Abstract
Salmonella enterica serovar Typhimurium is a common facultative intracellular pathogen that causes food-borne gastroenteritis in millions of people worldwide. Intracellular survival and replication are important virulence determinants and the bacteria can be found in a variety of phagocytic and non-phagocytic cells in vivo. Invasion of host cells and intracellular survival are dependent on two type III secretion systems, T3SS1 and T3SS2, each of which translocates a distinct set of effector proteins. However, other virulence factors including ion transporters, superoxide dismutase, flagella and fimbriae are also involved in accessing and utilizing the intracellular niche.
Introduction
Salmonella enterica are members of the Enterobacteriaceae family of bacteria, a large group of Gram-negative, facultative anaerobes many of which are a normal part of the gut microbiota in the intestines of vertebrates. Although there are well over 2000 serovars of S. enterica only a handful are commonly associated with disease in humans, which usually presents as a self-limiting gastroenteritis or the more severe enteric or typhoid fever. S. enterica serovar Typhimurium (S. Typhimurium) is one of the most frequent causes of food-borne gastroenteritis in humans, and is also an important pathogen of food-producing animals including cattle, pigs and chickens.
Salmonella are facultative intracellular bacteria that are found within a variety of phagocytic and non-phagocytic cells in vivo. Following intestinal colonization Salmonella enter enterocytes, M cells and dendritic cells (DCs) in the intestinal epithelium. Subsequently Salmonella that reach the submucosa can be internalized by resident macrophages and rapidly disseminate through the blood stream accumulating in mesenteric lymph nodes and, ultimately, the spleen (Salcedo et al., 2001). Altogether the ability of Salmonella to survive in a variety of host cells is vital to its success as a pathogen. The large assortment of bacterial and host factors that determine the outcome of infection is summarized here, focusing primarily on serovar Typhimurium.
Internalization into host cells
Internalization of Salmonella into host cells can occur via at least two distinct processes (Fig. 1). Professional phagocytes such as macrophages utilize phagocytic uptake to efficiently recognize and internalize bacterial pathogens. Salmonella can also actively invade both phagocytic and non-phagocytic cells using a type III secretion system (T3SS), T3SS1. Phagocytosis of Gram-negative bacteria is a complex mechanism that involves multiple receptors, some of which increase the efficiency of uptake and others that activate different signalling pathways in the phagocyte. Pattern-recognition receptors recognize pathogen-associated molecular patterns, including lipopolysaccharide (LPS) and flagellin, and binding to ligand, either on the cell surface or inside the phagosome, can affect phagosome maturation, signalling and gene expression (reviewed in Kumar et al., 2009). Whereas phagocytosis is an essential innate immune function that has developed to sample a potentially vast array of different pathogens, T3SS1-mediated invasion by Salmonella is a highly specific process that depends on the tightly regulated expression of a number of bacterial factors (Takaya et al., 2005; Kage et al., 2008). In a remarkably co-ordinated process a small group of effector proteins (SipA, SipC, SopB/SigD, SopD, SopE2 and SptP) induce dramatic rearrangement of the actin cytoskeleton resulting in massive localized membrane ruffles and rapid internalization of the bacteria (for review see McGhie et al., 2009). In addition to phagocytosis and T3SS1-mediated invasion, fimbriae and/or non-fimbrial adhesins on the surface of Salmonella may also mediate attachment and internalization via a T3SS1-independent process (Guo et al., 2007).
Fig. 1.
