Abstract
Cathepsin D (CD) is an enzyme that promotes breast cancer. CD is stored intracellularly; however, we demonstrated that IGF-II promotes CD secretion in estrogen receptor positive (ER+ ) breast cancer cells. We also showed that resveratrol (RSV) stimulates IGF-II in ER(+) breast cancer cells. Thus, we designed this study to determine whether RSV regulates CD in MCF-7, T47D (ER+ ) breast cancer cells as well as in Hs578t (cancer) and MCF-10A (normal) ER− cell lines. RSV (10−6 M) increased CD and IGF-II secretion in ER+ but not ER− cells. RSV treatment (10−4 M) inhibited CD in ER+ but not in ER− cells. Transfection of ER− cells with proIGF-II increased CD secretion. RSV (10−6 M) modulates CD secretion through IGF-II while RSV (10−4 M) inhibits CD in ER+ but not ER− cells. Regulation of CD by RSV represents a novel mechanism by which RSV may protect against breast cancer.
Keywords: Resveratrol, cathepsin D, insulin-like growth factor II, breast cancer
Introduction
Cathepsin D (CD) is an aspartyl protease regulated by estrogen and overexpressed in a variety of human cancers including estrogen receptor (ER) negative breast carcinomas (Rochefort et al. 1989). CD is synthesized as preprocathepsin D (pCD). The single chain procathepsin D (52 kDa) is autoactivated to CD (51 kDa) and further processed and glycosylated into several distinct forms (51–14 kDa). The CD gene has a mixed structure with the general features of housekeeping genes (constitutively secreted, high G + C content and potential Sp1 binding sites) and those of a regulated facultative gene that includes a TATAA sequence. In ER positive breast cancer cell lines, CD is regulated by 17βestradiol (E2). Transcription from the CD gene can be initiated at five sites spanning 52 bp; of the five start sites, estrogen stimulates transcription only from the site located downstream of the TATA box at −20; in contrast, low proportions of TATA-dependent transcription were observed in estrogen-unresponsive breast cancer cell lines, suggesting that factors other than estrogens may also stimulate TATA-dependent transcription. In TATA-independent transcription, like observed in ER(−) cell lines, the proportion of longer CD mRNA is increased. This effect might have biological consequences in terms of stability or initiation of translation and subcellular localization of the protein (Cavailles et al. 1993). ER targeting of upstream stimulatory factors 1 and 2 (USF-1, USF-2) is a critical step in hormone activation of CD transcription in human breast cancer cells (Xing and Archer 1998). CD secretion is associated with increase in metastasis, promoted by CD proteolytic and mitogenic activities (Rochefort et al. 1989; Garcia et al. 1990; Liaudet et al. 1994; Montcourrier et al. 1997; Glondu et al. 2001; Leto et al. 2004; Ruibal and Arias 2004). Our laboratory has demonstrated that expression of proIGF-II leads to an increase in CD secretion in MCF-7 breast cancer cells (De León et al. 1996). The IGF-II effect on CD routing is reversible since IGF-II antisense blocks CD secretion (De León et al. 1999). We also demonstrated that IGF-II does not change CD mRNA levels in MCF-7 cells, and that IGF-II binding to the IGF-II/M6P receptor is required for CD secretion (Faridi et al. 2004). More recently, we showed (Vyas et al. 2005) that IGF-II (not IGF-I) is regulated by the phytoestrogen resveratrol (RSV) in a concentration- and ER status-dependent manner. Treatment with RSV (10−6 M) stimulated IGF-II secretion in MCF-7 and T47D cells (but not in MCF-10A cells), increased cell proliferation and it also inhibited apoptosis. Since IGF-II interferes with CD routing, we hypothesized that RSV regulation of IGF-II will modulate CD. This is of great significance because our established cell model consists of IGF-II transfected MCF-7 cells. The present model represents modulation of endogenous IGF-II by RSV causing derouting of CD and increasing the secretion of this lysosomal enzyme. RSV is presently considered a potential chemopreventive treatment for breast cancer, and it acts as both mixed agonist/antagonist for ERs α and β and it also acts independent of ER. The mechanism of action for this phytoestrogen and its target genes are mostly unknown (Mgbonyebi et al. 1998; Damianaki et al. 2000; Bhat and Pezzuto 2002; Aziz et al. 2003; Dong 2003).
Thus, our study was designed to assess if RSV regulates CD in breast cancer cells; and whether this effect on CD is mediated by IGF-II.
Materials and methods
Cell culture
MCF-7, T47D, Hs578t breast carcinoma cell lines and MCF-10A breast epithelial cell line were obtained from the American Type Culture Collection (ATCC). MCF-7 cells were maintained in a 5% CO2 incubator at 37°C, using DMEM/F12 media (Cellgro) supplemented with 10 ml of 5000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro), 4 mM L-glutamine (Cellgro), 3 μg/ml β-amphotericin and 5% fetal bovine serum (Hyclone). T47D cells were grown in RPMI-1640 media supplemented with 10 μg/ml insulin, 2 mM L-glutamine (Cellgro), 5,000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro) and 10% fetal bovine serum (Hyclone). Hs578t DMEM media was supplemented with 0.01 mg/ml bovine insulin (Sigma), 10% fetal bovine serum (Hyclone), 4 mM L-glutamine (Cellgro) and 10 ml of 5000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro). MCF-10A DMEM/F12 media was supplemented with 10 ml of 5000 units penicillin/streptomycin (100 units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro), 4 mM L-glutamine (Cellgro), 3 μg/ml β-amphotericin, 10 μg/ml insulin (Sigma), 0.5 μg/ml hydrocortisone (Sigma), murine epidermal growth factor (EGF) 20 ng/ml (GIBCO-BRL), cholera toxin 100 ng/ml (Sigma) and 5% equine serum. Cells were detached by trypsinization (1 × Trypsin EDTA, Cellgro). RSV was purchased from Sigma Chemical Co. (St Louis, MO), and dissolved in dimethyl sulfoxide (DMSO, Fischer Scientific, Pittsburgh, PA). Media from RSV treated cells (CM) was collected, (24, 48 h), centrifuged (800 rpm for 5 min), and frozen (−20°C) until assayed.
