Abstract
Spatial memory formation is enabled through synaptic information processing, in the form of persistent strengthening and weakening of synapses, within the hippocampus. It is, however, unclear how relevant spatial information is selected for encoding, in preference to less pertinent information. As the noradrenergic locus coeruleus (LC) becomes active in response to novel experiences, we hypothesized that the LC may provide the saliency signal required to promote hippocampal encoding of relevant information through changes in synaptic strength. Test pulse stimulation evoked stable basal synaptic transmission at Schaffer collateral (SC)–CA1 stratum radiatum synapses in freely behaving adult rats. Coupling of these test pulses with electrical stimulation of the LC induced long-term depression (LTD) at SC–CA1 synapses and induced a transient suppression of theta-frequency oscillations. Effects were N-methyl-D-aspartate and β-adrenergic receptor dependent. Activation of the LC also increased CA1 noradrenalin levels and facilitated the encoding of spatial memory for a single episode via a β-adrenoceptor–dependent mechanism. Our results demonstrate that the LC plays a key role in the induction of hippocampal LTD and in promoting the encoding of spatial information. This LC–hippocampal interaction may reflect a means by which salient information is distinguished for subsequent synaptic processing.
Keywords: β-adrenergic, CA1, hippocampus, locus coeruleus, LTD, synaptic plasticity
Introduction
It is widely believed that hippocampus-dependent memory is encoded by means of a close interplay of different forms of synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD) (Bear and Abraham 1996; Martin et al. 2000; Kemp and Manahan-Vaughan 2007). The hippocampus is bombarded with sensory and spatial information from the entorhinal cortex (EC), which is filtered and stored, when appropriate, in the form of short- or long-term plasticity. This is believed to comprise a key step in the generation of short- and long-term memory. Although it is apparent that the intensity and frequency of an afferent signal can determine whether short- or long-term plasticity occurs (Alger and Teyler 1976), it remains unclear, however, how the hippocampus distinguishes novel or relevant information from irrelevant information. One possibility is that neuromodulatory systems may initiate or enhance the encoding of novel information through persistently modifying the strength of hippocampal synapses that are believed to be intrinsically involved in the formation of spatial and episodic memory (O'Keefe 1978; Eichenbaum 1996). The diffusely projecting noradrenergic system is one such neuromodulator (Kety 1970; Harley 1991).
Novelty detection is accompanied by increased hippocampal noradrenergic activity, driven by enhanced firing of the locus coeruleus (LC), the main source of noradrenalin (NA) in the brain (Sara et al. 1994; Kitchigina et al. 1997). Lesioning the afferents that carry neuromodulatory inputs (including NA) to the hippocampus impairs recollection of episodic-like memory (Easton 2006). LC stimulation improves memory retrieval (Devauges and Sara 1991), whereas vagus nerve stimulation enhances memory encoding and consolidation (Clark et al. 1995, 1999; Ghacibeh et al. 2006) via LC activation, suggesting that the LC, and the noradrenergic activity it mediates in the hippocampus, may be a key factor in driving synaptic plasticity in response to novel spatial events.
As the LC becomes active in response to novel experiences, we hypothesized that it may provide the saliency signal required to promote hippocampal encoding of relevant novel information through changes in synaptic strength. Recently, it has become apparent that hippocampal LTP and LTD may be responsible for the encoding of very different aspects of a spatial cognitive map (Kemp and Manahan-Vaughan 2007). Although LTP occurs at a variety of hippocampal synapses in response to changes in space (Kemp and Manahan-Vaughan 2007), it is facilitated by specific changes in spatial context, with the CA1 region responding to fine spatial detail and the dentate gyrus responding to landmark context (Kemp and Manahan-Vaughan 2008a). The possibility that the LC influences memory induction through its efferent activation of postsynaptic β-adrenergic receptors is supported by experiments showing the β-adrenergic receptor dependency of LTP (Dahl and Sarvey 1990; Munro et al. 2001; Straube et al. 2003) and LTD (Kemp and Manahan-Vaughan 2008b), also under conditions of novel information processing (Straube et al. 2003; Kemp and Manahan-Vaughan 2008b).
We observed that LC activation facilitates the induction of β-adrenergic and N-methyl-D-aspartate (NMDA) receptor–dependent LTD in the hippocampal CA1 region, a phenomenon that has been associated with the encoding of spatial information (Manahan-Vaughan and Braunewell 1999; Duffy et al. 2001; Li et al. 2003; Kemp and Manahan-Vaughan 2004; Etkin et al. 2006; Lemon and Manahan-Vaughan 2006; Oomura et al. 2006; Kemp and Manahan-Vaughan 2007), and may, thus, mediate a key saliency signal for hippocampal memory formation. In line with this possibility, we found that NA release in the hippocampus increases following LC stimulation and facilitates the encoding of spatial memory for a single episode via a β-adrenoceptor–dependent mechanism.
Materials and Methods
All experiments were performed according to the guidelines of the German Animal Protection Law and were approved by the North Rhine Westphalia State Authority.
Surgery
Male Wistar rats (Charles River, Sulzfeld, Germany, 7–8 weeks old) underwent implantation of hippocampal electrodes and a guide cannula under anesthesia, as described previously (Manahan-Vaughan 1997). Briefly, under sodium pentobarbitone (Synopharm, Barsbüttel, Germany) anesthesia (“Nembutal,” 52 mg/kg, intraperitoneally), animals underwent implantation of a monopolar recording and a bipolar stimulating electrode (made from 0.1-mm diameter Teflon-coated stainless steel wire [Biomedical Instruments, Zöllnitz, Germany] attached to cardboard). A drill hole (1 mm diameter) was made for the recording electrode, and a second drill hole (1 mm diameter) was made for the stimulation electrode. On the contralateral side, 2 holes were drilled (1.2 mm diameter) into which anchor screws were inserted. The anchor screws also served as reference or ground electrodes. The coordinates for CA1 recordings comprised 2.8 mm posterior to bregma and 1.8 mm lateral to the midline for the recording electrode and 3.1 mm posterior to bregma and 3.1 mm lateral to the midline for the stimulation electrode. The dura was pierced through both holes, and the recording and the stimulating electrodes were lowered into the CA1 stratum radiatum and the Schaffer collaterals (SCs), respectively, for CA1 recordings. The correct location of the electrodes was established by means of the electrophysiological characteristics of the field potentials evoked. A third drill hole (1 mm diameter) was made ipsilateral to the hippocampal electrodes for another stimulation electrode at 3.1 mm posterior and 1.3 mm lateral to lambda. This bipolar stimulation electrode was implanted in the LC (8.4 mm ventral to dura matter, entering at a 15° angle to the plane of the skull).
The entire assembly was sealed and fixed to the skull with dental acrylic (Paladur, Heraeus Kulzer GmbH, Hanau, Germany). In all, 7–10 days after surgery, recordings were obtained in the CA1 stratum radiatum by stimulation of SCs. Throughout the experiments, the animals moved freely within the recording chamber (40 × 40 × 40 cm) because the implanted electrodes were connected by a flexible cable and swivel connector to the stimulation unit and amplifier. Aside from the insertion of the connector cable at the start of the experiment, disturbance of the animals was kept to an absolute minimum. Throughout the experiments, the electroencephalogram (EEG) of each animal was continuously monitored.
