Abstract
Previous studies have proposed a variety of mechanisms by which attention influences neuronal activity. Here we investigated the mechanisms of attention in the striate cortex of monkeys performing a spatial or an object-based attention task at various stimulus contrasts and compared neuronal contrast response functions with and without attention. Our data are best described by an “additive” interaction: The influence of attention on the neuronal response is relatively independent of the stimulus contrast, at least when the stimulus has enough contrast to become visible. This shows that attention adds to the neuronal responses in a largely contrast invariant manner. These data support recent functional magnetic resonance imaging studies and suggest that feedback from higher areas exerts a constant attentional drive that is mostly task not stimulus driven.
Keywords: area V1, attentional modulation, contrast gain, contrast sensitivity, response gain
Introduction
Our ability to detect and discriminate a visual stimulus improves when we direct our attention to it, especially if the stimulus is faint or embedded in a cluttered scene (Bashinski and Bacharach 1980; Lu and Dosher 1998; Dosher and Lu 2000; Zenger et al. 2000). Over the last 2 decades, it has become clear that attention shifts are associated with changes in the activity of neurons in the visual cortex as well as in subcortical structures. Neurons that respond to an attended object increase their firing rate while the neuronal responses to other, unattended objects are suppressed. The attentional modulation of neuronal firing rates in cortex was initially described in higher visual areas (Bushnell et al. 1981; Moran and Desimone 1985; Spitzer et al. 1988; Treue and Maunsell 1996; Reynolds et al. 1999; Bisley and Goldberg 2003) but later also in low-level visual areas including the primary visual cortex (Motter 1993; Roelfsema et al. 1998; Vidyasagar 1998; Ito and Gilbert 1999; Li et al. 2004, 2006; Roberts et al. 2007).
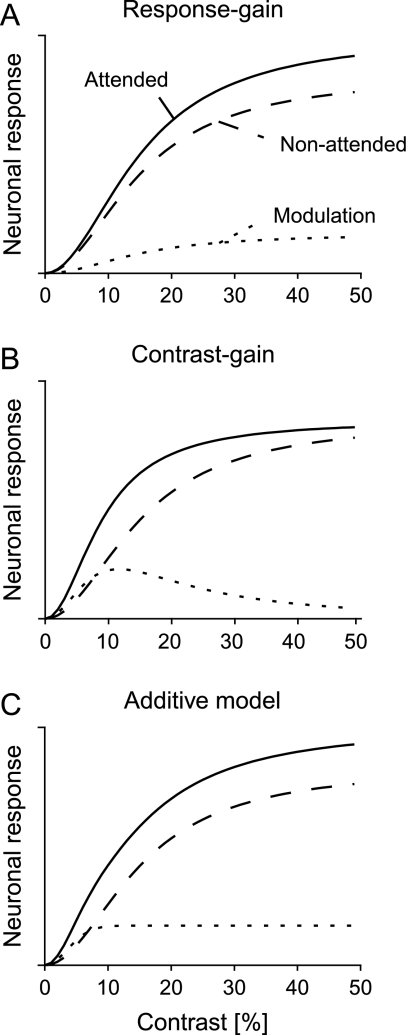
Despite a wealth of studies, important questions about the mechanism by which attention influences neuronal activity have remained unresolved. Some previous studies showed that attention scales neuronal responses in proportion to the response in the absence of attention: weak responses increase slightly, whereas strong responses increase more (Treue and Maunsell 1996; Treue and Trujillo 1999; McAdams and Maunsell 2000). The findings inspired a “multiplicative” or “response gain control” model of attention (Fig. 1A). However, this response gain model may not hold for all stimulus features as the effects of attention and stimulus contrast on a neuron's response do not always interact in a multiplicative manner.
Figure 1.
Three models for the effect of attention on contrast response functions. (A) According to the response gain model, attention increases the response in proportion to the response evoked in the absence of attention. The dashed curve represents the contrast response function without attention, the continuous curve the contrast response function with attention, and the dotted curve shows the response difference. Note that the effect of attention on neuronal firing rates is predicted to be strongest at the highest contrasts. (B) Contrast gain model that proposes that attention increases the effective contrast, causing a leftward shift of the contrast response function. This model predicts strongest effects of attention on the neuronal responses evoked by stimuli of lower contrast. (C) The additive model proposes that attention adds a fixed amount to the neuronal response once the stimulus has sufficient contrast to be detected by the animal. In this model, the effect of attention is relatively constant across a wide range of stimulus contrasts.
Specifically, Reynolds et al. (2000) demonstrated that attention enhances the weak response of neurons in area V4 evoked by low-contrast stimuli but that it has only little influence on the stronger response evoked by high-contrast stimuli (Fig. 1B). Attention thus appeared to shift the neurons’ contrast response function to the left as if it increased the effective contrast of the stimulus in their receptive field (RF). In a subsequent study, Martinez-Trujillo and Treue (2002) showed that neurons in motion sensitive area MT behave similarly: They also shift their contrast response function when the stimulus in their RF is attended, in support of what is now called the “contrast gain model” of attention. The idea emerging from these studies is that attention and contrast share the same neuronal code (Treue 2004). This hypothesis received support from a psychophysical study in human observers showing that an attended stimulus appears to have a higher contrast than a stimulus that is not attended (Carrasco et al. 2004).
The idea that the effects of attention are similar to an increase in stimulus contrast is not undisputed, however, as other psychophysical studies reported that attention has only little influence on perceived contrast (Prinzmetal et al. 1997; Liston and Stone 2008; Schneider and Komlos 2008), whereas another study demonstrated that attention and contrast can even have opposite effects (Roberts and Thiele 2008a, 2008b). Furthermore, observers are well able to direct their attention to low-contrast image regions and even give them priority if they are task relevant (Pashler et al. 2004; Einhauser et al. 2008). Also, a recent neurophysiological study by Williford and Maunsell (2006) found that attention does not necessarily result in a change of contrast gain of area V4 neurons. They observed that some neurons behaved according to the contrast gain model, whereas other neurons changed their response in accordance with the response gain model, and yet others displayed mixed effects. These results, taken together, suggest that attention and contrast may interact in multiple ways, but a single unifying picture has not yet emerged.
Imaging studies using functional magnetic resonance imaging (fMRI) in human observers inspired yet another type of model for the interaction between attention and contrast. In higher visual areas, such as the fusiform face area, the strength of the neuronal responses is relatively independent of the stimulus contrast once the stimulus has the necessary contrast to be perceived (Avidan et al. 2002). These contrast invariant responses are only observed, however, for attended objects, whereas the responses evoked by unattended objects depend monotonically on stimulus contrast (Murray and He 2006). If the attentional modulation of neuronal responses in lower visual areas depends on the feedback from higher areas, then it might be expected that the attentional modulation of neurons in low-level areas is also relatively independent of contrast. Recent fMRI studies (Buracas and Boynton 2007; Murray 2008) observed precisely such an “additive” interaction between attention and contrast in lower level visual areas V1, V2, and V3: Attention added an amount of blood oxygen level–dependent (BOLD) activity that did not depend strongly on stimulus contrast (Fig. 1C).