Cathepsin D antibodies
CD was purified from human placenta according to the method of Takahashi and Tang (1981), except that a Concanavalin A-Sepharose column purification step was used before affinity chromatography on Pepstatin–Sepharose. Analysis of protein purity by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) confirmed the presence of a major band of 30 kDa and a minor band of 14 kDa (visualized by silver and Coomassie blue staining of the gel). Two rabbits (# 4607 and 4608) were immunized with this preparation of CD. Sera was collected and titered by immunoprecipitation of [125I] CD as described by our laboratory (De Leon et al. 1996).
Construction of vectors
The human IGF-II (cDNA) was kindly provided by Dr Graeme I Bell (Howard Hughes Medical Institute, University of Chicago). The sense precursor IGF-II was generated by PCR and cloned into the HindIII-XbaI site of the pRc/CMV vector (Invitrogen, San Diego, CA) as previously detailed (De Leon et al. 1999).
Transfection of Hs578t and MCF-10A cells with IGF-II sense cDNA
Hs578t and MCF-10A cells were transfected with the sense IGF-II vector. Transfection was carried out by the liposome method (DOTAP, Boehringer). G418 (Geneticin, 560 μg/ml) was used to maintain stable transfectants. Stable transfectants were cloned by limiting dilution in 96 well plates. To identify sense clones, serum-free CM from 96 well plates was screened with a dot blot assay, which measures IGF-II without interference by IGF binding proteins (IGFBPs) (De Leon et al. 1997).
Western blot analysis
Total protein concentration (30 μg) of CM collected after 24 and 48 h of RSV treatment was used to load polyacrylamide-SDS gradient gels (10–20%) and transferred to a nitrocellulose membrane Bio-TraceRNT (Life Sciences, Ann Arbor, MI) using a Semi-Dry electrophoretic Transfer Cell (Bio-Rad Laboratories, Hercules, CA). Protein concentration was measured using the Coomassie Plus Protein Assay Reagent™(Pierce Biothechnology, Rockford, IL). Nitrocellulose membranes were blocked with 2% BSA IgG free (Sigma Chemical Co., St Louis, MO) in PBS/0.05% Tween for 2 h. Membranes were then incubated with Amano IGF-II monoclonal antibody, (clone S1-F2 1:1000 Amano, Mitsubishi, Troy, VA) which recognizes both the mature (mIGF-II) and the precursor (proIGF-II) forms, or with CD antisera (1:400), at 4°C overnight. The blots were also probed with β-actin antibody (1:10,000, Sigma Chemical Co., St Louis, MO) and used as a protein loading control. After 3 × 10 min washes in PBS/0.05% Tween, the corresponding biotinylated secondary antibodies (1:1000, Amersham, Arlington Heights, IL) were added to the membranes (1 h at RT), followed by 3 × 10 min washes and incubation with HRP complexes (1:1000 Amersham, Arlington Heights, IL). Protein visualization was achieved by using enhanced chemiluminescence (ECL) and autoradiography with Hyperfilm ECL film (Amersham, Arlington Heights, IL). The signals on the X-ray films were quantified using ChemiImager™ 4000 (Alpha Innotech Corporation).
Northern blot analysis
Total RNA was extracted using Tri Reagent™ (Molecular Research Center). RNA was then precipitated using isopropanol and solubilized in 0.5% SDS. Ten microgram of each sample RNA was then electrophoresed on a 1% agarose-formaldehyde gel, and transferred to a nylon membrane (Hybond™-N, Amersham, Arlington Heights, IL) followed by UV cross-linking. The membranes were pre-hybridized (Clontech expressHyb™ solution) for 1 h at 68°C, before 32P-labeled IGF-II or CD cDNA was added. Blots were hybridized at 42°C. The membranes were then washed and exposed to Kodak film with intensifying screens at −80°C for 2–3 days. Subsequently, the membranes were stripped and reprobed with 32P-labeled cyclophilin cDNA, which was used as a loading control. The signals were quantitated using densitometric analysis of the autoradiographs by Chemi-Imager™ 4000 (Alpha Innotech Corporation).
Statistical analysis
Values are expressed as the mean ± SE. Statistical differences between mean values were determined by one-way ANOVA, SPSS 11.0 software (SPSS, INC., Chicago, IL). A level of p < 0.05 was considered significant.
