003;23:5302–5307. doi: 10.1523/JNEUROSCI.23-12-05302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. Vision. San Francisco: Freeman; 1982. [Google Scholar]
- Marshall J, Pring T, Robson J, Chiat S. When ottoman is easier than chair: an inverse frequency effect in jargon aphasia. Brain Lang. 1998;65:78–81. doi: 10.1016/s0010-9452(08)70556-2. [DOI] [PubMed] [Google Scholar]
- Martin A, Caramazza A. Neuropsychological and neuroimaging perspectives on conceptual knowledge: an introduction. Cogn Neuropsychol. 2003;20:195–212. doi: 10.1080/02643290342000050. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory in the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/s0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Haxby JV, Lalonde FM, Wiggs CL, Ungerleider LG. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102–105. doi: 10.1126/science.270.5233.102. [DOI] [PubMed] [Google Scholar]
- Martin A, Ungerleider LG, Haxby JV. Category-specificity and the brain: the sensory-motor model of semantic representations of objects. In: Gazzaniga MS, editor. The new cognitive neurosciences. 2nd ed. Cambridge (MA): MIT Press; 2000. pp. 1023–1036. [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Masdeu JC, Schoene WC, Funkenstein H. Aphasia following infarction of the left supplementary motor area. Neurology. 1978;28:1220–1223. doi: 10.1212/wnl.28.12.1220. [DOI] [PubMed] [Google Scholar]
- Matsumoto A, Iidaka T, Haneda K, Okada T, Sadato N. Linking semantic priming effect in functional MRI and event-related potentials. Neuroimage. 2005;24:624–634. doi: 10.1016/j.neuroimage.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why are there complementary learning systems in the hippocampus and neocortex: insights from the success and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:409–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Crosson B, Valenstein E, Bowers D. Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase. 2001;7:407–417. doi: 10.1076/neur.7.5.407.16250. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, D'Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J Cogn Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Mesulam M. Patterns in behavioral neuroanatomy: association areas, the limbic system, and hemispheric specialization. In: Mesulam M, editor. Principles of behavioral neurology. Philadelphia (PA): F.A. Davis; 1985. pp. 1–70. [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (Area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Mitchell RL. The BOLD response during Stroop task-like inhibition paradigms: effects of task difficulty and task-relevant modality. Brain Cogn. 2005;59:23–37. doi: 10.1016/j.bandc.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Mohr JP. Broca's area and Broca's aphasia. In: Whitaker H, editor. Studies in neurolinguistics. New York: Academic Press; 1976. pp. 201–236. [Google Scholar]
- Moore CJ, Price CJ. A functional neuroimaging study of the variables that generate category specific object processing differences. Brain. 1999a;122:943–962. doi: 10.1093/brain/122.5.943. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Price CJ. Three distinct posterior basal temporal lobe regions for reading and object naming. Neuroimage. 1999b;10:181–192. doi: 10.1006/nimg.1999.0450. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11:2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J Neurophysiol. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci. 1998;10:766–777. doi: 10.1162/089892998563059. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJS. Generating ‘tiger’ as an animal name or a word beginning with T: differences in brain activation. Proc Roy Soc Lond B. 1996;263:989–995. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: a functional imaging perspective. Neuroimage. 1999;9:516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognitive psychology. New York: Appleton-Century-Crofts; 1967. [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer's disease) Eur J Neurosci. 2003;18:2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Newman SD, Just MA, Keller TA, Roth J, Carpenter PA. Differential effects of syntactic and semantic processing on the subregions of Broca's area. Cogn Brain Res. 2003;16:297–307. doi: 10.1016/s0926-6410(02)00285-9. [DOI] [PubMed] [Google Scholar]
