Abstract
Whether normal word reading includes a stage of visual processing selectively dedicated to word or letter recognition is highly debated. Characterizing pure alexia, a seemingly selective disorder of reading, has been central to this debate. Two main theories claim either that 1) Pure alexia is caused by damage to a reading specific brain region in the left fusiform gyrus or 2) Pure alexia results from a general visual impairment that may particularly affect simultaneous processing of multiple items. We tested these competing theories in 4 patients with pure alexia using sensitive psychophysical measures and mathematical modeling. Recognition of single letters and digits in the central visual field was impaired in all patients. Visual apprehension span was also reduced for both letters and digits in all patients. The only cortical region lesioned across all 4 patients was the left fusiform gyrus, indicating that this region subserves a function broader than letter or word identification. We suggest that a seemingly pure disorder of reading can arise due to a general reduction of visual speed and span, and explain why this has a disproportionate impact on word reading while recognition of other visual stimuli are less obviously affected.
Keywords: fusiform gyrus, number reading, reading, theory of visual attention, visual word form area
Pure alexia is an acquired reading disorder that leaves writing unaffected. Pure alexic reading is usually slow with single-word reading characterized by a pronounced word length effect; reaction times (RTs) in reading increase linearly with word length, often with hundreds of milliseconds per letter (Behrmann, Plaut, and Nelson 1998). Theories of pure alexia can be roughly divided into 2 groups: 1) Domain-specific accounts, suggesting that pure alexia arises due to damage to a cognitive system or cerebral area specialized for recognizing visual word forms (Warrington and Shallice 1980; Warrington and Langdon 1994; Cohen et al. 2003; Cohen and Dehaene 2004); 2) General visual accounts, claiming that pure alexia reflects a general deficit in visual perception (Farah and Wallace 1991; Behrmann, Nelson, and Sekuler 1998), often conceptualized as a primary impairment in simultaneous perception or parallel processing (Farah, 2004). The question of the relative selectivity of pure alexia is of great theoretical interest, as it bears on the issue of whether specialized perceptual brain areas can develop through learning, and whether normal reading includes a stage of processing selectively dedicated to visual letter recognition. This question has received a lot of attention in the neuroimaging literature, where the role of the putative visual word form area (VWFA) in normal reading is highly debated. Some argue that the VWFA, which is located the left mid-fusiform gyrus, is specialized for processing of letters and words (Cohen and Dehaene 2004), whereas others argue that this area is also involved in visual processing of other stimulus categories (Devlin et al. 2006; Joseph et al. 2006; Starrfelt and Gerlach 2007), and may even be involved in nonvisual tasks (Price and Devlin 2003). Lesions of the VWFA are thought to be important in causing pure alexia (Cohen et al. 2003; Cohen and Dehaene 2004; Leff et al. 2006, although see Hillis et al. 2005).
The 2 accounts of pure alexia predict different performance in tasks with 1) alphabetical versus nonalphabetical material; and 2) visual displays of single versus multiple items. Although a pure alphabetical deficit should affect letter and word processing only, a general visual deficit should affect visual recognition of other visual stimuli as well. A deficit in simultaneous perception should affect perception of multiple visual items regardless of stimulus category, whereas perception of single stimuli may be left intact. These predictions can be formally tested within the framework of a Theory of Visual Attention (TVA) (Bundesen 1990; Bundesen et al. 2005). This framework has proven effective for characterizing visual deficits after different types of brain damage (Duncan et al. 1999; Peers et al. 2005; Finke et al. 2006). TVA-based studies have been shown to be highly sensitive, as they can reveal subclinical visual deficits not evident on standard clinical tests (Habekost and Rostrup 2006), and highly specific, in that specific components of visual perception and attention can be singled out in TVA-based analyses (Duncan et al. 2003; Habekost and Rostrup 2007). Two measures of visual capacity, “processing speed” (the number of items processed per second) and the “visual apprehension span” (the maximum number of items that can be recognized in one view), can be modeled within this framework, and these 2 parameters can be assessed for different stimulus types. To test competing theories of pure alexia, we chose to investigate the visual capacity and stimulus specificity in 4 patients with this disorder using TVA-based assessment. Our first aim is to characterize the possible stimulus selectivity of pure alexia, whether only letter identification is affected, or if digit recognition is compromised also. Although letters and digits are visually similar and may be grouped as “alphanumeric symbols,” reading of either letters or digits can be selectively affected following damage to more central (i.e., nonperceptual) reading processes (Anderson et al. 1990; Cipolotti 1995; Starrfelt 2007). There is also behavioral evidence that letters and numbers are processed differently (Hamilton et al. 2006). A line of studies by Polk and Farah (1995, 1998) and Polk et al. (2002) has suggested that a dissociation between reading of letters and digits might also arise in the visual domain, but this suggestion has so far not been tested with patients. In addition, word-specific accounts (Gaillard et al. 2006) imply that pure alexia is specific to alphabetical material (words and/or letters), and comparing performance with letters and digits seems to be a stringent test of this hypothesis. Our second aim is to investigate whether pure alexia can be attributed to an impairment in simultaneous perception, that is, whether our patients' performance depends on the number of stimuli (one vs. many) in a display. Left posterior lesions may lead to deficits in simultaneous perception (Warrington and Rabin 1971), and the ventral type of simultanagnosia has been suggested as the cause of pure alexia (Farah 1990). However, not all patients with left posterior lesions have pure alexia (Binder and Mohr 1992; Leff et al. 2006), or deficits in simultaneous processing (Habekost and Starrfelt 2006) and the question of the relation between reading and simultaneous perception remains largely unresolved.
Methods
Subjects
Four patients with pure alexia participated in this investigation; all had English as their first language. Demographic and basic neuropsychological measures are presented in Table 1. None of the patients had any history of dyslexia, visual problems, psychiatric or neurological disease prior to their stroke/intracerebral hemorrhage, and all had normal or corrected to normal central visual acuity at the time of the investigation. In the background tests of reading, picture recognition, and auditory span, 6 control subjects were tested (3 female). Their mean age was 61 years (standard deviation, SD = 14). All had English as their first language. In the experimental tasks, 10 control subjects were tested (5 female), of whom 5 were British and 5 Danish. For this experimental control group, the mean age was 55 (SD = 11). None of the controls had any history of dyslexia, visual problems, psychiatric or neurological disease, and all had normal or corrected to normal vision. All control subjects were fully right handed, as assessed with the Edinburgh Handedness Inventory (mean LQ = + 100, Oldfield, 1971).
Table 1.
Background data for patients
| TJ | JT | BA | JH | |
| Age | 66 | 52 | 52 | 32 |
| Education | 3 | 0 | 6 | 8 |
| Handedness | +100 | +100 | +100 | −60 |
| WASI-2 IQ | 123 | 96 | 95 | 117 |
| Time since injury | 1.5 years | 5.5 years | 7.3 years | 3.3 years |
| Etiology | Infarct | Infarct | Intrahemispheric hematoma caused by head Injury | Intracerebral hemorrhage caused by AVM |
Note: Education refers to years of schooling after primary education. Handedness was assessed with Edinburgh Handedness Inventory (Oldfield 1971). Note that patient JH is left handed. WASI IQ is based on the 2-subtest form (Wechsler 1999).
To control for the nonspecific effects of a left posterior stroke on the experimental variables, we also include previously published data from a patient (NT) with a ventral left posterior stroke but who did not have pure alexia. Instead, he suffered from a mild form of hemianopic alexia. Background data as well as NT's pattern of performance on tests of reading and visual perception and attention are described in detail in an earlier publication (Habekost and Starrfelt 2006). NT's scores, as compared with a group of normal controls matched to him for age and education, will be presented for comparison where the relevant data are available.
Each subject gave informed consent to participate in the study that was approved by an NHS local research ethics committee (Royal Free Hospital). The Danish controls tested in the experimental investigation provided written informed consent according to the Helsinki Declaration to participate in the study and approval was given by ethical committees in Copenhagen (project no. KF 01-258988).