Results
RSV effect on cathepsin D secretion
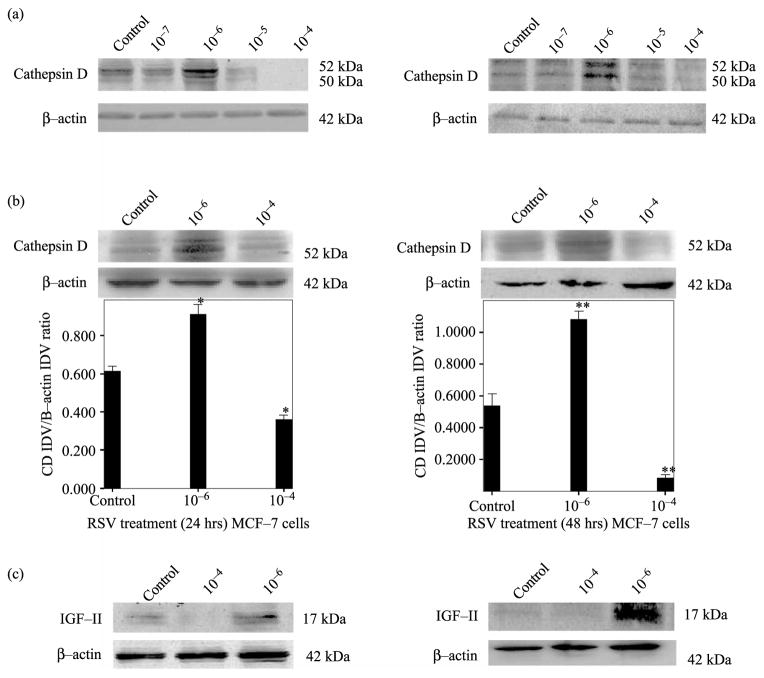
First we examined CD secretion by MCF-7 cells treated with varying concentrations of RSV (10−7– 10−4 M). Our results indicate that RSV exerts a concentration dependent effect on CD secretion 24 and 48 h post-treatment, as shown in Figure 1a. RSV (10−6) increases while RSV (10−4) decreases CD secretion. Thus, we concentrated our study on RSV treatment at 10−4 and 10−6 M. Figure 1b (upper panel) shows representative Western blots (WBs) (of three independent experiments). As seen on this figure, RSV (10−6 M) increased CD secretion ( p < 0.01) while RSV (10−4 M) inhibited CD secretion ( p < 0.01). Please note that only one WB is shown for representation purpose. Figure 1 (lower panel b) shows a bar graph representation of densitometric analysis of CD densitometry units (integrated density units, IDV) normalized to β-actin densitometry units of three separate experiments. As seen, RSV induced significant ( p < 0.01) changes in CD secretion when cells were treated at 10−4 and 10−6 M. CD changes correlated with IGF-II levels as observed in Figure 1c.
Figure 1.
RSV concentration-dependent effect on CD secretion by MCF-7 cells after 24 and 48 h treatment. Panel a shows a WB of CD secreted by MCF-7 cells, treated with different RSV concentrations (10−7–10−4). Panel b shows a representative WB of CD secreted by cells treated with RSV (10−6 M) or (10−4 M) dose of three separate experiments (upper panel). Lower panel b shows bar graph representations of CD data normalized to β-actin and presented as the mean ± standard error of three separate experiments. Asterisks indicate values significantly different from controls (*p < 0.05, **p < 0.01). Panel c shows WB analysis of IGF-II at 24 and 48 h of RSV treatment (10−6 and 10 −4 M).
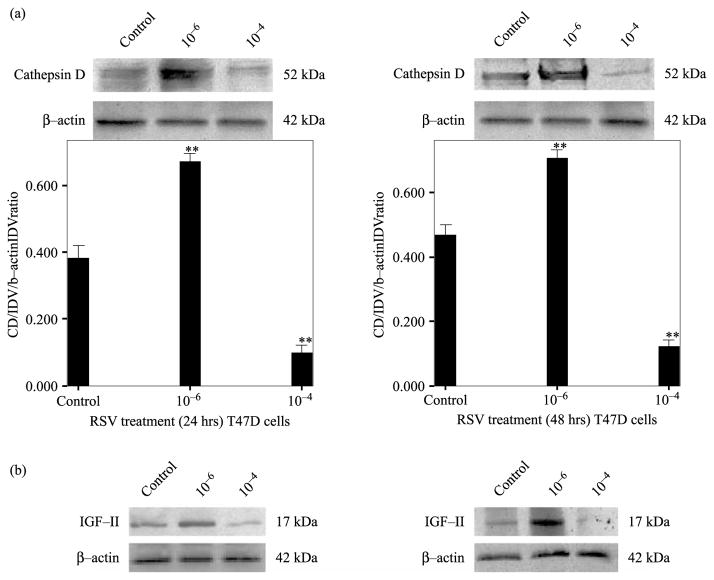
Similarly, Figure 2a (upper panels) shows RSV effect on CD secretion in T47D breast cancer cell after 24 and 48 h of treatment. RSV (10−6 M) induced an increase in CD secretion ( p < 0.01) while RSV (10−4 M) inhibited CD secretion ( p < 0.01). Figure 2a (lower panels) shows bar graph representation of densitometric analysis of CD normalized to β-actin densitometry units of three separate experiments. As seen with MCF-7 cells, RSV treatment (10−6) induced increased CD levels that correlated with increased IGF-II (Figure 2b).
Figure 2.