In an attempt to reconcile these discrepancies, we investigate the effect of attention on contrast tuning in the primary visual cortex of monkeys. We chose V1 as our target area because the previous electrophysiological studies on the interaction between attention and contrast were carried out in extrastriate areas. We conjectured that if attention and contrast are effectively interchangeable in affecting neuronal responses in area V1, at the lowest hierarchical level of visual cortical processing, then this type of interaction might be inherited by higher visual areas. Additionally, we specifically intended to test the possibility of an additive interaction between attention and contrast. Figure 1 illustrates that the predictions of the additive model are intermediate between the predictions of the contrast gain and response gain models. The response gain model predicts that attentional modulation is strongest for stimuli with a high contrast, the contrast gain model predicts strongest modulation at low contrast, whereas the additive model predicts relatively constant effects across a range of contrasts once the stimulus has enough contrast to become visible. To ensure the generality of our results, we studied the effects of attention in the primary visual cortex with 2 different tasks, a detection task probing aspects of top-down spatial attention and a curve-tracing task which probed aspects of object-based attention.
Methods
All experiments were carried out in accordance with the European Communities Council Directive 1986 (86/609/EEC) and the US National Institutes of Health Guidelines for the Care and Use of Animals for Experimental Procedures. The experiments performed at Newcastle University were additionally approved by the UK Home Office and in accordance with the UK Animals Scientific Procedures Act. Those performed at the Netherlands Institute for Neuroscience were approved by the institutional animal care and use committee of the Royal Netherlands Academy of Arts and Sciences.
Surgical Preparation
Experiments at Newcastle University
Following initial training, monkeys were implanted with a head holder, eye coil, and recording chambers above V1 under general anesthesia and sterile conditions. All details regarding surgical procedures, postoperative care, and the cleaning of the implant and recording chambers are published in detail elsewhere (Thiele et al. 2006).
Experiments at the Netherlands Institute for Neuroscience
Two macaque monkeys were implanted with a head holder, and a gold ring was inserted under the conjunctiva of one eye for the measurement of eye position.
In a separate operation, arrays of 4 × 5 or 5 × 5 electrodes (Cyberkinetics Neurotechnology Systems Inc., Foxborough, MA) were chronically implanted in area V1. The operations were performed under aseptic conditions and general anesthesia. Details of the surgical procedures and the postoperative care have been described elsewhere (Roelfsema et al. 1998).
Behavioral Tasks
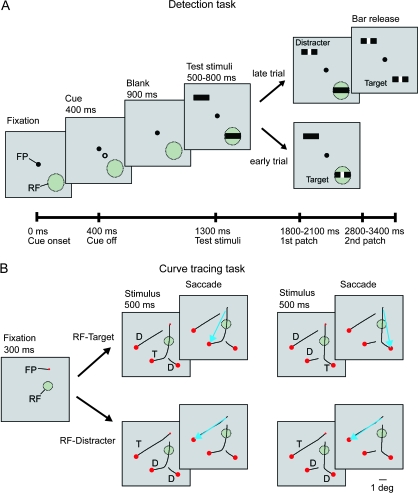
We employed 2 different tasks to determine the effect of attention on contrast response functions in V1. In one of the tasks, the animal's attention was directed by a visual cue on each trial. Their task was to detect a subtle change in luminance of the test stimulus presented at the cued location. We will refer to this task as the “detection task” for the remainder of the paper (Fig. 2A). These experiments were conducted at Newcastle University. In the other task, animals had to perform a mental curve-tracing task, in order to determine which of 3 peripherally located circular targets was connected to the fixation point (FP). We will refer to this task as the “curve-tracing task” for the remainder of the paper (Fig. 2B). The curve-tracing experiments were conducted at the Netherlands Institute for Neuroscience. We obtained behavioral and neuronal data from 2 animals in each of the 2 tasks, thus providing data from a total of 4 animals.
Figure 2.
Two tasks used to investigate the effect of attention on contrast response functions in area V1. (A) Detection task. Monkeys grasped a touch bar and fixated a fixation spot (FP) at the center of the monitor. After 250 ms of fixation, a cue was displayed indicating where the animal should attend (here we illustrate an attend RF trial). The cue was on for 400 ms, thereafter a period of 250 ms (monkey B) or 900 ms (monkey H) followed in which the animal maintained fixation until the test stimuli (black bars) appeared that had a varying luminance contrast. After an additional 500-800 ms a small patch with a higher luminance appeared either at the cued location (in which case it was a target) or at the distracter location. The animal had to report the target appearance by releasing the touch bar but to ignore distracter appearances. Dashed circle: receptive field (RF). (B) Curve-tracing task. The monkey fixated a red fixation point (FP) in the center of the screen. After 300 ms, 3 curves with 3 red circles at their ends were displayed. The monkey had to trace the target curve (T) that was connected to the FP and to ignore the distracter curves that were not connected (D), whereas he maintained fixation. Either the target curve or the distracter curve fell in the RF. After a delay of 500 ms, the FP disappeared and the monkey made an eye movement to the circle at the other end of the target curve (blue arrow).
Detection Task
Monkeys had to detect a small change in luminance at a cued (attended) location, while ignoring a luminance change that occurred at a noncued location (Fig. 2A outlines the basics of the task). Monkeys initiated trials by holding a touch bar and fixating a red FP (0.1° diameter) on a gray background (21 cd/m2) presented centrally on a 20″ analog cathode ray tube (CRT) monitor (75 Hz in monkey B, 100 Hz in monkey H, 1600 × 1200 pixels, 57 cm from the animal). The eye fixation window was ±0.3°–0.35° in monkey B and ±0.6°–0.7° in monkey H. Eye position was recorded with a scleral search coil in monkey B and with a scleral search coil or an infrared based camera system in monkey H (Thomas Recording GmBH, Giessen, Germany). A cue (blue annulus, 0.24° outer diameter, 0.18° inner diameter) was presented for 400 ms on one side of the fixation spot along the (invisible) line connecting the FP and the RF location. The cue was displaced either toward or away from the RF, at a distance from the FP of one-quarter of the eccentricity of the neuron's RF to indicate whether attention should be directed toward or away from the stimulus presented in the RF, respectively. After cue offset, a 250-ms blank (900 ms in monkey H) period occurred with just the FP present. Spatial and temporal separation of the cue from the test stimuli ensured that it had no direct effect on the neuronal response to the test stimulus. Thereafter, 2 identical stimuli were presented (test stimuli), one centered on the RF, the other at the same eccentricity in the opposite hemifield. Test stimuli were bars of the neuron's preferred orientation and subtended 0.4° × 0.1° of visual angle. The luminance of the bar was parametrically varied to measure the contrast response function when animals attended to the neuron's RF and when they attended away. We used 8 different contrasts, which were: 5.3%, 9.9%, 14.6%, 20.4%, 25.4%, 30.4%, 49.5%, and 100% Michelson contrast. The luminance of the bars was lower than the background, that is, a 100% contrast refers to a black bar on a gray background. After 500–800 ms (randomized in 1 ms steps), a brighter patch (0.1° × 0.1°) appeared at the center of one of the bars. If presented in the cued location, it is referred to as “target,” if presented in the noncued location, it is referred to as “distracter.” The target and distracter were brighter than the test stimuli and brighter than the background. The brightness difference to the test stimuli depended on the test stimulus contrast. The brightness difference to the background was constant. This means that behavioral performance in the detection task does not reflect the monkey's ability to detect the bar itself but to detect the target on top of the bar. Expressed in Michelson contrast, the brightness difference of the target to the background was 4.3% for monkey B and 23.1% for monkey H. After the presentation of a target, the monkey had to release the touch bar within 500 ms to receive a juice reward. If a distracter was presented first, the monkey had to continue to hold the touch bar and maintain fixation until target appearance, which was 1000–1300 ms (randomized in 1 ms steps) after distracter appearance. If the monkey made no response, the trial was terminated 500 ms after presentation of the target or distracter, whichever appeared last. Touch bar releases (correctly or incorrectly) or failure to maintain fixation resulted in immediate trial termination. For each stimulus contrast, the target occurred once at 500–800 ms after bar onset (early target condition) and once at 1000–1300 ms after distracter onset (late target condition).