Visual Field Tests
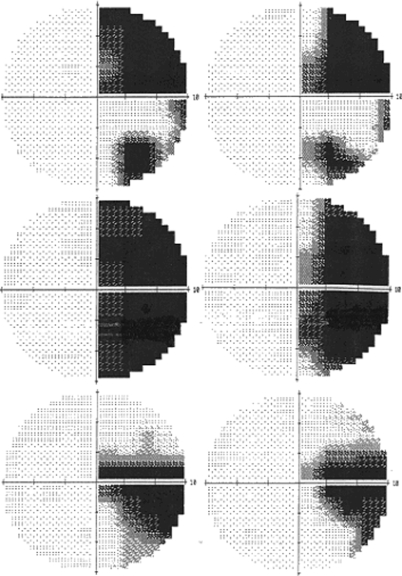
Three of the patients had static fields measured using the automated Humphrey field analyzer II (Carl Zeiss Group, CA) analysis of the central 10 degrees of vision (central 10-2 threshold test), as part of a previous experiment. We used a brief computerized binocular perimetry test to check the status of the patients' visual fields at the time of the current experimental investigation, using a program developed by Kasten et al. (1998). This confirmed that patient TJ had normal light sensitivity in the entire visual field (he correctly responded to 125/125 stimuli). The other 3 patients' perimetry remained unchanged so the more sensitive 10 degree fields are reported and are shown in Figure 1. JH has an incongruous, horizontal, homonymous sectoranopia with 2 degrees of sparing in the lower field of the better (right) eye. JT has a homonymous, predominantly upper, hemianopia with 8 degrees of sparing in the lower field. BA has a complete macular splitting homonymous hemianopia. NT (hemianopic control patient) has a homonymous, upper, quadrantopia that encroaches into parafoveal but not foveal vision.
Figure 1.
Ten degree static perimetry is shown for the 3 patients with visual field defects. JT top, BA middle, and JH bottom. Left eye fields on the left.
Magnetic Resonance Imaging Brain Scans
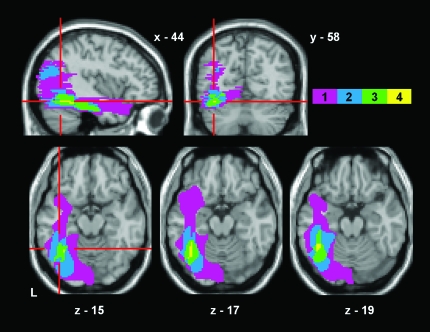
A single T1 weighted magnetic resonance imaging (MRI) brain scan was obtained for each subject. 1.5-T scanners were used at 2 different locations, both protocols collected data in 1-mm3 isotropic voxels. In order to produce a lesion overlap map, the images were spatially normalized using SPM5 software (http://fil.ion.ucl.ac.uk/spm). The unified segmentation algorithm was chosen as this has the best performance for lesioned brains (Crinion et al. 2007). After spatial normalization, the resultant images were imported into another software package, MRIcro (http://www.psychology.nottingham.ac.uk/staff/cr1/mricro.html), for lesion identification. Lesions were outlined by eye by one of us (APL), transformed into binary images and then overlaid on the single-subject canonical brain image available in SPM5. The resultant image was displayed at the mean coordinates for the VWFA as estimated by a meta-analysis of 27 functional imaging data sets (Jobard et al. 2003); see Figure 2. The only lesioned voxels common to all 4 patients are at the junction between the mid and posterior portion of the left fusiform gyrus, corresponding to the putative VWFA.
Figure 2.
Lesion maps from all 4 pure alexic patients have been overlaid and superimposed on a canonical single-subject MRI brain scan in MNI space. The colored scale refers to the number of voxels in common across the patients, with yellow voxels being common to all 4. All axial slices containing yellow voxels are shown (total volume of 100% overlap = 32 voxels or 256 mm3). The red crosshairs converge on the peak voxel identified in a meta-analysis as being at the center of the VWFA (−44 −58 −15). L = left.
NT's images have been published (see Habekost and Starrfelt 2006). He suffered a primary intracerebral hemorrhage that damaged the inferior and lateral part of the occipital lobe, with some extension into the posterior part of the temporal lobe. The lesion centers on the inferior and fourth occipital gyri (O3, O4), with the lingual gyrus (O5) and striate cortex (V1) spared. More anteriorly, the medial part of the posterior portion of the fusiform gyrus is just affected. The voxel at the center of the lesion overlap in the 4 pure alexic patients (−44 −58 −15, see Figure 2) is spared in NT; indeed, working posteriorly from this coordinate, the lesion does not appear until y = −78 (2 cm posterior to this point).
Statistical Analysis
To statistically compare patient performance in the behavioral and experimental tests with the control groups, we used 2 strategies. First, we compared the group of patients with the group of controls using independent samples t-tests as implemented in the SPSS software package (version 15.0). Second, to analyze individual patients' scores compared with the control group, we used a test devised by Crawford and Garthwaite (2002) and the accompanying software. Crawford and Garthwaite's test is based on the t-distribution rather than the standard normal distribution, which makes the test more appropriate for evaluating single-case results against control groups of limited size. The test has proven to be more statistically robust than the standard comparison to z-scores (see, e.g., Crawford and Garthwaite 2002) and has been used widely in neuropsychological single-case research. All reported P values are 1-tailed, unless otherwise specified.
Background Behavioral Measures
Reading and Writing
We first established the patients' reading deficit with a computerized word reading test, using a voice key attached to serial response box to measure RTs. Words were 3, 5, and 7 letters in length (25 examples of each), matched for frequency. Mean frequencies (SD in parentheses) from Kucera and Francis (1967) for 3, 5, and 7 letters words were 99 (85), 101 (95), and 100 (54), respectively. All words were selected from Osswald et al. (2002, Appendix A). Words were presented centrally on a computer screen in 36 point Times New Roman (white letters on a black background), one at a time. Errors were recorded by the experimenter. The interval between response and presentation of the next stimulus was 2 s. Subjects were instructed to read the words as quickly and accurately as possible, and the initiation of a verbal response terminated the presentation of the words and triggered the voice key. A practice version with 10 trials was administered first. Writing ability was assessed with subtests 24–26 from the Comprehensive Aphasia Test (Swinburn et al. 2004).
Object Naming and Object Decision
Object recognition was tested with a computerized naming task and an object decision task. For naming, 40 black and white line drawings from the set of Snodgrass and Vanderwart (1980) were presented centrally on a computer screen. The pictures subtended 3–5° of visual angle and remained on screen until the subject made a response. Subjects were asked to name the pictures as quickly and accurately as possible. RTs from picture onset were measured with a voice key. The interval between response and presentation of the next stimulus was 2 s. A practice version with 6 pictures was administered before the actual task.
For object decision, 40 black and white line drawings taken from the set of Snodgrass and Vanderwart (1980), and 40 nonobjects taken from the set of Lloyd Jones and Humphreys (1997) were presented centrally on a computer screen. Subjects were asked to decide if the stimulus represented a real object or a nonsense object. The nonobjects were chimeric line drawings of closed figures, constructed by exchanging single parts belonging to objects from the same category, which makes the discrimination between real objects and nonobjects quite demanding (see Gerlach et al. 2004). The pictures subtended 3–5° of visual angle and were presented until a response was made on a serial response box (index finger for real object, middle finger for nonobject). Subjects were instructed to respond as fast and as accurately as possible. A practice version with 16 stimuli was performed before the main task.
Auditory Digit and Letter Span
As Experiment 2 involved testing the limits of subjects’ visual short-term memory, we wanted to test whether a general reduction of short-term memory was present. We therefore tested the subjects’ short-term memory in a separate modality, audition, using both letters and digits (in separate blocks). All subjects received the digit version first. Stimuli were the same as in the following experiments; digits 0–9 and letters A–J. Sequences of 3–7 items (4 sequences in each condition) were read out, and the subject was asked to repeat the presented sequence. The items were presented approximately 1 per second, and the same item never appeared twice in the same sequence. Maximum total score in this test is 20 (4 sequences by 5 conditions). The maximum number of items repeated (maximum span = 7) was also scored.