RSV concentration-dependent effect on CD secretion by T47D cells after 24 and 48 h treatment. Panel a shows a representative WB of CD secreted by cells treated with RSV (10−6 M) or (10−4 M) of three separate experiments (upper panel). Figure 2a (lower panel) shows bar graph representations of CD data normalized to β-actin and presented as the mean ± standard error of three separate experiments. Asterisks indicate values significantly different from controls (**p < 0.01). Panel b shows WB analysis of IGF-II at 24 and 48 h of RSV treatment (10−6 and 10−4 M).
RSV effect on cathepsin D mRNA levels
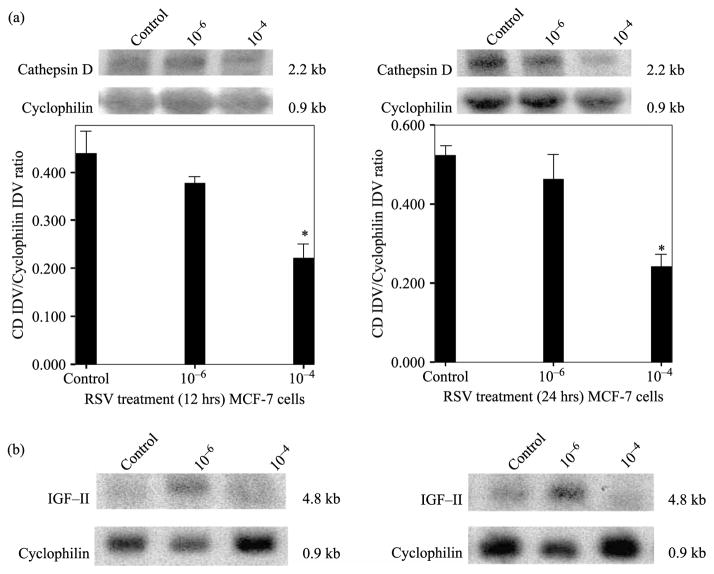
The effect of RSV on CD gene expression in MCF-7 cells was assessed by northern blot (NB) analysis. Figure 3a shows a representative NB (of three separate experiments) of CD at 12 and 24 h after RSV treatment. Since CD protein levels changed at 24 and 48 h, we reasoned that mRNA changes may occur earlier and chose to analyze mRNA levels at 12 and 24 h post-treatment. The decrease observed in CD mRNA when cells were treated with RSV (10−4 M) is significant at 12 ( p < 0.05) and 24 h ( p < 0.01). In contrast, there were no changes in CD mRNA when cells were treated with RSV (10−6 M). Figure 3a (lower panel) shows bar graph representations of densitometric analysis of CD densitometry units (integrated density units, IDV) normalized to cyclophilin mRNA densitometry units on three separate experiments. Please note that only one NB is shown for representation purposes, while data from three separate membranes of three different experiments were used in the bar graph below for the statistical analysis. Figure 3b shows that RSV treatment at high concentration (10−4 M) inhibited IGF-II mRNA at 12 and 24 h, while RSV (10−6 M) increased IGF-II mRNA. Furthermore, since the increase in CD secretion seen when cells are treated with RSV (10−6 M) does not correlate with increased CD mRNA levels, we conclude that treatment with RSV (10−6 M) modulated CD secretion by increasing the levels of IGF-II.
Figure 3.
RSV concentration-dependent effect on CD mRNA levels in MCF-7 cells (12 and 24 h). Upper panel a shows a representative CD NB from RSV treated (10−6 and 10−4 M) MCF-7 cells after 12 and 24 h treatment. The 2.2 kb band represents CD mRNA. Lower panel a shows bar graph representations of CD mRNA data normalized to Cyclophilin and presented as the mean ± standard error of three separate experiments. Asterisks indicate values significantly different from controls (*p < 0.05). Panel c shows NB analysis of IGF-II mRNA (the 4.8 kb band represents a transcript from IGF-II promoter) at 12 and 24 h of RSV treatment (10−6 and 10−4 M).