- Ni W, Constable RT, Mencl WE, Pugh KR, Fullbright RK, Shaywitz SE, Shaywitz BA, Gore JC. An event-related neuroimaging study distinguishing form and content in sentence processing. J Cogn Neurosci. 2000;12:120–133. doi: 10.1162/08989290051137648. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Friston KJ, Price CJ. Effects of visual deprivation on the organization of the semantic system. Brain. 2003;126:1620–1627. doi: 10.1093/brain/awg152. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Josephs O, Kiebel S, Friston KJ, Price CJ. Action selectivity in parietal and temporal cortex. Cogn Brain Res. 2005;25:641–649. doi: 10.1016/j.cogbrainres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss HE, Stamatakis EA, Bright P, Mummery CJ, Price CJ. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–1147. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Functional imaging of the semantic system: retrieval of sensory-experienced and verbally learned knowledge. Brain Lang. 2003;84:120–133. doi: 10.1016/s0093-934x(02)00525-4. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of abstract semantics. Neuroimage. 2004;22:164–170. doi: 10.1016/j.neuroimage.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson K, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Okada T, Tanaka S, Nakai T, Nishizawa S, Inui T, Sadato N, Yonekura Y, Konishi J. Naming of animals and tools: a functional magnetic resonance imaging study of categorical differences in the human brain areas commonly used for naming visually presented objects. Neurosci Lett. 2000;296:33–36. doi: 10.1016/s0304-3940(00)01612-8. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychol Rev. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Orban GA, Van Essen DC, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn Sci. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Orfanidou E, Marslen-Wilson WD, Davis MH. Neural response suppression predicts repetition priming of spoken words and pseudowords. J Cogn Neurosci. 2006;18:1237–1252. doi: 10.1162/jocn.2006.18.8.1237. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Soma Y, Koyama A, Yoshimura N, Furukawa H, Tsuji S. Transcortical sensory aphasia following left frontal infarction. J Neurol. 1998;245:69–76. doi: 10.1007/s004150050180. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore ET. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Seltzer B, Cusick CG. Architectonics and cortical connections of the upper bank of the superior temporal sulcus in the rhesus monkey: an analysis in the tangential plane. J Comp Neurol. 2003;467:418–434. doi: 10.1002/cne.10932. [DOI] [PubMed] [Google Scholar]
- Paivio A. A factor-analytic study of word attributes and verbal learning. J Verb Learn Verb Behav. 1968;7:41–49. [Google Scholar]
- Paivio A. 1986. Mental representations: a dual-coding approach. New York: Oxford University Press. [Google Scholar]
- Pandya DN, Seltzer B. Intrinsic connections and architectonics of the posterior parietal cortex in the rhesus monkey. J Comp Neurol. 1982;204:204–210. doi: 10.1002/cne.902040208. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Rev Neurosci. 2007;8:976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RSJ. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, et al. A cultural effect on brain function. Nature Neurosci. 2000;3:91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- Penfield W, Roberts L. Speech and brain-mechanisms. New York: Atheneum; 1959. [Google Scholar]
- Perani D, Cappa SF, Bettinardi V, Bressi S, Gorno-Tempini M, Matarrese M, Fazio F. Different neural systems for the recognition of animals and man-made tools. Neuroreport. 1995;6:1637–1641. doi: 10.1097/00001756-199508000-00012. [DOI] [PubMed] [Google Scholar]
- Perani D, Schnur T, Tettamanti M, Gorno-Tempini M, Cappa SF, Fazio F. Word and picture matching: a PET study of semantic category effects. Neuropsychologia. 1999;37:293–306. doi: 10.1016/s0028-3932(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Noppeney U, Humphreys GW, Price CJ. Can segregation within the semantic system account for category-specific deficits? Brain. 2002;125:2067–2080. doi: 10.1093/brain/awf215. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Pilgrim LK, Fadili J, Fletcher P, Tyler LK. Overcoming confounds of stimulus blocking: an event-related fMRI design of semantic processing. Neuroimage. 2002;16:713–723. doi: 10.1006/nimg.2002.1105. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich MM, Gabrieli JDE. Relations between the neural basis of dynamic auditory processing and phonological processing: evidence from fMRI. J Cogn Neurosci. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pope KS, Singer JL. Regulation of the stream of consciousness: toward a theory of ongoing thought. In: Schwartz GE, Shapiro D, editors. Consciousness and self-regulation. New York: Plenum Press; 1976. pp. 101–135. [Google Scholar]