Measurement of Visual Processing Speed and Apprehension Span
Mathematical modeling based on the TVA (Bundesen, 1990) enables performance on simple psychophysical tasks (single stimulus report, whole report, and partial report) to be analyzed into different functional components. The method is theoretically well founded, and the different parameters are clearly defined (Bundesen 1990; Bundesen et al. 2005). Two parameters are of special interest in the present investigation, the speed of visual processing C and the visual apprehension span K. In addition, the perceptual threshold t0 is measured, but this parameter is of less theoretical relevance here.
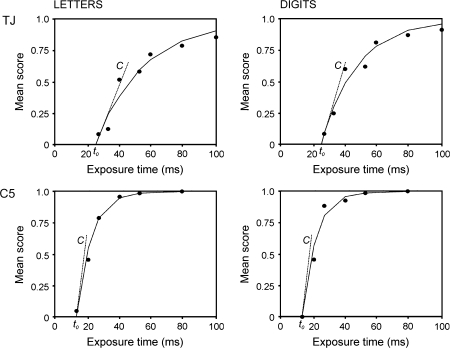
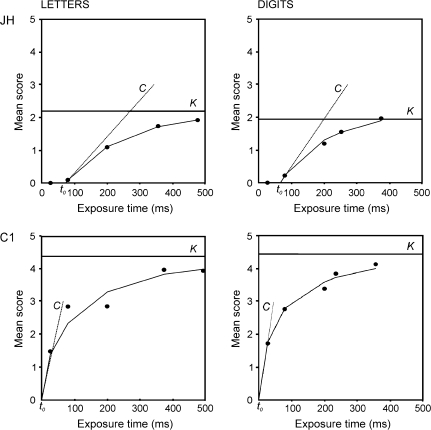
In single stimulus report experiments, the speed of visual processing C and the perceptual threshold t0 can be measured. At each trial, a single stimulus (e.g., a letter) is presented and then backward masked, and this is repeated for many trials at varying exposure durations. Subjects are instructed to report what they see, responses are unspeeded. Thus, the main test results are based on accuracy of performance at different exposure durations rather than measurement of reaction times, and therefore naming latency does not affect test scores. Exposure times (x-axis) are plotted against mean identification scores (y-axis), that is, how many times, on average, the subject is able to correctly identify the stimulus. A maximum likelihood curve is fitted to the data and the 2 TVA parameters (t0 and C) are calculated from this. The t0 is an extrapolated value of where this curve crosses the x-axis; it is an estimation of the period of exposure time (usually 10–20 ms in normal subjects), at or below which the subject is unable to report any items. C—processing speed—is taken as the slope of the curve at this point, its units are in s−1 and it is best conceived of as a measure of efficiency of visual recognition (the rate at which, as exposure time increases, the subject is able to report the stimulus better). Whole report paradigms, where subjects have to report elements from a display of multiple, unrelated stimuli (usually 5) also allow for the estimation of K—the visual apprehension span. K is calculated from the estimated asymptote of the subject's response data and corresponds to the maximum ability to perceive multiple items in one view. Only exposure durations below 200 ms are commonly used in whole report experiments, to prevent eye movements and serial encoding of items. If the stimulus display is not followed by a mask, the effective exposure duration is prolonged for several hundred ms (due to the visual afterimage), which is convenient for testing participants with relatively slow encoding rates. The prolongation of the effective exposure time can be modeled by TVA analysis (parameter μ). For a graphical example of how the main parameters are calculated, see Figures 5 and 6. Note that in Figure 6, exposure durations up to 500 ms are shown. These represent an unmasked exposure duration of 200 ms + μ.
Figure 5.
Plots to show how t0 and Ccentral are calculated for a normal control (C5) and a patient with pure alexia (TJ), from Experiment 1. See Table 3 for parameter estimates.
Figure 6.
Plots to show how t0 and Cperipheral and K are calculated for a normal control (C1) and a patient with pure alexia (JH), from Experiment 2. See Table 4 for parameter estimates.
We used 2 experimental types in our investigation: single stimulus report with central presentation (Experiment 1) and whole report with peripheral presentation (Experiment 2), both with 2 types of stimuli: letters and digits. In order to make the stimulus sets as similar as possible, we chose to use only 10 letters as there are only 10 digits. To make the letters as easy to remember as possible, the first 10 letters of the alphabet were chosen. The stimuli were computer generated and did not conform to a canonical typefont. An efficient mask was generated by superimposing all letters and digits, as well as 2 mirror images (1 “flipped” across the horizontal axis, 1 across the vertical). The stimulus sets and the mask are shown in Figure 3. In both experiments, a printed version of the relevant stimulus set (letters or digits) was placed in front of the subjects. Before each session, they were encouraged to name the printed stimuli one by one, and the patients could name the letters and digits without making errors. Both experiments were conducted in a semidarkened room, and subjects were seated approximately 100 cm from a 19” CRT monitor capable of 150 refreshes/s (6.7-ms resolution).
Figure 3.
Stimuli and mask used in Experiments 1 and 2.
Experiment 1: Single Stimulus Report of Letters and Digits
This experiment was designed to measure visual processing speed, C, and perceptual threshold, t0, for single letters or digits presented at the center of the visual field. Testing of letters and digits was performed in separate blocks. All subjects received 3 sessions with 2 blocks (letters and digits), in an ABBAAB design (digits first), interleaved with the other tests. To obtain highly reliable estimates of each TVA parameter, patients performed 288 repetitions for both the letter and digit version of the experiment, divided into 3 testing sessions (576 trials in total; in addition, 10 practice trials were included at the start of each session). For TJ, the number of trials in Experiment 1 was reduced to 264 × 2, because he also completed a second run of Experiment 2 in his right visual field. Controls performed either 288 or 360 repetitions per stimulus set. The first session included the same exposure durations for all subjects. In the second and third sessions, between 6 and 9 individual exposure durations were set to obtain the best TVA estimates, and these individually calibrated exposure durations ranged from 7 to 200 ms. Thus, subjects received a varying number of trials per exposure duration, and exposure durations varied between subjects but, importantly, this does not bias the TVA analysis. Within each testing block different exposure durations were chosen randomly from the individually set values, aiming to characterize the full performance span from floor to ceiling scores. In each trial, a single white letter or digit was chosen randomly from the set of 10 stimuli and flashed on a black background at the center of fixation. The stimulus was immediately followed by a white pattern mask, which remained on for 500 ms. Stimuli and mask subtended 1 × 1.5 degrees of visual angle. Participants were instructed to report the identity of the letter or digit only if “fairly certain.” Reports were unspeeded. To ensure central fixation before each trial, participants were required to focus on a centrally placed cross and indicate verbally when they were ready. Eye movements were monitored by the experimenter online. None of the subjects had any problems maintaining central fixation.
The best-fitting TVA parameter values to the observed data of each participant were estimated by a maximum likelihood algorithm. The model fitting procedure was the same as in previous TVA-based patient studies (see Duncan et al. 1999; Kyllingsbaek 2006 for mathematical details), but improved by a new fitting algorithm that corrects the TVA estimates for the influence of guessing. Using this modeling procedure, the TVA parameters Ccentral and t0 were estimated (separately for letters and digits).
For comparison, TVA estimates based on data from a single letter report task for patient NT and a group of age and education matched controls are also presented. These were based on a similar experiment using a slightly different stimulus set and modeling procedure (see Habekost and Starrfelt 2006 for details).