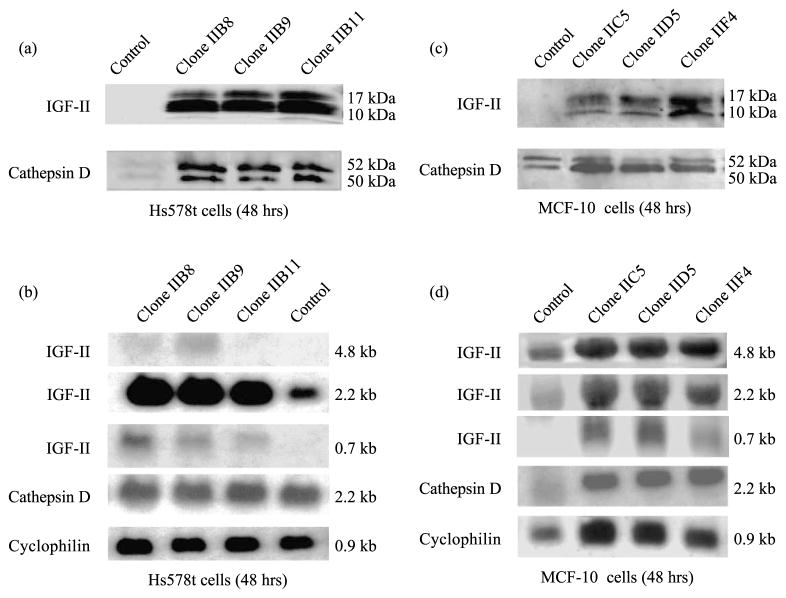
Transfection with proIGF-II sense cDNA induces increased CD secretion in Hs578t and MCF-10A cells
We also addressed the question of whether IGF-II modulates intracellular CD routing and CD secretion in hormone-independent ERα(−) breast cancer (Hs578t) and epithelial (MCF-10A) cells. We transfected Hs578t and MCF-10A cells with proIGF-II sense cDNA and measured CD protein secretion and mRNA levels after 48 h. Figure 4A shows IGF-II and CD WBs from IGF-II transfected cells (Clones IIB8, IIB9 and IIB11) or vector only (CMV) transfected Hs578t cells compared to non-transfected cells. As clearly seen, all transfected clones secreted high amounts of proIGF-II that correlated with increased CD secretion (4a). IGF-II was undetectable in control cells, although reactive CD bands were observed. Figure 4b shows corresponding NBs. Although three different IGF-II transcripts are seen (4.8, 2.2 and 0.7 kb), the major form transcribed by Hs578t cells corresponds to the 2.2 kb transcript. No significant change in CD mRNA is seen. Cyclophilin was used as a loading control. Similarly, Figure 4c and d shows MCF-10A cells WB and NB analyses of proIGF-II and CD from transfected (clones IIC5, IID5 and IIF4) and control cells (CMV). As observed in Figure 4d, all IGF-II transcripts (4.8, 2.2 and 0.7) increase in transfected MCF-10A cells. The 2.2 kb transcript represents mRNA from promoter 2 and 0.7 kb is the transfected IGF-II cDNA. Interestingly, CD mRNA also increased significantly in transfected MCF-10A cells when compared to control cells. Cyclophilin was used as a loading control.
Figure 4.
IGF-II and CD WB and NB analyses from Hs578t and MCF-10A cells 48 h after transfection with proIGF-II sense cDNA. Panel a shows WB of IGF-II (10 and 17 kDa immunoreactive bands) and CD (52 and 50 kDa bands) secreted by Hs578t cells transfected with proIGF-II sense cDNA. Panel b shows NB of IGF-II (4.8, 2.2 and 0.7 kb mRNA) and CD (2.2 kb mRNA) from transfected Hs578t cells. Cyclophilin was used as a loading control. Similarly, panels c and d show WB and NB, respectively, of IGF-II and CD secreted protein and mRNA levels from MCF-10A cells 48 h post-transfection.
Resveratrol effect on IGF-II and CD secretion in ER(−) human breast cancer and epithelial cells
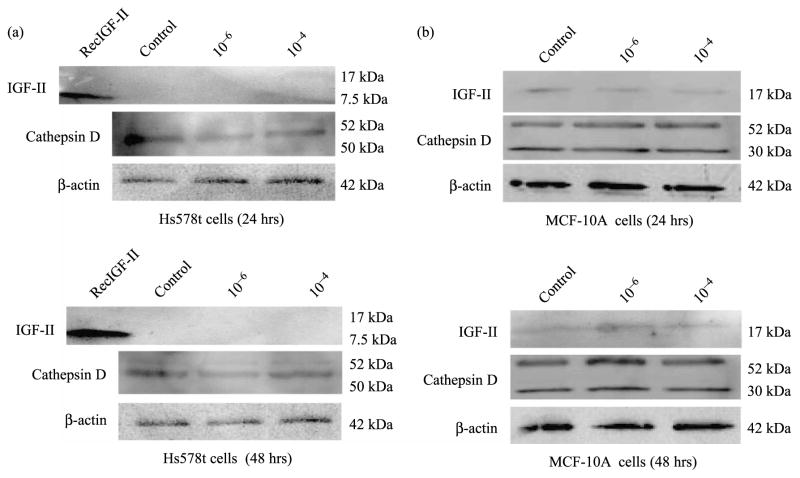
Since RSV exerts both ER-dependent and ER-independent effects on human breast cell lines and RSV (10−4) regulated CD gene expression in ER (+) breast cancer cells, we also studied IGF-II and CD secretion in Hs578t breast cancer and MCF-10A breast epithelial cell lines after RSV treatment (10−6 and 10−4 M). For this purpose, we measured IGF-II and CD protein secretion in RSV treated ERα(−) Hs578t breast cancer and MCF-10A breast epithelial cells. Figure 5a shows a representative WB (of three separate experiments) of IGF-II and CD secreted from Hs578t cells, at 24 and 48 h. As seen, control or RSV treated Hs578t cells did not secrete IGF-II (mature or precursor forms) and no changes in CD secretion were observed at any RSV concentration used. Figure 5b shows that MCF-10A cells secrete IGF-II, but RSV treatment does not change IGF-II or CD secretion from MCF-10A cells after 24 and 48 h.
Figure 5.
IGF-II and CD WB from RSV treated (10−6 and 10−4 M) Hs578t and MCF-10A cells. Panel a shows a representative WB of IGF-II (17 kDa) and CD (52, 50, 30 kDa) secreted by Hs578t cells after 24 and 48 h of RSV treatment, respectively. The 7.5 kDa band represents recombinant human IGF-II used as a positive control. Similarly, Figure 5b (upper panel) shows a representative WB of IGF-II and CD secreted by MCF-10A cells after 24 h of RSV treatment, while lower panel b shows IGF-II and CD WB 48 h after RSV treatment.
