Abstract
Coactivator-associated arginine methyltransferase 1 (CARM1), originally defined as a coactivator for steroid receptors, is a member of the protein arginine methyltransferases. Here, we report the discovery and characterization of an automethylation event by AgCARM1, a CARM1 homologue in the mosquito Anopheles gambiae, using top–down high resolution tandem mass spectrometry, which allows fine mapping of modifications in the intact protein accurately and quantitatively without priori knowledge. Unexpectedly, we found that AgCARM1 has already been predominantly dimethylated during its expression in Escherichia coli. A single arginine methylation site, R485, was identified which is conserved among CARM1 in insects. No methylation was observed in the intact AgCARM1R485K mutant where R485 is mutated to lysine, which confirms that R485 is the only detectable methylation site. Using AgCARM1 methyltransferase defective mutants, we confirmed that this is an automethylation event and show the automethylation of AgCARM1 occurs intermolecularly. This study represents the first comprehensive characterization of an automethylation event by top–down mass spectrometry. The unexpected high percentage of automethylated recombinant AgCARM1 expressed in E. coli may shed light on other bacterially expressed post-translational modifying enzymes, which could be modified but overlooked in biochemical and structural studies. Top–down high resolution tandem mass spectrometry thus provides unique opportunities for revealing unexpected protein modification, localizing specific modification to one amino acid, and delineating molecular mechanism of an enzyme.
Keywords: protein arginine methyltransferase, CARM1, automethylation, top–down mass spectrometry, Fourier transform mass spectrometry, post-translational modification, electron capture dissociation
Introduction
The methylation of arginine (R) residues is catalyzed by the protein arginine methyltransferase (PRMT) family of enzymes, which has been shown to play an important role in diverse cellular functions including transcription regulation, mRNA splicing, and DNA damage repair. The PRMTs are generally classified as either Type I or Type II enzymes: Type I PRMTs monomethylate and asymmetrically dimethylate the primary amine groups on the arginine side chain, whereas Type II PRMTs catalyze the formation of monomethyl arginine and symmetric dimethyl arginine.1
Coactivator-associated arginine methyltransferase 1 (CARM1), also known as PRMT4, is a Type I PRMT which was originally identified in a yeast two-hybrid screen as an associated protein for glucocorticoid receptor interacting protein 1, the p160 family steroid receptor coactivator.2 Mutation and structural studies have identified a number of functional domains in CARM1.3–5 CARM1's central catalytic core is characteristic of PRMT family proteins,6 which contain an S-adenosylmethionine (AdoMet) binding domain and a substrate binding domain. Within this central core, the AdoMet binding domain contains a valine, leucine, aspartic acid (VLD, amino acids 189–191) sequence that is essential for enzymatic function.2 This sequence is part of motif 1 (aliphatic-aliphatic-acidic-alphatic-G-X-G-X-G), the first of a set of shared motifs among methyltransferase enzymes.7 The central dimerization motif within the substrate binding domain makes up an “arm” that facilitates dimerization. The C-terminal catalytic core is the substrate binding domain, which is necessary for recognition of the arginine to be methylated. CARM1 has been shown to methylate both histone and non-histone substrates.8–13 In contrast to most PRMTs that methylate glycine-rich and arginine-rich patches (GAR motifs) within their substrates, CARM1 does not methylate the GAR motif but rather has a unique set of methylation targets,1 which have no apparent recognition sequence.
The function of CARM1 has recently been shown to be regulated by post-translational modifications (PTMs). Phosphorylation of serine 228 blocks CARM1 dimerization, likely interrupting proper binding of the dimerization “arm” in phosphorylated CARM1 and thus inhibits its methylation activity.14 Automethylation of CARM1 has been previously implicated in vitro10 in analog with other PRMT family members including PRMT1, PRMT6, and PRMT8,15,16 however, the automethylation of CARM1 and PRMT1 appeared to be significantly less than PRMT6 and PRMT8. The site(s) of CARM1 automethylation are unknown and the role of PRMT automethylation remains to be elucidated.
Mass spectrometry (MS) is the only technique that can universally provide information about protein PTMs without a priori knowledge of the modification.17 In the conventional “bottom-up” MS approach proteins of interest are digested with an enzyme prior to MS analysis providing only partial coverage of the protein sequence.18,19 In contrast, top–down MS analyzes intact protein directly, providing “a bird's eye view” of all possible protein modifications with full sequence coverage.20–33 The top–down MS approach subsequently fragments the protein ions of interest in the mass spectrometer to locate the modification site(s), which allows fine mapping of modifications in the intact protein. Moreover, the top–down MS approach is especially attractive for estimating the relative abundance of protein species with specific modifications since the ionization efficiency of intact proteins is much less affected by the presence of modifying groups in comparison with peptides.30,32,34,35 The newly developed MS/MS technique of electron capture dissociation (ECD)36 greatly improves both efficiency and sequence coverage in top–down analyses. ECD cleaves NH-CHR bonds to produce mainly c and z. ions, complementary to those from the well developed energetic dissociation methods such as collisionally activated dissociation (CAD).21 More importantly, ECD generates far more fragmentation ions as well as unique cleavages due to its nonergodic dissociation of covalent protein backbone bonds causing local fast (<10−12 s) cleavages of covalent bonds before energy randomization.21,23,37,38
In this study, we report the discovery and characterization of an unexpected automethylation event by AgCARM1, a CARM1 homologue in the mosquito model Anopheles gambiae, using top–down high resolution Fourier transform (FT) tandem mass spectrometry (MS/MS). AgCARM1 automethylation is shown to occur intermolecularly during expression in Escherichia coli as well as in vitro.