Experiment 2: Whole Report of Letters and Digits
This experiment was designed to measure the patients' ability to perceive multiple independent stimuli at the same time. This corresponds to the TVA parameter K, the visual apprehension span. The K parameter is best estimated by whole report experiments in which multiple, typically 5, unrelated stimuli are shown for variable exposure durations (which also allows for estimation of the visual processing speed, C). In order to display many items without crowding effects, the stimuli were placed in the peripheral visual field (thus the C measure in this experiment is termed Cperipheral). Because of the visual field deficits evidenced by 3 of the patients, presentations were limited to the left side for these subjects. Central fixation was controlled in the same way as in Experiment 1; again there was no indication that any of the subjects had difficulties maintaining central fixation. Note that the exposure durations were too brief for eye movements to be conducted between stimulus onset and offset. TJ and 4 controls (mean age 65, range 59–69) also performed a version of this task with stimuli presented in the right visual field, after the other experiments were completed. In alternating test blocks either 5 letters or 5 digits were chosen from the stimulus sets used in Experiment 1, and presented on the screen for 30–200 ms followed by either a blank screen (so that the effective exposure duration was prolonged by a visual afterimage) or by 5 bright pattern masks presented for 500 ms. Stimulus selection was random without replacement, so that the same letter/digit would never appear twice in the same display. Stimuli were shown at 5 locations at the circumference of an imaginary circle with a radius of 5 degrees centered on fixation. Similar to Experiment 1, the instruction was to report, unspeeded, the items the subject was fairly certain of having seen. For each of the 5 exposure durations (30, 80, 200, 30 ms + afterimage, 200 ms + afterimage; randomly intermixed) 24 repetitions were performed (i.e., 120 trials for each of the 2 stimulus sets), divided into 2 testing blocks (60 trials in each). Five controls performed an additional 105 trials per stimulus set. Blocks were presented in an ABBA design (letters first).
The data analysis was performed using the same TVA model fitting software as in Experiment 1. For each stimulus type 2 main parameters were estimated: visual apprehension span, K, and visual processing speed, Cperipheral (defined as the sum of the processing speeds at each of the 5 stimulus locations). The K parameter was estimated using noninteger values to improve the data fits. For example, a K value of 3.3 represents a probability mixture of visual short-term memory capacity at 3 and 4 elements, occurring with 70% and 30% probability, respectively.
NT and a group of age and education matched controls were tested using a slightly different whole report paradigm (they were tested in both visual fields within the same testing block, the stimulus set was larger, and guessing was controlled for by instruction rather than analytically; see Habekost and Starrfelt 2006 for details), which means that the model parameters are not directly comparable between experiments. However, as NT's scores were compared with a control group who performed the same experiment as him, the normality of his scores can be evaluated; the estimates of his K and Cperipheral for letters are therefore included.
Results
Background Behavioral Measures
Errors and mean RTs for patients and controls are presented in Table 2.
Table 2.
Results from the reading test, naming task, and object decision task, as well as the test of auditory letter and digit span for patients and controls (N = 6)
| TJ | JT | BA | JH | Control mean (SD) | |
| Reading mean RT | 1388** | 912** | 1351** | 1562** | 438 (43) |
| Reading errors | 7** | 14** | 2 | 2 | 1.2 (1.2) |
| Naming RT | 1189** | 1331** | 1732** | 1168** | 753 (91) |
| Naming errors | 1 | 3 | 4 | 0 | 1.7 (1.2) |
| Object decision RT | 1985** | 1001 | 1226* | 907 | 818 (176) |
| Object decision errors | 8 | 6* | 9 | 12 | 10.8 (1.5) |
| Digit span—max | 6 | 5* | 6 | 7 | 6.7 (0.82) |
| Digit span—total | 16 | 10 | 15 | 20 | 17.8 (2.9) |
| Letter span—max | 5 | 5 | 5 | 6 | 6.5 (0.84) |
| Letter span—total | 10 | 10 | 9 | 12 | 13.5 (3.45) |
Note: RTs reported in milliseconds.
*P < 0.05; **P < 0.01, Crawford and Garthwaite's test.
Reading and Writing
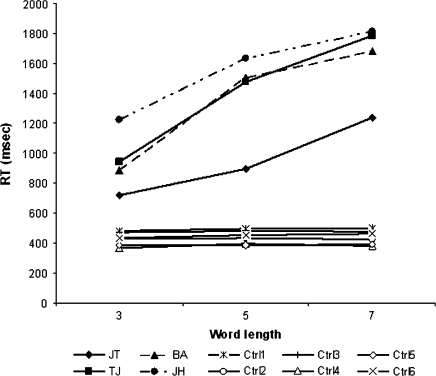
RTs to correctly named words, as well as reading errors, were analyzed. Three trials were excluded from analysis for JH, due to voice key error. Scores for individual patients and the control group are presented in Table 2, and an illustration of the RTs and word length effects is presented in Figure 3. The patient group mean RT of 1303 ms (SD = 277) was significantly different from the control group mean RT of 438 (SD = 43), t3.1 = −6.2, P = 0.004, and this difference was significant for all individual patients (see Table 2). On average, the patients error scores (mean = 6.3, SD = 5.7) did not differ significantly from controls (mean errors = 1.2, SD = 1.2, t3.2 = −1.8, n.s.). However, when compared individually, 2 patients made significantly more reading errors than controls (JT [14 errors] and TJ [7 errors], P < 0.01, Crawford and Garthwaite's test). These errors were mainly omitting or misreading of ends of words. Especially for JT, there was a clear relationship between word length and errors. He made no errors on 3-letter words, 5 errors on 5-letter words, and 9 errors on 7-letter words.
The mean RTs (range 912–1562 ms) as well as the word length effects, suggest that all patients have fairly mild alexia, but perform within the range commonly reported in pure alexia (e.g., Behrmann, Plaut, and Nelson 1998). For comparison, patient NT had a mean RT on words 3–7 letters in length of 699 ms, significantly higher than his matched controls’ mean RT of 487 ms (SD = 39), P = 0.004, Crawford and Garthwaite's test (Note that these data differ from the original publication, as words up to 12 letters were included in the original analysis. This also applies to his estimated word length effect (WLE) presented in the legend of Figure 4).
Figure 4.
Single-word reading speeds and word length effect for all 4 pure alexic patients and controls. The patients' word length effects, as estimated by linear regression, were TJ: 212 ms per letter (r2 = 0.198, F(1, 66) = 16.3, P < 0.001); JT: 176 ms/letter (r2 = 0.356, F(1, 59) = 32.5, P < 0.001); BA: 201 ms/letter (r2 = 0.449, F(1, 71) = 57.9, P < 0.001); JH 146 ms/letter (r2 = 0.269, F(1, 68) = 25.0, P < 0.001). For comparison, NT's word length effect was 36 ms/letter for words of 3–7 letters in length (r2 = 0.062, F(1,64) = 4.25, P = 0.043).
All 4 patients had a significant word length effect (Figure 4); control patient NT also had a word length effect, but this was only 36 ms per letter, 4 times lower than the fastest of the pure alexic patients and well within the range of that reported for hemianopic alexia (Leff et al. 2001).
In terms of their writing ability, all patients performed within the normal range. Raw scores (and T-scores in parentheses) were JH: 75 (65); TJ: 75 (65); BA 74 (64); and JT: 71 (60). This confirms that all 4 patients have alexia “without” agraphia, to use conventional neurological terminology, that is, pure alexia.
Object Naming and Object Decision
Errors and mean RTs on the object naming task for patients and controls are presented in Table 2. All 4r patients' accuracy in the naming task was within the normal range, whereas their RTs were significantly elevated compared with the control group, both on a group level (patient mean RT = 1355 ms, SD = 262, control mean RT = 753, SD = 91, t8 = − 5.3, P < 0.001), and when compared individually (Table 2).
For object decision, the overall error rate, as well as RTs to correctly categorized real objects, was analyzed (Table 2). On a group level, patients did not differ from controls with regards to accuracy (patient mean = 8.8, SD = 2.5; control mean = 10.8, SD = 1.5, t8 = 1.7, n.s.). Regarding RTs, the patient group mean of 1209 (SD = 355) differed significantly from the control mean RT of 818 (SD = 176, t8 = −2.3, P = 0.024). On an individual level, 1 patient (JT) made significantly “fewer” errors than controls in this task (2-tailed P = 0.016, Crawford and Garthwaite), whereas the other patients did not differ from controls with regards to accuracy. Two patients (TJ and BA) had mean RTs significantly slower than the control mean, whereas JT and JH had RTs within the normal range.
