Abstract
IGF-II plays a crucial role in fetal and cancer development by signaling through the IGF-I receptor. We have shown that inhibition of IGF-II by resveratrol (RSV) induced apoptosis and that proIGF-II (highly expressed in cancer) was more potent than mIGF-II in inhibiting this effect. Thus, we hypothesized that IGF-II differentially regulates the signaling cascade of the IGF-I receptor to stimulate the anti-apoptotic proteins Bcl-2 and Bcl-XL to prevent apoptosis. RSV treatment to breast cancer cells inhibited Bcl-2 and Bcl-XL expression and induced mitochondrial membrane depolarization. ProIGF-II was more potent than mIGF-II in: (1) activating the PI3/Akt pathway, (2) regulating Bcl-2 and Bcl-XL expression, and (3) inducing phosphorylation/nuclear translocation of Cyclic AMP-responsive element binding protein. Furthermore, IGF-II differentially regulated the intracellular translocation of Bcl-2 and Bcl-XL, a critical process in breast cancer progression to hormone-independence. Our study provides a novel mechanism of how proIGF-II promotes progression and chemoresistance in breast cancer development.
Keywords: Insulin-like growth factor II, Bcl-2, Bcl-XL, MCF-7, Akt, CREB
Introduction
IGF-II promotes proliferation, inhibits apoptosis, and stimulates angiogenesis and transformation of breast cancer cells (De León et al. 1992; Yang et al. 1996; Heffelfinger et al. 1999; Yee and Lee 2000). The IGF-II gene encodes a single transcript, which is translated into proIGF-II, a 156 amino acid protein including an 89 amino acid E-domain. ProIGF-II is expressed during fetal development and in tumors and is more potent than mIGF-II in stimulating thymidine incorporation in national institute for health (NIH) 3T3 (Yang et al. 1996). On the contrary, mIGF-II has been shown to be more effective in down-regulating the IGF-IR and decreasing cell survival in Caco2 colon cancer cells when compared to proIGF-II (Kim et al. 2005).
IGF-II mitogenic effects are mediated through the IGF-IR and IR-A, both members of the tyrosine kinase receptor family (Sciacca et al. 2002). The insulin receptor substrates 1 and 2 (IRS-1, IRS-2) are key cytoplasmic docking proteins that function as essential signaling intermediates downstream of the above mentioned receptors (Gibson et al. 2007). Upon activation by phosphorylation, specific residues become the docking site for multiple SH2-containing proteins such as PI3K, GRB-2 or SHP-2 phosphatase, thus, resulting in activation/inhibition of downstream pathways: the phosphatidylinositol 3′-kinase (PI3K) and the extracellular signal-regulated kinase (ERK; Myers et al. 1998; Hers et al. 2002; Dearth et al. 2007).
Bcl-2 and Bcl-XL, like IGF-II, are expressed in a variety of embryonic and post-natal tissues, which suggests their important roles in organogenesis and tissue homeostasis (González-García et al. 1994; Rossé et al. 1998). Bcl-XL is highly expressed in many types of cancer, including breast carcinoma, and has been associated with an increased risk of metastasis and resistance to treatment (Méndez et al. 2006). Bcl-XL has also been implicated in coupling redox pathways and glycolysis to protect breast cancer metastatic cells during transit from the primary tumor to the metastatic state (España et al. 2005). In MCF-7 cells, Bcl-2 and Bcl-XL have been found to differ substantially in the potency with which they inhibit apoptosis, possibly due to differences in the regulation of specific subcellular pathways (Fiebig et al. 2006). Furthermore, clinical studies have demonstrated that increased levels of Bcl-XL are associated with a poor outcome in breast cancer patients; conversely, Bcl-2 expression conferred a better prognosis (Lipponen et al. 1995; Olopade et al. 1997).
The transcription of IGF-II is regulated by estrogen (Lee et al. 1994), and IGF-I binding to the IGF-IR phosphorylates and activates the membrane estrogen receptor (ERα; Yee and Lee 2000). Thus, IGF-II signaling can stimulate estrogen regulated genes, including its own gene, by activating the ERα in the cell membrane. Previous studies in our laboratory demonstrated that the insulin-like growth factor II (IGF-II) is regulated by the chemopreventive agent resveratrol (RSV) in a dose-dependent manner in MCF-7 and T47D (ER + breast cancer cell lines), where the precursor form of IGF-II (proIGF-II), and not mature IGF-II (mIGF-II), is the predominant form secreted. This effect is mediated through the ER, as addition of tamoxifen blocked RSV effect on IGF-II expression (Vyas et al. 2005). Moreover, RSV (10−4 M) inhibitory effect on proIGF-II, leads to mitochondrial membrane depolarization and cell death. Since we also demonstrated that constitutively expressed IGF-II blocked cell death induced by RSV, we hypothesize that IGF-II must regulate pro-survival proteins and/or inhibit pro-apoptotic proteins.
Cyclic AMP-responsive element binding protein (CREB) and nuclear factor-KB (NF-KB) are transcription factors shown to be critical for a variety of cellular processes, including proliferation, differentiation and inflammation (Shaywitz and Greenberg 1999; Liu et al. 2001; Hirano et al. 2006). Both transcription factors have been shown to be able to induce Bcl-2 and/or Bcl-XL transcription following phosphorylation (by the Mitogen Activated Protein Kinase (MAPK) MAP/ERK kinase/(MEK)/extracellular signal regulated kinase (ERK) 1/2 or PI3K/Akt pathways) and nuclear translocation (Lee et al. 1999; Catz and Johnson 2001).
Therefore, the present study focuses on: (1) ProIGF-II and mIGF-II regulation of the anti-apoptotic proteins Bcl-2 and Bcl-XL; (2) The signaling pathways associated with IGF-II regulation of Bcl-2 and Bcl-XL; and (3) the role of the transcription factors NF-KB and CREB in IGF-II regulation of the above mentioned anti-apoptotic proteins.
Materials and methods
Cell culture
MCF-7 breast carcinoma cell line was obtained from the American Type Culture Collection. MCF-7 cells were maintained in a 5% CO2 incubator at 37°C, using Dulbecco’s modified eagle’s medium (DMEM) F12 media (Cellgro) supplemented with 10 ml of 5000 units penicillin/streptomycin (100units/ml penicillin and 100 units/ml streptomycin sulfate, Cellgro), 4 mM L-glutamine (Cellgro), 3 μg/ml β-amphotericin, and 5% fetal bovine serum (Hyclone). Recombinant human precursor IGF-II (proIGF-II, aa 1–156, non-glycosylated) and recombinant mature IGF-II were purchased from GroPep (Adelaide, Australia), and PeproTech (Rocky Hill, NJ, USA), respectively. RSV was purchased from Sigma Chemical Co. (St Louis, MO, USA), and dissolved in dimethyl sulfoxide (DMSO, Fischer Scientific Pittsburgh, PA, USA). The Akt 1/2 specific kinase inhibitor and MAPK specific inhibitor (LY294002 and PD98059, respectively) were obtained from Sigma Chemical Co. Cell lysates from RSV treated cells (CL) were collected (at 6 and 9 h), centrifuged (800 rpm for 5 min), and kept frozen (−20°C) until assayed.
siRNA transfection
MCF-7 cells were seeded at a density of 5 × 105 cells/well in six-well plates. siRNA (5 pmol) were added to the cells followed by 24 h incubation at 37°C in a 5% CO2 incubator. Cells were treated with RSV (10 −4 M) and IGF-II (mIGF-II, proIGF-II) for 9 h. Cell lysates were prepared using the Complete Lysis-M kit (Roche Applied Science, Germany), according to manufacturer protocol and stored at −20°C until assayed.