- Poremba A, Saunders RC, Sokoloff L, Crane A, Cook M, Mishkin M. Functional mapping of the primate auditory system. Science. 2003;299:568–572. doi: 10.1126/science.1078900. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Functional imaging studies of category-specificity. In: Forde EME, Humphreys G, editors. Category specificity in brain and mind. Hove, UK: Psychology Press; 2002. [Google Scholar]
- Price CJ, Mummery CJ, Moore CJ, Frackowiak RS, Friston KJ. Delineating necessary and sufficient neural systems with functional imaging studies of neuropsychological patients. J Cogn Neurosci. 1999;11:371–382. doi: 10.1162/089892999563481. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–797. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Neuroscience: the brain's dark energy. Science. 2006;314:1249–1250. [PubMed] [Google Scholar]
- Raichle ME, McLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapcsak SZ, Rubens AB. Localization of lesions in transcortical aphasia. In: Kertesz A, editor. Localization and neuroimaging in neuropsychology. San Diego: Academic Press; 1994. pp. 297–329. [Google Scholar]
- Reinholz J, Pollmann S. Differential activation of object-selective visual areas by passive viewing of pictures and words. Cogn Brain Res. 2005;24:702–714. doi: 10.1016/j.cogbrainres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE. An event-related fMRI investigation of implicit semantic priming. J Cogn Neurosci. 2003;15:1160–1175. doi: 10.1162/089892903322598120. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror–neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Robinson G, Blair J, Cipolotti L. Dynamic aphasia: an inability to select between competing verbal responses? Brain. 1998;121:77–89. doi: 10.1093/brain/121.1.77. [DOI] [PubMed] [Google Scholar]
- Roeltgen DP, Sevush S, Heilman KM. Phonological agraphia: writing by the lexical-semantic route. Neurology. 1983;33:755–765. doi: 10.1212/wnl.33.6.755. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci. 2001;13:829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Bullmore ET, Williams SCR, David AS. Brain activation during automatic and controlled processing of semantic relations: a priming experiment using lexical-decision. Neuropsychologia. 2001;39:1167–1176. doi: 10.1016/s0028-3932(01)00049-5. [DOI] [PubMed] [Google Scholar]
- Rossell SL, Price CJ, Nobre AC. The anatomy and time course of semantic priming investigated by fMRI and ERPs. Neuropsychologia. 2003;41:550–564. doi: 10.1016/s0028-3932(02)00181-1. [DOI] [PubMed] [Google Scholar]
- Rothi LJG, Ochipa C, Heilman KM. A cogn neuropsycholological model of limb praxis. Cogn Neuropsychol. 1991;8:443–458. [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114:349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Humphreys GW. Recognition by action: dissociating visual and semantic routes to action in normal observers. J Exp Psychol Human Percep Perform. 1998;24:631–647. doi: 10.1037//0096-1523.24.2.631. [DOI] [PubMed] [Google Scholar]
- Ruschmeyer S-A, Brass M, Friederici AD. Comprehending prehending: neural correlates of processing verbs with motor stems. J Cogn Neurosci. 2007;19:855–865. doi: 10.1162/jocn.2007.19.5.855. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Nixon PD, Passingham RE. Parietal cortex and movement I. Movement selection and reaching. Exp Brain Res. 1997;117:292–310. doi: 10.1007/s002210050224. [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2007;506:659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nature Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkissov SA, Filimonoff IN, Kononowa EP, Preobraschenskaja IS, Kukuew LA. Atlas of the cytoarchitectonics of the human cerebral cortex. Moscow: Medgiz; 1955. [Google Scholar]
- Schroeder CE, Foxe JJ. Multisensory convergence in early cortical processing. In: Calvert G, Spence C, Stein BE, editors. The handbook of multisensory processes. Cambridge (MA): MIT Press; 2004. pp. 295–309. [Google Scholar]
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26:100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 1988;8:4049–4068. doi: 10.1523/JNEUROSCI.08-11-04049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Some cortical projections to the parahippocampal gyrus in the rhesus monkey. Exp Neurol. 1976;50:146–160. doi: 10.1016/0014-4886(76)90242-9. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Further observations on parieto-temporal connections in the rhesus monkey. Exp Brain Res. 1984;55:301–312. doi: 10.1007/BF00237280. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989;281:97–113. doi: 10.1002/cne.902810108. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Sethi NK, Burke L, Torgovnick J, Arsura E. Transcortical sensory aphasia as a result of left frontal cortical–subcortical infarction. A case report. Eur Neurol. 