Attentional cueing was done in a blocked design; blocks were counterbalanced in random order. Conditions of cueing toward the location of the RF are labeled “attend-RF,” conditions of cueing attention toward the opposite hemifield are labeled “attend away.” Conditions (early or late target) were presented in pseudorandom order within each block. If the monkey made an error, the condition would be repeated later in the block. Twenty trials per stimulus and attention condition were recorded in most recordings. Cells were excluded if fewer than 10 trials per stimulus and contrast were available.
Curve-Tracing Task
The monkeys sat at a distance of 75 cm from a monitor (CRT monitor, 21″, with a resolution of 1024 × 768, and frame rate of 75 Hz). The eye position was monitored with the double induction technique (Bour et al. 1984) and sampled at a rate of 900 Hz. A trial started as soon as the monkey's eye position was within a 1° × 1° window centered on the FP (0.2° diameter). After an interval of 300 ms, circular targets (0.6° diameter) and curves appeared on the screen, but the monkey had to maintain fixation (Fig. 2B). The background display was gray (luminance 16.3 cd/cm2), the circular targets and the FP were red, and the curves were darker than the background. The contrast of the curves was parametrically varied between 2% and 100% Michelson contrast (2%, 4%, 8%, 12%, 18%, 24%, 48%, and 100%).
In each trial, 3 curves were presented (Fig. 2B), one of the curves was the target curve that was connected to the FP. The other 2 curves that were not connected to the FP were distracters. Within a trial, all the points (pixels) of the 3 curves had the same contrast and only differed in their connection to the FP. After 500 ms, the FP disappeared and the monkey made an eye movement to one of the larger circles. An eye movement to the circle at the end of the target, curve was counted as correct and rewarded with apple juice. Eye movements to the other circles were counted as errors, and no reward was given. Trials in which the monkey failed to maintain fixation until the disappearance of the FP were terminated immediately. We presented 4 stimuli in an interleaved fashion. The stimuli shown above each other in Figure 2B are identical except for the connection to the FP. For one stimulus of such a pair, the RF of the multiunit recording site was on the target curve, and for the other stimulus, it was on the distracter curve. In our analysis, we pooled the neuronal responses across the 2 stimuli with the RF on the target curve and across the 2 stimuli with the RF on the distracter curve. Note that the stimulation of the classical RF is the same for the responses evoked by the target and distracter curve. All stimulus conditions (4 stimuli at the 8 contrast levels) were randomly interleaved and were presented equally often. In a recording session, we recorded at least 30 correct trials for every stimulus.
Assessing Behavioral Performance
To determine behavioral performance and stimulus visibility as a function of contrast, we fitted the following Weibull function to the psychophysical data of our monkeys:
| (1) |
In this function, Perfmax corresponds to the performance at the highest contrasts, c are the different contrast levels used, α is the threshold contrast, and β determines the slope of the function.
Neuronal Data Analysis
Due to the different nature of the recorded signals (discrete single unit spikes in the detection task and multiunit activity in the curve-tracing task), the initial processing differed for the 2 data sets. For the detection task, spike times in relation to stimulus and behavioral events were analyzed and converted into spike frequencies within periods of interest. These single-trial spike frequencies were used for the statistical assessment of contrast sensitivity functions and the effects of attention on neuronal activity. The MUA recorded in the curve-tracing task is a continuous signal. We calculated peristimulus time histograms (PSTHs) for the various contrast and attention conditions in a time window from 500 ms before stimulus onset until 1000 ms thereafter and smoothed these PSTHs with a 5-point moving average (5 data points correspond to 6.58 ms) to measure the peak response (Pe) that occurred when the stimulus of the highest contrast was presented. We computed the average spontaneous activity (Sp) in a window of 300–0 ms before stimulus onset and normalized the responses by first subtracting Sp and dividing the result by the peak response. We applied the same normalization to the activity on single trials for the statistical assessment of the contrast sensitivity functions and to quantify the effects of attention on neuronal activity in the curve-tracing task.
RF Mapping
The RFs and orientation tuning of single units that were recorded in the detection task were characterized before the main task. The RFs were mapped by presenting a 0.1° black (100% contrast) square at pseudorandom locations on a 10 × 10 grid (i.e., a 1° × 1° area; 5 repetitions at each location; 100 ms presentation time with 100 ms gaps), while monkeys fixated centrally on the CRT. To prevent the monkey from attributing a “special status” to the RF location, an identical stimulus was simultaneously presented in the opposite hemifield. The resulting space dependent response distributions were displayed online to determine the RF location. Stimuli used in additional cell characterization and the contrast tuning function were presented at the center of the RF.
For monkey B, the preferred orientation was measured by varying test stimuli orientations in 8 steps of 22.5° between 0° and 157.5° (stimulus size: 0.4° × 0.1°, 100% contrast), while the monkey performed the detection task as described above. Each stimulus was presented 8 times for both attention conditions. The preferred orientation was taken as the orientation with the highest mean response in either attention condition. In monkey H, we determined the preferred orientation (in conjunction with the preferred spatial frequency and phase) by employing a reverse correlation technique (DeAngelis et al. 1994; Ringach et al. 1997). Stimuli were 336 circular patches of static sinusoidal gratings (1.0° diameter) varying in orientation (12 orientations 0°–165°), spatial frequency (1, 3, 5, 7, 8, 9, 10 cycle/°), and phase (0, 0.5π, π, 1.5π). Gratings were presented for 60 ms in a pseudorandomized order centered over the RF. Responses were averaged over a 60-ms time window following stimulus onset at +30 ms and at +60 ms. In all, 5–10 repetitions of each stimulus were averaged. The stimulus that yielded the peak response was taken to represent the preferred orientation.
The MUA that was recorded in the curve-tracing task provides an instantaneous measure of the number and the size of action potentials of neurons in the vicinity of the electrode tip (Super and Roelfsema 2005). MUA represents the pooled activity of a number of single units in the vicinity of the tip of the electrode, and the population response obtained with this method is expected to be identical to the population response obtained by pooling across single units. We recently compared MUA with single unit data in the curve-tracing task and found that the signal provides a reliable estimate of the average single unit response (Super and Roelfsema 2005). We obtained recordings with sufficient signal-to-noise ratio from ∼75% of the electrode sites. For these sites, RF dimensions were measured by determining the onset and offset of the visual response to a slowly moving light bar for each of the 8 movement directions (Super and Roelfsema 2005). The median area of the RFs was 0.8 deg2 (range 0.12 degree2 to 3.9 degree2). RF eccentricity ranged from 0.9° to 4.4° with an average of 2.5°.