Results
AgCARM1 is the A. gambiae homologue of CARM1
CARM1 contains a conserved PRMT catalytic core,39 which is highly conserved across species even between mammals and insects. The Drosophila CARM1 homologue CARMER (or DART4) has been reported to have similar substrate specificity to mouse CARM1 (mCARM1) but is lacking the C-terminal activation domain found in other CARM1 sequences (Supporting Fig. S1).40,41 To develop an animal model for CARM1 function, we chose to study CARM1 in the mosquito model A. gambiae since this model organism has a sequenced genome and a simplified hormone signaling pathway. We have cloned a sequence that is highly similar to known CARM1 genes from the A. gambiae genome (AgCARM1, Genbank: FJ391182) and generated highly purified AgCARM11–509 protein in large quantities [Fig. 1(A)]. To verify AgCARM11–509 is the functional homologue of CARM1, we performed in vitro methylation assays using purified AgCARM11–509 and AgCARM1VLD (catalytic inactive mutant where the amino acids in the AdoMet binding domain, VLD, were mutated to AAA) and compared their methylation specificity to mCARM1 and mPRMT1. These were incubated with known CARM1 substrates polyA binding protein 1 (PABP1), core histones from Hela cells, as well as GAR, a common substrate for PRMT family members including PRMT1 but not CARM1. AgCARM11–509 was found to methylate the CARM1 specific targets exclusively [Fig. 1(B,C)], while exhibiting no activity towards GAR, which is methylated by PRMT1 [Fig. 1(D)]. Analogous to the mCARM1VLD mutant,2 the AgCARM1VLD mutant showed a complete loss of methylation activity [Fig. 1(B,C)]. AgCARM11–509 also exhibits automethylation activity in vitro [Fig. 1(E)] as reported previously for mCARM1.10 These results confirm that AgCARM11–509 is the CARM1 homologue in A. gambiae.
Figure 1.
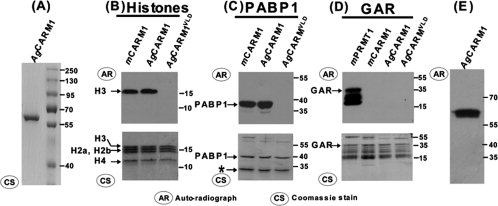
In vitro methylation studies of AgCARM11–509 show similar substrate specificity to mCARM1. (A) Coomassie stain of Ni-NTA purified AgCARM1.1–509 (B,C) In vitro methylation of core histones (B) and PABP1 (C) by CARM1 variants in the presence of 3H-AdoMet. Top panel is an autoradiograph showing radiolabel incorporation of the methyl group. Bottom panel is the commassie stained gel after exposure to film. (D) In vitro methylation of the fibronectin fragment GAR by various PRMTs in the presence of 3H-AdoMet. (E) Autoradiograph of AgCARM11–509 incubated without substrate in an otherwise identical in vitro methylation assay as seen in B–D. *Band that copurifies with PABP1, likely a degradation product. GST-GAR also exhibits significant degradation consistent with previous publications of this protein fragment. CS, Coomassie Stain; AR, Autoradiograph.
AgCARM11–509 overexpressed in E. coli is dimethylated at R485 in vivo
We used top–down MS to characterize AgCARM11–509 expressed in E. coli, later referred to as AgCARM1WT. After purification, the AgCARM1WT protein sample was directly analyzed by LTQ FT ultra high resolution mass spectrometer. Electrospray ionization (ESI)/FTMS spectrum revealed two molecular forms of AgCARM1WT (Fig. 2, top). The most abundant molecular weight of the minor form is 58549.36–58549.38, which is consistent with the DNA predicted mass value for AgCARM1WT with the removal of its N-terminal methionine (Calc'd Mr 58549.31–58549.38, 0.9 ppm). Surprisingly, the molecular weight of the major form, 58577.37–58577.38, is 28 Da more than the minor form suggesting a dimethylated form of AgCARM1WT (Fig. 2, bottom). Eighty-three percentage of the total AgCARM1WT was dimethylated and 17% was unmethylated, with no detectable amount of monomethylated AgCARM1WT based on peak height. This relative quantification is based on the assumption that the ionization efficiency of intact proteins is much less affected by a modifying group in comparison to peptides.30 Since the analyzed sample was freshly purified after over-expression, it is conceivable that this methylation event occurred in vivo after expression in E. coli.
Figure 2.
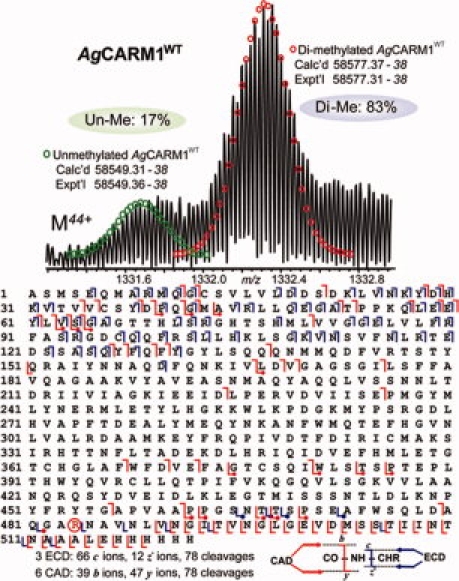
High resolution ESI/FTMS analysis of AgCARM1WT expressed in E. coli. Top, ESI/FTMS spectrum of AgCARM1WT intact protein ions (M44+), suggesting AgCARM1WT is predominantly dimethylated. Bottom, fragmentation map from 3 ECD and 6 CAD spectra of both un- and di-methylated AgCARM1WT matched with assignments to the DNA-predicted sequence of AgCARM1WT with the removal of N-terminal methionine, localizing the dimethylation site to R485 (highlighted in circle). The fragmentation ions containing the dimethylated form were indicated in dots.
