Abstract
The human DNA damage responses are modulated by both nonessential and essential pathways. The extensively studied ATM kinase and p53 are examples of the former. While loss-of-function mutations in genes that encode ATM and p53 cause marked predispositions to cancer, the loss of these proteins does not appear to impact basic cell growth and proliferation. In contrast, the checkpoint kinase Chk1 and its upstream activator ATR are essential.1-4 What do these proteins do in undamaged cells?
Keywords: Chk1, ATR, mitosis, cell cycle, phosphorylation, ionizing radiation
The ATR-Chk1-Cdc25A Pathway Monitors DNA Replication
Chk1 and ATR are strongly activated by agents that inhibit DNA replication.5-7 Perhaps not surprisingly, both of these proteins are required for efficient completion of S phase in unperturbed cells. Experimental depletion of Chk1 causes replication fork slowing and stalling8,9 and the appearance of replication-associated DNA strand breaks.10 At the cytogenetic level, deficiency of ATR leads to the expression of fragile sites that are believed to correspond to replication “slow zones”.11 Several lines of evidence thus converge on the conclusion that the ATR-Chk1 pathway both monitors and controls the progress of DNA replication forks.
A key downstream substrate of Chk1 during DNA replication is the protein phosphatase Cdc25A. Cdc25A stimulates DNA replication by activating Cdk2, which then promotes recruitment of replicative DNA polymerases by the activation of Cdc45.12,13 During DNA replication, Chk1 triggers the rapid turnover of Cdc25A by phosphorylating it on at least four sites, including serines 123, 178, 278 and 292.14 Chemical inhibition of Chk1 accordingly leads to accumulation of Cdc25A. Interestingly, DNA damage caused by ionizing radiation triggers robust, Chk1-dependent Cdc25A phosphorylation—on the same four sites that are basally modified by Chk1,14—and causes accelerated Cdc25A proteolysis.15 The fundamental insight gained from these experimental observations is that Chk1-mediated DNA damage responses represent an amplification of physiological processes that occur in unperturbed cells.
This concept has recently been validated and expanded by genetics. By targeting the human CHK1 locus, we have recently derived a human cell line that exclusively expresses a mutant form of Chk1 that cannot be phosphorylated by ATR.16 Mutation of a single DNA damage responsive serine residue, codon 317, led to defective degradation of Cdc25A after IR, a corresponding loss of Chk1-mediated checkpoints and impairment of survival after treatment with a DNA replication inhibitor. This cell line, designated DLD-Chk1S317A, also exhibited striking DNA replication abnormalities in the absence of exogenous stimuli, including slowed DNA replication forks and increased levels of spontaneous fork stalling. Thus, both Chk1-mediated DNA damage responses and control of DNA replication could be unambiguously linked to a single phosphorylation site mutation.16 This genetic linkage provides solid support for the model of S-phase regulation originally proposed by Bartek, Lukas and colleagues.14
An Alternate Mode of Chk1 Phosphorylation During Mitosis
Perhaps the most informative aspect of the DLD-Chk1S317A cell line is its very existence. Human cells that lose Chk1-mediated checkpoints and exhibit DNA replication defects are in fact fully viable. Similarly, CHK1-null mouse cells can be rescued by exogenous expression of a homologous serine 317 Chk1 mutant.17 One might have assumed that a critical role for ATR-Chk1 during S phase would explain why both of these proteins are encoded by essential genes. However, the DNA replication defects caused by CHK1 S317A mutation are clearly not lethal.
These new data can be interpreted in at least two different ways. It is possible that the serine 317 mutation partially disables the DNA replication function of Chk1, but does not eliminate it entirely. If this is in fact the case, the essential role of Chk1 in unperturbed cells may indeed be the regulation of DNA replication. Chk1 is phosphorylated by ATR on multiple neighboring serine residues in the regulatory domain of its C-terminus.3,18 One might predict that mutation of only one of these sites may lead to a partial loss of function, perhaps consistent with the nonlethal DNA replication defects that were observed. However, serine 317-mutant Chk1 was found to be defective in signaling not only to that residue, but also to the flanking residues serines 296 and 345.16 Chk1 serine 317 appears to be a key trigger that facilitates broader phosphorylation of Chk1 in response to DNA strand breaks. It may be that mutation of serine 317 does in fact cause a complete loss of Chk1-mediated checkpoint and DNA replication control, and that cells are viable in spite of this loss.