Measurement of Evoked Potentials
The field excitatory postsynaptic potential (fEPSP) slope was employed as a measure of excitatory synaptic transmission in the CA1 region. To obtain these measurements, an evoked response was generated in the stratum radiatum by stimulating with single biphasic square wave pulses of 0.1-ms duration per half wave, generated by a constant current isolation unit (WPI, Sarasota, FL). Five such evoked responses were recorded every 40 s and averaged to provide a representative average recording for each 5-min time period. After 90 min of recording, the period between samples of evoked potentials was extended to 15 min. The signals from the recording electrodes were amplified using an A-M-Systems 1700 differential amplifier (100×), filtered (0.1 Hz–10 KHz bandpass) and digitized at 10 KHz through a DA/AD converter (CED 1401-plus, micro CED; Cambridge Electronic Design, Cambridge, UK). Waveforms, displayed and analyzed on a PC with data acquisition software (Pwin, Leibniz-Institute, Magdeburg, Germany), were stored in the hard disk online. The fEPSP was measured as the maximal slope through the 5 steepest points obtained on the first negative deflection of the potential. By means of input–output curve determination, the maximum fEPSP was found, and during experiments, all potentials used as baseline criteria were evoked at a stimulus intensity that produced 40% of this maximum (100–400 μA). The mean fEPSP slope of the first 6 time periods recorded in each experiment served as baseline. For data comparison, one-way analysis of variance (ANOVA) or Fisher’s least significance difference (LSD) test was used. All data periods were expressed as a mean percentage ± standard error of the mean (SEM) of the average baseline value. High-frequency stimulation (4 trains of 30 pulses at 100 Hz, with an intertrain interval of 5 min) was used to induce LTP defined as lasting over 4 h. LTD was defined as a persistent depression of synaptic strength that endured for over 4 h (Manahan-Vaughan 1997). This is in contrast to in vitro studies, where depression of approximately 40 min is typically referred to as LTD.
Analysis of Network Activity
The intracortical EEG was obtained via recordings from the stratum radiatum layer of hippocampal area CA1. EEG was unfiltered and sampled at 0.5 kHz and stored on a hard disc for additional off-line analysis. To evaluate delta (1–3.5 Hz), theta (4–10 Hz), alpha (10–13 Hz), beta1 (13.5–18 Hz), beta2 (18.5–30 Hz), and gamma (30–100 Hz) oscillatory activity during the course of the experiment, 4-s long epochs, 1 s after each test pulse, were selected. Fourier analysis of artifact-free epochs was performed with a Hanning window function using Spike2 software (Cambridge Electronic Design). The absolute values of spectral power for each individual animal were transformed into relative ones (with the mean value for the baseline preinjection period taken as 100%) that were then used further for statistics. For each time point, the results of Fourier analysis of 5 epochs were averaged. The statistical treatment and analysis of data included the calculation of descriptive statistics (mean, SEM) and ANOVA. ANOVA was used to estimate the effects of LC stimulation in the presence or absence of drugs. For the correlation analyses, we used Pearson's coefficient of comparison.
LC Stimulation
The current level for stimulation of the LC of each animal was chosen through a preliminary input/output assessment (referring current injection to behavioral response) performed 1 week prior to experimental recording. Initial experiments established that 200 μA of current applied within our 100-Hz stimulation protocol consistently resulted in behavioral stress to the animal as displayed by freezing and the production of fecal boli and at times a slight head twitch. By reducing the amount of current to between 60 and 100 μA, no observable behavioral stress was induced and it did not affect the amount of time spent by the animal exploring an open field, subsequent to stimulation. LC stimulation consisted of 2 trains of 100 pulses at 100 Hz with each train lasting 1 s with a 20-s intertrain interval. Stimulus strength was 60–100 μA with single biphasic square wave pulses of 0.1-ms duration per half wave.
Histology
At the end of the study, brains were removed for histological verification of electrode and cannula localization. Brain sections (16 μm) were embedded in paraffin, stained according to the Nissl method using 1% toluidine blue, and then examined using a light microscope. Brains in which incorrect electrode localization was found were discarded from the study.
Compounds and Drug Treatment
±Propranolol (2 μg), ifenprodil (4.9 μg) (all from Biomol, Eching, Germany), or vehicle (0.9% NaCl) were injected via the ipsilateral cerebral ventricle (i.c.v.) in a 5-μl volume over 5 min, 25 min before LC stimulation.
Microdialysis and High-Performance Liquid Chromatography Electrochemical Detection
A guide cannula for microdialysis (Microbiotech, Arsta, Sweden) was implanted at the same location in area CA1, as the recording electrode for electrophysiology experiments. The exposed membrane portion of the probe was 2.0 mm, with the tip positioned at the depth corresponding to the recording electrode for stratum radiatum of CA1. The probe had a polyethersulfone (PES) membrane with a 6-KDa cutoff. For collection of dialysates, the probes (Microbiotech) were perfused at 2 μl/min with Ringer's solution by using a microinfusion pump (11 Plus; Harvard Apparatus GmbH, Hugstetten, Germany). Dialysates were collected every 10 min (20 μl) into vials containing 5 μl of the internal standard solution (4000 pg dihydroxybenzylamine in 20 μl 0.1 M perchloric acid) and were immediately stored at −80 °C C until high-performance liquid chromatography electrochemical detection analysis. Baseline concentrations for all substances were determined as the mean of the 5 dialysates samples taken prior to LC stimulation. Extracellular monoamine concentrations are expressed as the mean ± SEM percentage of the baseline levels. For data comparison, data were normalized to percent baseline, followed by one-way ANOVA and a post hoc one-tailed Tukey's honest significant difference test for those data that were found to be significant by ANOVA.
Behavioral Testing
The episodic-like memory task was performed as described by Kart-Teke et al. (2006) with modifications as described below. Thirty-seven naive male Wistar rats between the ages of postnatal day 70 and day 355 were used. Rats were used for behavioral experimentation 10 days after they were tested for LC stimulation–induced SC–CA1 LTD. Rats were individually housed and maintained in a temperature- and humidity-controlled room (20–22 °C) on a 12-h light–dark cycle and had free access to rodent chow and tap water. Testing occurred between 3:00 and 8:00 PM at the time corresponding to dusk.
In order to reduce possible stress from the testing procedure, rats were habituated to the procedure of being plugged in, having the internal cannula inserted for i.c.v. injections and given 10 min of exploration time in both the empty test field and the holding container for the 3 days preceding the experiment. For animals that would receive i.c.v. injections, test saline injections were given while the animal was in the holding container during the habituation trials. In the habituation sessions that immediately preceded the actual experiments, no saline was administered.