To locate the specific site of dimethylation, multiple charge states of AgCARM1WT were individually isolated and then fragmented by CAD and ECD. The six combined CAD spectra generated 39 b and 47 y ions whereas 3 ECD spectra generated 66 c and 12 z. fragment ions representing 78 cleavages each. These covered 26% of the total 521 NH-CH available backbone bonds in AgCARM1WT and confirmed removal of methionine at the N-terminus. Fragmentation ions were consistent with dimethylation of R485, showing a +28 Da shift in those fragments containing the R485 residue. MS/MS spectra of a mixture of unmethylated and dimethylated AgCARM1WT show predominantly (∼83%) dimethylated form of fragment ions containing R485 (Supporting Fig. S2) suggesting R485 is the only site for dimethylation. Large b and y ions were observed in CAD spectra including several complementary pairs although fragmentation occurred most frequently in the terminal regions of the protein (Fig. 2, bottom). The overlapping b and y ions span the entire sequence giving confidence that the assigned primary structure is correct. The Asn39-Pro40 bond was very sensitive to CAD dissociation, resulting in not only the complementary ion pair (y483 and b39) but also a series of internal ions with Asn39 as the C-terminus (data not shown). This pattern was observed in all AgCARM1 mutants analyzed hereafter.
To validate that R485 is the sole methylation site on AgCARM1WT, R485 was mutated to lysine to maintain the positive charge while blocking methylation. The AgCARM1R485K mutant was expressed and purified as for wild-type protein. Only one molecular form was shown in ESI/FTMS spectrum, matching the mass of unmethylated AgCARM1R485K (Fig. 3, top). Similarly, CAD and ECD data confirmed the lack of detectable methylation in AgCARM1R485K, which is estimated to be <3% of the total protein ion population (Fig. 3, bottom). Two ECD spectra generated 63 c and 26 z. fragment ions representing 89 cleavages and two CAD spectra generated 24 b and 26 y ions representing 39 cleavages. This result confirms that R485 is the only significant methylation site on CARM1 during its expression in E. coli. Similar to the AgCARM1WT data, very large fragments were observed in broadband CAD spectra (see Fig. 4). The largest b/y fragmentation ions observed were b495 (Calc'd 55576.66–55576.37; Expt'l 55576.57–55576.37) and y483 (Calc'd 54169.99–54169.36; Expt'l 54169.94–54169.36), which is, to our knowledge, the largest fragmentation ions isotopically resolved in a MS/MS spectrum.
Figure 3.
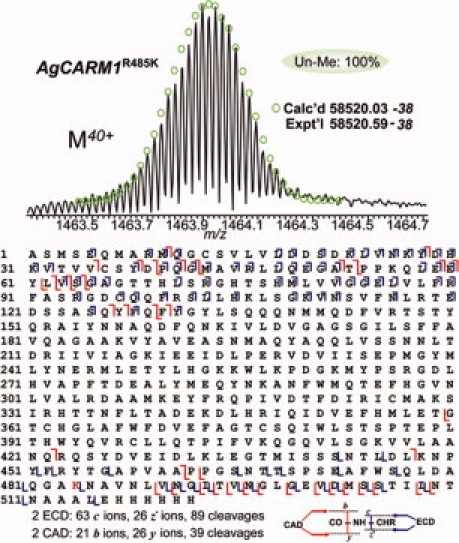
High resolution ESI/FTMS analysis of AgCARM1R485K mutant expressed in E. coli. Top, ESI/FTMS spectrum of AgCARM1R485K intact protein ions (M40+), suggesting AgCARM1R485K is not methylated. Bottom, fragmentation map from 2 ECD and 2 CAD spectra of AgCARM1R485K matched with assignments to the DNA-predicted sequence of AgCARM1R485K without the N-terminal methionine.
Figure 4.
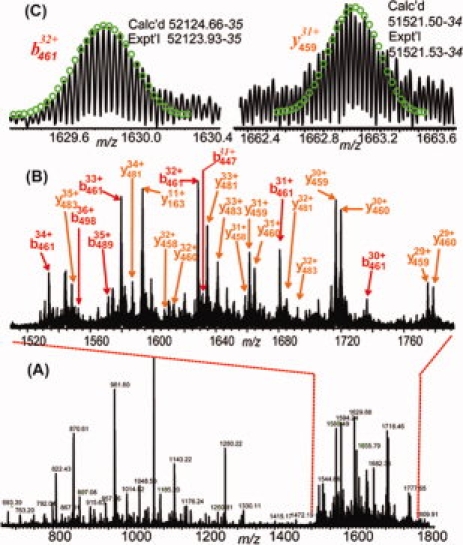
CAD spectrum of AgCARM1R485K intact protein ions. (A) CAD spectrum of isolated single charge state of AgCARM1R485K (M40+). (B) Expanded spectrum of (A) showing large fragmentation ions observed at m/z 1500–1800. (C) Representative isotopically resolved large fragmentation ions.
AgCARM1R485K exhibits normal methylation activity towards other CARM1 substrates
To examine whether methylation of R485 affects the methyltransferase activity of AgCARM1, either AgCARM1WT or AgCARM1R485K was incubated with substrates PABP1, p300 and core histones in the presence of 3H-AdoMet in an in vitro methylation assay. Methylation of substrates was determined to be at the same level between AgCARM1WT and AgCARM1R485K [Fig. 5(A)]. These results suggest that the methyltransferase activity of AgCARM1 is independent of its methylation status at R485.
Figure 5.
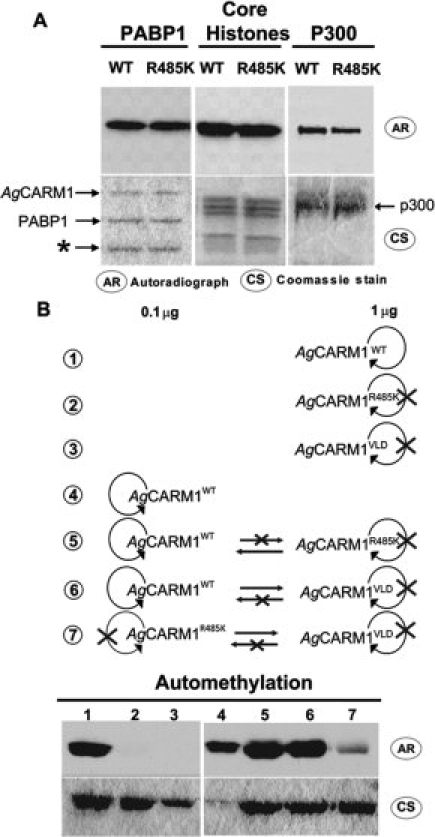
In vitro methylation assays characterizing AgCARM1 automethylation and its affect on enzyme activity. 3H-AdoMet was incubated with purified protein followed by SDS-PAGE, coomassie staining, and exposure to film. (A) In vitro methylation of known CARM1 substrates by AgCARM1WT and AgCARM1R485K. (B) Isolated and mixed automethylation reactions with 0.1 μg and/or 1 μg of AgCARM1. Top shows a cartoon representation of reaction contents and possible interactions. Looping arrows indicate possible intramolecular and/or intermolecular automethylation. Straight arrows indicate possible intermolecular automethylation. Bottom shows in vitro methylation results of these reaction conditions, with lane numbers corresponding to the same numbered cartoon above. *Band copurified with PABP1, likely due to degradation. CS, Coomassie Stain; AR, Autoradiograph.