Discussion
CD is a prognostic indicator of metastasis and survival for breast cancer patients (Rochefort et al. 2000), making this protease an important target for chemotherapeutic agents. Several studies reported that RSV treatment stimulated cell proliferation of ER positive breast cancer cells (Gehm et al. 2004; Pozo-Guisado et al. 2004). In contrast, other reports have shown that RSV treatment inhibits cell growth of not only ER positive but also ER negative breast epithelial and cancer cells (Mgbonyebi et al. 1998; Damianaki et al. 2000). Recent studies in our laboratory (Vyas et al. 2005) showed that RSV effect on MCF-7 and T47D cells growth was mediated by a concentration- and ER-dependent regulation of IGF-II levels. Because both proteins, IGF-II and CD, bind the IGF-II/M6PR and binding of one ligand interferes with the binding of the other ligand (Mathieu et al. 1990), we explored the effect of RSV on CD secretion.
The present study demonstrates that RSV modulates CD secretion in a dose dependent manner in breast cancer cells. Treatment with RSV (10−6 M) caused an increase in CD secretion in MCF-7 and T47D cells without a corresponding increase in CD mRNA. In contrast, increased IGF-II secretion was associated with an increase in IGF-II mRNA levels in cells treated with RSV (10−6 M). Although CD is regulated by E2, our results showed that RSV-induced increase in CD secretion did not correlate with changes in CD mRNA, indicating that increased CD secretion did not require an increase in gene expression.
Our previous study demonstrated that RSV effect on IGF-II is ER (α) mediated since no effect was observed when ER (α − ) ERβ(+) MCF-10 cells were treated with RSV at 10−6 M (Vyas et al. 2005). In that study, we also showed that the effect of RSV on IGF-II promoted growth was significantly abolished by blocking the IGF-I receptor with an IGF-I receptor antibody. Thus, we believe that RSV-induced increase in endogenous IGF-II levels is responsible for the increase in CD secretion in the present study.
To further confirm this observation, we transfected ER(−) breast cell lines (Hs578t and MCF-10) with precursor IGF-II (proIGF-II) sense cDNA. An increase in CD secretion was observed in the Hs578t and MCF-10 transfected cells. This data provides evidence, that the expression of IGF-II in ERα (−) Hs578t cells and in the “non-transformed” MCF-10 cells increases the secretion of CD independent of ER status. Furthermore, since the MCF-10 cells are non-transformed, the increased secretion of CD caused by IGF-II expression is not unique to cancer cells. Since no changes in CD secretion were observed when non-transfected Hs578t and MCF-10 were treated with RSV, we conclude that RSV increase in CD secretion is observed only in ERα(+) cells and it requires IGF-II expression. In the context of CD gene activation, it is known that ERα interaction with USF-1 and USF-2 in the CD promoter is a critical step in the estrogen activation of CD gene transcription, a step that might not take place when RSV (10−6 M) binds the ERα.
Thus, changes in CD secretion are related to the derouting of intracellular CD caused by IGF-II expression. Since normal mammary cells should not secrete CD, we were surprised by the high levels of CD secreted by the non-transformed MCF-10 cells. As shown in our study, MCF-10A cells express the 4.8 and 2.2 kb IGF-II mRNAs. The expression of the 4.8 kb IGF-II mRNA is derived from the IGF-II gene promoter 3, active only in fetal development and in cancer (Daimon et al. 1992; Mineo et al. 2000). IGF-II expression in these “normal” mammary cells may result in CD secretion. This finding is of great significance, because in addition to inducing degradation of the extracellular matrix (ECM), CD also causes proteolysis of IGFBPs leading to increased IGF bioavailability and stimulated cell growth (Conover and DeLeon 1993; Claussen et al. 1997). Secreted CD is involved in cancer cell growth and differentiation. Several studies have shown that when CD is increased, it autoactivates itself by cleavage of a 1 kDa N-terminal peptide that acts as a mitogen for breast epithelial cells (Vetvika et al. 2002). Since MCF-10A cells were derived from tissue with fibrocystic changes, we propose that they represent the earlier steps in the development of breast cancer. The lack of IGF-II secretion by the MCF-10A cells is probably due to its binding to the IGF-II/M6P receptor and its routing to the lysosomes where it is degraded. Nevertheless, by promoting CD secretion IGF-II expression alters the cell microenvironment increasing cell proliferation. Thus, both IGF-II and CD may be associated with the increased risk of breast cancer observed in women with fibrocystic disease.
In contrast to the effects of RSV treatment at 10−6 M, treatment with RSV (10−4 M) had an inhibitory effect on CD gene expression and protein secretion in ER(+) MCF-7 and T47D cells. RSV has also been shown to exert ER-independent effects on both cells expressing the ER and cells that do not express the ER-α. Recently, RSV has been shown to activate adenylyl cyclase (AC)/kinase-A pathway in ER(+) MCF-7 as well as in ER(−) MDA-MB-231 breast cancer cell lines (El-Mowafy and Alkhalaf 2003). This data indicates that recruitment of AC by RSV is not confined to ER(+)MCF-7 cells; thus RSV’s effect on AC is independent of the cell ER status. Our study also showed that RSV effect on CD secretion is only present in ERα (+) human breast cancer cells and not in ERα (−) cells. Of note, the 52 and 30 kDa CD forms were present in the conditioned media from MCF-10A cells, suggesting that a sufficiently acidic pH, which is required for CD processing and activation and is normally found in the microenvironment of malignant cells, might also be found in the MCF-10A microenvironment, a significant finding in terms of potential malignant transformation.