Auditory Digit and Letter Span
For digits, patients' mean total score was 15.3 (SD = 4.1), not significantly different from the control group mean of 17.8 (SD = 2.9, t8 = 1.2, n.s.). The patients' average maximum span was 6.0 (SD = 0.82), again not significantly different from the control mean of 6.7 (SD = 0.82, t8 = 1.3, n.s.). See Table 2 for individual scores. For letters, the patients' average total score was 10.3 (SD = 1.26), not different from the control group mean of 13.5 (SD = 3.45, t8 = 1.8, n.s.). The patients' average maximum span was 5.25 (SD = 0.50), which was reduced compared with the control mean of 6.5 (SD = 0.84, t8 = 2.7, P = 0.015). On an individual level, none of the patients' scores differed significantly from the control group.
Measurement of Visual Processing Speed and Apprehension Span
Experiment 1: Single Stimulus Report of Letters and Digits
Individual parameter estimates for patients, as well as control group mean scores, are presented in Table 3. The model fits were close, correlating on average 98.1% with the observed data. See Figure 5 for a graphical comparison of a representative patient (TJ) and a control subject. One control subject was excluded from this analysis, based on Chauvenet's criterion (Barnett and Lewis 1994) because his Ccentral scores for letters and numbers were significantly superior to the rest of the controls. His scores deviated from the control group mean (including his own data) by 2.35 SDs for letters, and 2.61 SDs for digits.
Table 3.
Results from Experiment 1
| TJ | JT | BA | JH | Control mean (SD) | |
| Single letter Ccentral | 31** | 27** | 25** | 22** | 117 (23) |
| Single letter t0 central | 25** | 31** | 17 | 10 | 13.0 (3.1) |
| Single digit Ccentral | 44** | 47** | 29** | 79* | 119 (16) |
| Single digit t0 central | 25** | 26** | 20* | 15 | 11.9 (2.8) |
Note: Processing speed (Ccentral given in s−1) and perception threshold (t0, given in milliseconds) for single letters and digits presented at fixation for individual patients, and control group (N = 9) mean results (SD in brackets).
*P < 0.05; **P < 0.01 by Crawford and Garthwaite's test.
For single letters, the patient group's mean processing speed (Ccentral = 26, SD = 4) was significantly reduced compared to control performance (Ccentral = 117, SD = 23, t11 = 7.5, P < 0.001). The patients individual Ccentral estimates (range 22–31) were all significantly different from the control group mean (see Table 3). The control mean perception threshold (t0) for letters was 13.0 ms (SD = 3.1), which did not differ significantly from the patients' mean t0 of 20.8 (SD = 9.2, t3.3 = −1.65, n.s.). On an individual level, patient JT's and TJ's thresholds for letter perception were significantly elevated compared with controls. For comparison, patient NT's (Habekost and Starrfelt 2006) processing speed for centrally presented letters (Ccentral) was 84, and his t0 value was 13 ms, both within the normal range compared with his matched controls (control Ccentral = 95, SD = 20; t0 = 10, SD = 2).For single digits, the mean processing speed of the patient group (Ccentral = 50, SD = 21) was again significantly different from the control group mean (Ccentral = 119, SD = 16, t11 = 6.6, P < 0.001). This difference was significant on an individual level for all patients (see Table 3). The patients average perception threshold for digits was t0 = 21.5 (SD = 5.1), also significantly different from the control group mean of t0 = 11.9 ms (SD = 2.9; t11 = −4.5, P < 0.001). Individually, the perception thresholds for BA, JT, and TJ were significantly elevated compared with controls.
Experiment 2: Whole Report of Letters and Digits
The average correlation of parameter estimates with observed data from the whole report experiment was 94.4%. With regards to estimates of visual apprehension span, K, patients differed significantly from controls both for letters and digits on a group level as well as individually. For letters, the patients mean was K = 2.3 (SD = 0.28), significantly different from the control group mean of K = 4.5 (SD = 0.30; t12 = 12.2, P < 0.001). For digits, the patient mean was K = 2.4 (SD = 0.51), also significantly different from the control mean of K = 4.6 (SD = 0.30; t3.9 = 8.0, P = 0.001). This difference was significant on an individual level for both letters and digits for all patients, as can be seen Table 4a. See also Figure 6 for a graphical comparison of a representative patient's (JH) performance and a control subject. TJ was the best performing patient, with a K = 3.0 for digits in the left visual field, which is still significantly reduced compared with controls (P < 0.001, Crawford and Garthwaite's test). The K-estimates for the controls reflect that they could all report 5 items from the display in some instances. The patients, on the other hand, reported a maximum of 3 items.With regard to the processing speed (Cperipheral) in this experiment, the patients differed from controls on a group level with both stimulus types. For letters, the patients mean Cperipheral = 17 (SD = 7) was significantly different from the control group mean of Cperipheral = 38 (SD = 15; t12 = 2.5, P = 0.013). For digits, the patients average Cperipheral was 20 (SD = 11), again significantly different from the control mean of Cperipheral = 54 (SD = 25; t12 = 2.6, P = 0.011). Comparing the individual patients with the control group, even the lowest scoring patients JH and JT, who both had a Cperipheral value for letters of 11, only showed a nonsignificant trend away from the control mean of 38 (P = 0.060, Crawford and Garthwaite's test). As patient TJ had no visual field defect, he could be tested in both visual fields in this experiment. His performance in the right visual field was compared with that of 4 controls (Table 4b). TJ's performance showed the same pattern in both visual fields, his visual apprehension span (K) was significantly reduced for both letters and digits (P < 0.001, Crawford and Garthwaite's test), whereas his processing speed (Cperipheral) was not significantly different from controls in this individual comparison. For both TJ and controls, there was a nonsignificant trend toward higher processing speed in the right visual field for both letters and digits.
Table 4a.
Whole report results from Experiment 2
| TJ | JT | BA | JH | Control mean (SD) | |
| K—letters | 2.6** | 2.5** | 2.0** | 2.2** | 4.5 (0.30) |
| K—digits | 3.0** | 2.7** | 2.1** | 1.9** | 4.6 (0.30) |
| Cperipheral letters | 24 | 11 | 23 | 11 | 38 (15) |
| Cperipheral digits | 36 | 17 | 13 | 15 | 54 (25) |
Note: Parameter estimates of visual span of apprehension (K) and processing speed (Cperipheral) for letters and digits, as measured in the left visual field for individual patients and the control group (N = 10).
**P < 0.01 (Crawford and Garthwaite's test).
Table 4b.
Whole report results from presentation in the right visual field for patient TJ and controls (N = 4)
| TJ | Control mean (SD) | |
| K—letters right | 2.8** | 4.9 (0.1) |
| K—digits right | 2.9** | 4.8 (0.1) |
| Cperipheral letters right | 33 | 60 (24) |
| Cperipheral digits right | 45 | 146 (118) |
**P < 0.01 (Crawford and Garthwaite's test).
In comparison, patient NT's (Habekost and Starrfelt 2006) K value for letters, as measured in the left visual field, was estimated to be 3.7, which was within the normal range compared with a group of matched controls (mean K = 4.3, SD = 0.43). His Cperipheral for letters was estimated at 13, again not significantly different from the controls (mean Cperipheral = 34.4, SD = 14.5).
Discussion
Using sensitive psychophysical measures and analyses based on a TVA (Bundesen 1990), we have investigated 2 central questions regarding the main deficit in pure alexia: 1) whether their deficit in visual recognition was selective to alphabetic characters, that is, letters, and 2) whether their performance was influenced by the number of items in a display.