Western blot analysis
Total protein (60 μg) of cell lysates or subcellular compartment fractions (cytosol (F1), membrane/organelle (F2) and nucleus (F3)) obtained by ProteoExtract® Subcellular Proteome Extraction Kit (Calbiochem) were collected after 6 and 9 h of RSV treatment and used to load polyacrylamide sodium dodecyl sulfate (SDS) gradient gels (4–12%), transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen, Carlsbad, CA, USA) using a X-Cell SureLock® electrophoretic transfer module (Invitrogen). Protein concentration was measured using the Coomassie Plus Protein Assay Reagent™ (Pierce Biotechnology, Rockford, IL, USA). PVDF membranes were blocked with 5% non-fat milk in PBS/0.05% Tween for 2 h. Membranes were then incubated with IGF-II monoclonal antibody (Amano), Bcl-2 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or Bcl-XL polyclonal antibody (BD Biosciences, San Diego, CA, USA), followed by overnight incubation at 4°C. The blots were also probed with β-actin (whole cell lysates) or IGF-I antibodies (Sigma Chemical Co.) and used as a protein loading control (organelles and nuclear fractions). Specific markers were used to verify each cell fraction; nucleus (lens-epithelium derived growth factor (LEGDF), nuclear pore), organelles (voltage-dependent anion channel (VDAC), MnSOD, COX-2 and COX-4). To address loading, we added human recombinant IGF-I peptide (which was added at equimolar concentration in F2, F3 cell fractions before aliquoting the samples), as loading control for F2 and F3. After 3 × 10 min washes in phosphate buffered saline (PBS)/0.05% Tween, the corresponding biotinylated secondary antibodies (1:1000, Amersham, Arlington Heights, IL, USA) were added to the membranes (1 h at room temperature (RT)), followed by 3 × 10 min washes and incubation with horseradish peroxidase (HRP) complexes (1:1000 Amersham). Protein visualization was achieved by using enhanced chemiluminescence (ECL) and autoradiography with Hyperfilm ECL film (Amersham). The signals on the X-ray films were quantified using ChemiImager™ 4000 (Alpha Innotech Corporation, San Leandro, CA, USA).
Phosphorylation studies
MCF-7 cells were plated at a density of 5 × 105 cells/well in six-well plates, and grown in serum free media (SFM). Cells were treated with RSV (10−4 M) and/or IGF-II (precursor or mature forms, 100 ng/ml). Total cell lysates and cellular fractions were prepared, as previously described, 0, 5, 10, 15, and 20 min post-treatment. PVDF membranes were incubated with IRS-1/phospho-IRS-1 (Tyr1179, Ser 641 and Tyr 632), Pan-Akt/phospho-Akt (threonine 308 or serine 473, R&D Systems Inc., Minneapolis, MN, USA), ERK/phospho-ERK1/2 (Santa Cruz Biotechnology), CREB/phospho-CREB and NFKB/phospho-NF-KB (Cell Signaling) polyclonal antibodies and incubated at 4°C overnight. The blots were also probed with β-actin (whole cell lysates) or IGF-I antibodies (Sigma Chemical Co.) and used as a protein loading control (organelles and nuclear fractions).
Real time PCR
One step SYBR real-time-PCR (RT-PCR) was performed to assess survivin expression in RSV (10−4) treated cells at 3 and 6 h, using the primers Bcl-2-forward (5′-ATG TGT GTG GAG AGC GTC AAC C-3′), and Bcl-2-reverse (5′-AGC CAG GAG AAA TCA AAC AGA GG-3′); Bcl-XL (5′-GGA AAG CGT AGA CAA GGA GAT GC-3′), and Bcl-XL-reverse (5′-TCC ACA AAA GTA TCC CAG CCG-3′). PCR amplifications were performed using the iCycler (BIO-RAD, Hercules, CA, USA). Reactions were performed in a mixture consisting of a 50 μl volume solution containing 1 × SYBR Green supermix PCR buffer (BIO-RAD; 100 mM KCl, 6 mM MgCl2, 40 mM Tris–HCl, PH 8.4, 0.4 mM of each dNTP [dATP, dCTP, dGTP and dTTP], iTaq DNA Polymerase 50 U/ml, SYBR Green I, 20 mM Fluorescein) 300 nM of each primer, 0.25 U/ml MultiScribe Reverse Transcriptase (Promega, Madison, WI, USA) and 0.4 U/ml RNAse Inhibitor (Promega). The RT-PCR protocol starts with 30 min at 42°C for the RT. Prior to the PCR step iTaq DNA polymerase activation at 95°C for 10 min was performed. Followed by 30 s denaturation at 95°C, 15 s annealing at 57°C, and 1.5 min elongation at 72°C for 40 cycles. Fluorescence was detected at the end of every 72°C extension phase. To exclude the contamination by non-specific PCR products such as primer dimers, melting curve analysis was applied to all final PCR products after the cycling protocol.
Cell viability assay
Cell viability was measured by the 3-[4,5-dimethylthiazolyl] 2, 5-diphenyltetrazolium bromide (MTT) assay; cells (1 × 104/well) were seeded in 96-well plates and grown in SFM. Cell viability was assessed at 6 and 9 h post-treatment with RSV and/or IGF-II (precursor or mature forms, 100 ng/ml) by measuring the rate of tetrazolium salts reduction to formazan (MTT, Sigma), which is proportional to the number of living cells. At the end of incubation, the absorbance was read at 540 nm.
Statistical analysis
* Values are expressed as the mean ± SEM of three or more replicate experiments. Statistical differences between mean values were determined by one-way ANOVA, SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). A level of p < 0.05 was considered significant.