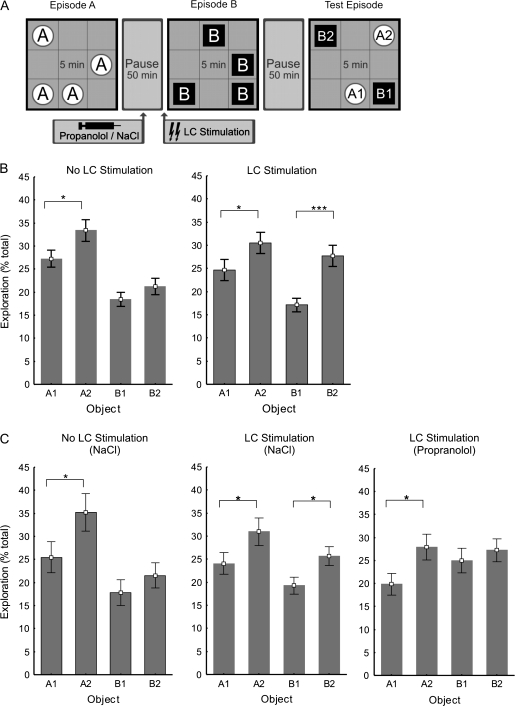
The open field (80 × 80 × 80 cm) was made of gray Perspex and illuminated by two 75W bulbs producing a light intensity of approximately 3.0 lux. A white circle (20 cm diameter) made of construction paper was fixed 35 cm above the floor on one of the walls. The floor was virtually divided into 9 quadrants of equal size. The implanted electrodes and cannula were connected via a flexible cable and a flexible tube to the stimulation, recording, and drug application equipment. A swivel connector ensured that the animal was not restricted in its movement in the chamber. Although not in the chamber but still in an experimental session, the animal was placed in a holding container. Different objects were used for the set of experiments with and without i.c.v. injections. Four different objects in quadruplicate were used for the experiments. Objects differed in terms of height (22–26 cm), base diameter (8–11 cm) and color (transparent, white, or black), shape (rectangle, cylinder, octagon or cone), and surface texture (plain or grooved). Objects had sufficient weight to ensure that they could not be displaced by the rat. Before each trial, the chamber and the objects were thoroughly cleaned with a 1:1 mixture of water and ethanol to rid them of odor cues. The objects had not been introduced to the animals before the first experimental session. Previous trials on another group of rats ensured that animals showed no significant preference for either object. A camera was mounted above the chamber to record the experiments on video for off-line analysis. On the day of the experiment, 30 min before the first sample trial, the rat was habituated to the empty open field for 5 min and then placed in the container for 25 min. After this preliminary habituation session, each animal underwent 2 sample trials and a test trial (Fig. 5). On the first sample trial, the rat was placed in the center of the open field in which identical novel objects had been placed in 4 of the 8 possible locations (the middle square was not used as to avoid entanglement of the cable). After 5 min of exploration (Episode A), the animal was placed back into the holding container. It remained in the holding container for 50 min. In the experiments in which i.c.v. injections were given, after 25 min in the holding container, a total of 5 μl saline or propranolol, respectively, was administered (0.5 μl every 30 s) using a Hamilton syringe. At the end of the interval between Episode A and Episode B, the LC was stimulated as previously described. Immediately after stimulation, the rat was placed into the open field, where 4 new identical objects were randomly positioned. The rat was allowed to explore for 5 min, after which it was removed from the open field and placed in the container for an additional 50 min until the test trial. The test trial was identical to the 2 sample trials except that 2 of the objects from each sample trial were placed in the field. One of the familiar objects was positioned in the same place as previously encountered (A1 and B1) and one of the familiar objects was placed in a new position (A2 and B2) (Fig. 3A). Objects were therefore categorized as; “old familiar” stationary = A1, “old familiar” displaced = A2, “recent familiar” stationary = B1, and “recent familiar” displaced = B2. For each rat, the time spent exploring the objects during sample and test trials was scored offline from videotapes. Exploration of an object was assumed when the rat approached an object and had physical contact with it, either with its snout and/or forepaws. At the end of each test trial, the level of liquid in the tube was checked as an index of successful injection. The scoring of behavioral exploration was done blind to the experimenter.
Figure 5.
LC stimulation prior to an episode results in a β-adrenoceptor–dependent enhancement of spatial memory for that particular episode. (A) Schematic summary of the experimental design for the episodic-like memory task. (B) In control experiments, during the Test Episode, rats preferred exploring old displaced objects (A2) over old stationary objects (A1). They showed no spatial preference in the exploration of the more recently seen objects (B1 vs. B2) (n = 22). In experiments in which rats received LC stimulation prior to Episode B, there was a distinct exploration preference for the new displaced object (B2) over the new stationary object (B1) (n = 22). (C) Vehicle (NaCl 0.9%) injected i.c.v. 25 min prior to Episode B did not alter the rats exploration pattern during the Test Episode (n = 17). Vehicle was also injected 25 min prior to LC stimulation and Episode B. The rats again exhibited a preference for the displaced B2 objects over the stationary B1 objects (n = 17). When the β-adrenoceptor antagonist propranolol (2 μg) was injected i.c.v. prior to LC stimulation and Episode B, the preference for B2 over B1 was blocked leaving the preference for A2 over A1 intact (n = 17). Error bars indicate SEM. *P < 0.05, ***P < 0.001.
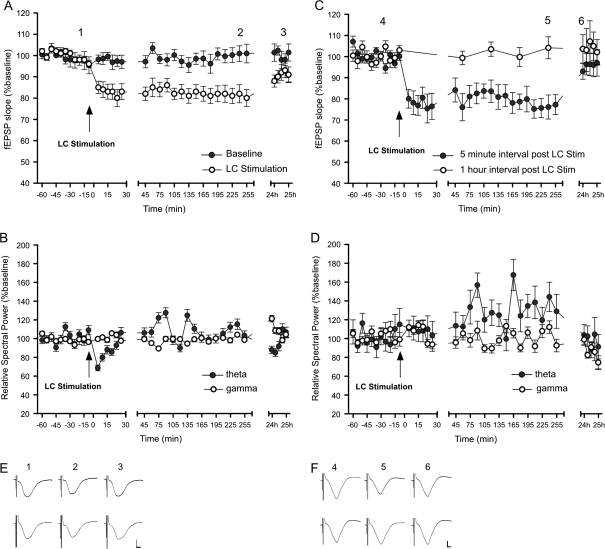
Figure 3.