All patients demonstrated elevated RTs in single-word reading and had a typical word length effect (see Figure 4), whereas their writing ability was intact, consistent with a diagnosis of pure alexia. The control patient with hemianopic alexia, NT, also had a mild WLE effect of 36 ms/letter. The much milder WLE seen in hemianopic alexia is likely to be due to extra rightward saccades being required to read words that are too long to fall into residual foveal/parafoveal vision (Upton et al. 2003). Patients with hemianopic alexia are considerably slower at text reading than their single-word reading speed would imply, as their field defect interferes with generating an efficient reading scanpath (Zihl 1995; McDonald et al. 2006). Conversely, patients can have pure alexia with no visual field defect, such as TJ reported here and AR reported in Leff et al. (2001); their slow text reading speeds are largely a reflection of their slow single-word reading speeds.
All patients were within the normal range on an object naming task with regards to accuracy, whereas their RTs in this task were elevated compared with controls. In a difficult object decision task, all patients were within the normal range with regards to accuracy, whereas 2 patients (TJ and BA) had elevated RTs compared with controls. Only the latter had a hemianopia that may have interfered with RTs on this task. It should be noted that the main experimental tasks were not based on RT measurement, and therefore, latencies in naming of visual items did not affect the results in Experiments 1 and 2. The patients all had lesions affecting the ventral portion of the posterior left hemisphere, and the only damaged region common to all 4 patients was the left fusiform gyrus; the area corresponding to the putative VWFA (see Figure 2).
The experimental investigation revealed that recognition efficiency (Ccentral) for single letters and single digits presented at fixation was severely reduced in all patients (Experiment 1). In addition, visual apprehension span (K) was markedly reduced in all patients (Experiment 2). These impairments were clearly evident for both letters and digits. On a group level, the patients also differed from controls with respect to peripheral visual processing speed (Cperipheral), both for letters and digits (Experiment 2). In comparison, we have found normal efficiency of single letter recognition (Ccentral) as well as visual apprehension span (K) for letters in patient NT, who had a ventral occipital lesion sparing the putative VWFA, and who did not have pure alexia (Habekost and Starrfelt 2006). Thus, the impairments observed in the group of pure alexic patients cannot be explained as a general, nonspecific effect of a left posterior lesion.
The impairment in single letter recognition apparent in our group of pure alexic patients is consistent with findings in most other studies of pure alexia (Behrmann, Plaut, and Nelson 1998). This deficit could potentially be explained by damage to a cognitive system or cerebral area specialized for extracting abstract letter identities, as suggested for the VWFA (Cohen et al. 2003). This area, localized in or just lateral to the left mid-fusiform gyrus (Cohen et al. 2003; Jobard et al. 2003; Cohen and Dehaene 2004), has been suggested to be the critical region damaged in pure alexia. The only region of lesion overlap between our patients was found in the fusiform gyrus, at and surrounding the coordinates of the putative VWFA (see Figure 2), and hence damage to this region is likely to be the major cause of our patients' reading problems. Indeed, patients like NT (Habekost and Starrfelt 2006) with left ventral occipital lesions that do not affect the putative VWFA, do not have pure alexia, but commonly have reading deficits attributable to their visual field defects (Leff et al. 2006). This supports the claim that the VWFA is of crucial importance for normal visual word recognition (Cohen and Dehaene 2004). However, a deficit in a system specialized for extracting abstract letter identities does not seem sufficient to explain our patients' deficits, which affected processing of digits as well as letters. In all 4 pure alexic patients reported here a deficit in single digit perception was clearly evident, and although this finding may not at first seem very surprising, it is not trivial. On the contrary, it is sometimes assumed that number reading can be spared in pure alexia, and reading of multidigit numbers in free vision has been reported to be preserved in some patients (Warrington and Shallice 1980; Leff et al. 2001). In other patients with pure alexia, number reading has been reported to be impaired (Henderson 1987), although commonly not to the same degree as letter identification and word reading (Cohen and Dehaene 1995; Miozzo and Caramazza 1998). Our results support the notion that number reading is impaired in pure alexia, a finding that suggests that a more general impairment in visual processing is at the core of this disorder. As patient NT was not tested with number stimuli, it remains a possibility that he would be impaired with numbers although his performance with letters was normal in both the single stimulus and whole report tasks. To our knowledge, there are no previous case reports of patients who have impaired number reading with normal letter/word reading due to occipital damage or perceptual deficits, although this dissociation has been reported in a patient with more widespread brain pathology (Cipolotti, 1995). Indeed although anatomical specificity is commonly suggested for letter or word perception, few claim the existence of a specialized perceptual “visual number form area.” Our results cannot strictly rule out that there are separate brain modules for the early visual processing of letters and numbers, and that our pure alexic patients' lesions were large enough to damage both. Our results show an association between deficits, which leaves a possibility that reading of letters and numbers can be dissociated in other pure alexic patients with lesions affecting the VWFA. However, although our study only involves 4 patients, whose lesion sizes vary, we consider this unlikely: The region of lesion overlap between our patients is discrete (∼256 mm3, see Figure 2) and includes the central coordinate of the VWFA as identified by a meta-analysis of 27 functional imaging studies (Jobard et al. 2003). This location is almost identical to that of the N200 response to letter stimuli reported in a study using cortical surface electrodes in patients undergoing brain surgery (Allison et al. 1994). Interestingly, this same study identified an N200 source for numbers at a site 20 mm more anterior and medial to the letter peak. None of our 4 patients had damage to this region and none demonstrated a dissociation for processing of letter and number stimuli, as would be predicted by a dual early-visual-processing module hypothesis.
Turning to our patients' performance in the whole report experiment (Experiment 2), the most notable finding is that all patients had severely reduced visual apprehension span compared with normal controls, and that this reduction was not specific to letters in any patient. As the patients' maximum auditory span for letters and digits was 5 or more items, a reduction in amodal short-term memory cannot account for the reduced apprehension span in the visual domain. The result points to a form of simultanagnosia, which has been postulated to contribute to or even be the root cause of pure alexia (Kinsbourne and Warrington 1962; Levine and Calvanio 1978; Farah 1990). However, the simultanagnosia hypothesis cannot account for our patients' reduced processing speed for single items presented at fixation, and thus a simultanagnosic deficit fails to fully explain our patients' pattern of performance.
How might we then account for the observed results? Reductions in the C or K parameter are functionally specific: They represent impairment in the visual speed or span, respectively. However, deficits in visual speed or span are not anatomically specific, in the sense that each of the 2 behavioral impairments can be produced by damage to structurally distinct neural networks. Full conscious recognition of visual stimuli presumably depends on a widely distributed brain network including both visual areas in the posterior cortex as well as fronto-parietal structures (Dehaene et al. 2003). Damage to different parts of this network may compromise perceptual efficiency, leading to reduced C and/or K values, but for different reasons. For example, patients with dorsal simultanagnosia after bilateral parietal lesions may have even more reduced C values for letter stimuli (Duncan et al. 2003) than those reported in the present study, but may nonetheless be able to read single words without resorting to a letter-by-letter strategy (Coslett and Saffran 1991; Baylis et al. 1994; Vinckier et al. 2006). This suggests that the visual deficit in dorsal simultanagnosia is of a different nature than in pure alexia, although slow processing of letters is characteristic of both patient groups. One possibility is that bilateral parietal lesions severely impair the efficiency of fronto-parietal loops supporting conscious recognition, but spare the basic sensory analysis and grouping of items performed in the ventral visual stream.