Results
RSV and/or IGF-II effects on Bcl-2 and Bcl-XL protein levels
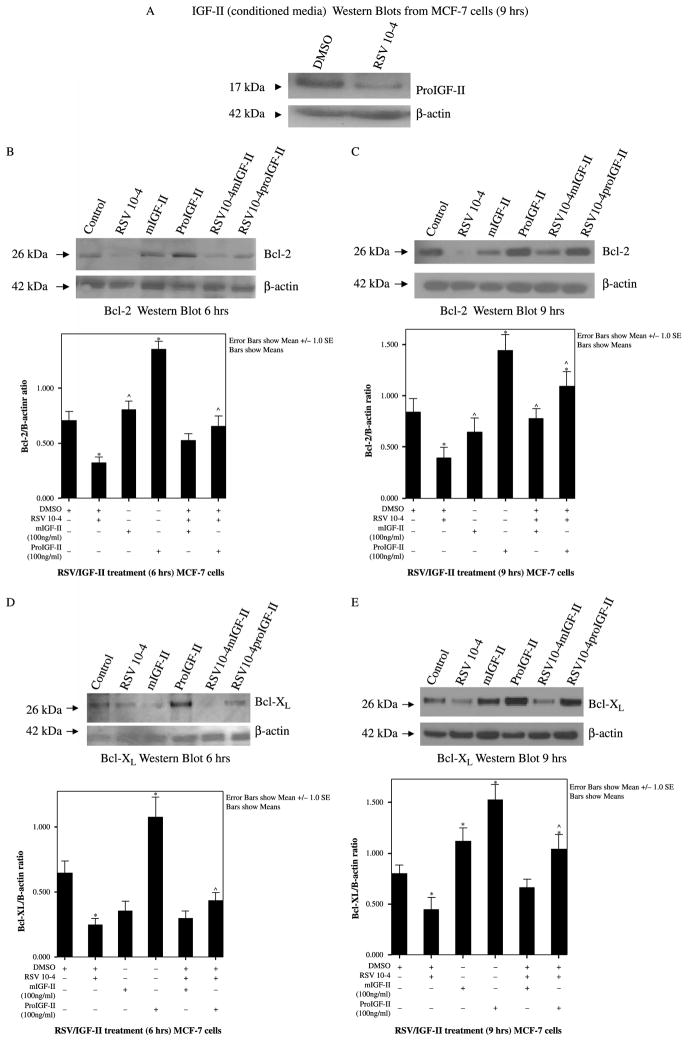
We have previously shown that RSV regulates IGF-II in a concentration-dependent manner in MCF-7 breast cancer cells. Figure 1(A) shows IGF-II Western blot from MCF-7 cells treated with RSV (10−4 M) showing RSV regulation of IGF-II (17 kDa bands correspond to proIGF-II). Figure 1(B)–(C) show representative Bcl-2 Western blots from three independent experiments done in triplicate 6 and 9 h post-treatment. Cells were treated with RSV (10−4 M), IGF-II (proIGF-II or mIGF-II, 100 ng/ml), or IGF-II added to RSV-treated cells. As seen, RSV induces inhibition of Bcl-2 protein levels (60 and 70% as compared to vehicle-only (DMSO) cells), 6 and 9 h post-treatment (26 kDa band). Of note, treatment with proIGF-II alone was associated with a significant time-dependent increase in Bcl-2 (60 and 90% as compared to vehicle cells) 6 and 9 h post-treatment. Furthermore, addition of recombinant proIGF-II to RSV-treated cells maintained Bcl-2 levels comparable to vehicle cells as early as 6 h, while mIGF-II blocked RSV inhibitory effect on Bcl-2, only 9 h post-treatment. Figure 1(E) shows that RSV decreases Bcl-XL protein levels by 50%, 9 h post-treatment. ProIGF-II alone was associated with a significant increase (50 and 70% as compared to vehicle cells) 6 and 9 h post-treatment (Figure 1(D)–(E)), while mIGF-II treatment alone increased Bcl-XL by 30%, 9 h post-treatment. Figure 1(B)–(E) (lower panels) show bar graphs of densitometric analysis of Bcl-2 and Bcl-XL densitometry units (integrated density units) normalized to β-actin densitometry units on three separate experiments. Please note that, although, only one Western blot is depicted as a representative experiment, the bar graphs represent three separate experiments done in triplicate (three Western blots per experiment). Asterisks indicate values significantly different from vehicle (★p < 0.05) or RSV-treated cells (^p < 0.05).
Figure 1.
Western blot of IGF-II (A), Bcl-2 and Bcl-XL from MCF-7 cells treated with RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml). 1A, Western blot of proIGF-II secretion 48 h after RSV treatment. The 17 kDa band represents proIGF-II and is the only IGF-II form secreted by MCF-7 cells. (B) and (C) represent Bcl-2 and Bcl-XL Western blots (whole cell lysates) 6 and 9 h post-treatment. β-actin was used as loading control (42 kDa). Lower panels (B)–(C) show bar graphs of Bcl-2 and Bcl-XL data normalized to β-actin and presented as the mean ± SE of three separate experiments. * indicate values significantly different from vehicle (★p < 0.05) or RSV-treated cells (^p < 0.05).
RSV and/or IGF-II effects on Bcl-2 and Bcl-XL mRNA levels
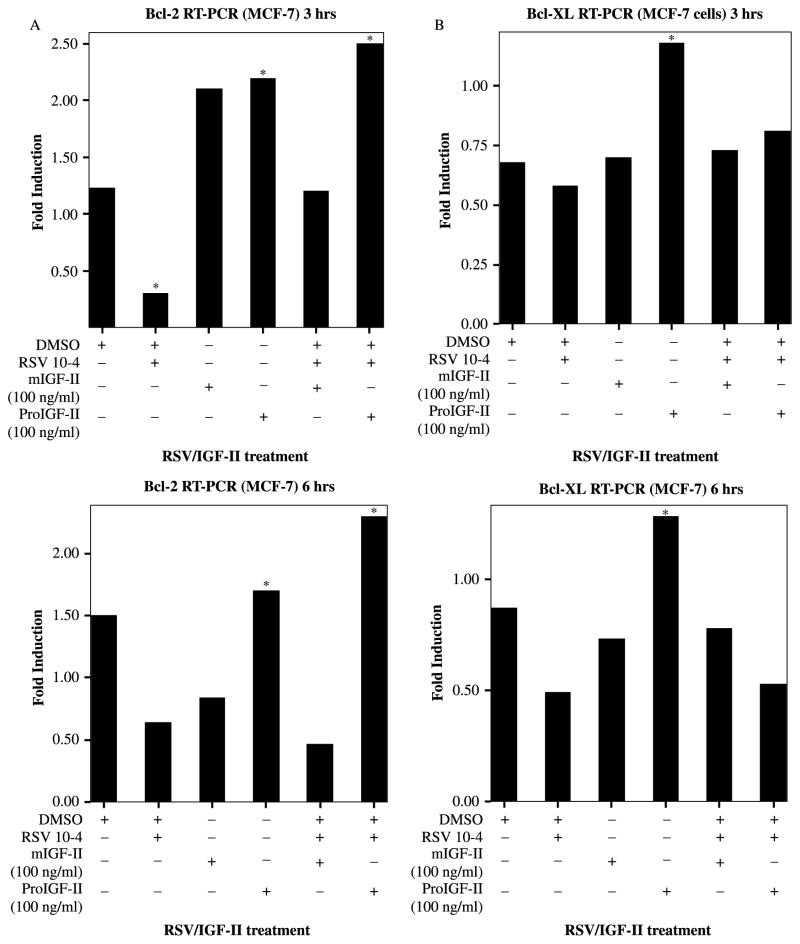
Next, we studied RSV (10−4 M) and IGF-II effects on Bcl-2 and Bcl-XL gene expression by Real Time-PCR following 3 and 6 h treatment. Because, Bcl-2 and Bcl-XL protein levels changed at 6 and 9 h, we reasoned that mRNA changes may occur earlier and chose to analyze mRNA levels at 3 and 6 h after treatment. As seen on Figure 2(A) (upper panel) RSV induces a decrease in Bcl-2 mRNA (70%) as early as 3 h post-treatment. Treatment with mIGF-II or proIGF-II (100 ng/ml) increased Bcl-2 mRNA (60 and 70%, respectively) 3 h post-treatment. Addition of recombinant proIGF-II to RSV-treated cells significantly increased Bcl-2 mRNA (80 and 