LC stimulation induces LTD at SC–CA1 synapses. LC burst stimulation (100 Hz) coupled with test pulse stimulation of SC–CA1 synapses induces CA1 LTD that lasts for over 4 h. No depression occurs in the baseline group that did not receive LC stimulation. (B) Induction of LTD is accompanied by a reduction in the spectral power of theta-frequency network oscillations lasting 15 min. The spectral power of gamma frequency oscillations is unaffected. (C) LC stimulation–induced CA1 LTD requires activation of the SC–CA1 pathway following LC stimulation. Application of SC test pulses at 1-h intervals after LC stimulation does not induce LTD. (D) Without regular SC test pulse stimulation after LC stimulation, there is no observable suppression of theta oscillations. (A, C, insets) Analog traces representing SC–CA1 field potentials before LC stimulation, 4 h and 24 h after LC stimulation. (E) Top row: no LC stimulation baseline group. Bottom row: LC stimulation group. (F) Top row: group with 5-min SC test pulse intervals after LC stimulation. Bottom row: group with 1 h SC test pulse intervals after LC stimulation. Calibration: vertical bar, 1 mV; horizontal bar, 3 ms. The mean EPSP slope is shown along with the corresponding SEM.
Animals were reused for both electrophysiology and behavior experiments. Animals were first included in a baseline recording session 7–10 days after surgery to ensure that stable baseline recordings could be made for at least 4 h. Subsequent to baseline recordings, the animals were tested for LC stimulation, and a level of stimulation was chosen between 60 and 100 μA for subsequent experiments. Animals were then tested for their response to LC stimulation 7 days later. All subsequent electrophysiology experiments were performed 7 days after the initial LC stimulation experiment.
The design of the behavioral experiments was a randomized block with repeated measures. All animals had been previously handled extensively and had undergone the initial baseline and LC stimulation experiments.
Results
LC Stimulation Increases NA Levels in the CA1 Region
To examine whether LC stimulation has direct effects on NA release in the hippocampal CA1 region, we used microdialysis analysis to measure extracellular hippocampal monoamine levels, by means of a probe placed in the CA1 stratum radiatum of freely moving adult male Wistar rats. Recordings were taken prior to and following brief (1 s), high-frequency (100 Hz) burst stimulation of the LC. Data were normalized to a percent of the pre-LC stimulation baseline.
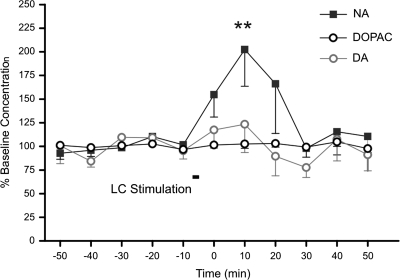
LC stimulation caused a significant increase in the level of NA detected in CA1 (Fig. 1) (ANOVA F1,10 = 6.267; P = 0.013) (Tukey's test, P = 0.002). The concentration of NA increased from 4.76 ± 0.73 pg/20 μl in the period prior to LC stimulation to 8.94 ± 1.38 pg/20 μl for the 10-min period following LC stimulation. No change in the levels of dopamine, or its metabolite 3,4-dihydroxyphenylacetic acid, was observed (Fig. 1).
Figure 1.
Effect of LC stimulation on monoamine levels in CA1. Monoamine levels in hippocampal CA1 were measured before and after LC stimulation using in vivo microdialysis (n = 8). Graph represents the outcome of high-performance liquid chromatography analysis of NA, dopamine (DA), and 3,4-dihydroxyphenylacetic acid (DOPAC) levels (mean ± SEM, n = 8). LC stimulation resulted in a selective increase in the concentration of NA measured in the dialysate.
LC Stimulation Induces CA1 LTD
A primary goal of this study was to assess how LC activation modulates SC–CA1 synaptic transmission. Bipolar stimulating electrodes were chronically implanted in the LC and SC pathway, and a recording electrode was implanted in the stratum radiatum of hippocampal area CA1 (Fig. 2A,B,C,E). To enable i.c.v. application of pharmacological agents, a guide cannula was also implanted in the lateral cerebral ventrical (Fig. 2D). Synaptic transmission was evoked in the CA1 stratum radiatum by test pulse stimulation (5 pulses at 0.0025 Hz every 5 min) of the SCs. LC stimulation (1 s, 100 Hz) resulted in a persistent synaptic depression in CA1, lasting more than 4 h (Fig. 3A, n = 29, F1,24 = 115.33; P < 0.0001), which had recovered 24 h later (F1,4 = 1.3; P = 0.255).
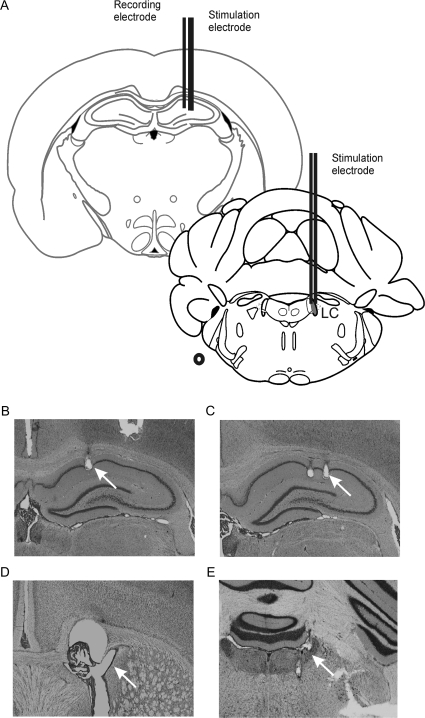
Figure 2.
Placement of electrodes and cannula. (A) Schematic representation of electrode placement in the hippocampus and LC (redrawn from Paxinos and Watson's Atlas, 1997). (B) Microphotograph showing recording electrode placement in the stratum radiatum of CA1. Microphotograph of bipolar stimulation electrode placement in (C) the SC input from CA3 to CA1 and the (E) LC. (D) A cannula was inserted into the lateral cerebral ventricle to enable drug or vehicle solution administration.
Hippocampal theta-frequency oscillations occur as a rat investigates novel stimuli in the environment and are believed to be critical for both the encoding and retrieval of spatial information (Manns et al. 2007). The onset of LTD, which immediately followed LC stimulation, was coincident with a selective suppression of theta-frequency oscillations lasting 15 min (ANOVA F1,24 = 5.865, P < 0.01) (Fisher’s LSD: 5 min [P = 0.0008], 10 min [P = 0.009], and 15 min [P = 0.014] after stimulation) (Fig. 3B). The spectral power of gamma frequency oscillations was unaffected by LC stimulation (Fig. 3B).
The persistence of LTD following LC stimulation was dependent on the subsequent test pulse stimulation interval. LC stimulation resulted in LTD when followed by low-frequency SC test pulse stimulation (5 test pulses at 0.025 Hz every 5 min) (n = 9). However, when fEPSPs were measured at a longer 1-h interval, no LTD was observed (Fig. 3C) (n = 9) (F1,3 = 47.7; P < 0.001). Additionally, the selective suppression of theta activity was absent without regular SC–CA1 test pulse activity after LC stimulation (Fig. 3D) (P = 0.285). This suggests that after LC stimulation, a suppression of theta oscillations is locally induced at SC–CA1 synapses by the subsequent low-frequency test pulse stimulation. The local suppression of theta power at the junction of CA3 input to CA1 (stratum radiatum) may confer predominance to inputs of a more sensory nature coming directly from the EC to CA1 stratum lacunosum. This enhanced temperoammonic pathway input would then facilitate the encoding of new information from the EC over the retrieval of older information from CA3.