2007;57:52–53. doi: 10.1159/000097012. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Meizin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simmons A, Miller D, Feinstein JS, Goldberg TE, Paulus MP. Left inferior prefrontal cortex activation during a semantic decision-making task predicts the degree of semantic organization. Neuroimage. 2005;28:30–38. doi: 10.1016/j.neuroimage.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Ramjee V, Beauchamp MS, McRae K, Martin A, Barsalou LW. A common neural substrate for perceiving and knowing about color. Neuropsychologia. 2007;45:2802–2810. doi: 10.1016/j.neuropsychologia.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: I. During cognitive task performance. Proc Natl Acad Sci. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JL. Experimental and theoretical studies of consciousness. Chichester: John Wiley & Sons; 1993. Experimental studies of ongoing conscious experience; pp. 100–122. [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18:633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA. Components of verbal working memory: evidence from neuroimaging. Proc Natl Acad Sci. 1998;95:876–882. doi: 10.1073/pnas.95.3.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Goulding PJ, Neary D. Semantic dementia: a form of circumscribed temporal atrophy. Behav Neurol. 1989;2:167–182. [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, Kamo H, Ohkawa Y, Akiguchi I, Kimura J. A case of retrosplenial amnesia. Brain. 1991;110:331–333. [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT. Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta-analysis. Hum Brain Mapp. 2005;25:83–91. doi: 10.1002/hbm.20134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Saito H, Fukada Y, Moriya M. Coding visual images of objects in the inferotemporal cortex of the macaque monkey. J Neurophysiol. 1991;66:170–189. doi: 10.1152/jn.1991.66.1.170. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Dritschel BH, Taylor MJ, Proctor L, Lloyd CA, Nimmo-Smith I, Baddeley AD. Stimulus-independent thought depends on central executive resources. Mem Cognit. 1995;23:551–559. doi: 10.3758/bf03197257. [DOI] [PubMed] [Google Scholar]
- Teasdale JD, Proctor L, Lloyd CA, Baddeley AD. Working memory and stimulus-independent thought: effects of memory load and presentation rate. Eur J Cogn Psychol. 1993;5:417–433. [Google Scholar]
- Tettamanti M, Buccino G, Saccuman MC, Gallese V, Danna M, Scifo P, Fazio F, Rizzolatti G, Cappa SF, Perani D. Listening to action-related sentences activates fronto-parietal motor circuits. J Cogn Neurosci. 2005;17:273–281. doi: 10.1162/0898929053124965. [DOI] [PubMed] [Google Scholar]
- Thioux M, Pesenti M, Costes N, De Volder A, Seron X. Task-independent semantic activation for numbers and animals. Cogn Brain Res. 2005;24:284–290. doi: 10.1016/j.cogbrainres.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Aguirre GK, D'Esposito M, Farah MJ. A neural basis for category and modality specificity of semantic knowledge. Neuropsychologia. 1999;37:671–676. doi: 10.1016/s0028-3932(98)00126-2. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Werner C, Weiss PH, Fink GR. Stimulus properties matter more than perspective: an fMRI study of mental imagery and silent reading of action phrases. Neuroimage. 2007;36:T128–T141. doi: 10.1016/j.neuroimage.2007.03.035. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35:1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Kemmerer D, Adolphs R, Damasio H, Damasio AR. Neural correlates of conceptual knowledge for actions. Cogn Neuropsychol. 2003;20:409–432. doi: 10.1080/02643290244000248. [DOI] [PubMed] [Google Scholar]
- Tregallas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage. 2006;32:307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Bright P, Dick P, Tavares P, Pilgrim LK, Fletcher P. Do semantic categories activate distinct cortical regions? Evidence for a distributed neural semantic system. Cogn Neuropsychol. 2003;20:541–559. doi: 10.1080/02643290244000211. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Russell R, Fadili J, Moss HE. The neural representation of nouns and verbs: PET studies. Brain. 2001;124:1619–1634. doi: 10.1093/brain/124.8.1619. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Stamatakis EA, Dick E, Bright P, Fletcher P, Moss HE. Objects and their actions: evidence for a neurally distributed semantic system. Neuroimage. 2003;18:542–557. doi: 10.1016/s1053-8119(02)00047-2. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110:1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The parahippocampal gyrus: new observations regarding its cortical connections in the monkey. Trends Neurosci. 1982;5:345–350. [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Vandenbulcke M, Peeters R, Fannes K, Vandenberghe R. Knowledge of visual attributes in the right hemisphere. Nature Neurosci. 2006;9:964–970. doi: 10.1038/nn1721. [DOI] [PubMed] [Google Scholar]