60%, respectively) 3 and 6 h post-treatment as compared to vehicle cells, and induced 5- and 4-folds increase in Bcl-2 mRNA (at 3 and 6 h, respectively) when compared to RSV-only treated cells. Of note, ProIGF-II was three times more potent than mIGF-II in increasing Bcl-2 mRNA levels after RSV treatment. MIGF-II kept Bcl-2 mRNA comparable to vehicle cells 3 h post-treatment only, with a further decrease at 6 h. Figure 2(B) shows RSV-induced decrease in Bcl-XL mRNA (40%) 6 h post-treatment. Although, proIGF-II alone increased Bcl-XL mRNA significantly 3 and 6 h post-treatment (60 and 40%, respectively), it did not block RSV inhibitory effect on Bcl-XL mRNA levels 6 h post-treatment. The discrepancy observed between Bcl-XL protein levels (9 h) and mRNA (6 h) in the RSV/proIGF-II treatment group (lane 6), could be explained by increased protein/mRNA stability/translation induced by proIGF-II instead of increased Bcl-XL transcription. On the contrary, although, mIGF-II was able to maintain Bcl-XL mRNA levels comparable to vehicle group at all treatment times (30% increase when compared to RSV-treated cells), this effect did not translate into increased Bcl-XL protein levels (mIGF-II/RSV, lane 5) suggesting that mIGF-II does not affect Bcl-XL mRNA and/or protein stability.
Figure 2.
RSV (10−4 M) and/or IGF-II (mature and precursor forms, 100 ng/ml) effect on survivin gene expression in MCF-7 cells and assessed by Real Time-PCR (RT-PCR). Panels (A)–(B) represent Bcl-2 and Bcl-XL gene expression, expressed as fold increase/decrease relative to corresponding control groups 3 and 6 h post-treatment. GAPDH was used as internal control. ★ indicate values significantly different from vehicle or RSV-treated cells (★p < 0.05).
Effect of IGF-II siRNA on Bcl-2 and Bcl-XL expression in MCF-7 breast cancer cells
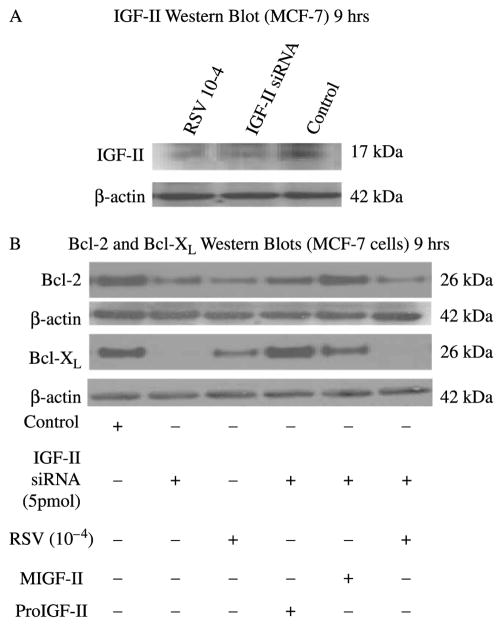
Since we hypothesize that RSV effect on the anti-apoptotic proteins are mediated through IGF-II regulation, and proIGF-II blocked RSV-inhibitory effect on these anti-apoptotic proteins, we examined the effect of IGF-II siRNA on Bcl-2 and Bcl-XL protein levels in MCF-7 cells. As seen on Figure 3(A), IGF-II siRNA blocked 70% of IGF-II expression. Likewise, RSV (10−4 M) also inhibited IGF-II expression in MCF-7 cells (please note that the 17 kDa band shown corresponds to proIGF-II, no mIGF-II or 7.5 kDa band was seen). Figure 3(B) shows Bcl-2 and Bcl-XL Western blot were protein levels decreased by 50 and 90%, respectively, in MCF-7 cells transfected with IGF-II siRNA. Interestingly, treatment with RSV alone decreased both proteins by 50%. Addition of recombinant proIGF-II to siRNA-transfected cells had a more significant effect on increasing Bcl-XL, a protein associated with increased risk of metastasis and high grade tumors, than on Bcl-2 which is associated with a better prognosis in breast cancer patients. On the contrary, mIGF-II had a more significant effect on maintaining Bcl-2 levels comparable to control group.
Figure 3.
Western blot of IGF-II, Bcl-2 and Bcl-XL following IGF-II siRNA treatment in MCF-7 cells. (A) Western blot of proIGF-II secretion 9 h after RSV and IGF-II siRNA treatment. The 17 kDa band represents proIGF-II and is the only IGF-II form secreted by MCF-7 cells. (B) Shows IGF-II siRNA and RSV effects on Bcl-2 and Bcl-XL protein levels in the presence or absence of recombinant IGF-II (mature or precursor forms, 100 ng/ml).