LC Stimulation–Induced CA1 LTD is NMDA and β-Adrenoceptor Dependent
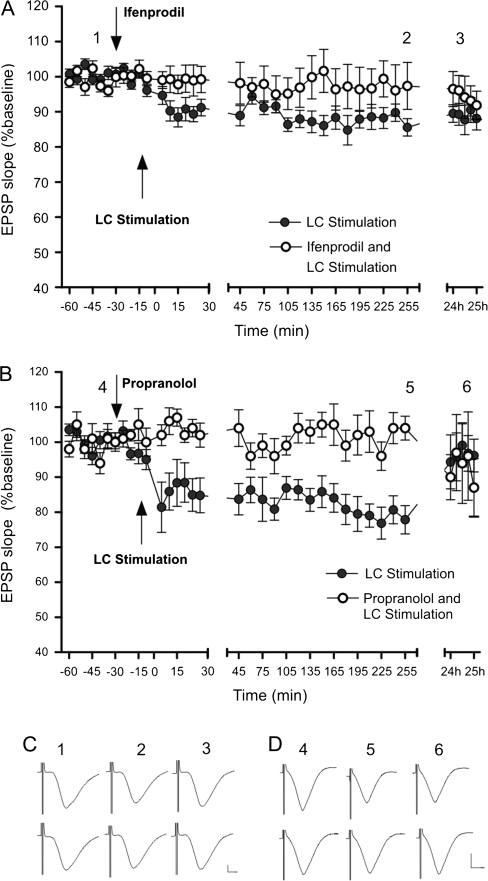
LC stimulation–induced CA1 LTD was blocked by i.c.v. application of an NMDA receptor NR2B subunit–selective antagonist ifenprodil (4.9 μg) (Fig. 4A; F1,24 = 47.4; P < 0.0001) and by the β-adrenergic antagonist propranolol (2 μg) (Fig. 4B; F1,24 = 182.4; P < 0.0001). Ifenprodil, not only eliminated the theta oscillation suppression observed following LC stimulation but also resulted in a persistent increase in theta power (F1,24 = 77.4; P < 0.0001). Propranolol also blocked the selective suppression of theta power observed following LC stimulation (P = 0.437). LC stimulation–induced SC–CA1 LTD and theta oscillation power suppression, thus, requires not only low-frequency SC stimulation but also NR2B subunit and β-adrenoceptor activation.
Figure 4.
LC stimulation–induced CA1 LTD is NR2B and β-adrenoceptor dependent. (A) NMDA receptor subunit 2B (NR2B)–selective antagonist ifenprodil (4.9 μg) and (B) β-adrenoceptor antagonist propranolol (2 μg) block LC stimulation–induced SC–CA1 LTD. (C, D, insets), Analog traces representing SC–CA1 field potentials before LC stimulation, 4 h and 24 h after LC stimulation. Top row: LC stimulation control. Bottom row: LC stimulation with drug application. Calibration: vertical bar, 1 mV; horizontal bar, 3 ms. The mean fEPSP slope is displayed along with the corresponding SEM.
Display of Spatial Memory for an Episode
The synaptic plasticity responses described above may reflect processes underlying hippocampal encoding of novel spatial information. We therefore examined novel learning behavior in our rats and its relationship to NA neuromodulation. Rodents display novelty preference: they innately prefer to explore objects that are in new locations or objects they have not recently encountered. We used this innate preference to test the rats memory for the “what, where, and when” relationship of spatial information (Clayton and Dickinson 1998; Eacott et al. 2005; Babb and Crystal 2006; Dere et al. 2006; Ergorul and Eichenbaum 2006) and to investigate if this is regulated by LC and NA activation.
The experiment was composed of 2 consecutive learning episodes (Episode A and Episode B) followed by a test episode (Fig. 5A). During Episode A and Episode B, the animal was free to explore an open field in which 4 identical immovable objects were randomly positioned. The animal, thus, had the opportunity to learn “what” object was located “where” and during which trial (“when”). During the Test Episode, the open field contained 2 objects from each learning episode, of which one was in the same place as before and one which had been displaced. The animal's memory was evaluated by measuring its relative level of exploration of the stationary (A1) and displaced (A2) objects from Episode A and the stationary (B1) and displaced (B2) objects from Episode B.
We found that the rats clearly preferred to explore the objects to a different degree (n = 22, ANOVA F1,3 = 22.48; P < 0.001). A preference was shown to explore the earlier encountered “A” objects over the more recently encountered “B” objects (n = 22; Fisher’s LSD, P < 0.001). A preference to explore older displaced objects (A2) over older stationary objects (A1) was also evident, in accordance with the novelty preference paradigm (Fig. 3B, n = 22; Fisher’s LSD; P < 0.05). However, the rats do not appear to distinguish between the spatial location of the more recently encountered objects (B1 and B2, Fig. 3A, n = 22, Fisher’s LSD; P = 0.286). This lack of spatial discrimination for the B objects (what) may be attributed to the recency of Episode B. The temporal order (when) of presentation appears to have a stronger effect on memory than the spatial location of the object (where) in this task. This interaction between what, where, and when information suggests that the what, where, and when memory was integrated (Kart-Teke et al. 2006).
LC Stimulation Enhancement of Spatial Memory Is β-Adrenoceptor Dependent
A 2-way ANOVA revealed a significant interaction effect between LC stimulation (stimulation vs. no stimulation) and type of object (displaced vs. stationary) (n = 22, F1,2 = 4.6905; P = 0.032048).
When the animals received LC stimulation immediately preceding Episode B, they showed a significant change in their exploration of the objects from Episode B in the Test Episode (2-way ANOVA [stimulation × type of object], n = 22, F1,2 = 4.6905; P < 0.05), showing greater exploration of the spatially displaced object (B2) than of the stationary object (B1) during the test trial (Fig. 5B, n = 22, Fisher’s LSD; P < 0.001). There was no significant difference in the relative exploration of the objects from Episode A due to LC stimulation (2-way ANOVA [stimulation × type of object], n = 22, F1,3 = 0.0032; P = 0.9547).
The preference for the older “A” objects over the newer “B” objects was no longer statistically significant in the group that received LC stimulation (n = 22, F1,2 = 2.906; P = 0.090). No differences in object exploration levels during Episodes A and B were observed that could account for differences seen in the exploration of objects in the Test Episode. There were also no significant differences in exploration during Episodes A and B due to NaCl (vehicle) injection (P = 0.255), NaCl injection and stimulation (P = 0.254), or propranolol (β-adrenoceptor antagonist) injection and stimulation (P = 0.790).
These findings suggest that LC stimulation facilitates memory of object–place associations for a particular episode while simultaneously impairing temporal order memory. The question remains as to whether the improved spatial memory is due to the effect of LC stimulation on encoding, consolidation, or retrieval. To answer this question, we repeated the tests in the presence of the β-adrenoceptor antagonist propranolol. We used new subjects and 2 new sets of objects for these experiments (Fig. 5C).