Contrary to this, we suggest that our patients' deficits in C and K reflect degradation in the basic sensory representations necessary for visual recognition of letters and digits. In addition to its suggested role in visual word form processing, the visual ventral stream (including the left fusiform gyrus) has been suggested to be of particular importance for: processing of foveal stimuli (Devlin et al. 2006); extracting medium to high range spatial frequencies (Fiset et al. 2006); rapid perception of multiple visual forms (Farah 2004); and for the integration of visual elements into perceptual wholes (Starrfelt and Gerlach 2007), all of which are extremely important in reading. Of particular interest to the current findings is the suggestion that a loss of sensitivity to medium to high range spatial frequencies may be the “low level visual deficit” giving rise to effects of word length and letter confusability in pure alexia (Fiset et al. 2006). Such an impairment in the use of “the optimal spatial frequency band for letter and word recognition” (Fiset et al. 2006, p. 1466) would be expected to degrade the sensory representations of both letters and digits, and thus affect perception of these symbols in single or multiple displays: Visual processing speed, C, should be markedly reduced by low signal-to-noise ratios for both stimulus types. Similarly, the ability to perceive multiple stimuli at the same time, K, should be impaired due to increased interference between the weaker (concurrent) stimulus representations. If visual processing speed for letters is very low, one needs to fixate longer at each segment of text to derive the same information as a normal reader. Further, if the visual span is impaired, less of the surrounding text can be apprehended, which prohibits the normal pattern of relatively large amplitude saccades between content words. In combination, severe deficits in visual speed and span should therefore result in a very slow and laborious reading process with longer fixations and shorter saccades, precisely what is found in patients with pure alexia (Behrmann et al. 2001). In this way, our results may explain the central symptom of the disorder.
Fiset et al. (2006) have suggested that there may be hemispheric differences in sensitivity to spatial frequencies, and that this may explain why pure alexia arises from damage to left but not right posterior cortex. One interesting avenue for future research lies in investigating how damage to the right hemisphere homologue of the VWFA affects visual processing capacity, and how this may be affected by physical characteristics of the stimuli including spatial frequency. Future studies of VWFA function could select patients based on lesion anatomy rather than the presence of a given deficit (i.e., pure alexia). In the present study, we found that patients with pure alexia and VWFA damage consistently had impaired TVA values for letters and digits; however, it is theoretically possible that damage to the VWFA can occur without pure alexia or a reduction of the patient's TVA scores. An interesting follow-up to the present study would be to investigate a series of patients selected purely on the basis of damage to the left VWFA and surrounding areas.
One important question remaining is why patients with pure alexia rarely complain about other visuo-perceptual problems. None of the 4 patients reported here complained of any cognitive deficits save reading. This is probably because reading is a high capacity skill that places different demands on the visual system than other visual tasks. Although the patients' RTs in picture naming were elevated, such a problem may be less obvious in everyday life than the corresponding pattern (slow but accurate) in reading. In visual agnosia, real objects are often recognized better than photographs of objects, which again are recognized better than line drawings (Farah 1990), suggesting that line drawings, as used in the present study, are more difficult to visually recognize than real objects or photographs. This has also been shown in normal subjects, where naming latency decreases when color and texture is added to simple line drawings (Rossion and Pourtois 2004). Thus, the impairment on the naming and object decision tasks observed in our patients possibly reflects a real perceptual problem, but one that may not be very noticeable in the patients' everyday life, where other cues to aid object recognition are present. Similarly, although impaired with single digits, the patients reported here were able to identify single digits accurately when perceived. Normal subjects show a “number length effect” on RT when reading multidigit numbers, at least numbers that exceed 2 digits (Brysbaert 2005), indicating that they parse the number into its constituent digits. As our patients were able to recognize single digits accurately, albeit more slowly than controls, they should be able to read multidigit numbers without resorting to an abnormal strategy. This may explain the observation that patients with pure alexia seem to read multidigit numbers normally when presented in free vision (Warrington and Shallice 1980; Leff et al. 2001). For word reading, such a parsing strategy will have a more devastating effect. The explanation for the material specific complaints of patients with pure alexia is thus most likely to be found in the unique demands imposed on the visual system by reading, rather than at a brain level in the sense of an area specialized for visual word recognition. Even slight reductions in the efficiency of letter recognition and discrimination will have a disproportional impact on reading and we suggest that this is the case in pure alexia. This does not imply a word-specific deficit, but merely reflects that for the purposes of fluent reading, patients with pure alexia see too little, too late.
Funding
Danish Research Council for the Humanities to R.S.; Copenhagen University's research priority area “Brain and Mind” to T.H.; The Wellcome Trust (ME033459MES) to A.P.L. Institutional level funding: National Institute for Health Research Comprehensive Biomedical Research Centre at University College London Hospitals.
Acknowledgments
Thanks to Christian Gerlach for providing the object recognition tests and for helpful comments on an earlier version of this manuscript. The first author is indebted to Fakutsi for enthusiastic support during this study. Conflict of Interest: None declared.
References
- Allison T, McCarthy G, Nobre A, Puce A, Belger A. Human extrastriate visual cortex and the perception of faces, words, numbers, and colors. Cereb Cortex. 1994;4:544–554. doi: 10.1093/cercor/4.5.544. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio AR, Damasio H. Troubled letters but not numbers. Domain specific cognitive impairments following focal damage in frontal cortex. Brain. 1990;113:749–766. doi: 10.1093/brain/113.3.749. [DOI] [PubMed] [Google Scholar]
- Barnett V, Lewis T. Outliers in statistical data. Chichester: John Wiley & Sons; 1994. [Google Scholar]
- Baylis GC, Driver J, Baylis LL, Rafal RD. Reading of letters and words in a patient with Balint's syndrome. Neuropsychologia. 1994;32:1273–1286. doi: 10.1016/0028-3932(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Nelson J, Sekuler EB. Visual complexity in letter-by-letter reading: “pure” alexia is not pure. Neuropsychologia. 1998;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Plaut DC, Nelson J. A literature review and new data supporting an interactive account of letter-by-letter reading. Cogn Neuropsychol. 1998;15:7–51. doi: 10.1080/026432998381212. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Shomstein SS, Black SE, Barton JJ. The eye movements of pure alexic patients during reading and nonreading tasks. Neuropsychologia. 2001;39:983–1002. doi: 10.1016/s0028-3932(01)00021-5. [DOI] [PubMed] [Google Scholar]
- Binder JR, Mohr JP. Topography of callosal reading pathways. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- Brysbaert M. Number recognition in different formats. In: Campbell JID, editor. Handbook of mathematical cognition. Hove: Psychology Press; 2005. [Google Scholar]
- Bundesen C. A theory of visual attention. Psychol Rev. 1990;97:523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Bundesen C, Habekost T, Kyllingsbaek S. A neural theory of visual attention: bridging cognition and neurophysiology. Psychol Rev. 2005;112:291–328. doi: 10.1037/0033-295X.112.2.291. [DOI] [PubMed] [Google Scholar]
- Cipolotti L. Multiple routes for reading words, why not numbers? Evidence from a case of arabic numeral dyslexia. Cogn Neuropsychol. 1995;12:313–342. [Google Scholar]
- Cohen L, Dehaene S. Number processing in pure alexia: the effects of hemispheric asymmetries and task demands. Neurocase. 1995;1:121–137. [Google Scholar]