Statistical Assessment of Stimulus-Driven Responses and Attentional Effects
To determine whether neurons responded (differently) to the different stimulus contrasts and whether attention had a significant effect on neuronal activity, we used the single-trial data averaged over the period from 200 to 500 ms after stimulus onset for each cell (site) as previous studies demonstrated that attentional effects in V1 and V4 neurons are most pronounced during the sustained response phase (Roelfsema et al. 1998; Reynolds et al. 2000; Roberts et al. 2007). We then performed a 2-Factor analysis of variance (ANOVA) to determine whether stimulus contrast (Factor 1) or attention (Factor 2) significantly affected neuronal activity and whether there was an interaction between the 2 factors. Only cells (sites) that showed a significant effect of contrast on firing rates were included in the study.
Determination of Contrast Sensitivity and Modeling of Contrast Response Functions
To investigate whether the effect of attention on the contrast response function was best described by a contrast gain, response gain, or an additive model, we adopted and modified an approach introduced by Williford and Maunsell (2006), who fitted functions to the contrast response functions of the following general form:
| (2) |
where R(c) is the response as function of contrast, Rmax is the saturated response, c50 is the contrast at which the half maximal response is reached, n determines the slope of the contrast response function, and M corresponds to the spontaneous activity. We used multidimensional unconstrained nonlinear minimization (Nelder–Mead) to minimize the summed squared difference between the data and the model (Matlab 7.1, Mathworks, Natick, MA). The above model has been shown to provide a good approximation of contrast response functions in monkey visual cortex (Albrecht and Hamilton 1982; Thiele et al. 2004; Williford and Maunsell 2006).
To determine the effect of attention on contrast tuning, we fitted 3 different models to the data. The contrast gain model holds that attention increases the effective contrast and was modeled as follows:
| (3) |
where a determines the effect of attention on the shift of the contrast response function (i.e., the effect on c50). Using this function, we performed a combined fit to the data from the attend RF and attend away conditions where a was the only difference between the 2 attention conditions.
We also determined whether the response gain model gave an adequate description of the effect of attention on the contrast response function by fitting the following function:
| (4) |
Thus, the response gain model holds that attention (a) increases the response magnitude by a constant factor.
As a third model, we fitted the additive model, which assumes that attention adds a constant amount of activity to the neuronal response once the stimulus has sufficient contrast to become visible:
| (5) |
where stimulus visibility was determined independently by fitting equation (1) to the animals’ behavioral performance (the visibility function was rescaled so that it ranged from 0 to 1). The term “Visibility” was derived individually for monkeys B, G, and A from fitting equation (1) to the performance data. Because in monkey H performance was fairly constant across all contrast levels (for reasons described in the Results), we used the Visibility function from monkey B (who performed the same task as monkey H) to fit neuronal data from monkey H.
To determine which of the models describes the effect of attention best, we calculated the percent variance accounted for and Pearson's correlation coefficient between the data and the model. Given the similarity of the different models, we calculated the partial correlation coefficient, that is, the correlation of the data with the model after taking into account the effect of the comparison model. Partial correlations were calculated as previously described (Movshon et al. 1985; Smith et al. 2005).
Results
Behavioral Performance
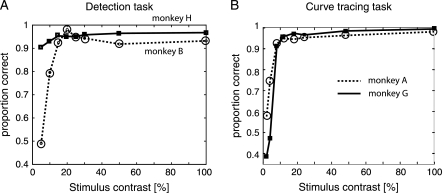
The performance of the animals in the 2 different tasks as a function of stimulus contrast is shown in Figure 3. The animals were proficient in both tasks, provided the stimulus had sufficient contrast. In the detection task, the performance of monkey B fell to ∼50% correct at low contrast because the luminance increment was difficult to detect if the test bar contrast was low (Fig. 3A). This effect was not observed in monkey H because this animal had to detect a luminance increment that was well above the background luminance (23.1% Michelson contrast), and he could even successfully complete the task in the absence of test bars visibility. Behavioral contrast threshold was quantified by fitting a Weibull function to the performance data (see Methods). For monkey B, the threshold contrast (α) was 11.03%, whereas the slope of the curve (β) was 3.03. Due to the relatively constant performance of monkey H, a Weibull fit was not performed. The performance of the 2 monkeys in the curve-tracing task fell to chance level at low luminance contrast (Fig. 3B) because this task could not be solved when the curves were difficult to discriminate from the background. Quantifying stimulus visibility by fitting equation (1) to their average performance revealed threshold values of 7.85% and 5.58%, respectively, and slopes of 28.7 and 1.44.
Figure 3.
Performance as a function of stimulus contrast. (A) Performance of the 2 animals in the detection task on trials that were not aborted due to a fixation break. Note that performance of monkey B fell to chance level for test bars of low contrasts because the luminance increment was difficult to detect. In monkey H, the performance was also good with low-contrast bars because the luminance increase relative to the background was higher and could even be detected in the absence of the test bar. (B) Performance of the 2 animals in the curve-tracing task on trials not aborted due to a fixation break. The performance fell to chance level for the lower contrasts because the animals could not do the task if the curves were difficult to detect.
Neuronal Data from the Detection Task
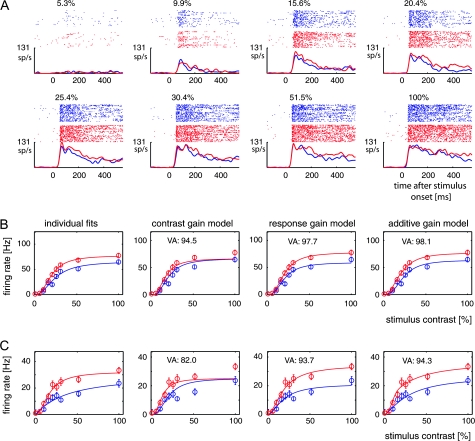
In the detection task, we recorded from 109 cells that were well driven by the higher contrast stimuli (41 cells from monkey B and 68 from monkey H). The RFs of the neurons were located in the lower quadrant, at an eccentricity of 2°–7°. Figure 4A illustrates data from a typical recording session. The response of the example V1 cell increased as a function of the stimulus contrast, whereas attention also influenced the responses: the neuronal activity was stronger when attention was directed to the stimulus inside the RF than when attention was directed to the stimulus in the opposite hemifield. For this example neuron, the effects of attention were observed across all the contrast levels above 10%, that is, the contrast levels where the test bar was easily perceived.
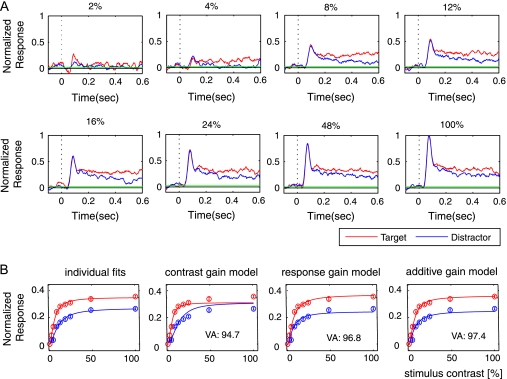
Figure 4.