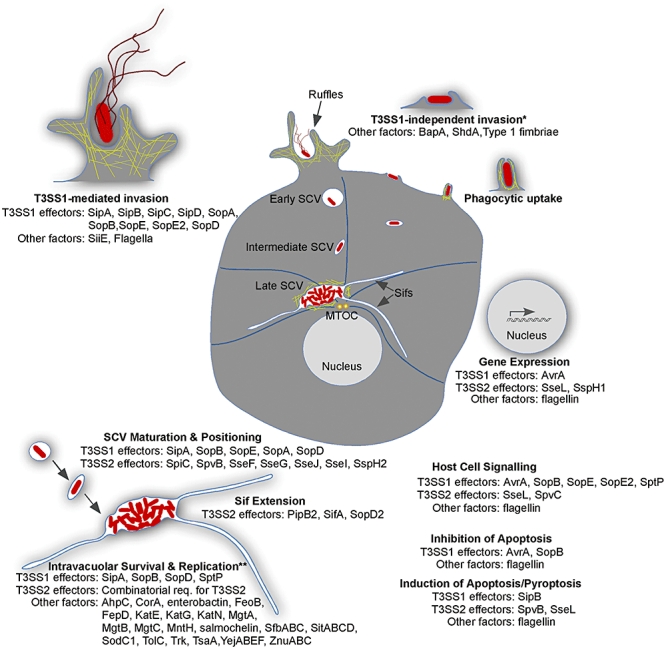
Virulence factors involved in the intracellular survival of Salmonella. Salmonella can enter host cells by invasion (T3SS1-mediated) or phagocytosis. In addition, a T3SS1-independent invasion* has been shown to occur in several cell types that may be mediated by fimbriae or non-fimbrial adhesins. Following internalization Salmonella remain within a modified phagosome known as the SCV (Salmonella-containing vacuole). Biogenesis of the SCV and its translocation to the MTOC (microtubule-organizing centre) involves interactions with the host cell endocytic pathway and microtubules and is mediated by a variety of T3SS1 and T3SS2 effector proteins. Survival and replication within the SCV are dependent on a number of factors including nutrient acquisition and avoidance of host antibacterial activities. **Listed are a number of factors implicated but not necessarily proven to be required for intracellular survival. Yellow and blue lines indicate actin, associated with invasion and the SCV, and microtubules, required for positioning of the SCV and Sif extension respectively.
The Salmonella-containing vacuole
Following internalization Salmonella survive and replicate within a modified phagosome known as the Salmonella-containing vacuole (SCV), which initially is marked by the accumulation of early endosome markers. These ‘early’ markers are then rapidly removed and within 60–90 min post invasion (p.i.) SCVs become highly enriched in markers of late endosomes and lysosomes particularly lysosomal glycoproteins (Steele-Mortimer et al., 1999). Concomitantly, the SCV moves from the cell periphery to a juxtanuclear position at the microtubule-organizing centre (MTOC) (Salcedo and Holden, 2003; Deiwick et al., 2006). The onset of intracellular replication is accompanied in some cell types by the appearance of Sifs (Salmonella-induced filaments), a network of dynamic membrane tubules that radiate from the SCV (Drecktrah et al., 2008).
Salmonella virulence determinants affecting intracellular survival
In addition to two T3SSs, Salmonella have a type I secretion system and other factors such as fimbriae, flagella and ion transporters that have important roles in establishing and maintaining the intracellular niche. Many virulence factors are encoded on Salmonella Pathogenicity Islands (SPI) on the chromosome. Most notably, T3SS1 and T3SS2 are encoded on SPI1 and SPI2 respectively. Invasion and early post-invasion processes are modulated by T3SS1, flagella, fimbriae and non-fimbrial adhesins; subsequently the T3SS2 and factors involved in nutrient acquisition and avoidance of antibacterial mechanisms are induced. In reality the system is rather more complex and there is considerable temporal overlap.
Type I secretion systems
BapA and SiiE are two huge surface-associated proteins that have been implicated in invasion/adhesion and are secreted via dedicated type I secretion systems BapBCD and SiiCDF respectively (Latasa et al., 2005; Gerlach et al., 2007). SiiE, which has multiple 90-amino-acid repeats and a C-terminal secretion signal, is encoded on SPI4, which is co-regulated with SPI1 (Morgan et al., 2007; Main-Hester et al., 2008).
Fimbriae
Salmonella have 13 predicted fimbrial loci, many of which are induced in vivo and are required for biofilm formation, attachment to host cells and colonization but not intracellular survival per se (Humphries et al., 2001). The type 1 fimbrial adhesin FimH mediates T3SS1-independent uptake in murine DCs (Guo et al., 2007).
