Abstract
We present a novel multi-compartment neuron co-culture microsystem platform for in vitro CNS axon-glia interaction research, capable of conducting up to six independent experiments in parallel for higher-throughput. We developed a new fabrication method to create microfluidic devices having both micro and macro scale structures within the same device through a single soft-lithography process, enabling mass fabrication with good repeatability.
The multi-compartment microfluidic co-culture platform is composed of one soma compartment for neurons and six axon/glia compartments for oligodendrocytes (OLs). The soma compartment and axon/glia compartments are connected by arrays of axon-guiding microchannels that function as physical barriers to confine neuronal soma in the soma compartment, while allowing axons to grow into axon/glia compartments. OLs loaded into axon/glia compartments can interact only with axons but not with neuronal soma or dendrites, enabling localized axon-glia interaction studies. The microchannels also enabled fluidic isolation between compartments, allowing six independent experiments to be conducted on a single device for higher throughput.
Soft-lithography using poly(dimethylsiloxane) (PDMS) is a commonly used technique in biomedical microdevices. Reservoirs on these devices are commonly defined by manual punching. Although simple, poor alignment and time consuming nature of the process makes this process not suitable when large numbers of reservoirs have to be repeatedly created. The newly developed method did not require manual punching of reservoirs, overcoming such limitations. First, seven reservoirs (depth: 3.5 mm) were made on a poly(methyl methacrylate) (PMMA) block using a micro-milling machine. Then, arrays of ridge microstructures, fabricated on a glass substrate, were hot-embossed against the PMMA block to define microchannels that connect the soma and axon/glia compartments. This process resulted in macro-scale reservoirs (3.5 mm) and micro-scale channels (2.5 μm) to coincide within a single PMMA master. A PDMS replica that served as a mold master was obtained using soft-lithography and the final PDMS device was replicated from this master.
Primary neurons from E16-18 rats were loaded to the soma compartment and cultured for two weeks. After one week of cell culture, axons crossed microchannels and formed axonal only network layer inside axon/glia compartments. Axons grew uniformly throughout six axon/glia compartments and OLs from P1-2 rats were added to axon/glia compartments at 14 days in vitro for co-culture.
Protocol
Part 1: Preparing a master mold for multi-compartment neuron co-culture device fabrication
First step to fabricate the PMMA master is to make 3.5 mm deep compartments. One soma compartment and six axon/glia compartments are defined on a PMMA block using a micro-milling machine (MDX-40, Roland or any other CNC milling machine). PMMA master is then sonicated in isopropyl alcohol for 10 minutes to remove debris.
Arrays of 2.5 μm high ridge structures are patterned on a 50.8 mm x 50.8 mm glass slide by a standard photolithography process, followed by wet etching of the glass.First, array of ridge structures are patterned on a cleaned glass substrate with positive photoresist S1818 using photolithography. S1818 is spin-coated on the substrate (4000 rpm), soft based at 110°C for 5 min, and then exposed to UV light (84 mJ/cm2) using a mask aligner followed by developing of the photoresist in MF319 for 40 seconds. Next, the photoresist patterned glass substrate is immersed in buffered oxide etch (BOE) at room temperature for 6-7 minute to obtain a 2.5 μm tall ridge structures. Finally, the photoresist layer is removed by acetone and isopropyl alcohol, followed by thorough rinsing in de-ionized (DI) water.
Glass substrate with micro-ridge structures is hot-embossed against the PMMA master to transfer its patterns.First, PMMA mold master and glass substrates are manually aligned and placed on a hot-press. Then, temperature is raised to 115 °C. Once desired temperature is reached, 0.5 tons of weight is applied for 5 minutes. After 5 minutes, temperature is cooled down to 40 °C and pressure is released. The PMMA master is then cut into a 50.8 mm x 50.8 mm size block.
PDMS pre-polymer (Sylgard 184) is mixed with curing agent at the ratio of 10: 1 by weight.
PMMA master is placed inside a cask and PDMS mixture is poured on top of the PMMA master to fabricate a PDMS master. It is then placed in a desiccator connected to a vacuum pump. PDMS poured on top of PMMA master is then degassed for 15 minutes to remove bubbles generated during the PDMS mixing process.
When all bubbles are removed, PDMS is put inside a leveled 85 °C oven for one hour for polymerization.
Once PDMS is fully cured, PDMS master is peeled off from the PMMA master and placed inside a desiccator with 2-3 drops of trichlorosilane and vacuumed for 15 minutes to vapor coat the PDMS master surface. Trichlorosilane coating facilitates the release of the final PDMS neuron co-culture devices from the PDMS master. After coating, the PDMS master is briefly rinsed with isopropyl alcohol to remove excessive trichlorosilane, and dried with N2 gas.
Part 2: Neuron culture device replication
PDMS pre-polymer (Sylgard 184) is mixed with curing agent at the ratio of 10: 1 by weight.
PDMS mixture is poured on top of the PDMS master and degassed inside a desiccator connected to a vacuum pump for 15 minutes. It is important not to pour too much PDMS so that the 3.5 mm high PDMS structure on the master is not fully immersed.
When all bubbles are removed, PDMS is put inside a leveled 85 °C oven for one hour for polymerization.
PDMS is taken out from the oven after one hour and left at room temperature to cool down. Then, PDMS neuron culture devices are gently peeled off from the PDMS master. Peeled off PDMS devices are wrapped with parafilm to protect them from dusts or debris.
PDMS devices, wrapped with parafilm, are cut into individual pieces using a drill press equipped with a blade.