Activity of V1 neurons in the detection task. (A) Example cell that showed an increased response when attention was directed to the RF. Note that the influence of attention is most pronounced in the sustained response phase, after the initial response transient evoked by the appearance of the test bar in the RF. (B) Average activity of the neuron in (A) evoked by test bars of various contrasts that were attended (red) or not attended (blue). We fitted the data with a contrast response function individually (individual fits) and with a contrast gain model, a response gain model, and an additive model. Error bars show standard error of the mean. VA denotes the percentage of variance accounted for by each of the 3 models. (C) A second example V1 neuron in the detection task.
To investigate whether the effect of attention on the contrast response function was best described by a contrast gain, response gain, or an additive model, we fitted our data from the 200- to 500-ms response period with the 3 different models outlined in Methods. It can be seen for the example shown in Figure 4B that the contrast gain model captured some aspects of the contrast response function but failed to account for attentional effects at higher luminance contrast (especially at 50% and 100% contrast). The fitted functions accounted for 94.5% of the variance in the data. The response gain model gave slightly better fits to the example cell. The fits of this model to the contrast response functions accounted for 97.7% of the variance (Fig. 4B). The additive model gave the best description of the effect of attention on the contrast response function and accounted for 98.1% of the variance in the data.
Figure 4C shows another example neuron that was recorded in the detection task. It can be seen that also for this neuron, the additive model accounted for most of the variance in the data, closely followed by the response gain model, which, in turn, gave a better description of the data than the contrast gain model. Although the additive model explained the largest amount of variance in the data, it is worth emphasizing that the 2 alternative models were by no means poor model descriptors, both explaining at least 82% of the variance for the 2 example neurons. Similar results were obtained for the entire data set, that is, the amount of variance explained by the different models was generally fairly large (usually >70%).
Population Analysis
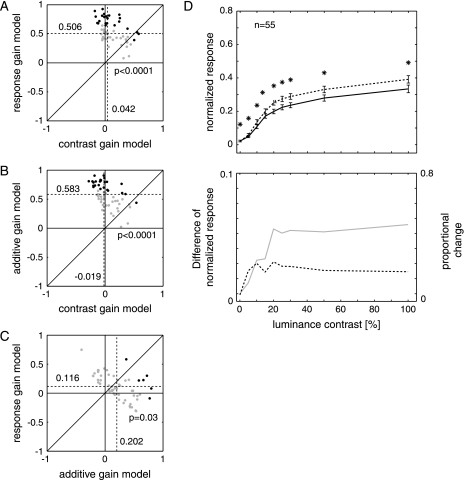
Attention significantly altered the response of 18 of 41 (43.9%) cells from monkey B and 37 of 68 (54.4%) cells in monkey H in the detection task. Given that we are interested in the effects of attention on the neuronal responses, we focused our analysis on cells that were significantly affected by attention. We wished to determine which of the models provides the best fit across the population of cells. It is convenient that the 3 models have the same number of free parameters, so we can directly compare the quality of the fits. The examples of Figure 4 illustrated that all 3 models provided reasonable fits to the data, and we therefore focused our analysis on the extra variance that one of the models can account for when the predictions of one of the other 2 models are taken into account. To this end, we calculated partial correlations (Movshon et al. 1985; Smith et al. 2005; Williford and Maunsell 2006) that are shown in Figure 5A. The upper panel compares the pairwise partial correlations between the contrast gain and the response gain model. Points along the ordinate represent neurons that were best described by the response gain model, whereas points along the abscissa correspond to neurons best fitted by the contrast gain model. It can be seen that the partial correlations of the response gain model were on average much larger than those of the contrast gain model (P < 0.001, rank sum test). Thus, the response gain model gave a better account of the effect of attention on the contrast response functions than the contrast gain model.
Figure 5.
Population analysis of the detection task. (A–C) Distributions of partial correlations between fits of 2 models. (A) Comparison of the contrast gain and response gain model. Abscissa (ordinate), residual correlation coefficient between contrast gain (response gain) model and data after the correlation between the data and response gain model (contrast gain) is taken into account. The medians of the distributions are indicated by the numbers next to the dashed lines. Black dots denote data points where partial correlations were significant for one model and significantly larger than the partial correlations for the comparison model (P < 0.05). P values denote whether the distributions of partial correlation coefficients were significantly different from another (rank sum test). (B) Comparison between the contrast gain model and the additive model. (C) Comparison between the additive model and the response gain model. (D) Average responses in the detection task evoked by the attended (dashed curve) and unattended test bars (continuous curve) of varying contrasts. Error bars show standard error of the mean. Black stars denote data points where the activity in the attend RF condition was significantly stronger than the activity in the attend away condition (P < 0.05, Wilcoxon signed-rank test). The lower panel shows the absolute activity difference (thick gray curve, ordinate to the left) as well as the proportional difference, that is, the response difference divided by the activity in the attend RF condition (black curve with ordinate on the right).
The middle panel (Fig. 5B) presents the equivalent comparison between the contrast gain model and the additive model. It can be seen that the additive model also gave a better fit to the data than the contrast gain model. The final comparison was between the response gain model and the additive model, and it is shown in Figure 5C. The additive model gave significantly better fits to the data than the response gain model (P = 0.03, rank sum test). The same finding held true if partial correlations were calculated on all cells, irrespective of whether they were significantly affected by attention or not (P < 0.001, rank sum test).
We next computed the contrast response functions with and without attention by averaging across the activity of all the significantly affected neurons (Fig. 5D). Attention increased the responses at medium as well as at high-contrast levels. This result is not compatible with the contrast gain model, which predicts that the effects of attention are small for stimuli with a high contrast, and thereby supports the findings from the partial correlation analysis. The lower panel of Figure 5D shows the absolute and proportional response difference caused by attention. The absolute difference (gray curve) increased with luminance contrast and reached a plateau at 20% luminance contrast, whereas the proportional change (dashed curve) reached a peak at 20% luminance contrast and showed a slight decrease at the higher contrasts.
Influence of Attention on Ongoing Activity
There is a variant of the response gain model called “activity gain model” that proposes that attention also increases the ongoing activity (Williford and Maunsell 2006). A number of previous studies in extrastriate visual cortex reported that attention increases the ongoing activity in addition to its effect on stimulus-driven activity (Luck et al. 1997; Williford and Maunsell 2006). To the best of our knowledge, no such effect has been reported for area V1. We therefore compared the ongoing activity in the attend RF and attend away condition. Interestingly, we found that attending to the RF actually slightly but significantly reduced the ongoing activity (ongoing activity attend away: 2.34 ± 3.44 spikes/s; attend RF: 2.09 ± 3.07 spikes/s; P < 0.003; paired t-test). This implies that the activity gain model cannot give a better account of our data than the response gain model, and we thus did not consider it further.
Curve-Tracing Task
Previous studies gave conflicting results on how attention influences contrast response functions, with some studies supporting the contrast gain model and others supporting the response gain model. Our analyses so far revealed that the additive model significantly outperforms the contrast and the response gain models. We decided that it is important to replicate this result with another task to investigate the generality of these findings, and we therefore studied neuronal responses in area V1 during a curve-tracing task.