AgCARM1WT is automethylated during its expression in E. coli
There are no known arginine methyltransferases in E. coli. To ensure that methylation of AgCARM1 in bacteria is due to automethylation activity and not a previously unidentified endogenous bacterial enzyme, the methyltransferase defective AgCARM1VLD mutant was bacterially expressed and analyzed by top–down MS. This mutation is not proximal to arginine 485, and so will likely maintain the sequence and structural integrity of the methylation site, thus serving as an effective control. A single molecular form was observed at 58433.83–58433.38 (Calc'd 58433.97–58433.38, 2 ppm), consistent with the unmethylated mass of AgCARM1VLD with the removal of N-terminal methionine. The overlapping b and y ions fragmentation ions from CAD of AgCARM1VLD span the entire sequence, which were consistent with an unmethylated parent ion (see Fig. 6). A single ECD spectrum generated 77 c and 38 z. fragment ions representing 115 cleavages; in contrast, one CAD spectrum generated 24 b and 24 y ions representing 47 cleavages. ECD fragmentation data showed a very rich fragmentation pattern mainly located on the two termini (Supporting Fig. S3). Lack of fragmentation of the central region of the protein was consistent among the AgCARM1VLD, AgCARM1R485K, and AgCARM1WT proteins. These results suggest that methylation is dependent on AgCARM1 activity and AgCARM11–509 is automethylated when expressed in bacteria.
Figure 6.
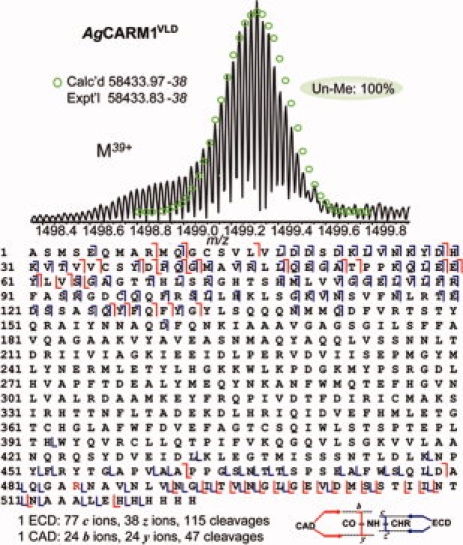
Top–down MS analysis of AgCARM1VLD mutant. Top, ESI/FTMS spectrum of AgCARM1VLD (M39+), intact protein ions, suggesting AgCARM1VLD is not methylated. Bottom, Fragmentation map from both 1 CAD and 1 ECD spectra with assignments to the DNA-predicted sequence of AgCARM1VLD with the removal of N-terminal Met. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Automethylation of AgCARM1 occurs intermolecularly
In contrast to AgCARM1WT, whose automethylation indicates that it acts as both enzyme and substrate, no automethylation was observed in vitro when AgCARM1R485K or AgCARM1VLD mutants were incubated alone with 3H-SAM [Fig. 5(B), compare lane 1 with lane 2 and 3]. AgCARM1R485K shows a lack of auto-methylation activity due to its inability to act as substrate in this reaction, while AgCARM1VLD has no enzymatic activity but still contains the methylation site arginine residue. To determine how the AgCARM1 mutants and wild-type proteins interact, a number of mixed in vitro methylation reactions were performed. Although the AgCARM1R485K and AgCARM1VLD mutants do not automethylate in isolated reactions, mixing AgCARM1WT either with AgCARM1R485K or AgCARM1VLD at a 1:10 ratio in vitro results in increased methylation above that of AgCARM1WT alone [Fig. 5(B), compare lanes 5 and 6 with lane 4]. Mixing of AgCARM1R485K and AgCARM1VLD shows a weak methylation in vitro even though neither form shows automethylation activity by itself [Fig. 5(B), lane 7]. These data support an intermolecular mechanism for the automethylation of AgCARM1 since these increases in methylation must be due to the interaction between different AgCARM1 proteins.
Discussion
We have biochemically characterized a novel CARM1 homologue from the mosquito A. gambiae. AgCARM1 shows conserved substrate specificity for CARM1 methylation targets [Fig. 1(B,C)] as well as exhibiting automethylation activity both in vivo during recombinant expression (see Fig. 2) and in vitro [Fig. 1(E)]. Unexpectedly, our top–down MS results indicate that AgCARM1WT has already been predominantly automethylated during its expression in E. coli. Recombinant proteins expressed in E. coli are generally thought to be lacking methylation and other PTMs; however, our study clearly shows protein arginine methyltransferase can utilize bacterial AdoMet and be automethylated when expressed in bacteria, suggesting that the same principle may apply to other post-translational modifying enzymes. Since PTMs can affect the activity or binding specificity of a given protein,34,42,43 the possibility of PTM during recombinant expression may create structural and functional diversity that would affect characterization of these proteins.44 This phenomenon may apply to other methyltransferases and specifically PRMT enzymes. Previous observation of weakly methylated CARM1 and PRMT1 in vitro has been logically attributed to weak automethylation activity.16 Our results indicate that a weak in vitro automethylation signal can also be attributed to near saturated automethylation in vivo prior to purification of the enzyme. Thus, it may be inaccurate to compare the activity of these enzymes through in vitro methylation without prior characterization of their methylation states.