Rats given vehicle injections prior to Episode B (n = 17) showed a similar pattern of Test Episode exploration as those in control experiments done without injections (Fig. 5B,C). There was no significant difference in the pattern of exploration during the Test Episode between the subject groups that did not receive LC stimulation based on the presence or absence of NaCl injections (2-way ANOVA [injection × type of object], F1,3 = 0.737, P = 0.5309). Likewise, there was no significant difference due to NaCl injection between the groups that did receive LC stimulation (2-way ANOVA [injection × type of object], F1,3 = 1.117, P = 0.3429).
Interestingly, there was a significant difference in the exploration pattern between the rats that received LC stimulation depending on whether they received NaCl or propranolol injected before Episode B (2-way ANOVA, F1,3 = 3.06, P < 0.05). When propranolol (2 μg) was administered, prior to LC stimulation and Episode B, LC-mediated enhanced spatial memory was completely eliminated (Fig. 5C, n = 17, Fisher’s LSD; P = 0.663). Critically, the rats continued to prefer the displaced object for the session that preceded administration of propranolol (A2 > A1) (n = 17, Fisher’s LSD; P < 0.05). The LC stimulation–induced increase in spatial discrimination for a specific episode therefore appears to be β-adrenoceptor dependent. This improved spatial memory also appears to be specific for the encoding of that particular episode in memory (Episode B) as opposed to enhancing consolidation or retrieval of the previous episode.
Discussion
Our data support that the LC plays an intrinsic role in both synaptic information storage and memory processing in the hippocampus. We observed that LC stimulation results in a significant increase of NA in the CA1 region. LC stimulation also modified SC–CA1 stratum radiatum synaptic transmission such that LTD is enabled. To our knowledge, this is the first time that LC activation has been shown to depress CA1 synaptic transmission. This novel form of synaptic plasticity is NMDA and β-adrenoceptor dependent and is associated with a transient suppression of theta-frequency network oscillations. In addition, LC activation facilitated the encoding of spatial memory for a single episode via a β-adrenoceptor–dependent mechanism. These findings suggest that the LC plays a key role in the induction of hippocampal LTD and in facilitating the encoding of spatial information. This modulation of hippocampal function may reflect a means by which salient information is distinguished by the hippocampus for subsequent synaptic processing.
The primary source of NA in the brain is the LC. LC neurons fire phasically in response to novel information and changes in the environment (Sara et al. 1994; Vankov et al. 1995). These findings led to the hypothesis that the phasic activation of LC neurons in time with cognitive shifts could provoke or facilitate dynamic reorganization of target neural networks, permitting rapid behavioral adaptation to changing environmental information (Bouret and Sara 2005). Kety (1970) postulated that noradrenergic activation fulfills a dual role in regulating synaptic activity: “suppressing most, but permitting or even accentuating activity in those [synapses] that are transmitting novel or significant stimuli.” The hippocampal formation receives abundant NA input from the LC (Loy et al. 1980). We therefore investigated whether LC activation can directly influence synaptic strength in the hippocampus. Test pulse stimulation of SC–stratum radiatum synapses in the CA1 region enabled recording of highly stable basal synaptic transmission that could be followed for over 24 h. If test pulse stimulation was coupled with LC activation, LTD was immediately induced. This finding is intriguing, given recent reports that LTD is associated with the encoding of novel spatial information in the hippocampus (Manahan-Vaughan and Braunewell 1999; Duffy et al. 2001; Li et al. 2003; Kemp and Manahan-Vaughan 2004; Lemon and Manahan-Vaughan 2006; Oomura et al. 2006; Kemp and Manahan-Vaughan 2007).
LTD induced in the CA1 stratum radiatum by LC activation is dependent on activation of NMDA receptors, for which a role in learning has already been described (Morris et al. 1986; Morris 2006). This form of LTD is also β-adrenoceptor dependent. β-Adrenoceptors are G-protein–coupled receptors that activate adenylyl cyclase, producing cyclic adenosine 3′,5′-monophosphate (cAMP) that binds to regulatory subunits of cAMP-dependent protein kinase A holoenzymes (Johnson 1998; Gelinas and Nguyen 2007). β-Adrenoceptor activation facilitates the expression of both LTP and LTD at SC–CA1 synapses (Thomas et al. 1996; Katsuki et al. 1997; Gelinas and Nguyen 2005; Gelinas et al. 2007). Furthermore, activation of β-adrenoceptors lowers the threshold for LTP and LTD induction at SC–CA1 synapses (Katsuki et al. 1997). Our data support that coincident β-adrenoceptor and glutamatergic receptor activation, via LC and SC stimulation, respectively, results in a shift of the frequency-response relationship of stratum radiatum synapses, thereby lowering the threshold for LTD induction. This shift, following LC stimulation, permits the induction of LTD at very low stimulation frequencies (5 test pulses at 0.025 Hz every 5 min), which normally do not affect synaptic strength.
Concomitant with the induction of hippocampal LTD via LC stimulation was the transient onset of a suppression of theta-frequency network activity. In the hippocampus, one of the most prominent patterns of network oscillatory activity is theta rhythm (4–10 Hz), which is highest during behavioral states such as spatial exploration or rapid eye movement (REM) sleep and which may reflect active information processing (Kahana et al. 2001; Buzsaki 2002, 2005). The transient suppression of theta oscillatory power, which was associated with the induction of LC stimulation–induced CA1 LTD, may be the manifestation of a network reset function in the hippocampus (Pavlides et al. 1988; Huerta and Lisman 1993, 1995; Givens 1996) that serves to amplify incoming information and possibly facilitate LTD induction.
Our observation that theta activity was reduced following LC stimulation is in contrast to an earlier report where an increase was reported (Berridge and Waterhouse 2003; Brown et al. 2005). However, Berridge and Foote recorded theta between a narrow range of 2.7–6.8 Hz and saw this range increase in spectral power after LC stimulation via bethanechol injection, whereas all other frequencies were decreased. Brown et al. (2005) defined theta as oscillations occurring between 4 and 8 Hz. They observed a significant increase in 8- to 12-Hz theta in the dentate gyrus (and not in the CA1), and only within the first minute after LC stimulation, whereas between 8 and 12 Hz they observed a small insignificant decrease in theta within the first minute after LC stimulation. They used glutamate injection to the LC to trigger responses. We defined theta as oscillations that occurred between 4 and 12 Hz and used electrical stimulation. This may explain the differences in the results seen.
The persistence of LTD following LC stimulation was dependent on the subsequent test pulse stimulation interval. LC stimulation resulted in LTD when followed by low-frequency SC test pulse stimulation. However, when fEPSPs were measured at a longer 1-h interval, no LTD was observed. Additionally, the selective suppression of theta activity was absent without regular SC–CA1 test pulse activity after LC stimulation. This suggests that after LC stimulation, a suppression of theta oscillations is locally induced at SC–CA1 synapses by the subsequent low-frequency test pulse stimulation. The local suppression of theta power at the junction of CA3 input to CA1 (stratum radiatum) may confer predominance to inputs of a more sensory nature coming directly from the EC to CA1 stratum lacunosum. This enhanced temperoammonic pathway input would then facilitate the encoding of new information from the EC over the retrieval of older information from CA3.