RSV and/or IGF-II effects on Bcl-2 and Bcl-XL subcellular localization and mitochondrial membrane polarization
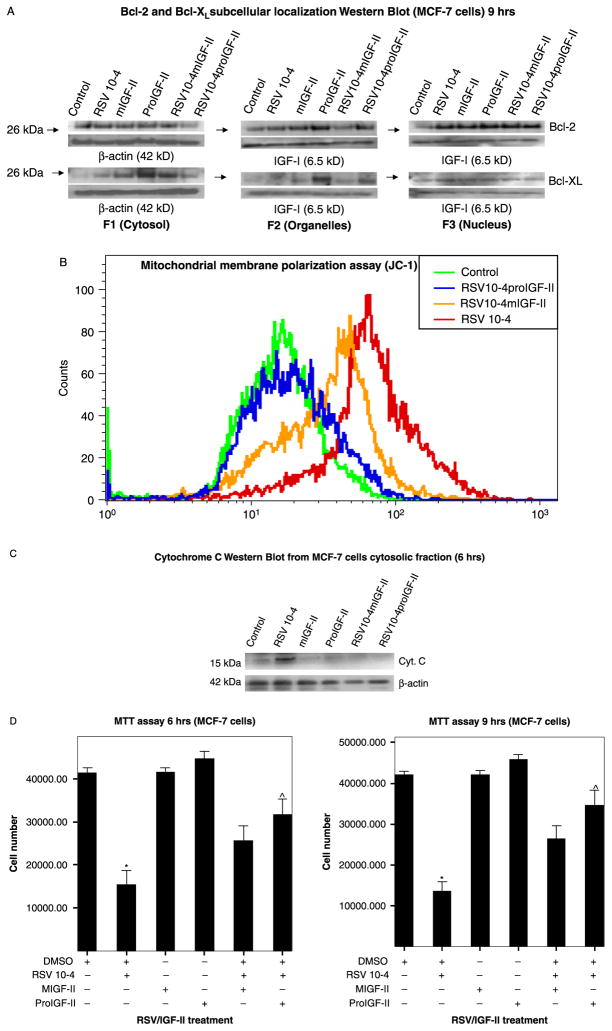
Recent studies have suggested that Bcl-2 and Bcl-XL differ in the potency with which they inhibit apoptosis, in part, by differences in the regulation of specific subcellular pathways. In this regard, we examined the effects of RSV and IGF-II on Bcl-2 and Bcl-XL subcellular localization 9 h post-treatment. As seen on Figure 4(A), in RSV-treated cells Bcl-2 and Bcl-XL preferentially localize to the cytosol and/or nuclear compartments. Of note, proIGF-II was able to keep higher levels of Bcl-2 in the organelles fraction when compared to vehicle-only, RSV and mIGF-II-treated cells; while Bcl-XL was seen in the cytosol and organelles compartments following proIGF-II treatment. Next, we compared Bcl-2 and Bcl-XL subcellular localization with mitochondrial membrane polarization status; as seen on Figure 4(B), RSV treatment depolarized the mitochondrial membrane, an effect that was completely blocked by addition of proIGF-II keeping it comparable to control cells, while mIGF-II only partially blocked RSV-induced depolarization.
Figure 4.
(A) Western blot of Bcl-2 and Bcl-XL subcellular localization (F1 (cytosol), F2 (organelles), F3 (nucleus)). β-actin was used as loading control for F1, and recombinant IGF-I was used as loading control for F2 and F3 (specific markers were used to verify each cell fraction (LEGDF, nuclear pore protein for F3, VDAC, MnSOD, COX-2 and COX-4 for F2), and found to be regulated/modulated by IGF-II/RSV treatment). (B) Shows mitochondrial membrane depolarization used as an indicator of apoptotic cell death and assessed by potential-dependent lipophilic cation JC-1 (5 μg/ml). The JC-1 assay measured the red/green fluorescence in a quantitative approach using the fluorescence-activated cell sorter. The FL-1 channel associates with membrane depolarization and indicates apoptotic cell death. (C) Shows a Western blot analysis of Cytochrome c (cytosolic fraction, F1) 6 h after RSV and/or IGF-II treatment (precursor and mature forms) and, (D) Shows MTT assay of MCF-7 cells treated with RSV and co-incubated with IGF-II (mIGF-II or proIGF-II, 100 ng/ml), 6 and 9 h post-treatment. Data are presented as the mean ± SE of three independent experiments. ★ indicate values significantly different from controls (★p < 0.05) or RSV-treated cells (^p < 0.05).
RSV and/or IGF-II (proIGF-II and mIGF-II) effect on MCF-7 cells survival and Cytochrome c release
Figure 4(C) shows a Western blot analysis of Cytochrome c (cytosolic fraction, F1) 6 h after RSV and/or IGF-II treatment (precursor and mature forms). As seen RSV induced a significant increase in Cytochrome c levels, while proIGF-II and mIGF-II maintained levels comparable to control.
The MTT cell viability assay was used to confirm that RSV inhibitory effect on MCF-7 cell survival can be partially or completely blocked by mIGF-II and/or proIGF-II. Figure 4(D) shows that RSV (10−4 M) caused a significant decrease in cell viability (65 and 70%, respectively) 6 and 9 h post-treatment. In contrast, proIGF-II blocked RSV inhibitory effect on cell viability (p < 0.01) 6 and 9 h post-treatment, while mIGF-II blocked RSV inhibitory effect on cell survival 9 h after treatment (p < 0.05).
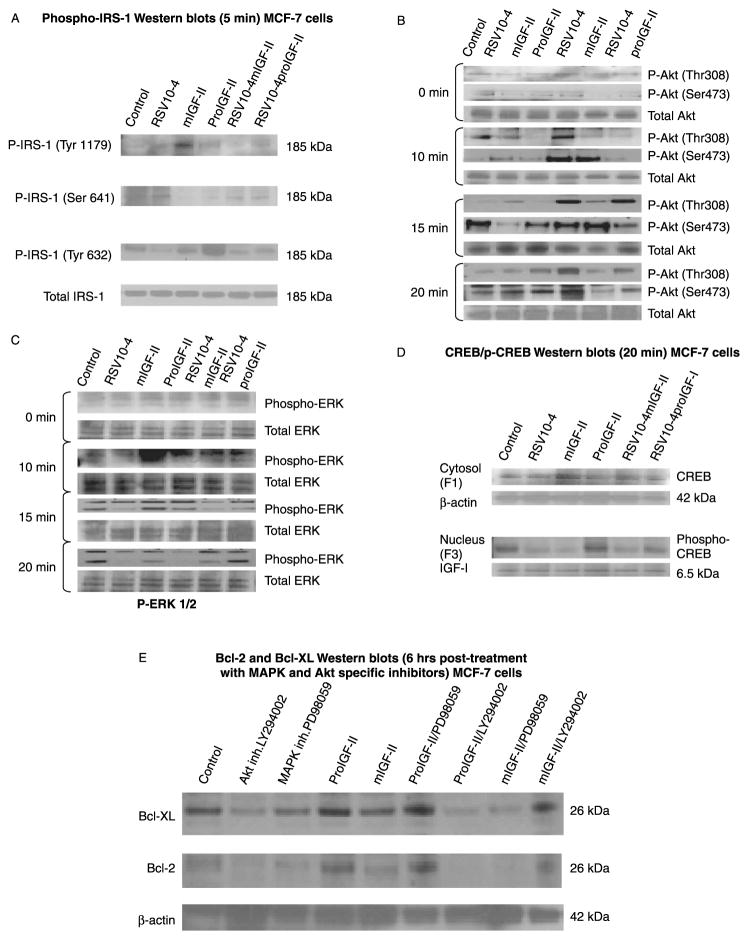
ProIGF-II and mIGF-II effects on IRS-1, Akt, ERK 1/2 phosphorylation
Since, both proIGF-II and mIGF-II can bind to the IGF-I and IR-A receptors and induce differential expression of Bcl-2 and Bcl-XL, we reasoned that different signaling pathways might be involved in the regulation of these anti-apoptotic proteins by IGF-II. We started by conducting IRS-1 phosphorylation studies based on the fact that this downstream adaptor molecule can be activated by either receptor. As seen on Figure 5(A), mIGF-II treatment increased IRS-1 phosphorylation at tyrosine 1179; phosphorylation at this residue is associated with IRS-1 binding to the phosphatase SHP-2 leading to less activation of PI3K/Akt pathway. On the contrary, no difference was seen on Ser 641 phosphorylation after mIGF-II or proIGF-II treatment, although, RSV significantly increased this residue’s phosphorylation. Serine 641 is a known binding site for the protein 14–3–3, causing less activation/inhibition of the Akt pathway. ProIGF-II was more potent than mIGF-II in phosphorylating IRS-1 at tyrosine 632, which is a site predicted to bind SH2 domains in the p85 regulatory subunit of PI3K, resulting in activation of p110 catalytic subunit. Next, we examined Akt and ERK 1/2 phosphorylation at 0, 10, 15, and 20 min post-treatment (Figure 5(B)). Although, both IGF-II forms (pro- and mIGF-II) are known activators of MAPK and GT pase protein (RAS)/MEK/ERK signaling pathways, exposure of MCF-7 to proIGF-II lead to rapid activation of Akt (at threonine 308 and serine 473), remaining fully active for up to 20 min. In contrast, mIGF-II induced 2 peaks of ERK activity at 10 and 20 min post-stimulation (Figure 5(B)–(C)). No changes on total IRS-1, Akt or ERK were observed after RSV and or IGF-II treatments.