- Varney NR, Damasio H. Locus of lesion in impaired pantomime recognition. Cortex. 1987;23:699–703. doi: 10.1016/s0010-9452(87)80061-8. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. Neuroimage. 2006;30:1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal–parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Erg ebrisse unserer Hirnforschung. J Psychol Neurol. 1919;25:279–461. [Google Scholar]
- von Bonin G. The evolution of the human brain. Chicago: University of Chicago Press; 1962. [Google Scholar]
- von Bonin G, Bailey P. The neocortex of macaca mulatta. Urbana (IL): University of Illinois Press; 1947. [Google Scholar]
- von Economo K, Koskinas G. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Vienna: Springer; 1925. [Google Scholar]
- von Kriegstein K, Eger E, Kleinschmidt A, Giraud AL. Modulation of neural responses to speech by directing attention to voices or verbal content. Cogn Brain Res. 2003;17:48–55. doi: 10.1016/s0926-6410(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cereb Cortex. 2000;10:1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides semantic retrieval. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Lund TE, Ostergaard S, Ostergaard L, Roepstorff A. Motion verb sentences activate left posterior middle temporal cortex despite static context. Neuroreport. 2005;16:649–652. doi: 10.1097/00001756-200504250-00027. [DOI] [PubMed] [Google Scholar]
- Wallentin M, Østergaarda S, Lund TE, Østergaard L, Roepstorff A. Concrete spatial language: see what I mean? Brain Lang. 2005;92:221–233. doi: 10.1016/j.bandl.2004.06.106. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Agnosia: the impairment of object recognition. In: Frederiks JAM, editor. Handbook of clinical neurology. New York: Elsevier; 1985. pp. 333–349. [Google Scholar]
- Warrington EK. Concrete word dyslexia. Br J Psychol. 1981;72:175–196. doi: 10.1111/j.2044-8295.1981.tb02175.x. [DOI] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exp Psychol. 1975;27:635–657. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110:1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Multiple meaning systems in the brain: a case for visual semantics. Neuropsychologia. 1994;32:1465–1473. doi: 10.1016/0028-3932(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107:829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Wernicke C. Der aphasische Symptomenkomplex. Breslau: Cohn & Weigert; 1874. [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40:174–186. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Verret L, Fung D, Chertkow H. Common and contrasting areas of activation for abstract and concrete concepts: an H2 15O PET study. J Cogn Neurosci. 2004;16:1211–1226. doi: 10.1162/0898929041920540. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci. 2005;17:1871–1885. doi: 10.1162/089892905775008689. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor P. Connectivity among semantic associates: an fMRI study of semantic priming. Brain Lang. 2006;97:294–305. doi: 10.1016/j.bandl.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Wise RJS, Howard D, Mummery CJ, Fletcher P, Leff A, Büchel C, Scott SK. Noun imageability and the temporal lobes. Neuropsychologia. 2000;38:985–994. doi: 10.1016/s0028-3932(99)00152-9. [DOI] [PubMed] [Google Scholar]
- Wise RSJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within ‘Wernicke's area’. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Zhang JX, Wang X, Wu R, Hu X, Weng X, Tan LH. Differential activity in left inferior frontal gyrus for pseudowords and real words: an event-related fMRI study on auditory lexical decision. Hum Brain Mapp. 2005;25:212–221. doi: 10.1002/hbm.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Wattanabe O, Ono Y. Dissociation between semantic and autobiographical memory: a case report. Cortex. 1997;33:623–638. doi: 10.1016/s0010-9452(08)70721-4. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Nishikawa T, Tokunaga H, Yoshiyama K, Nakagawa Y, Ikejiri Y, Oku N, Hashikawa K, Tanabe H, Shinozaki K, et al. The neural basis of perceptual and conceptual word priming – a PET study. Cortex. 2000;36:59–69. doi: 10.1016/s0010-9452(08)70836-0. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Evans AC, Meyer E, Gjedde A. Lateralization of phonetic and pitch discrimination in speech processing. Science. 1992;256:846–849. doi: 10.1126/science.1589767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.