In the curve-tracing task, we recorded from a total of 38 multiunit recording sites in area V1 (15 sites in monkey G, 23 sites in monkey A) with RFs at eccentricities ranging from 0.8° to 4.5°. Figure 6A illustrates the responses evoked at an example recording site by curves with various levels of luminance contrast. It can be seen that the response amplitude increased with contrast and also that the target curve generally evoked stronger responses than the distracter curve. The attentional modulation was pronounced at the higher contrast levels and basically absent when the curves had a very low contrast so that they were hardly visible. Accordingly, the contrast gain model did not fit the contrast response functions as well, although it still accounted for 94.7% of the variance (Fig. 6B). The response gain model fitted better (96.8% explained variance), whereas the additive model gave the best fit to the data (97.4% explained variance).
Figure 6.
Activity in area V1 in the curve-tracing task. (A) Responses evoked at an MUA recording site in area V1 by target and distracter curves of varying contrasts during the curve-tracing task. (B) Average activity in window from 200 to 500 ms evoked by the target (red symbols) and distractor curves (blue symbols). The red and blue curves represent best fits contrast response functions fitted individually (left panel) of the contrast gain model, the response gain model, and the additive model (right panel). VA, the percentage of the variance accounted for by each of the models.
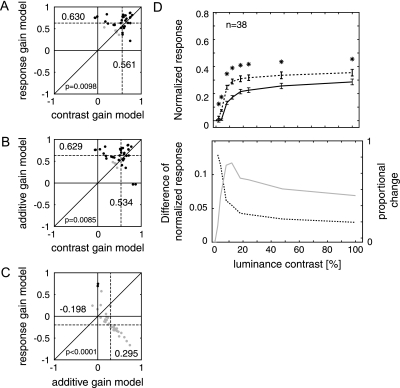
Across the population of recording sites, the effects of attention were widespread in the curve-tracing task as the responses at 23/23 (100%) of the recording sites in monkey A and at 15/15 (100%) of sites in monkey G were significantly modulated by the difference between the target and distracter curve. We compared the 3 models by computing the partial correlation coefficients that are shown in Figure 7A. The results of this analysis were in line with the data from the detection task. The additive model gave the best fit to the data and it significantly outperformed the response gain (P < 0.001, rank sum test) as well as the contrast gain model (P = 0.0085, rank sum test). The response gain model, in turn, gave a better fit to the data than the contrast gain model (P = 0.0098, rank sum test).
Figure 7.
Population analysis of neuronal responses in the curve-tracing task. (A–C) Partial correlations between neuronal responses and models for the effects of attention on contrast response functions. (A) Compares the contrast gain and response gain model. Abscissa (ordinate), remaining correlation coefficient between contrast gain (response gain) model and data after the correlation between the data and response gain model (contrast gain) has been taken into account. (B) Comparison of the contrast gain model to the additive model, and (C) comparison of the response gain model to the additive model. The additive model gave the best fit, followed by the response gain model, which in turn fitted better than the contrast gain model. Median partial correlations are indicated by dashed lines and the adjacent numbers. P values denote whether the distributions of partial correlation coefficients were significantly different from another (rank sum test). Black dots denote data points where partial correlations were significant for one model and significantly larger than the partial correlations for the comparison model (P < 0.05). (D) Average neuronal activity evoked by the attended (dashed line) and unattended curve (continuous line) in the curve-tracing task for stimuli with various contrasts. Error bars denote standard error of the mean. Black stars denote data points where attention significantly enhanced the neuronal response (P < 0.05, Wilcoxon signed-rank test). The lower panel shows the activity difference (thick gray curve, ordinate on the left) and the proportional difference (dashed black curve, ordinate on the right).
We next computed the contrast response functions at the population level by averaging across the responses evoked at individual recording sites (Fig. 7D). It can be seen that attention increased the activity across all contrast levels, although a small peak in the effect of attention can be seen at ∼10% contrast. The difference in the response between target and distracter curve reached a plateau at ∼20% contrast. The dashed curve in Figure 7B shows the proportional change in the response due to attention. The proportional changes were largest at low luminance contrast (the peak proportional change of 70% occurred at 2.6% luminance contrast) and decreased to a level between 15% and 20% at ∼ 8% luminance contrast. The dependence of the proportional change on stimulus contrast is in accordance with the poorer fit of the response gain model, which holds that the proportional increase is relatively independent of contrast.
So far, we tested the predictions from 3 models of attentional modulation and assumed that only 1 of the 3 models was at work. It is possible that attention acts to increase the contrast gain as well as amplifies the response, whereas the precise mixture of these effects varies across neurons. We note that such a model requires 2 fitting parameters for the influence of attention (one influencing c50 and the other influencing Rmax). The reason for the additional parameter is that attention increases the Rmax value, whereas it decreases the c50 value in the contrast gain model, that is, a single attention parameter cannot account for both simultaneously. Due to the additional fitting parameter, it is not possible to compare the partial correlations in an unbiased manner. However, we were able to compare the goodness of fit by comparing the normalized χ2 values obtained by fitting the models to our data (χ2 normalized by the number of fitting parameters; Roberts et al. 2007). We found that the combined contrast and response gain model yielded significantly larger normalized χ2 values than the additive model in both data sets (P < 0.05, RM-ANOVA on ranks), whereas it was not significantly different from the simple contrast gain or the response gain model. This additional analysis shows that the additive model is a better descriptor of the data than a combined contrast-response gain model where attention has independent effects on c50 and Rmax.
Discussion
Here we have investigated how attention influences contrast response functions in area V1. We used 2 different behavioral paradigms to ensure that our results did not depend on the specific demands of the task at hand. Our results are clear and consistent across the 2 tasks: attention increases neuronal firing rates in area V1 at low and at high stimulus contrast and the additive model gives a better description of our results than the contrast gain and response gain models. We anticipated to find robust effects of attention at the higher contrast levels because we and others have used high-contrast stimuli to study the neuronal correlates of attention shifts in area V1 (Motter 1993; Roelfsema et al. 1998; Vidyasagar 1998; Roberts et al. 2007). However, we now found that the effects of attention on the responses evoked by low to medium contrast stimuli in area V1 are equally strong, and we conclude that the effects of attention on the V1 firing rates are relatively independent of luminance contrast, at least once the stimulus has sufficient contrast to become visible (once animal performance reaches ∼ 82% detection).
Comparison to Studies Proposing Contrast Gain and Response Gain Models
Although our results are not in accordance with previous neurophysiological studies on the effect of attention on contrast response functions, we feel confident about the validity of our results that were obtained with 2 different behavioral tasks. We noted in relation to Figure 1 that the predictions of the additive model are intermediate between those of the contrast gain and response gain models. The previous neurophysiological studies focused on the contrast gain and response gain models and did not consider the possibility of an additive interaction. A predominance of additive effects may, however, also provide an explanation for the variable results obtained in previous studies in area V4 that tried to distinguish between the response gain and contrast gain models (Reynolds et al. 2000; Williford and Maunsell 2006), and it appears from Figure 6 in Williford and Maunsell (2006) that the additive gain model would perform at least as well as the other models tested in that study.