Figure 5.
Western blots of (A) phospho-IRS-1 (Tyr1179 and Ser641), (B) phospho-Akt (T308 and T473), (C) phospho-ERK 1/2, from MCF-7 cells treated with RSV (10−4 M) and/or IGF-II (mature or precursor forms, 100 ng/ml). Total IRS-1, Akt and ERK are shown for each treatment time, and (D) shows Western blots of CREB (cytosolic fraction), phospho-CREB (nuclear fraction) following 20 min RSV and IGF-II treatments. β-actin and recombinant IGF-I were used as loading controls for the cytosolic and nuclear fractions, respectively.
ProIGF-II and mIGF-II effects on CREB phosphorylation and nuclear translocation
Since Bcl-2 and Bcl-XL expression is regulated, in part, by the transcription factors CREB and NF-KB, and these transcription factors can be activated by the PI3K/Akt and/or MAPK pathways, we examined the effect of RSV, mIGF-II and proIGF-II treatments on CREB and NF-KB phosphorylation and nuclear translocation. As seen on Figure 5(D), although, no significant changes were seen in total CREB protein, treatment with RSV decreased CREB phosphorylation and nuclear translocation, while proIGF-II treatment increased phosphorylated CREB in the nuclear fraction alone or when added to RSV treated cells (20 min). No significant changes were observed on NF-KB phosphorylation levels (data not shown).
ProIGF-II and mIGF-II effects on Bcl-2 and Bcl-XL following treatment Akt/PI3K and MAPK specific inhibitors (LY294002 and PD98059)
In order to correlate the increased and sustained Akt and ERK phosphorylation induced by proIGF-II and mIGF-II with the increase in Bcl-2 and Bcl-XL expression, we used the specific inhibitors LY294002 and PD98059. Addition of proIGF-II to LY294002 treated cells failed to increase Bcl-2 and Bcl-XL proteins levels, as seen in Figure 5(E), while the MAPK-specific inhibitor PD98059 had no significant effect on proIGF-II-induced regulation of these anti-apoptotic proteins. On the contrary, mIGF-II effects on Bcl-2 and Bcl-XL expression were completely blocked by the MAPK-inhibitor PD98059, but and only partially inhibited by LY294002. Our results suggest that in the case of proIGF-II the Akt/PI3K pathway preferentially mediates this mitogen effects on Bcl-2 and Bcl-XL expression, while for mIGF-II, although, both pathways seem to be involved, the MAPK plays a more significant role in the regulation of Bcl-2 and Bcl-XL expression by this growth factor.
Discussion
Elucidating the molecular mechanisms that enable breast cancer cells to proliferate, progress to a hormone-independent state, and migrate from the primary tumor to invade distant tissues is crucial for developing new and more efficient therapies. IGF-II is a growth factor known to be expressed in fetal tissues and in many types of cancer. IGF-II binds and activates the IGF-IR, IR-A, and IGF-I/IR-A hybrid receptors and regulates several signaling pathways, thus it constitutes a potential target for new breast cancer therapies. Interestingly, studies focusing on the differences between proIGF-II and mIGF-II functions and receptor binding affinities are lacking. Both precursor and mature IGF-II are expressed in a variety of human cancers, although, proIGF-II is the predominant form secreted (breast, colon cancers and Wilms tumor among others; Lee et al. 1994; Korn et al. 1995; Kim et al. 2005). Several mechanisms might be involved in the differential effects of the two IGF-II forms: mIGF-II bound to the IGF-IR has been shown to cause a significant downregulation of this receptor in Caco-2 human colon cancer cells (Kim et al. 2005), while proIGF-II has been shown to be more potent than mIGF-II in inducing thymidine incorporation in NIH-3T3 cells (Yang et al. 1996). Similarly, binding of mIGF-II to the IGF-IR may cause downregulation of this receptor in breast cancer cells resulting in less receptor activation and decreased stimulation, contributing to the significant enhanced effect of proIGF-II. A correlation between an increased half-life of the receptor–ligand complex could also cause sustained activation of the IGF-IR and/or IR-A receptor leading to higher mitogenic potency (Hansen et al. 1996). Another interesting possibility focuses on differential mIGF-II and proIGF-II binding affinity and/or phosphorylation to the IGF-I and IR-A receptors leading to differential regulation of key survival proteins.
Previous studies in our laboratory demonstrated that RSV (10−6 M) effects on cell growth of MCF-7 cells are mediated by proIGF-II (Vyas et al. 2005). Moreover, since RSV regulated proIGF-II in ER(+) (not ER(−)) breast cancer cells and this effect is blocked by tamoxifen, we concluded that RSV regulation of IGF-II is mediated through the ER. In contrast, the inhibitory effect of RSV is independent of the ER presence since RSV (10−4 M) inhibited proliferation and cell survival of ER(−) cells. We also showed that proIGF-II (the predominant form expressed in cancer, not mature IGF-II) blocked RSV (10−4 M) induced cell death in MCF-7 cells, in part, by regulating the antiapoptotic protein survivin (Kalla Singh et al. 2008). The present study demonstrates that proIGF-II regulates Bcl-2 and Bcl-XL as part of its mechanism to block RSV inhibitory effect on cell survival.