Mutation of R485 to lysine appears to have no effect on AgCARM1's enzymatic activity, as CARM1 substrates are methylated equally by AgCARM1WT and AgCARM1R485K. This suggests that dimerization, AdoMet binding, and substrate specificity are not affected by loss of automethylation. R485 is located in the C-terminal domain adjacent to the substrate-binding site and, consistent with the above results, the corresponding region in mCARM1 is unstructured and not essential for the enzymatic activity of CARM1.3 The AgCARM1R485K mutant shows a loss of automethylation both in bacteria (see Fig. 3) and when isolated in vitro [Fig. 5(A)], suggesting that R485 is the only detectable automethylation site on AgCARM1. R485 is highly conserved in other sequenced insect species (Supporting Fig. S1).
The mechanism of automethylation has yet to be well characterized in PRMTs or other methyltransferases. An automethylation site on the lysine methyltransferase G9a has been determined,45,46 and this work suggests that G9a automethylation may occur by an intramolecular mechanism. While we were not able to confirm or rule out intramolecular activity by AgCARM1, we do show direct evidence of intermolecular methylation using a series of mixed in vitro methylation reactions [Fig. 5(B)]. Mixing AgCARM1WT with the catalytically inactive AgCARM1VLD, which can act only as a substrate in an automethylation reaction, increases radiolabeling above that of AgCARM1WT alone. Since AgCARM1VLD cannot contribute to the enzymatic activity of the reaction, this result indicates that AgCARM1VLD is being methylated by AgCARM1WT. Similar experiments show that AgCARM1R485K is able to methylate AgCARM1VLD in a similar manner. Mixing AgCARM1WT with AgCARM1R485K also shows an increase in the intensity of radiolabeling above AgCARM1WT alone, suggesting that AgCARM1R485K is methylating AgCARM1WT, since it does not appear to methylate itself. Altogether these data support an intermolecular mechanism for automethylation.
The ESI/FTMS analysis of AgCARM1 expressed in bacteria showed predominant dimethylated protein, while the remaining AgCARM1WT was unmethylated. This observation was supported by fragmentation spectrum (Supporting Fig. S2), which showed a similar ratio of dimethylated and unmethylated forms with no detectable monomethylated form in fragmentation ions containing the dimethylation site, R485. We speculate that the unmethylated AgCARM1WT represents the freshly expressed protein, while the dimethylated AgCARM1WT may be expressed earlier and automethylated over time. This notion is supported by the top–down MS result that AgCARM1WT can be further dimethylated when incubated with cold AdoMet in vitro (data not shown). The specific lack of monomethylated AgCARM1WT suggests that AgCARM1 may bind its substrate continuously during methylation, which is known as a processive mechanism. If validated, this would be in contrast to data from other Type I PRMTs such as PRMT1, PRMT3, and PRMT6. PRMT6 has been shown to methylate synthetic peptide substrates using a distributive mechanism, where a majority of substrate is monomethylated before dimethylation occurs.47 PRMT1's mechanism is still under debate, as recent works suggest both a partially processive mechanism,48 and a distributive mechanism.49 These studies focused on the methylation of peptides instead of intact proteins. Thus, the role of extended substrate structure and the unique character of automethylation in defining the mechanism of PRMT activity remain poorly defined. Although not conclusive, our results indicate that automethylation may have a different mechanism than other substrates' methylation by PRMT family members.
The top–down MS used in our study allows us to unambiguously determine any and all detectable modifications of AgCARM1. We were able to resolve the intact AgCARM1 protein (>58 kDa) in a LTQ/FT mass spectrometer and achieved extensive fragmentation by CAD and ECD. Extremely large fragmentation ions (>55 kDa) were isotopically resolved in CAD spectra. The overlapping b and y ions span the entire sequence giving confidence that the assigned primary structure is correct. ECD provided more backbone cleavages than CAD; here one ECD spectrum of one isolated single charge state of a 58 kDa protein generated as many as 115 cleavages out of 521 bonds. Therefore, top–down MS shows great promise in the elucidation and quantitation of large intact proteins.20–33 Recently, Webb et al. characterized methylation of lysine residues in yeast ribosomal protein Rpl42ab (12 kDa) via such a top–down MS approach. Despite its enormous promise, top–down MS has yet to be popularized mainly due to the high-resolution instrument requirement and technical difficulties in handling large intact proteins. Desalting is required before a protein sample can be analyzed on a mass spectrometer, however, proteins are more likely to precipitate in a solution free of salt. To overcome this dilemma, we found that it is critically important to analyze proteins freshly purified and desalted. Another limitation to top–down MS is the sensitivity of currently commercially available mass spectrometers. We need to acquire 1000–3000 transients per spectrum to ensure high quality MS and MS/MS, which required a relatively large amount of high purity proteins (>20 μg) and a highly stable electrospray. Hence, improvements in the instrument (i.e., sensitivity and resolution of a mass spectrometer) and software for automatic data processing50 will greatly facilitate the use of top–down MS for large proteins.
Materials and Methods
Cloning, expression, and purification
AgCARM1 was amplified from A. gambiae cDNA. The PCR product of the forward primer: (5′CGACTTGCTAGCATGAGTGAACAAATGGCTCG 3′) and reverse primer: (5′ATGGCAGCGGCCGCGTTGGTGTTGATGATTGTCGACG 3′) was restriction digested and ligated into pET21a, adding seven N-terminal amino acids (MARMSEQ) and an C-terminal His tag AgCARM11–509. AgCARM1R485K, AgCARM1VLD, GST-PABP1, and GST-GAR were expressed in BL21 cells and purified using tag affinity chromatography (Qiagen). mCARM1 and mPRMT1 were purified from insect cells as described previously.10
Site-directed mutagenesis
pET21a-AgCARM11–509 was mutated by amplification with complementary primers containing mutations to induce codon changes. AgCARM1VLD forward primer (5′CGCAAGACTTCCAGAATAAGATCGCGGCAGCTGTGGGGGCCGGATCCG 3′) and AgCARM1R485K forward primer: (5′CAGCTGGACGCACAGGGCGCCAAAAATGCGGTCAACCTCGTCAAC3′) (bold = mutated).