- Cohen L, Dehaene S. Specialization within the ventral stream: the case for the visual word form area. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Cohen L, Martinaud O, Lemer C, Lehericy S, Samson Y, Obadia M, Slachevsky A, Dehaene S. Visual word recognition in the left and right hemispheres: anatomical and functional correlates of peripheral alexias. Cereb Cortex. 2003;13:1313–1333. doi: 10.1093/cercor/bhg079. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Saffran E. Simultanagnosia. To see but not two see. Brain. 1991;114:1523–1545. doi: 10.1093/brain/114.4.1523. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Garthwaite PH. Investigation of the single case in neuropsychology: confidence limits on the abnormality of test scores and test score differences. Neuropsychologia. 2002;40:1196–1208. doi: 10.1016/s0028-3932(01)00224-x. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Gonnerman LM, Matthews PM. The role of the posterior fusiform gyrus in reading. J Cogn Neurosci. 2006;18:911–922. doi: 10.1162/jocn.2006.18.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Chavda S, Shibuya H. Systematic analysis of deficits in visual attention. J Exp Psychol Gen. 1999;128:450–478. doi: 10.1037//0096-3445.128.4.450. [DOI] [PubMed] [Google Scholar]
- Duncan J, Bundesen C, Olson A, Humphreys G, Ward R, Kyllingsbaek S, van Raamsdonk M, Rorden C, Chavda S. Attentional functions in dorsal and ventral simultanagnosia. Cogn Neuropsychol. 2003;20:675–701. doi: 10.1080/02643290342000041. [DOI] [PubMed] [Google Scholar]
- Farah MJ. Visual agnosia: disorders of object recognition and what they tell us about normal vision. Cambridge (MA): MIT Press; 1990. [Google Scholar]
- Farah MJ. Visual agnosia. Cambridge (MA): MIT Press; 2004. [Google Scholar]
- Farah MJ, Wallace MA. Pure alexia as a visual impairment: a reconsideration. Cogn Neuropsychol. 1991;8:313–334. [Google Scholar]
- Finke K, Bublak P, Dose M, Muller HJ, Schneider WX. Parameter-based assessment of spatial and non-spatial attentional deficits in Huntington's disease. Brain. 2006;129:1137–1151. doi: 10.1093/brain/awl040. [DOI] [PubMed] [Google Scholar]
- Fiset D, Gosselin F, Blais C, Arguin M. Inducing letter-by-letter dyslexia in normal readers. J Cogn Neurosci. 2006;18:1466–1476. doi: 10.1162/jocn.2006.18.9.1466. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gerlach C, Law I, Paulson OB. Structural similarity and category-specificity: a refined account. Neuropsychologia. 2004;42:1543–1553. doi: 10.1016/j.neuropsychologia.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Habekost T, Rostrup E. Persisting asymmetries of vision after right side lesions. Neuropsychologia. 2006;44:876–895. doi: 10.1016/j.neuropsychologia.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Habekost T, Rostrup E. Visual attention capacity after right hemisphere lesions. Neuropsychologia. 2007;45:1474–1488. doi: 10.1016/j.neuropsychologia.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Habekost T, Starrfelt R. Alexia and quadrant-amblyopia: reading disability after a minor visual field deficit. Neuropsychologia. 2006;44:2465–2476. doi: 10.1016/j.neuropsychologia.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Mirkin M, Polk TA. Category-level contributions to the alphanumeric category effect in visual search. Psychonom Bull Rev. 2006;13:1074–1077. doi: 10.3758/bf03213928. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Is number reading selectively spared in pure alexia. J Clin Exp Neuropsychol. 1987;9:41. [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker P, Herskovits E, Degaonkar M. The roles of the “visual word form area” in reading. Neuroimage. 2005;24:548–559. doi: 10.1016/j.neuroimage.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Jobard G, Crivello F, Tzourio-Mazoyer N. Evaluation of the dual route theory of reading: a meta analysis of 35 neuroimaging studies. Neuroimage. 2003;20:693–712. doi: 10.1016/S1053-8119(03)00343-4. [DOI] [PubMed] [Google Scholar]
- Joseph JE, Cerullo MA, Farley AB, Steinmetz NA, Mier CR. fMRI correlates of cortical specialization and generalization for letter processing. Neuroimage. 2006;32:806–820. doi: 10.1016/j.neuroimage.2006.04.175. [DOI] [PubMed] [Google Scholar]
- Kasten E, Wust S, Behrens-Baumann W, Sabel BA. Computer-based training for the treatment of partial blindness. Nat Med. 1998;4:1083–1087. doi: 10.1038/2079. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M, Warrington EK. A disorder of simultaneous form perception. Brain. 1962;85:461–486. doi: 10.1093/brain/85.3.461. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis W. A computational analysis of present day American English. Providence (RI): Brown University Press; 1967. [Google Scholar]
- Kyllingsbaek S. Modeling visual attention. Behav Res Methods. 2006;38:123–133. doi: 10.3758/bf03192757. [DOI] [PubMed] [Google Scholar]
- Leff AP, Scott SK, Rothwell JC, Wise RJ. The planning and guiding of reading saccades: a repetitive transcranial magnetic stimulation study. Cereb Cortex. 2001;11:918–923. doi: 10.1093/cercor/11.10.918. [DOI] [PubMed] [Google Scholar]
- Leff AP, Spitsyna G, Plant GT, Wise RJ. Structural anatomy of pure and hemianopic alexia. J Neurol Neurosurg Psychiatry. 2006;77:1004–1007. doi: 10.1136/jnnp.2005.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DN, Calvanio R. A study of the visual defect in verbal alexia-simultanagnosia. Brain. 1978;101:65–81. doi: 10.1093/brain/101.1.65. [DOI] [PubMed] [Google Scholar]
- Lloyd Jones TJ, Humphreys GW. Perceptual differentiation as a source of category effects in object processing: evidence from naming and object decision. Mem Cogn. 1997;25:18–35. doi: 10.3758/bf03197282. [DOI] [PubMed] [Google Scholar]
- McDonald SA, Spitsyna G, Shillcock RC, Wise RJ, Leff AP. Patients with hemianopic alexia adopt an inefficient eye movement strategy when reading text. Brain. 2006;129:158–167. doi: 10.1093/brain/awh678. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Caramazza A. Varieties of pure alexia: the case of failure to access graphemic representations. Cogn Neuropsychol. 1998;15:203–238. doi: 10.1080/026432998381267. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osswald K, Humphreys GW, Olson A. Words are more than the sum of their parts: evidence for detrimental effects of word-level information in Alexia. Cogn Neuropsychol. 2002;19:675–695. doi: 10.1080/02643290244000103. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, Driver J, Antoun N, Duncan J. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;15:1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. Late experience alters vision [letter] Nature. 1995;376:648–649. doi: 10.1038/376648a0. [DOI] [PubMed] [Google Scholar]
- Polk TA, Farah MJ. The neural development and organization of letter recognition: evidence from functional neuroimaging, computational modeling, and behavioral studies. Proc Natl Acad Sci USA. 1998;95:847–852. doi: 10.1073/pnas.95.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk TA, Stallcup M, Aguirre GK, Alsop DC, D'Esposito M, Detre JA, Farah MJ. Neural specialization for letter recognition. J Cog Neurosci. 2002;14:145–159. doi: 10.1162/089892902317236803. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol [Hum Learn] 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Starrfelt R. Selective alexia and agraphia sparing numbers: a case study. Brain Lang. 2007;102:52–63. doi: 10.1016/j.bandl.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Starrfelt R, Gerlach C. The visual what for area: words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Swinburn K, Porter G, Howard D. Comprehensive aphasia test. Hove, UK: Psychology Press; 2004. [Google Scholar]
- Upton NJ, Hodgson TL, Plant GT, Wise RJ, Leff AP. “Bottom-up” and “top-down” effects on reading saccades: a case study. J Neurol Neurosurg Psychiatry. 2003;74:1423–1428. doi: 10.1136/jnnp.74.10.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinckier F, Naccache L, Papeix C, Forget J, Hahn-Barma V, Dehaene S, Cohen L. “What” and “where” in word reading: ventral coding of written words revealed by parietal atrophy. J Cogn Neurosci. 2006;18:1998–2012. doi: 10.1162/jocn.2006.18.12.1998. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Langdon D. Spelling dyslexia: a deficit in the visual word-form. J Neurol Neurosurg Psychiatry. 1994;57:211–216. doi: 10.1136/jnnp.57.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, Rabin P. Visual span of apprehension in patients with unilateral cerebral lesions. Q J Exp Psychol. 1971;23:423–431. doi: 10.1080/14640747108400254. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Word-form dyslexia. Brain. 1980;103:99–112. doi: 10.1093/brain/103.1.99. [DOI] [PubMed] [Google Scholar]
- Wechsler D. San Antonio: Harcourt; 1999. Wechsler Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Zihl J. Eye movement patterns in hemianopic dyslexia. Brain. 1995;118:891–912. doi: 10.1093/brain/118.4.891. [DOI] [PubMed] [Google Scholar]