CD is not only associated with cancer progression, but appears to be a rate limiting factor in stimulating in vitro and in vivo tumor growth; CD stimulates directly or indirectly both, cancer cell proliferation and tumor angiogenesis independently of its proteolytic activity and may provide some protection against tumor apoptosis by proteolysis (Berchem et al. 2002).
Thus, our results indicate that high RSV concentrations (10−4 M) decrease CD mRNA and protein levels. Although CD secretion is increased by treatment with RSV at low concentration (10−6 M), this effect is only seen in cell lines expressing the ERα. Furthermore, our previous study showed that in the presence of E2 and tamoxifen, RSV treatment at all concentrations studied (10−4–10−6 M) inhibited IGF-II secretion in all breast cancer cell lines studied (Vyas et al. 2005). This implies, that RSV increase in IGF-II secretion in vitro may be of no consequence in vivo, particularly in pre-menopausal patients where estrogen is present and antiestrogens may be used as treatment. This is of great significance since the use of RSV is a promising chemical in the chemoprevention of breast cancer treatment and it is currently being used in clinical trials phase 1 at the National Cancer Institute (NCI protocol). Thus, inhibition of CD by RSV represents a novel mechanism by which RSV may protect against breast cancer.
References
- Aziz MH, Kumar R, Ahmad N. Cancer chemoprevention by resveratrol: In vitro and in vivo studies and the underlying mechanisms. Int J Oncol. 2003;23:17–28. [PubMed] [Google Scholar]
- Berchem G, Glondu M, Gleizes M, Brouillet JP, Vignon F, Garcia M, Liaudet-Coopman E. Cathepsin D affects multiple tumor progression steps in vivo: Proliferation, angiogenesis and apoptosis. Oncogene. 2002;21:5951–5955. doi: 10.1038/sj.onc.1205745. [DOI] [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. Cancer chemopreventive activity of resveratrol. Ann NY Acad Sci. 2002;957:210–229. doi: 10.1111/j.1749-6632.2002.tb02918.x. [DOI] [PubMed] [Google Scholar]
- Cavailles V, Augereau P, Rochefort H. Cathepsin D gene is controlled by a mixed promoter, and estrogens stimulate only TATA-dependent transcription in breast cancer cells. Proc Natl Acad Sci. 1993;90:203–207. doi: 10.1073/pnas.90.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claussen M, Kubler B, Wendland M, Neifer C, Schmidt B, Zapf J, Braulke T. Proteolysis of insulin-like growth factors (IGF) and IGF binding proteins by cathepsin D. Endocrinology. 1997;138:3797–3803. doi: 10.1210/endo.138.9.5418. [DOI] [PubMed] [Google Scholar]
- Conover CA, De León DD. Acid-activated insulin-like growth factor binding protein-3 proteolysis in normal and transfected cells. Role of Cathepsin D. J Biol Chem. 1994;269:7076–7080. [PubMed] [Google Scholar]
- Daimon M, Johnson TR, Ilan J. The third IGF-II promoter specifies transcription of three transcripts out of five in human placenta. Mol Reprod Dev. 1992;33:413–417. doi: 10.1002/mrd.1080330407. [DOI] [PubMed] [Google Scholar]
- Damianaki A, Bakogeorgou E, Kampa M, Notas G, Hatzoglou A, Panagiotou S, Gemetzi C, Kouroumalis E, Marti PM, Castanas E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J Cell Biochem. 2000;78:429–441. doi: 10.1002/1097-4644(20000901)78:3<429::aid-jcb8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- De León DD, Asmerom Y. Quantification of insulin-like growth factor I without interference by IGF binding proteins. Endocrinology. 1997;138:2199–2202. doi: 10.1210/endo.138.5.5237. [DOI] [PubMed] [Google Scholar]
- De Leon D, Issa N, Nainani S, Asmerom Y. Reversal of cathepsin D routing modulation in MCF-7 breast cancer cells expressing antisense insulin-like growth factor II. Horm Metab Res. 1999;31:142–147. doi: 10.1055/s-2007-978712. [DOI] [PubMed] [Google Scholar]
- De Leon D, Terry C, Asmerom Y, Nissley SP. Insulin-like growth factor II modulates the routing of cathepsin D in MCF-7 breast cancer cells. Endo. 1996;137:1851–1859. doi: 10.1210/endo.137.5.8612524. [DOI] [PubMed] [Google Scholar]
- Dong Z. Molecular mechanism of the chemopreventive effect of resveratrol. Mutat Res. 2003;523–524:145–150. doi: 10.1016/s0027-5107(02)00330-5. [DOI] [PubMed] [Google Scholar]
- El-Mowafy AM, Alkhalaf M. Resveratrol activates adenylyl-cyclase in human breast cancer cells: A novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis. 2003;24:869–873. doi: 10.1093/carcin/bgg015. [DOI] [PubMed] [Google Scholar]