In vitro methylation assay
Various enzymes and substrates were incubated in 15 μL of 5 mM MgCl2, 20 mM Hepes pH 7.9, 1 mM EDTA, 1 mM DTT, 10% glycerol containing 1 μL of 3H-S-adenosylmethionine (GE Healthcare) for 1 h. This was then separated by SDS-PAGE and fixed in methanol for 1 h. Fixed gels were incubated in “Amplify” (Amersham Biosciences) scintillation fluid for 30 min before exposure to film.
Top–down mass spectrometry
All the protein samples (20–100 μg) were desalted by ultrafiltration using microcon centrifugal filter devices (Millipore, Billerica, MA), dissolved in 10–50% ACN and 1% acetic acid in water at 0.2–1 μg/μL, and introduced to the mass spectrometer using an automated chip-based nanoESI source, the Triversa NanoMate (Advion BioSciences, Ithaca, NY) with a spray voltage of 1.25–1.6 kV versus the inlet of the mass spectrometer, resulting in a flow of 50–200 nL/min. Intact protein molecular ions were analyzed using a linear trap/FT-ICR (LTQ FT Ultra) hybrid mass spectrometer (Thermo Scientific, Bremen, Germany). Capillary temperature was 200°C, capillary voltage was 36 V, and the tube lens voltage was 190 V. Ion transmission into the linear trap and further to the FT-ICR cell was automatically optimized for maximum ion signal with the target values (the approximate number of accumulated ions) for a full MS scan linear trap (LT) scan, FT-ICR cell (FT) scan, MSn FT-ICR, ECD scan were 3 × 104, 106, 106, and 8 × 106, respectively. The resolving power of the FT-ICR mass analyzer was set at 100,000 or 400,000 m/Δm50% at m/z 400, resulting in one scan/s acquisition rate. Individual charge states of the protein molecular ions were first isolated and then dissociated by ECD using 2–3% “electron energy” and a 50–70 ms duration time with no delay. Isolated charge states were also dissociated by CAD using 15–20% collision energy. Up to 3000 transients were averaged per spectrum to ensure high quality ECD/CAD spectra. All FT-ICR spectra were processed with Xtract Software (FT programs 2.0.1.0.6.1.4, Xcallibur 2.0.5, Thermo Scientific, Bremen, Germany) using a signal to noise threshold of 1.5 and fit factor of 60% and validated manually. The resulting monoisotopic mass lists were further assigned using in-house “Ion Assignment” software (Version 1.0) based on the recombinant CARM1 protein sequence generated from DNA sequencing with consideration of the static (mono-, di-) methylation and 10 ppm monoisotopic precursor and fragment tolerance. The Mr value reported in the study are all most abundant masses and the mass difference (in units of 1.00235 Da) between the most abundant isotopic peak and the monoisotopic peak is denoted in italics after each Mr value. The top 10 most abundant isotopic peak heights were integrated to calculate the relative abundance of the intact protein.
Conclusions
We present, here, the discovery and comprehensive characterization of an automethylation event by AgCARM1 using top–down high resolution MS/MS. Unexpectedly, we found AgCARM1WT has already been predominantly automethylated on a single site during its expression in E. coli via this top–down MS approach which could easily have been missed in traditional bottom–up MS. Mutation of this site reveals automethylation occurs intermolecularly and is consistent with a processive model for AgCARM1WT automethylation. This study may shed light on other bacterially expressed post-translational modification enzymes, which could be modified but overlooked in biochemical and structural studies. Top–down high resolution MS/MS shows great promise for revealing unexpected protein modification and localizing such modification to one amino acid.
Acknowledgments
The authors thank Susan Paskewitz (UW-Madison) for the generous gift of A. gambiae cDNA, Mark Bedford (University of Texas MD Anderson Center) for the generous gift of GST-PABP1 and GST-GAR plasmids, Dick Burgess (UW-Madison) for helpful discussions, Yongna Xing (UW-Madison) and Tadhg Begley (Cornell University) for critical reading of the manuscript. Financial support was kindly provided by Shaw Scientist Award from Greater Milwaukee Foundation and NIH (W.X.) and the Wisconsin Partnership Fund for a Healthy Future (Y.G.).
Glossary
Abbreviations:
- AdoMet
S-adenosylmethionine
- AgCARM1
A. gambiae CARM1
- CAD
collisionally activated dissociation
- ECD
electron capture dissociation
- ESI
electrospray ionization
- FT
Fourier transform
- LTQ
linear ion trap
- MS
mass spectrometry
- MS/MS
tandem mass spectrometry
- PABP1
polyA binding protein 1
- PRMT
protein arginine methyltransferase
- PTM
post-translational
- modification R
arginine.