Alternatively, Chk1 may play an important—and perhaps underappreciated—role in a distinct phase of the cell cycle: mitosis. Several tantalizing clues suggest that Chk1 might be required for progression of mitosis in unperturbed cells. Tang and colleagues observed that knockdown of Chk1 in G1 synchronized cells caused an arrest when cells reached metaphase.19 Previously, Bartek, Lukas and colleagues had discovered that Chk1 localizes to the centrosome, the organizer of mitosis, where it is thought to control mitotic entry.20,21 These important studies revealed that Chk1 localizes to interphase centrosomes, where it shields Cdk1 from premature activation by Cdc25B.22 Chk1 may directly contribute to the controlled timing of the early steps of mitotic entry, such as mitotic spindle formation. The regulatory mechanism by which Chk1 localizes to the centrosome remains undefined.
Our recent study16 demonstrated that Chk1 is phosphorylated on the highly conserved serine 345 in untreated cells. Interestingly, the onset of Chk1 phosphorylation on the centrosome in prophase that we observed coincides with the ongoing activation of Cdk1 after entry into mitosis23 and suggests that Chk1 phosphorylation is a regulatory event. Importantly, the physiological phosphorylation of Chk1 serine 345 is clearly independent of serine 317 status,16 suggesting that a mechanism distinct from the DNA damage response is at play during unperturbed mitosis. In contrast, DNA damage induces multisite phosphorylation of Chk1and its relocalization to centrosomes, where it may negatively regulate mitotic entry by inducing G2/M arrest.21 Centrosomal relocalization of Chk1 after DNA damage would appear to be an important checkpoint response.
In summary, recent data suggest a model (Fig. 1) in which two distinct phosphorylation patterns differentially affect the association of Chk1 with the centrosome, resulting in one of two different outcomes: progression into and through mitosis, or cell cycle arrest at G2. While the precise role of Chk1 during mitosis remains to be defined, it is becoming clear that ongoing studies of Chk1 and its activation will have broad relevance.
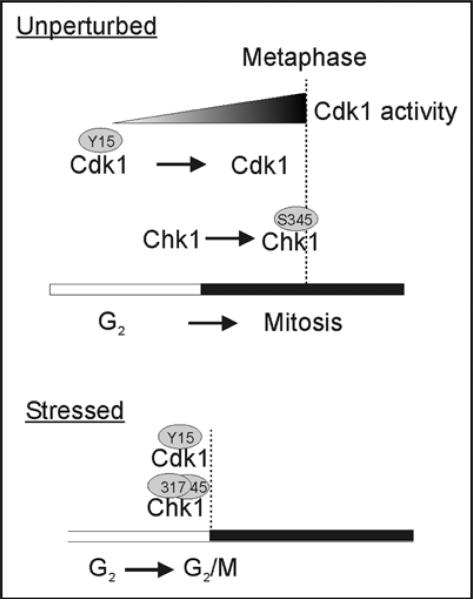
Figure 1.
Timing of Cdk1 and Chk1 phosphorylation and activation. During unperturbed cell growth, Chk1 becomes phosphorylated on the highly conserved serine 345 (S345) during late prophase. Chk1 phospho-protein is first evident at the centrosome. Later in mitosis, phosphorylated Chk1 is no longer concentrated at the centrosome. Cdk1 is activated upon dephosphorylation at tyrosine 15 (Y15) by Cdc25 proteins. The activation of Cdk1 begins in G2 at the centrosome and continues during early mitosis. Cdk1 is then deactivated during the metaphase to anaphase transition. Phosphorylation of Chk1 at the centrosome is thus temporally offset from the dephosphorylation/activation of Cdk1 during unperturbed cell growth. In response to DNA damage, the cell cycle is halted by the simultaneous phosphorylation of Chk1 on multiple residues, including S317 and S345, and loss of Cdk1 dephosphorylation.
Acknowledgements
The authors are supported by grants from the Flight Attendant Medical Research Institute and the NIH (CA104253 and CA119724).