Our finding that the contrast gain model gave the worst description of the effect of attention on contrast response functions implies that the effects of attention in area V1 are not equivalent to an increase in stimulus contrast and do not support previous findings in area V4 (Reynolds et al. 2000) and area MT (Martinez-Trujillo and Treue 2002). We found that the response gain model also gave a poorer description of the data than the additive model. In contrast to the prediction of the response gain model, the increase in the neuronal activity due to attention was not a constant proportion of the response. Instead, the attentional response modulation was already large for low to medium luminance contrast stimuli, especially in the curve-tracing task where the responses of the majority of neurons were better described by the additive model. Another model that has been proposed previously is the so-called activity gain model that proposes that the spontaneous activity also increases if attention is directed to the RF. In contrast, we observed a significantly reduced level of ongoing activity with attention in the detection task, which is incompatible with the activity gain model. To our knowledge, no other study has reported a reduction of ongoing activity with attention, although this could provide a mechanism to increase the signal-to-noise ratio and thereby aid in stimulus detection.
Previous studies have shown that task difficulty affects neuronal responses (Spitzer et al. 1988; Chen et al. 2008). In both tasks, performance varied with stimulus contrast (albeit only in one of the monkeys in the detection task) and the task was more difficult for low-contrast stimuli. If task difficulty is the major determinant of neuronal activity, we expect the largest attentional modulations for the low-contrast stimuli and less modulation for the high-contrast stimuli. Such a scenario would predict that the contrast gain model would result in a better fit than either of the alternative models and is not supported by our data. However, we also have to consider the possibility that task difficulty interacts with other effects of attention. If the task difficulty increases the attentional modulation more strongly for low-contrast stimuli than for high-contrast stimuli, then this effect might combine with a response gain effect to generate an overall additive effect of attention on the neuronal responses or result in a combined contrast/response gain model. However, 2 lines of evidence argue against such a confounding effect of task difficulty. First, we found that a combined contrast/response gain model gave a poorer fit to our data than the additive model and could thus be discounted. Second, in monkey H (which performed the detection task), task difficulty did not vary with stimulus contrast, but we still found the same pattern of attentional modulation, namely that the additive model explained our data best. From these results, we infer that it is not simply a combination of multiplicative gain control with varying task difficulty that results in the superiority of the additive gain model.
Contrast Response Functions in Striate and Extrastriate Visual Cortex
The first studies of contrast sensitivity in area V1 were carried out in anesthetized monkeys and reported median c50 values of 24% (Albrecht and Hamilton 1982) and 33% (Sclar et al. 1992). In our study, the c50 values generally fell between 11% and 17%, which is lower than in the anesthetized animals but at the same time higher than the value of 7% that was recently reported by Palmer et al. (2007) in the awake monkey. An important difference between the present study and the one by Palmer et al. (2007) is that we used stimuli with a negative luminance contrast (i.e., with a higher background luminance). We have also tested the MUA recording sites with bright curves on a dark background in the curve-tracing task and obtained c50 values of 5–6% (data not shown), which implies that the contrast sensitivity of V1 neurons may depend on the contrast polarity.
Some of the V1 neurons had c50 values that were smaller than the c50 values of ∼15% that have been reported for area V4 (Williford and Maunsell 2006). This result is noteworthy as it suggests that pooling the activity across multiple V1 neurons either does not increase contrast sensitivity as is often thought (Sclar et al. 1992; Thiele et al. 2000; Williford and Maunsell 2006) or that the contribution of area V4 to the detection of low-contrast stimuli is limited.
Strength of Attentional Modulation in Area V1 and in Higher Areas
We observed robust effects of attention on neuronal firing rates in area V1 in each of our 4 monkeys in 2 different tasks. These effects were small or absent during the initial transient response but were profound during the sustained response period as has been observed previously in area V1 (Roelfsema et al. 1998; Roberts et al. 2007) and area V4 (Reynolds et al. 2000). We found that attention increased firing rates by ∼10–20% at medium and high luminance contrast. These attentional effects on the strength of neuronal responses in area V1 are comparable to the effects that have been observed in area V4 (Williford and Maunsell 2006; see their Fig. 6E and H) or even larger (Reynolds et al. 2000; see their Fig. 5A). Our results are therefore compatible with a previous study (Motter 1993), which demonstrated that the effects of selective attention on the neuronal responses in areas V1, V2, and V4 have a similar magnitude.
The magnitude of attentional modulation in area V1 is also similar to the strength of the effects of attention in areas MT and MST (Treue and Maunsell 1996, 1999). All these results, taken together, suggest that the magnitude of attentional effects remains relatively constant when ascending the cortical hierarchy up to the level of areas V4 and MT. The strength of attentional modulation may thus not be determined by the cortical hierarchy but rather by the visual stimulus that requires attention (Roberts et al. 2007) and by how useful neuronal selectivity in an area is for the task at hand (Roelfsema and Spekreijse 2001).
Additive Effects of Attention
To summarize, we observed that attention increases the neuronal response by a relatively constant amount once the stimulus has enough contrast to become visible. This finding agrees to some extent with a recent fMRI study in human observers showing that the effect of attention on the BOLD response in area V1 is relatively independent of stimulus contrast (Buracas and Boynton 2007; Murray 2008). However, there are also important differences. In our data, the additive component only becomes active once the stimulus reaches detection threshold, whereas the fMRI data show significant effects on baseline responses, that is, in the absence of a stimulus. Thus, the fMRI data, which measure BOLD activity might reflect a change in subthreshold membrane potential of V1 neurons, or even a predominant effect on blood supply when no stimulus is presented. Buracas and Boynton (2007) also reported that the effect of attention on the contrast response functions in areas V2 and V3 is equally well described by an additive model. In a yet higher visual area, the lateral occipital complex, Murray and He (2006) observed that the neuronal responses evoked by an attended stimulus are relatively invariant across variations in contrast, whereas the responses evoked by an unattended stimulus do not exhibit the same degree of contrast invariance. We suggest that the neurons that represent the attended stimulus in higher areas might feedback to earlier areas in a manner that is relatively independent of contrast. In this view, the extra activity in earlier areas due to the attentional feedback depends little on stimulus contrast once the stimulus has sufficient visibility to be registered in the higher visual areas.
Funding
Biotechnology and Biological Sciences Research Council (BBS/B/09325); Wellcome Trust (070380/Z/03/Z) to A.T. P. Roelfsema's European Union (EU IST Cognitive Systems, project 027198 “Decisions in Motion”); NWO-ALW.
Acknowledgments
Conflict of Interest: None declared.