The Bcl-2 family of proteins constitutes a critical intracellular checkpoint in the intrinsic apoptotic pathway by regulating permeabilization and mitochondrial membrane potential (España et al. 2005). Moreover, metastatic cells become resistant to treatment by modifying the expression levels of Bcl-XL or Bcl-2. In the present study, we first evaluated the effect of mIGF-II and proIGF-II on the expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL. We demonstrated that proIGF-II was more potent than mIGF-II in up-regulating these proteins in RSV-treated and untreated cells. Although, no Bcl-XL mRNA (6 h)/protein levels (9 h) correlation was found for the proIGF-II/RSV treatment group, this finding could suggest a possible role of proIGF-II as a regulator of Bcl-XL mRNA and/or protein stability allowing this growth factor to maintain higher Bcl-XL protein levels when compared to mIGF-II-treated cells. Furthermore, it has been shown that Bcl-2 can potentially form complexes with the p50 subunit of NF-KB in the nucleus leading to blockage of gene expression, while mitochondrial Bcl-XL plays a central role in preventing alteration of mitochondrial dysfunction, Cytochrome c release and caspase activation
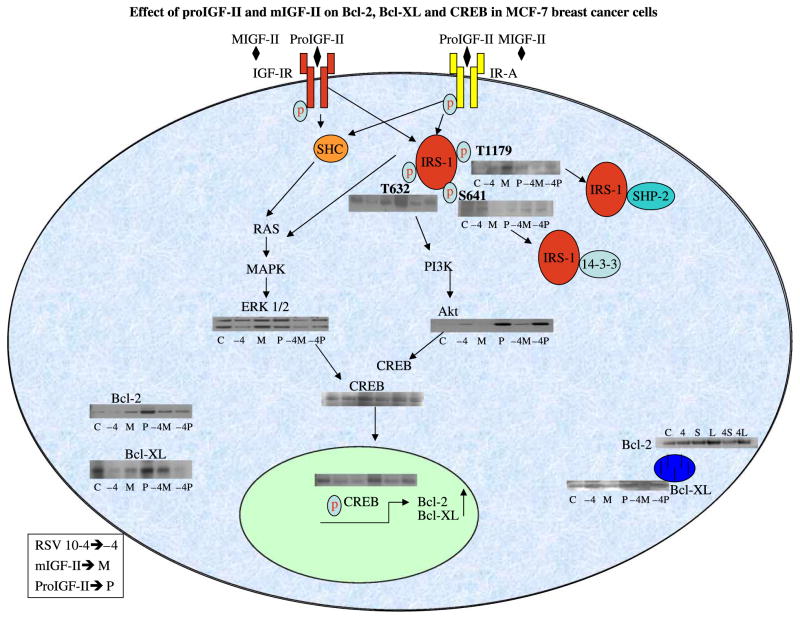
To have a more complete understanding of how different IGF-II forms regulate Bcl-2 and Bcl-XL expression, we next focused on phosphorylation/activation studies of intracellular signaling pathways including the docking protein IRS-1, whose activity levels are determined by phosphorylation of specific residues that subsequently bind to specific domains of downstream signaling molecules; this constitutes one mechanism of regulating post-receptor signaling. In our study RSV treatment increased Serine 641 phosphorylation leading to decreased PI3K/Akt activation, possibly by binding the phosphatase 14–3–3. On the contrary, proIGF-II increased IRS-1 phosphorylation at tyrosine 632, which is predicted to bind SH2 domains in the p85 regulatory subunit of PI3K. These observations are in agreement with our findings of increased Akt phosphorylation by proIGF-II but not mIGF-II. Furthermore, previous studies have linked MCF-7 cells proliferation to activation of PI3K/Akt pathway with only transient activation of the Raf-MEK-ERK pathway. However, prolonged activation of the Raf cascade inhibited growth of these cells, determining whether, the response is proliferation or growth arrest (Zimmermann and Moelling 1999). Consistent with these studies, our results showed that proIGF-II induced a more potent activation of the Akt pathway, with an earlier and transient activation of the Raf-MEK-ERK cascade, while mIGF-II preferentially induced a sustained activation of the Raf-MEK-ERK pathway (Figure 6.). Moreover, activation of the PI3K/Akt pathway has been shown to inhibit the pro-apoptotic protein Bax translocation from cytoplasm to mitochondria, thus, promoting cell survival (Tsuruta et al. 2002).
Figure 6.
Schematic representation of our proposed model for the differential mechanism of mIGF-II and proIGF-II on Bcl-2 and Bcl-XL regulation in MCF-7 breast cancer cells.
Since the transcription factors CREB and NF-KB not only regulate Bcl-2 and Bcl-XL, but are also linked to the development of hormone-independent breast cancer (Shaywitz and Greenberg 1999; Zhou et al. 2005), our study also addresses the effects of proIGF-II and mIGF-II on phosphorylation and nuclear translocation of these transcription factors. ProIGF-II induced increased phosphorylation of CREB (Serine 133) when compared to control, mIGF-II-treated cells and also when added to RSV-treated cells. Because CREB functions in the nucleus, phosphorylation at Serine 133 might be required for translocation; within the nucleus phospho-Serine 133 might lead to CRE binding or transcriptional activation by promoting interaction with components of the basal transcription machinery, such as transcription factor IID (TFIID) and RNA polymerase II (Shaywitz and Greenberg 1999).
In addition to the mechanisms of action, described above, IGF-II can also activate the membrane ER. Studies by Benz (2004) showed that activation of receptor tyrosine kinase family, phosphorylate the ER in the membrane stimulating rapid genomic and non-genomic responses: membrane–receptor initiated signaling through MAPK and PI3K/Akt pathways results in phosphorylation of ER (S167, S118, S104, S106) and leads to both ligand-dependent and ligand-independent ER-mediated gene activation via “classical” (direct ER–DNA binding at promoters containing EREs) and “non-classical” (ER tethering and co-activation of other DNA-bound transcription factor complexes such as AP-1, Sp1, C/EBPβ and CREB; Benz 2004). This mechanism, currently under investigation, may also contribute to the differential effect of proIGF-II and mIGF-II in the regulation of cell survival proteins in breast cancer cells. Furthermore, the ability of proIGF-II to induce CREB phosphorylation indicates that expression of this growth factor may play a key role in the development of estrogen independent breast cancer.
In summary, our study provides novel evidence demonstrating how different IGF-II forms binding to the same receptors can differentially activate intra-cellular signaling pathways. Since proIGF-II is the main form expressed in cancer, it represents a potential key target in the development of new and more efficient breast cancer therapies.