Our data show that LTD triggered by LC activation depends critically upon coincident (test pulse) stimulation of afferent fibers to the CA1 region and is consistent with other reports as to the necessity of coincident information signals for the induction of synaptic plasticity. In a previous paper, we reported that a certain threshold of postsynaptic activity must be passed in order for LTD to occur (Manahan-Vaughan and Braunewell 1999). Stimulation of the SCs (during exposure to a novel environment) at a frequency of once every 60 min will not induce LTD, whereas stimulation at once every 40 s is effective. This suggests that a coincidence of the novel informational signal with the activation of a specific set of synapses is sufficient to induce LTD. Our LC data are an extension of this: It suggests that a combination of LC activation and postsynaptic (CA1) activity is essential for the induction of input-specific LTD. This is an important finding as it suggests that LC activation does not elicit a global and generalized suppression of synaptic transmission in the CA1 region.
LTP is believed to be one of the mechanisms that underlie learning and memory (Martin et al. 2000; Whitlock et al. 2006). Previous studies have shown that LC stimulation can restore decaying SC–CA1 LTP, thus, contributing to LTP maintenance (Ezrokhi et al. 1999). In vitro studies have also shown that NA, and specifically β-adrenoceptor activation, promotes LTP in adult Wistar rats (Thomas et al. 1996; Katsuki et al. 1997; Izumi and Zorumski 1999; Gelinas and Nguyen 2005). We have shown here that stimulating the LC in freely moving rats lowers the threshold for the induction of hippocampal LTD and depotentiation. The increased range of the SC–CA1 synaptic response following LC stimulation may increase the signal to noise ratio in this pathway and enhance information processing (Aston-Jones and Cohen 2005). Thus, synapses that receive a low-frequency afferent stimulation would be more likely to become depressed, whereas those receiving higher frequency stimulation would be relatively more potentiated.
We have observed that LTP occurs at all synapses of the trisynaptic circuit when the environment changes in a global manner. LTD occurs in the dentate gyrus in response to novel configurations of landmark cues, whereas LTD occurs in the CA1 region in response to novel configurations of small contextual cues (Kemp and Manahan-Vaughan, 2007, 2008a). Given the temporal dynamics of LTP versus LTD induction (rapid induction via high-frequency afferent activation vs. gradual induction via prolonged low-frequency afferent activation), we hypothesize that LTP may comprise a fundamental encoding response to general changes in a spatial environment. Whether LTD participates in this encoding is determined by the saliency of the contextual information. LTD is believed to play an important role in the encoding of novel contextual space (Manahan-Vaughan and Braunewell 1999; Etkin et al. 2006; Lemon and Manahan-Vaughan 2006; Kemp and Manahan-Vaughan 2007, 2008a, 2008b). In line with this, LC activation not only facilitated LTD but also facilitated the encoding of spatial memory for a single episode via a β-adrenoceptor–dependent mechanism. Taken together, these data suggest that LC activation in response to novel spatial exploration may confer informational saliency such that information storage by means of hippocampal LTD occurs.
To test the effects of LC stimulation on learning and remembering unique episodes, we employed an open-field task designed to test the rat's object recognition memory (what), memory for object location (where), and temporal order memory (when) (Kart-Teke et al. 2006). The task exploited the animals’ innate preference to explore objects which have not been recently encountered or that are in different locations. Our research confirms the ability of rats to form spatial memories for discrete episodes (Dere et al. 2006). We also show for the first time that LC activation preceding exposure to a novel environment facilitates memory for the spatial details of that environment while interfering with temporal order memory. A number of different explanations are possible. The LC stimulation may facilitate spatial memory for Episode B, it may interfere with the recency effect displayed for the memory of Episode B, or it may do both. Eliminating the recency effect in this task would increase the exploration of B objects in the Test Episode. Once the strong temporal order effect is diminished, the weaker spatial memory may become more prevalent resulting in B2 being explored more than B1. Importantly, memory for the previously explored Episode A, which was not preceded by LC stimulation remained unaffected. Thus, facilitation of spatial memory for an episode occurred following LC stimulation, whereas temporal order memory was unaffected.
The finding that LC activation facilitated spatial memory for an episode in a β-adrenoceptor–dependent manner, combined with the observation that LC-mediated hippocampal LTD was also β-adrenoceptor dependent, provides an interesting link to reports that support a role for LTD in encoding spatial context in the hippocampus (Heynen et al. 1996; Lemon and Manahan-Vaughan 2006; Kemp and Manahan-Vaughan 2007, 2008a). Recently, it was shown that LTD at CA1 synapses that is facilitated by novel learning behavior, but not LTD that is elicited solely by electrical activation of SCs (via low frequency, i.e., 1 Hz, stimulation), depends on β-adrenoceptor activation (Kemp and Manahan-Vaughan 2008b). The facilitation of short-term depression (STD) into LTD produced through spatial exploration may be analogous to the β-adrenoceptor–dependent, LC stimulation–induced SC–CA1 LTD that we observed. β-Adrenoceptor activation in area CA1 initiates local protein synthesis required for late LTP (Gelinas and Nguyen 2005), suggesting that activation of translation machinery may play a role in the induction of β-adrenoceptor–dependent LTD seen in the present study.
The release of NA by the LC may provoke or facilitate dynamic reorganization of neural networks through changes in synaptic strength (Bouret and Sara 2005). Using the rat as a model, we have explored how LC stimulation affects hippocampal SC–CA1 synaptic plasticity and learning and memory. Stimulating the LC in freely moving rats facilitates the induction of LTD. LC stimulation immediately prior to a learning episode also selectively increases memory for the spatial aspects of that episode. Memory for a nonstressful odor–award association has previously been shown to be impaired at the late stages of consolidation by a β-adrenoceptor antagonist (Sara et al. 1999). We have shown that memory “induction” is also reliant upon β-adrenoceptor activation. Propranolol administration prior to an episode impairs the spatial memory for that particular episode, whereas leaving the spatial memory for the previous episode intact. LC stimulation therefore not only induces SC–CA1 LTD but also facilitates memory encoding for the spatial details of an event.
Conclusion
We have found that the formation of spatial contextual memory in rats is heavily influenced by activation of the adrenergic system. Activation of the LC elevates NA levels in the hippocampus, suppresses theta-frequency network activity, and also enables the induction of hippocampal long-term synaptic depression, that is, β-adrenoceptor and NMDA receptor dependent. Our data support that the LC novelty response triggers persistent synaptic plasticity in the hippocampus and enduring spatial memory. The activity of the LC may, thus, confer saliency upon novel spatial information such that hippocampal LTD and spatial memory formation result.
Funding
International Graduate School of Neuroscience (IGSN) scholarship (to N.L.) and the German research foundation (Deutsche Forschungsgemeinschaft [DFG] grant Ma1843 to D.M-V.).