References
- Albrecht DG, Hamilton DB. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982;48:217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Avidan G, Harel M, Hendler T, Ben-Bashat D, Zohary E, Malach R. Contrast sensitivity in human visual areas and its relationship to object recognition. J Neurophysiol. 2002;87:3102–3116. doi: 10.1152/jn.2002.87.6.3102. [DOI] [PubMed] [Google Scholar]
- Bashinski HS, Bacharach VR. Enhancement of perceptual sensitivity as the result of selectively attending to spatial locations. Percept Psychophys. 1980;28:241–248. doi: 10.3758/bf03204380. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bour LJ, van Gisbergen JA, Bruijns J, Ottes FP. The double magnetic induction method for measuring eye movement—results in monkey and man. IEEE Trans Biomed Eng. 1984;31:419–427. doi: 10.1109/TBME.1984.325281. [DOI] [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. The effect of spatial attention on contrast response functions in human visual cortex. J Neurosci. 2007;27:93–97. doi: 10.1523/JNEUROSCI.3162-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell CM, Goldberg ME, Robinson DL. Behavioral enhancement of visual responses in monkey cerebral cortex.I. Modulation in posterior parietal cortex related to selective attention. J Neurophysiol. 1981;46:755–771. doi: 10.1152/jn.1981.46.4.755. [DOI] [PubMed] [Google Scholar]
- Carrasco M, Ling S, Read S. Attention alters appearance. Nat Neurosci. 2004;7:308–313. doi: 10.1038/nn1194. Epub 2004 Feb 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Martinez-Conde S, Macknik SL, Bereshpolova Y, Swadlow HA, Alonso JM. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nat Neurosci. 2008;11:974–982. doi: 10.1038/nn.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis GC, Freeman RD, Ohzawa I. Length and width tuning of neurons in the cat's primary visual cortex. J Neurophysiol. 1994;71:347–374. doi: 10.1152/jn.1994.71.1.347. [DOI] [PubMed] [Google Scholar]
- Dosher BA, Lu ZL. Noise exclusion in spatial attention. Psychol Sci. 2000;11:139–146. doi: 10.1111/1467-9280.00229. [DOI] [PubMed] [Google Scholar]
- Einhauser W, Rutishauser U, Koch C. Task-demands can immediately reverse the effects of sensory-driven saliency in complex visual stimuli. J Vis. 2008;8(2):1–19. doi: 10.1167/8.2.2. [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD. Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron. 1999;22:593–604. doi: 10.1016/s0896-6273(00)80713-8. [DOI] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci. 2004;7:651–657. doi: 10.1038/nn1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Piech V, Gilbert CD. Contour saliency in primary visual cortex. Neuron. 2006;50:951–962. doi: 10.1016/j.neuron.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Liston DB, Stone LS. Effects of prior information and reward on oculomotor and perceptual choices. J Neurosci. 2008;28:13866–13875. doi: 10.1523/JNEUROSCI.3120-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZL, Dosher BA. External noise distinguishes attention mechanisms. Vision Res. 1998;38:1183–1198. doi: 10.1016/s0042-6989(97)00273-3. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo J, Treue S. Attentional modulation strength in cortical area MT depends on stimulus contrast. Neuron. 2002;35:365–370. doi: 10.1016/s0896-6273(02)00778-x. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JHR. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol. 2000;83:1751–1755. doi: 10.1152/jn.2000.83.3.1751. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Movshon JA, Adelson EH, Gizzi M, Newsome WT. The analysis of moving visual patterns. In: Chagas T, Gattass R, Gross CG, editors. Study group on pattern recognition. Vatican City: Pontifica Academia Scientiarum; 1985. pp. 117–151. [Google Scholar]
- Murray SO. The effects of spatial attention in early human visual cortex are stimulus independent. J Vis. 2008;8(10):1–11. doi: 10.1167/8.10.2. [DOI] [PubMed] [Google Scholar]
- Murray SO, He S. Contrast invariance in the human lateral occipital complex depends on attention. Curr Biol. 2006;16:606–611. doi: 10.1016/j.cub.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Palmer C, Cheng SY, Seidemann E. Linking neuronal and behavioral performance in a reaction-time visual detection task. J Neurosci. 2007;27:8122–8137. doi: 10.1523/JNEUROSCI.1940-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H, Dobkins K, Huang L. Is contrast just another feature for visual selective attention? Vision Res. 2004;44:1403–1410. doi: 10.1016/j.visres.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Prinzmetal W, Nwachuku II, Bodanski L, Blumenfeld L, Shimizu N. The phenomenology of attention. Conscious Cogn. 1997;6:372–412. [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- Ringach DL, Sapiro G, Shapley R. A subspace reverse-correlation technique for the study of visual neurons. Vision Res. 1997;37:2455–2464. doi: 10.1016/s0042-6989(96)00247-7. [DOI] [PubMed] [Google Scholar]
- Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nat Neurosci. 2007;10:1483–1491. doi: 10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Thiele A. Attention and contrast differently affect contextual integration in an orientation discrimination task. Exp Brain Res. 2008a;187:535–549. doi: 10.1007/s00221-008-1322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Thiele A. Spatial integration and its moderation by attention and acetylcholine. Front Biosci. 2008b;13:3742–3759. doi: 10.2741/2963. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H. Object-based attention in the primary visual cortex of the macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Spekreijse H. The representation of erroneously perceived stimuli in the primary visual cortex. Neuron. 2001;31:853–863. doi: 10.1016/s0896-6273(01)00408-1. [DOI] [PubMed] [Google Scholar]
- Schneider KA, Komlos M. Attention biases decisions but does not alter appearance. J Vis. 2008;8(3):1–10. doi: 10.1167/8.15.3. [DOI] [PubMed] [Google Scholar]
- Sclar G, Maunsell JHR, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 1992;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- Smith MA, Majaj NJ, Movshon JA. Dynamics of motion signaling by neurons in macaque area MT. Nat Neurosci. 2005;8:220–228. doi: 10.1038/nn1382. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Super H, Roelfsema PR. Chronic multiunit recordings in behaving animals: advantages and limitations. Prog Brain Res. 2005;147:263–282. doi: 10.1016/S0079-6123(04)47020-4. [DOI] [PubMed] [Google Scholar]
- Thiele A, Delicato LS, Roberts MJ, Gieselmann MA. A novel electrode-pipette design for simultaneous recording of extracellular spikes and iontophoretic drug application in awake behaving monkeys. J Neurosci Methods. 2006;158:207–211. doi: 10.1016/j.jneumeth.2006.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Distler C, Korbmacher H, Hoffmann K-P. Contribution of inhibitory mechanisms to direction selectivity and response normalization in macaque middle temporal area. Proc Natl Acad Sci USA. 2004;101:9810–9815. doi: 10.1073/pnas.0307754101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Dobkins KR, Albright TD. Neural correlates of contrast detection at threshold. Neuron. 2000;26:715–724. doi: 10.1016/s0896-6273(00)81207-6. [DOI] [PubMed] [Google Scholar]
- Treue S. Perceptual enhancement of contrast by attention. Trends Cogn Sci. 2004;8:435–437. doi: 10.1016/j.tics.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Treue S, Maunsell JH. Effects of attention on the processing of motion in macaque middle temporal and medial superior temporal visual cortical areas. J Neurosci. 1999;19:7591–7602. doi: 10.1523/JNEUROSCI.19-17-07591.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treue S, Maunsell JHR. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- Treue S, Trujillo JCM. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR. Gating of neuronal responses in macaque primary visual cortex by an attentional spotlight. Neuroreport. 1998;9:1947–1952. doi: 10.1097/00001756-199806220-00006. [DOI] [PubMed] [Google Scholar]
- Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. J Neurophysiol. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Zenger B, Braun J, Koch C. Attentional effects on contrast detection in the presence of surround masks. Vision Res. 2000;40:3717–3724. doi: 10.1016/s0042-6989(00)00218-2. [DOI] [PubMed] [Google Scholar]