References
- 1.Bedford MT, Richard S. Arginine methylation: an emerging regulator of protein function. Mol Cell. 2005;18:263. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284:2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 3.Teyssier C, Chen D, Stallcup MR. Requirement for multiple domains of the protein arginine methyltransferase CARM1 in its transcriptional coactivator function. J Biol Chem. 2002;277:46066–46072. doi: 10.1074/jbc.M207623200. [DOI] [PubMed] [Google Scholar]
- 4.Troffer-Charlier N, Cura V, Hassenboehler P, Moras D, Cavarelli J. Functional insights from structures of coactivator-associated arginine methyltransferase 1 domains. EMBO J. 2007;26:4391–4401. doi: 10.1038/sj.emboj.7601855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yue WW, Hassler M, Roe SM, Vale VT, Pearl LH. Insights into histone code syntax from structural and biochemical studies of CARM1 methyltransferase. EMBO J. 2007;26:4402–4412. doi: 10.1038/sj.emboj.7601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin JL, McMillan FM. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr Opin Struc Biol. 2002;12:783–793. doi: 10.1016/s0959-440x(02)00391-3. [DOI] [PubMed] [Google Scholar]
- 7.Katz JE, Dlakic M, Clarke S. Automated identification of putative methyltransferases from genomic open reading frames. Mol Cell Proteomics. 2003;2:525–540. doi: 10.1074/mcp.M300037-MCP200. [DOI] [PubMed] [Google Scholar]
- 8.Ma H, Baumann CT, Li H, Strahl BD, Rice R, Jelinek MA, Aswad DW, Allis CD, Hager GL, Stallcup MR. Hormone-dependent, CARM1-directed, arginine-specific methylation of histone H3 on a steroid-regulated promoter. Curr Biol. 2001;11:1981. doi: 10.1016/s0960-9822(01)00600-5. [DOI] [PubMed] [Google Scholar]
- 9.Schurter RT, Koh SS, Chen D, Bunick GJ, Harp JM, Hanson BL, Henschen-Edman A, Mackay DR, Stallcup MR, Aswad DW. Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry. 2001;40:5747–5756. doi: 10.1021/bi002631b. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Chen H, Du K, Asahara H, Tini M, Emerson BM, Montminy M, Evans RM. A transcriptional switch mediated by cofactor methylation. Science. 2001;294:2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Bedford MT. PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays. EMBO Rep. 2002;3:268–273. doi: 10.1093/embo-reports/kvf052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Q, Yi P, Wong J, O'Malley BW. Signaling within a coactivator complex: methylation of SRC-3/AIB1 is a molecular switch for complex disassembly. Mol Cell Biol. 2006;26:7846–7857. doi: 10.1128/MCB.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naeem H, Cheng D, Zhao Q, Underhill C, Tini M, Bedford MT, Torchia J. The activity and stability of the transcriptional coactivator p/CIP/SRC-3 is regulated by CARM1-dependent methylation. Mol Cell Biol. 2006;27:120–134. doi: 10.1128/MCB.00815-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higashimoto K, Kuhn P, Desai D, Cheng X, Xu W. Phosphorylation-mediated inactivation of coactivator-associated arginine methyltransferase 1. Proc Natl Acad Sci USA. 2007;104:12318–12323. doi: 10.1073/pnas.0610792104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel A, Yadav N, Lee J, Branscombe TL, Clarke S, Bedford MT. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specificity. J Biol Chem. 2002;277:3537–3543. doi: 10.1074/jbc.M108786200. [DOI] [PubMed] [Google Scholar]
- 16.Sayegh J, Webb K, Cheng D, Bedford MT, Clarke SG. Regulation of protein arginine methyltransferase 8 (PRMT8) activity by its N-terminal domain. J Biol Chem. 2007;282:36444–36453. doi: 10.1074/jbc.M704650200. [DOI] [PubMed] [Google Scholar]
- 17.Nedelkov D, Kiernan UA, Niederkofler EE, Tubbs KA, Nelson RW. Population proteomics—the concept, attributes, and potential for cancer biomarker research. Mol Cell Proteomics. 2006;5:1811–1818. doi: 10.1074/mcp.R600006-MCP200. [DOI] [PubMed] [Google Scholar]
- 18.Chait BT. Mass spectrometry: bottom-up or top–down? Science. 2006;314:65–66. doi: 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- 19.Kjeldsen F, Savitski MM, Nielsen ML, Shi L, Zubarev RA. On studying protein phosphorylation patterns using bottom-up LC-MS/MS: the case of human alpha-casein. Analyst. 2007;132:768–776. doi: 10.1039/b701902e. [DOI] [PubMed] [Google Scholar]
- 20.Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, Fridriksson EK, McLafferty FW. Top down versus bottom up protein characterization by tandem high-resolution mass spectrometry. J Am Chem Soc. 1999;121:806–812. [Google Scholar]
- 21.Ge Y, Lawhorn BG, ElNaggar M, Strauss E, Park JH, Begley TP, McLafferty FW. Top down characterization of larger proteins (45 kDa) by electron capture dissociation mass spectrometry. J Am Chem Soc. 2002;124:672–678. doi: 10.1021/ja011335z. [DOI] [PubMed] [Google Scholar]
- 22.Sze SK, Ge Y, Oh H, McLafferty FW. Top–down mass spectrometry of a 29-kDa protein for characterization of any posttranslational modification to within one residue. Proc Natl Acad Sci USA. 2002;99:1774–1779. doi: 10.1073/pnas.251691898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge Y, ElNaggar M, Sze SK, Bin Oh H, Begley TP, McLafferty FW, Boshoff H, Barry CE. Top down characterization of secreted proteins from Mycobacterium tuberculosis by electron capture dissociation mass spectrometry. J Am Soc Mass Spectrom. 2003;14:253–261. doi: 10.1016/s1044-0305(02)00913-3. [DOI] [PubMed] [Google Scholar]
- 24.Ge Y, Lawhorn BG, Elnaggar M, Sze SK, Begley TP, McLafferty FW. Detection of four oxidation sites in viral prolyl-4-hydroxylase by top–down mass spectrometry. Protein Sci. 2003;12:2320–2326. doi: 10.1110/ps.03244403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zabrouskov V, Giacomelli L, van Wijk KJ, McLafferty FW. New approach for plant proteomics—characterization of chloroplast proteins of Arabidopsis thaliana by top–down mass spectrometry. Mol Cell Proteomics. 2003;2:1253–1260. doi: 10.1074/mcp.M300069-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Kelleher NL. Top–down proteomics. Anal Chem. 2004;76:196A–203A. [PubMed] [Google Scholar]