Part 3: Device assembly
PDMS neuron culture device is placed inside the plasma cleaner and exposed to air plasma for 90 seconds to make the surface of the PDMS device hydrophilic. This plasma treatment will facilitate microchannels to be filled with culture media after assembly to poly-d-lysine coated substrate.
Devices treated with plasma are then immersed in 70 % ethanol for 30 minutes for sterilization and moved inside the sterilized bio-hood.
Sterilized devices are taken out from 70 % ethanol and dried with Millipore filtered N2 gas. Dried devices are placed on top of poly-d-lysine coated polystyrene 6-well culture plate and gentle pressure is applied to ensure the seal between the substrate and the device.
Culture media is then filled into the soma compartment, followed by filling the six axon/glia compartments. It is better to give 1-2 minutes pause before filling axon/glia compartments after adding culture media to the soma compartment to ensure that no bubbles are trapped within the microchannels.
Devices filled with culture media is incubated inside an 37 °C incubator overnight to rinse any possible debris or toxic residue.
Part 4: Cell loading and neuron-oligodendrocyte co-culture
On the first day of the cell culture, primary forebrain neuron cells from embryonic day 16-18 rats are loaded into the soma compartment at an areal density of 500 cells/mm2. First, culture media inside both the soma compartment and axon/glia compartments are aspirated using pipette prior to cell loading. Then, 38500 cells, diluted in 40 μl of culture media, are loaded to the soma compartment around the microchannel inlets. It is important not to touch the PDMS device while loading the cells, since it can break the seal between the device and the substrate.
Incubate the cell loaded devices for 30 minutes inside an 37 °C incubator to enhance cell adhesion to the substrate. After incubation, all compartments are filled up with culture media.
Cells are cultured at 37 °C in a humidified 5 % CO2 incubator and only half of culture media is changed out every 3-4 days.
After 14 days of neuron culture in vitro, oligodendrocytes are added to the axon/glia compartments for co-culture. Oligodendrocytes, dissected from the cerebral hemispheres of Sprague-Dawley rats at postnatal day 1-2, are added to each axon/glia compartment at an area density of 1000 cells/mm2. Prior to loading cells, approximately 60 % of culture media inside the compartments are aspirated with pipette. Caution must be made not to touch the bottom substrate, since it can damage the axonal network formed on the substrate. Then, 6700 oligodendrocyte cells, diluted in 5 μl of culture media, are added to the axon/glia compartments. Cells are incubated for 1 hour and compartments are filled up with culture media.
Cells are cultured at 37 °C in a humidified 5 % CO2 incubator and only half of culture media is changed out every 3-4 days for two more weeks.
Part 5: Representative Results:
When experiments are carried out properly, neuron cells loaded to the soma compartment locate close to microchannel inlets and axonal layer start to form inside the axon/glia compartments after 1 week of culture. Sign of neuron cell aggregation inside the soma compartment can be an indication that cells are not healthy. When loading oligodendrocytes after two weeks of neuron culture, axon/glia compartments should be covered with dense layer of axons and oligodendrocytes should be loaded on top of the axonal layer.
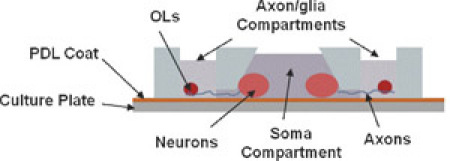 Figure 1. Schematic illustrations of the high-throughput microfluidic multi-compartment CNS neuron co-culture platform. Cross-sectional view showing isolation of axons from neuronal soma and dendrites as well as fluidic isolation between compartments with minute fluidic level difference. Please click here to see a larger version of figure 1.
Figure 1. Schematic illustrations of the high-throughput microfluidic multi-compartment CNS neuron co-culture platform. Cross-sectional view showing isolation of axons from neuronal soma and dendrites as well as fluidic isolation between compartments with minute fluidic level difference. Please click here to see a larger version of figure 1.
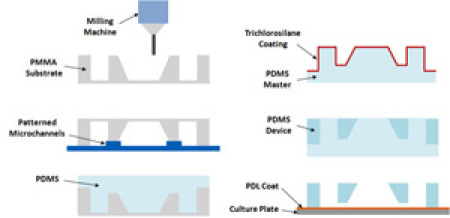 Figure 2. Fabrication and assembly steps for the multi-compartment PDMS microfluidic co-culture device. Each device fits into one well of a conventional 6-well polystyrene cell culture plate. Please click here to see a larger version of figure 2.
Figure 2. Fabrication and assembly steps for the multi-compartment PDMS microfluidic co-culture device. Each device fits into one well of a conventional 6-well polystyrene cell culture plate. Please click here to see a larger version of figure 2.
Discussion
We have developed a multi-compartment co-culture platform for studying mammalian CNS axon-glia interaction. CNS axons were successfully isolated from neuronal cell bodies/dendrites, and oligodendrocytes were successfully co-cultured inside the device. Neuron density can be varied by applications, but too low or too high concentration can cause cells to die. Also, it is important to change only half of culture media so that the cells or axonal layer on the substrate are not damaged. We expect that this system will be a powerful tool for studying CNS axon-glia signaling networks in vitro and provide a path toward a high-throughput platform for screening growth factors or potential drug candidates that promote myelination and myelin repair.
Acknowledgments
This work was supported by the National Institutes of Health / National Institute of Mental Health (NIH/NIMH) grant #1R21MH085267.
References
- Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature Reviews. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- Xia Y, Whitesides G. Soft Lithography. Angew. Chem. 1998;37:550–575. doi: 10.1002/(SICI)1521-3773(19980316)37:5<550::AID-ANIE550>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Taylor A. Microfluidic culture platform for neuroscience research. Nature Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA, A P. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proceedings of the National Academy of Sciences. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]