References
- 1.Brown EJ, Baltimore D. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 2000;14:397–402. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown EJ, Baltimore D. Essential and dispensable roles of ATR in cell cycle arrest and genome maintenance. Genes Dev. 2003;17:615–28. doi: 10.1101/gad.1067403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 4.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: Partners in checkpoint signaling. Science. 2001;294:1713–76. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–296. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 6.Osborn AJ, Elledge SJ, Zou L. Checking on the fork: The DNA-replication stress-response pathway. Trends Cell Biol. 2002;12:509–16. doi: 10.1016/s0962-8924(02)02380-2. [DOI] [PubMed] [Google Scholar]
- 7.Hurley PJ, Bunz F. ATM and ATR: Components of an integrated circuit. Cell Cycle. 2007;6:414–7. doi: 10.4161/cc.6.4.3886. [DOI] [PubMed] [Google Scholar]
- 8.Maya-Mendoza A, Petermann E, Gillespie DA, Caldecott KW, Jackson DA. Chk1 regulates the density of active replication origins during the vertebrate S phase. EMBO J. 2007;26:2719–31. doi: 10.1038/sj.emboj.7601714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petermann E, Maya-Mendoza A, Zachos G, Gillespie DA, Jackson DA, Caldecott KW. Chk1 requirement for high global rates of replication fork progression during normal vertebrate S phase. Mol Cell Biol. 2006;26:3319–26. doi: 10.1128/MCB.26.8.3319-3326.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syljuasen RG, Sorensen CS, Hansen LT, Fugger K, Lundin C, Johansson F, et al. Inhibition of human Chk1 causes increased initiation of DNA replication, phosphorylation of ATR targets, and DNA breakage. Mol Cell Biol. 2005;25:3553–62. doi: 10.1128/MCB.25.9.3553-3562.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casper AM, Nghiem P, Arlt MF, Glover TW. ATR regulates fragile site stability. Cell. 2002;111:779–89. doi: 10.1016/s0092-8674(02)01113-3. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo V, Robertson K, Ying CY, Kim E, Avvedimento E, Gottesman M, et al. Reconstitution of an ATM-dependent checkpoint that inhibits chromosomal DNA replication following DNA damage. Mol Cell. 2000;6:649–59. doi: 10.1016/s1097-2765(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 13.Falck J, Petrini JH, Williams BR, Lukas J, Bartek J. The DNA damage-dependent intra-S phase checkpoint is regulated by parallel pathways. Nat Genet. 2002;30:290–4. doi: 10.1038/ng845. [DOI] [PubMed] [Google Scholar]
- 14.Sorensen CS, Syljuasen RG, Falck J, Schroeder T, Ronnstrand L, Khanna KK, et al. Chk1 regulates the S phase checkpoint by coupling the physiological turnover and ionizing radiation-induced accelerated proteolysis of Cdc25A. Cancer Cell. 2003;3:247–58. doi: 10.1016/s1535-6108(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 15.Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, et al. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288:1425–9. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- 16.Wilsker D, Petermann E, Helleday T, Bunz F. Essential function of Chk1 can be uncoupled from DNA damage checkpoint and replication control. Proc Natl Acad Sci USA. 2008;105:20752–7. doi: 10.1073/pnas.0806917106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niida H, Katsuno Y, Banerjee B, Hande MP, Nakanishi M. Specific role of Chk1 phosphorylations in cell survival and checkpoint activation. Mol Cell Biol. 2007;27:2572–81. doi: 10.1128/MCB.01611-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H, Piwnica-Worms H. ATR-mediated checkpoint pathways regulate phosphorylation and activation of human Chk1. Mol Cell Biol. 2001;21:4129–39. doi: 10.1128/MCB.21.13.4129-4139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang J, Erikson RL, Liu X. Checkpoint kinase 1 (Chk1) is required for mitotic progression through negative regulation of polo-like kinase 1 (Plk1). Proc Natl Acad Sci USA. 2006;103:11964–9. doi: 10.1073/pnas.0604987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer A, Mailand N, Lukas C, Syljuasen RG, Wilkinson CJ, Nigg EA, et al. Centrosome-associated Chk1 prevents premature activation of cyclin-B-Cdk1 kinase. Nat Cell Biol. 2004;6:884–91. doi: 10.1038/ncb1165. [DOI] [PubMed] [Google Scholar]
- 21.Loffler H, Bochtler T, Fritz B, Tews B, Ho AD, Lukas J, et al. DNA damage-induced accumulation of centrosomal Chk1 contributes to its checkpoint function. Cell Cycle. 2007;6:2541–8. doi: 10.4161/cc.6.20.4810. [DOI] [PubMed] [Google Scholar]
- 22.Loffler H, Rebacz B, Ho AD, Lukas J, Bartek J, Kramer A. Chk1-dependent regulation of Cdc25B functions to coordinate mitotic events. Cell Cycle. 2006;5:2543–7. doi: 10.4161/cc.5.21.3435. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist A, van Zon W, Karlsson Rosenthal C, Wolthuis RM. Cyclin B1-Cdk1 activation continues after centrosome separation to control mitotic progression. PLoS Biol. 2007;5:123. doi: 10.1371/journal.pbio.0050123. [DOI] [PMC free article] [PubMed] [Google Scholar]