Acknowledgments
We gratefully acknowledge the assistance of Tim Parker, Stoyan Popkirov, Arthur Bikbaev, Jens Klausnitzer, and Beate Krenzek.
Conflict of Interest: None declared.
References
- Alger BE, Teyler TJ. Long-term and short-term plasticity in the CA1, CA3, and dentate regions of the rat hippocampal slice. Brain Res. 1976;110:463–480. doi: 10.1016/0006-8993(76)90858-1. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. doi: 10.1002/cne.20723. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brown RAM, Walling SG, Milway JS, Harley CW. Locus ceruleus activation suppresses feedforward interneurons and reduces {beta}-{gamma} electroencephalogram frequencies while it enhances {theta} frequencies in rat dentate gyrus. J Neurosci. 2005;25:1985–1991. doi: 10.1523/JNEUROSCI.4307-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta rhythm of navigation: link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15(7):827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Dahl D, Sarvey JM. Beta-adrenergic agonist-induced long-lasting synaptic modifications in hippocampal dentate gyrus require activation of NMDA receptors, but not electrical activation of afferents. Brain Res. 1990;526:347–350. doi: 10.1016/0006-8993(90)91245-c. [DOI] [PubMed] [Google Scholar]
- Dere E, Kart-Teke E, Huston JP, De Souza Silva MA. The case for episodic memory in animals. Neurosci Biobehav Rev. 2006;30:1206–1224. doi: 10.1016/j.neubiorev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Devauges V, Sara SJ. Memory retrieval enhancement by locus coeruleus stimulation: evidence for mediation by beta-receptors. Behav Brain Res. 1991;43:93–97. doi: 10.1016/s0166-4328(05)80056-7. [DOI] [PubMed] [Google Scholar]
- Duffy SN, Craddock KJ, Abel T, Nguyen PV. Environmental enrichment modifies the PKA-dependence of hippocampal LTP and improves hippocampus-dependent memory. Learn Mem. 2001;8:26–34. doi: 10.1101/lm.36301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learn Mem. 2005;12:221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- Easton A, Eacott MJ, Zinkivskay A, Jimenez-Rodriguez M. 2006. Fornix lesions and episodic-like memories: Recollection impaired, familiarity intact. In: Soc. Neurosci. Abstr. 66.5. Atlanta. [Google Scholar]
- Eichenbaum H. Is the rodent hippocampus just for ‘place'? Curr Opin Neurobiol. 1996;6:187–195. doi: 10.1016/s0959-4388(96)80072-9. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. Essential role of the hippocampal formation in rapid learning of higher-order sequential associations. J Neurosci. 2006;26:4111–4117. doi: 10.1523/JNEUROSCI.0441-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Alarcón JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER. A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron. 2006;50:127–143. doi: 10.1016/j.neuron.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Ezrokhi VL, Zosimovskii VA, Korshunov VA, Markevich VA. Restoration of decaying long-term potentiation in the hippocampal formation by stimulation of neuromodulatory nuclei in freely moving rats. Neuroscience. 1999;88:741–753. doi: 10.1016/s0306-4522(98)00232-2. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. {beta}-Adrenergic receptor activation facilitates induction of a protein synthesis-dependent late phase of long-term potentiation. J Neurosci. 2005;25:3294–3303. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Nguyen PV. Neuromodulation of hippocampal synaptic plasticity, learning, and memory by noradrenaline. Cent Nerv Syst Agents Med Chem. 2007;7:17–33. [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of vagus nerve stimulation on memory. Cogn Behav Neurol. 2006;19:119–122. doi: 10.1097/01.wnn.0000213908.34278.7d. [DOI] [PubMed] [Google Scholar]
- Givens B. Stimulus-evoked resetting of the dentate theta rhythm: relation to working memory. Neuroreport. 1996;8:159–163. doi: 10.1097/00001756-199612200-00032. [DOI] [PubMed] [Google Scholar]
- Harley C. Noradrenergic and locus coeruleus modulation of the perforant path-evoked potential in rat dentate gyrus supports a role for the locus coeruleus in attentional and memorial processes. Prog Brain Res. 1991;88:307–321. doi: 10.1016/s0079-6123(08)63818-2. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364:723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Zorumski CF. Norepinephrine promotes long-term potentiation in the adult rat hippocampus in vitro. Synapse. 1999;31:196–202. doi: 10.1002/(SICI)1098-2396(19990301)31:3<196::AID-SYN4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158(5 Pt 3):S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem. 2006;85:173–182. doi: 10.1016/j.nlm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Katsuki H, Izumi Y, Zorumski CF. Noradrenergic regulation of synaptic plasticity in the hippocampal CA1 region. J Neurophysiol. 1997;77:3013–3020. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. The hippocampal CA1 region and dentate gyrus differentiate between environmental and spatial feature encoding through long-term depression. Cereb Cortex. 2008a;18:968–977. doi: 10.1093/cercor/bhm136. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. {beta}-Adrenoreceptors comprise a critical element in learning-facilitated long-term plasticity. Cereb Cortex. 2008b;18:1326–1334. doi: 10.1093/cercor/bhm164. [DOI] [PubMed] [Google Scholar]
- Kety SS. The biogenic amines in the central nervous system: their possible roles in arousal, emotion, and learning. In: Schmitt FO, editor. The Neurosciences: Second Study Program. New York: The Rockefeller University Press; 1970. pp. 324–336. [Google Scholar]
- Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–47. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Loy R, Koziell DA, Lindsey JD, Moore RY. Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol. 1980;189:699–710. doi: 10.1002/cne.901890406. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci USA. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol Learn Mem. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Morris RG. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 2006;23:2829–2846. doi: 10.1111/j.1460-9568.2006.04888.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Munro CA, Walling SG, Evans JH, Harley CW. Beta-adrenergic blockade in the dentate gyrus in vivo prevents high frequency-induced long-term potentiation of EPSP slope, but not long-term potentiation of population spike amplitude. Hippocampus. 2001;11:322–328. doi: 10.1002/hipo.1046. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. The hipocampus as a cognitive map. Oxford: Clarendon; 1978. [Google Scholar]
- Oomura Y, Hori N, Shiraishi T, Fukunaga K, Takeda H, Tsuji M, Matsumiya T, Ishibashi M, Aou S, Li XL, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Greenstein YJ, Grudman M, Winson J. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Vankov A, Herve A. Locus coeruleus-evoked responses in behaving rats: a clue to the role of noradrenaline in memory. Brain Res Bull. 1994;35:457–465. doi: 10.1016/0361-9230(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: beta-adrenergic receptor involvement in the late phase. Learn Mem. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- Straube T, Korz V, Balschun D, Frey JU. Requirement of beta-adrenergic receptor activation and protein synthesis for LTP-reinforcement by novelty in rat dentate gyrus. J Physiol. 2003;552:953–960. doi: 10.1113/jphysiol.2003.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Vankov A, Herve-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci. 1995;7:1180–1187. doi: 10.1111/j.1460-9568.1995.tb01108.x. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]