- Faridi JS, Mohan S, DeLeon DD. Modulation of cathepsin D routing by IGF-II involves IGF-II binding to the IGF-II/M6P receptor in MCF-7 breast cancer cells. Growth Factors. 2004;22:169–177. doi: 10.1080/08977190410001725531. [DOI] [PubMed] [Google Scholar]
- Garcia D, Derocq D, Pujol P, Rochefort H. Overexpression of transfected cathepsin D in transformed cells increases their malignant phenotype and metastatic potency. Oncogene. 1990;5:1809–1814. [PubMed] [Google Scholar]
- Gehm BD, Levenson AS, Liu A, Lee EJ, Amudsen BM, Cushman M, Jordan VC, Jameson JL. Estrogenic effects of resveratrol in breast cancer cells expressing mutant and wild-type estrogen receptors: Role of AF-1 and AF-2. J Steroid Biochem Mol Biol. 2004;88:223–234. doi: 10.1016/j.jsbmb.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Glondu M, Coopman P, Laurent-Matha V, Garcia M, Rochefort H, Liaudet-Coopman E. A mutated cathepsin D devoid of its catalytic activity stimulates the growth of cancer cells. Oncogene. 2001;20:6920–6929. doi: 10.1038/sj.onc.1204843. [DOI] [PubMed] [Google Scholar]
- Leto G, Tumminello FM, Crescimanno M, Flandina C, Gebbia N. Cathepsin D expression levels in non-gynecological solid tumors: Clinical and therapeutic implications. Clin Exp Metastasis. 2004;21:91–106. doi: 10.1023/b:clin.0000024740.44602.b7. [DOI] [PubMed] [Google Scholar]
- Liaudet E, Garcia M, Rochefort H. Cathepsin D maturation and its stimulatory effect on metastasis are prevented by addition of KDEL retention signal. Oncogene. 1994;9:1145–1154. [PubMed] [Google Scholar]
- Mathieu M, Rochefort H, Barenton B, Prebois C, Vignon F. Interactions of cathepsin D and insulin-like growth factor II (IGF-II) on the IGF-II/mannose-6-phosphate receptor in human breast cancer cells and possible consequences on mitogenic activity of IGF-II. Mol Endocrinol. 1990;4:1327–1335. doi: 10.1210/mend-4-9-1327. [DOI] [PubMed] [Google Scholar]
- Mgbonyebi OP, Russo J, Russo IH. Antiproliferative effect of synthetic resveratrol on human breast epithelial cells. Int J Oncol. 1998;12:865–869. [PubMed] [Google Scholar]
- Mineo R, Fichera E, Liang SJ, Fujita-Yamaguchi Y. Promoter usage for insulin-like growth factor II in cancerous and benign human breast, prostate, and bladder tissues and confirmation of a 10th exon. Biochem Biophys Res Commun. 2000;268:886–892. doi: 10.1006/bbrc.2000.2225. [DOI] [PubMed] [Google Scholar]
- Montcourrier P, Silver IA, Farnoud R, Bird I, Rochefort H. Breast cancer cells have a high capacity to acidify extracellular milieu by a dual mechanism. Clin Exp Metastasis. 1997;15:382–3892. doi: 10.1023/a:1018446104071. [DOI] [PubMed] [Google Scholar]
- Pozo-Guisado E, Lorenzo-Benayas MJ, Fernandez-Salguero PM. Resveratrol modulates the phosphoinositide 3-kinase pathway through an estrogen receptor alpha-dependent mechanism: Relevance in cell proliferation. Int J Cancer. 2004;109:167–173. doi: 10.1002/ijc.11720. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Cavailles V, Augereau P, Capony F, Maudelonde T, Touitou I, Garcia M. Overexpression and hormonal regulation of pro-cathepsin D in mammary and endometrial cancer. J Steroid Biochem. 1989;34:177–182. doi: 10.1016/0022-4731(89)90080-0. [DOI] [PubMed] [Google Scholar]
- Rochefort H, Garcia M, Glondu M, Laurent V, Liaudet E, Rey JM, Roger P. Cathepsin D in breast cancer: Mechanisms and clinical applications, a 1999 overview. Clinica Quimica Acta. 2000;291:157–170. doi: 10.1016/s0009-8981(99)00226-0. [DOI] [PubMed] [Google Scholar]
- Ruibal A, Arias JI. Axillary lymph node involvement in hormone-independent breast carcinomas. Med Clin. 2004;123:50–52. doi: 10.1016/s0025-7753(04)74408-5. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Tang J. Cathepsin D from porcine and bovine spleen. Methods Enzymol. 1981;80:565–581. doi: 10.1016/s0076-6879(81)80045-6. [DOI] [PubMed] [Google Scholar]
- Vetvika V, Benes P, Fusek M. Procathepsin D in breast cancer: What do we know? Effects of ribozymes and others inhibitors. Cancer Gene Ther. 2002;9:854–863. doi: 10.1038/sj.cgt.7700508. [DOI] [PubMed] [Google Scholar]
- Vyas S, Asmerom Y, De Leon DD. Resveratrol regulates IGF-II in breast cancer cells. Endocrinology. 2005 doi: 10.1210/en.2004–1344. published July 21, 2005. [DOI] [PubMed] [Google Scholar]
- Xing W, Archer T. Upstream stimulatory factors mediate estrogen receptor activation of the cathepsin D promoter. Mol Endocrinol. 1998:1310–1321. doi: 10.1210/mend.12.9.0159. [DOI] [PubMed] [Google Scholar]