Acknowledgments
This research was supported by 5P20 MD001632, and NIGMS 5R25GM060507.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Benz CC. HER2 and endocrine response in breast cancer: the evidence for clinical application of estrogen receptor status. Oncol Exchange. 2004;3(21):8–11. [Google Scholar]
- Catz SD, Johnson JL. Transcriptional regulation of Bcl-2 by NF-KB and its significance in prostate cancer. Oncogene. 2001;20(50):7342–7351. doi: 10.1038/sj.onc.1204926. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Cui X, Kim H-J, Hadsell DL, Lee AV. Oncogenic transformation by the signaling adaptor proteins insulin receptor substrate (IRS)-1 and IRS-2. Cell Cycle. 2007;6(6):705–713. doi: 10.4161/cc.6.6.4035. [DOI] [PubMed] [Google Scholar]
- De León DD, Wilson DM, Powers M, Rosenfeld RG. Effects of IGFs and IGF receptor antibodies on the proliferation of breast cancer cells. Growth Factors. 1992;6(4):327–336. doi: 10.3109/08977199209021544. [DOI] [PubMed] [Google Scholar]
- España L, Martín B, Aragués R, Chiva C, Oliva B, Andreu D, Sierra A. Bcl-XL-mediated changes in metabolic pathways of breast cancer cells: From survival in the blood stream to organ-specific metastasis. Am J Pathol. 2005;167(4):1125–1137. doi: 10.1016/S0002-9440(10)61201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig AA, Zhu W, Hollerbach C, Leber B, Andrews DW. Bcl-XL is qualitatively different and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer. 2006;6(213) doi: 10.1186/1471-2407-6-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle. 2007;6(6):631–637. doi: 10.4161/cc.6.6.3987. [DOI] [PubMed] [Google Scholar]
- González-García M, Pérez-Ballestero R, Ding L, Duan L, Boise LH, Thompson CB, Nuñez G. Bcl-XL is the major Bcl-X mRNA form expressed during murine development and its product localizes to mitochondria. Development. 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- Hansen BF, Danielsen GM, Drejer K, Sørensen AR, Wiberg FC, Klein HH, Lundemose AG. Sustained signaling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J. 1996;315:271–279. doi: 10.1042/bj3150271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffelfinger SC, Miller MA, Yassin R, Gear R. Angiogenic growth factors in preinvasive breast disease. Clin Cancer Res. 1999;5:2867–2876. [PubMed] [Google Scholar]
- Hers I, Bell CJ, Poole AW, Jiang D, Denton RM, Schaefer E. Reciprocal feedback regulation of insulin receptor and insulin substrate tyrosine phosphorylation by PI3K in primary adipocytes. Biochem J. 2002;368:875–884. doi: 10.1042/BJ20020903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Furutama D, Hanafusa T. Physiologically high concentrations of 17-B estradiol enhance NF-KB activity in human T cells. Am J Physiol Regul Integr Comp Physiol. 2006;292:465–471. doi: 10.1152/ajpregu.00778.2006. [DOI] [PubMed] [Google Scholar]
- Kalla Singh S, Moretta D, Almaguel F, Wall NR, De Léon M, De Léon D. Differential effect of proIGF-II and mIGF-II on resveratrol induced cell death by regulating survivin cellular localization and mitochondrial depolarization in breast cancer cells. Growth Factors. 2008;25(6):363–372. doi: 10.1080/08977190801886905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Holthuizen PE, Kim J, Park JH. Overexpression of mature IGF-II leads to growth arrest in Caco-2 human colon cancer cells. Growth Horm IGF Res. 2005;15(1):64–71. doi: 10.1016/j.ghir.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Korn E, Van Hoff J, Buckley P, Daughaday WH, Carpenter TO. Secretion of a large molecular-weight form of insulin-like growth factor by a primary renal tumor. Med Pediatr Oncol. 1995;24(6):392–396. doi: 10.1002/mpo.2950240610. [DOI] [PubMed] [Google Scholar]
- Lee AV, Darbre P, King RJ. Processing of IGF-II by human breast cancer cells. Mol Cell Endocrinol. 1994;99(2):211–220. doi: 10.1016/0303-7207(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Lee HH, Dadgostar H, Cheng Q, Shu J, Cheng G. NF-KB mediated up-regulation of Bcl-X and Bfl-1/A1 is required for CD40survival signaling in B lymphocytes. Proc Natl Acad Sci USA. 1999;96(16):9136–9141. doi: 10.1073/pnas.96.16.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipponen P, Pietilainen T, Kosma VM, Aaltomaa S, Eskelinen M, Syrjanen KJ. Apoptosis suppressing protein Bcl-2 is expressed in well differentiated breast carcinomas with favourable prognosis. J Pathol. 1995;177:49–55. doi: 10.1002/path.1711770109. [DOI] [PubMed] [Google Scholar]
- Liu W, Liu Y, Lowe WL. The role of PI3K and MAPK in IGF-I mediated effects in vascular endothelial cells. Endocrinology. 2001;142(5):1710–1719. doi: 10.1210/endo.142.5.8136. [DOI] [PubMed] [Google Scholar]
- Méndez O, Martín B, Sanz R, Aragüés R, Moreno V, Oliva B, Stresing V, Sierra A. Underexpression of transcriptional regulators is common in metastatic breast cancer cells over-expressing Bcl-XL. Carcinogenesis. 2006;27(6):1169–1179. doi: 10.1093/carcin/bgi363. [DOI] [PubMed] [Google Scholar]
- Myers MG, Mendez R, Shi P, Pierce JH, Rhoads R, White MF. The COOH-terminal tyrosine phosphorylation sites on IRS-1 bind SHP-2 and negatively regulates insulin signaling. J Biol Chem. 1998;273(41):26908–26914. doi: 10.1074/jbc.273.41.26908. [DOI] [PubMed] [Google Scholar]
- Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB, Recant WM. Overexpression of Bcl-X protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am. 1997;3(4):230–237. [PubMed] [Google Scholar]
- Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Sciacca L, Mineo R, Pandini G, Murabito A, Vigneri R, Belfiore A. In IGF-I receptor deficient leiomyosarcoma cells autocrine IGF-II induces cell invasion and protection from apoptosis via the insulin receptor isoform A. Oncogene. 2002;21(54):8240–8250. doi: 10.1038/sj.onc.1206058. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: A stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Tsuruta F, Masuyama N, Gotoh Y. The PI3K-Akt pathway suppresses Bax translocation to mitochondria. J Biol Chem. 2002;277(16):14040–14047. doi: 10.1074/jbc.M108975200. [DOI] [PubMed] [Google Scholar]
- Vyas S, Asmerom Y, DeLeon DD. Resveratrol regulates IGF-II in breast cancer cells. Endocrinology. 2005;146(10):4224–4233. doi: 10.1210/en.2004-1344. [DOI] [PubMed] [Google Scholar]
- Yang CQ, Zhan X, Hu X, Kondepudi A, Perdue JF. The expression and characterization of recombinant proIGF-II and a mutant that is defective in the O-glycosylation of its E-domain. Endocrinology. 1996;137(7):2766–2773. doi: 10.1210/endo.137.7.8770896. [DOI] [PubMed] [Google Scholar]
- Yee D, Lee AV. Crosstalk between the IGFs and estrogens in breast cancer. J Mammary Gland Biol Neoplasia. 2000;5:107–115. doi: 10.1023/a:1009575518338. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Moelling K. Phosphorylation and regulation of Raf by Akt. Science. 1999;286:1741–1744. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Eppenberger-Castori S, Eppenberger U, Benz CC. The NF-KB and endocrine-resistant breast cancer. Endocr Relat Cancer. 2005;12:S37–S46. doi: 10.1677/erc.1.00977. [DOI] [PubMed] [Google Scholar]