- 27.Meng FY, Forbes AJ, Miller LM, Kelleher NL. Detection and localization of protein modifications by high resolution tandem mass spectrometry. Mass Spec Rev. 2005;24:126–134. doi: 10.1002/mas.20009. [DOI] [PubMed] [Google Scholar]
- 28.Zhai HL, Dorrestein PC, Chatterjee A, Begley TP, McLafferty FW. Simultaneous kinetic characterization of multiple protein forms by top down mass spectrometry. J Am Soc Mass Spectrom. 2005;16:1052–1059. doi: 10.1016/j.jasms.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Han XM, Jin M, Breuker K, McLafferty FW. Extending top–down mass spectrometry to proteins with masses greater than 200 kilodaltons. Science. 2006;314:109–112. doi: 10.1126/science.1128868. [DOI] [PubMed] [Google Scholar]
- 30.Pesavento JJ, Mizzen CA, Kelleher NL. Quantitative analysis of modified proteins and their positional isomers by tandem mass spectrometry: human histone H4. Anal Chem. 2006;78:4271–4280. doi: 10.1021/ac0600050. [DOI] [PubMed] [Google Scholar]
- 31.Xie YM, Zhang J, Yin S, Loo JA. Top–down ESI-ECD-FT-ICR mass spectrometry localizes noncovalent protein-ligand binding sites. J Am Chem Soc. 2006;128:14432–14433. doi: 10.1021/ja063197p. [DOI] [PubMed] [Google Scholar]
- 32.Zabrouskov V, Han XM, Welker E, Zhai HL, Lin C, van Wijk KJ, Scheraga HA, McLafferty FW. Stepwise deamidation of ribonuclease A at five sites determined by top down mass spectrometry. Biochemistry. 2006;45:987–992. doi: 10.1021/bi0517584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siuti N, Kelleher NL. Decoding protein modifications using top–down mass spectrometry. Nat Methods. 2007;4:817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabrouskov V, Ge Y, Schwartz J, Walker JW. Unraveling molecular complexity of phosphorylated human cardiac troponin I by top down electron capture dissociation/electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2008;7:1838–1849. doi: 10.1074/mcp.M700524-MCP200. [DOI] [PubMed] [Google Scholar]
- 35.Webb KJ, Laganowsky A, Whitelegge JP, Clarke SG. Identification of two SET domain proteins required for methylation of lysine residues in yeast ribosomal protein Rpl42ab. J Biol Chem. 2008;283:35561–35568. doi: 10.1074/jbc.M806006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal Chem. 2000;72:563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 37.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissociation of multiply charged protein cations. A nonergodic process. J Am Chem Soc. 1998;120:3265–3266. [Google Scholar]
- 38.Breuker K, Oh HB, Lin C, Carpenter BK, McLafferty FW. Nonergodic and conformational control of the electron capture dissociation of protein cations. Proc Natl Acad Sci USA. 2004;101:14011–14016. doi: 10.1073/pnas.0406095101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bedford MT. Arginine methylation at a glance. J Cell Sci. 2007;120:4243–4246. doi: 10.1242/jcs.019885. [DOI] [PubMed] [Google Scholar]
- 40.Boulanger M, Miranda T, Clarke S, Di Fruscio M, Suter B, Lasko P, Richard S. Characterization of the Drosophila protein arginine methyltransferases DART1 and DART4. Biochem J. 2004;379:283–289. doi: 10.1042/BJ20031176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cakouros D, Daish TJ, Mills K, Kumar S. An arginine-histone methyltransferase, CARMER, coodinates Ecdysone-mediated apoptosis in Drosophila cells. J Biol Chem. 2004;279:18467–18471. doi: 10.1074/jbc.M400972200. [DOI] [PubMed] [Google Scholar]
- 42.Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nat Biotechnol. 2003;21:255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 43.Karanam B, Jiang L, Wang L, Kelleher NL, Cole PA. Kinetic and mass spectrometric analysis of p300 histone acetyltransferase domain autoacetylation. J Biol Chem. 2006;281:40292–40301. doi: 10.1074/jbc.M608813200. [DOI] [PubMed] [Google Scholar]
- 44.Olivares-Illana V, Meyer P, Bechet E, Gueguen-Chaignon V, Soulat D, Lazereg-Riquier S, Mijakovic I, Deutscher J, Cozzone AJ, Laprévote O, Morera S, Grangeasse S, Nessler S. Structural basis for the regulation mechanism of the tyrosine kinase CapB from staphylococcus aureus. PLoS Biol. 2008;6:e143. doi: 10.1371/journal.pbio.0060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chin HG, Esteve PO, Pradhan M, Benner J, Patnaik D, Carey MF, Pradhan S. Automethylation of G9a and its implication in wider substrate specificity and HP1 binding. Nucleic Acids Res. 2007;35:7313–7323. doi: 10.1093/nar/gkm726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sampath SC, Marazzi I, Yap KL, Sampath SC, Krutchinsky AN, Mecklenbräuker I, Viale A, Rudensky E, Zhou MM, Chait BT, Tarakhovsky T. Methylation of a histone mimic within the histone methyltransferase G9a regulates protein complex assembly. Mol Cell. 2007;27:596–608. doi: 10.1016/j.molcel.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 47.Lakowski TM, Frankel A. A kinetic study of human protein arginine N-methyltransferase 6 reveals a distributive mechanism. J Biol Chem. 2008;283:10015–10025. doi: 10.1074/jbc.M710176200. [DOI] [PubMed] [Google Scholar]
- 48.Osborne TC, Obianyo O, Zhang X, Cheng X, Thompson PR. Protein arginine methyltransferase 1: positively charged residues in substrate peptides distal to the site of methylation are important for substrate binding and catalysis. Biochemistry. 2007;46:13370–13381. doi: 10.1021/bi701558t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolbel K, Ihling C, Bellmann-Sickert K, Neundorf I, Beck-Sickinger AG, Sinz A, Kühn U, Wahle E. Type I arginine methyltransferases PRMT1 and PRMT-3 act distributively. J Biol Chem. 2009;284:8274–8282. doi: 10.1074/jbc.M809547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng FY, Cargile BJ, Miller LM, Forbes AJ, Johnson JR, Kelleher NL. Informatics and multiplexing of intact protein identification in bacteria and the archaea. Nat Biotechnol. 2001;19:952–957. doi: 10.1038/nbt1001-952. [DOI] [PubMed] [Google Scholar]