Flagella and flagellin
Flagellar-based motility can increase the invasiveness of Salmonella (Schmitt et al., 2001), although this remains somewhat controversial especially since flagellin monomers are potent inducers of innate immunity (Franchi et al., 2006; Miao et al., 2006). In Salmonella-infected macrophages flagellin is translocated into the cytosol by T3SS1 resulting in activation of the inflammasome and caspase-1-mediated cell death (pyroptosis) (Ren et al., 2006; Miao et al., 2007; Sun et al., 2007). In the intestinal epithelium flagellin induces inflammation while inhibiting apoptosis also via TLR5, but the flagellin must be translocated to the basolateral side of the epithelial cells, since TLR5 is not expressed on the apical surface (Gewirtz et al., 2001; Vijay-Kumar et al., 2006). Flagella are usually downregulated inside the host, although inside macrophages it has been suggested they may be induced with T3SS1 and used for escape (Sano et al., 2007).
T3SS1
At least 15 effectors can be translocated by T3SS1 into the host cell (reviewed in McGhie et al., 2009). Four of these, SopE/SopE2, SopB and SipA, cooperatively induce the actin rearrangements required for invasion but almost all of the others have been implicated in a variety of post-invasion processes, including host cell survival, SCV biogenesis and modulation of the inflammatory response. Accumulating evidence suggests that many effector proteins have multiple activities within host cells. For example, the inositol phosphatase SopB is involved in: invasion, Akt activation, fluid secretion and SCV formation/biogenesis/positioning (Terebiznik et al., 2002; Hernandez et al., 2004; Drecktrah et al., 2005; Knodler et al., 2005; Mallo et al., 2008; Patel et al., 2009). Each of these activities is presumably dependent on the generation of specific phosphoinositides where SopB is localized, namely the plasma and SCV membranes. One intriguing possibility is that intracellular localization of SopB determines its specificity, since the subcellular localization of SopB, and therefore presumably its activity, is controlled by ubiquitination (Knodler et al., 2009; Patel et al., 2009).
In addition to SopB several other T3SS1 and T3SS2 effector proteins intersect with host cell ubiquitin pathways. The T3SS1 effector AvrA is a member of a family of ubiquitin-like acetyltransferases/cysteine proteases produced by bacterial pathogens including the Yersinia effector YopJ. AvrA removes ubiquitin from two inhibitors of the NF-κB pathway, IκBα and beta-catenin, thus inhibiting the inflammatory response (Ye et al., 2007; Jones et al., 2008), activating beta-catenin signalling (Sun et al., 2004) and preventing apoptosis in intestinal epithelial cells (Jones et al., 2008). SopA in contrast, another effector that can contribute to invasion (Raffatellu et al., 2005), is one of several bacterial effectors that have HECT-like E3 ubiquitin-ligase activity (Zhang et al., 2006).
Other T3SS1 effectors implicated in SCV/Sif biogenesis are the tyrosine phosphatase SptP, which dephosphorylates the AAA+ ATPase VCP (Humphreys et al., 2009) and is required for switching off ruffle formation following invasion (Fu and Galan, 1999), and SipA which is implicated in SCV morphology and juxtanuclear positioning and has been shown to cooperate with the SPI2 effector SifA (Brawn et al., 2007).
T3SS2
T3SS2 is required for systemic virulence in the mouse and survival within macrophages (Hensel et al., 1998). Although the roles of individual T3SS2 effectors remain ill defined, several are involved in SCV positioning and the formation of Sifs that extend from the surface of late SCVs (≥ 6 h p.i.) in epithelial cells. SseF and SseG are required for maintenance of the SCV at the MTOC and intracellular replication (Kuhle and Hensel, 2002; Salcedo and Holden, 2003). SifA is essential for Sif formation, a process apparently linked to SCV membrane integrity since mutants lacking SifA are released into the cytosol (Beuzon et al., 2002; Brumell et al., 2002). Two other T3SS2 effectors, PipB2 and SseJ, cooperate with SifA via a process involving several mammalian proteins. PipB2 interacts with kinesin light chain, a subunit of the kinesin-1 motor complex that drives anterograde transport along microtubules, recruiting it to the surface of the SCV (Henry et al., 2006). This interaction drives the extension of Sif tubules from the juxtanuclear SCV towards the periphery of the host cell (Knodler and Steele-Mortimer, 2005). Recent studies focusing on the interaction between SifA and the mammalian protein SKIP have identified small GTPases as potential targets. Thus SKIP interacts directly with rab9, a GTPase involved in lysosome and late endosome function and positioning, and SifA may displace rab9 from this complex (Barbero et al., 2002; Ganley et al., 2004; Jackson et al., 2008). SifA can also bind directly to rab7 and acts as an exchange factor (GEF) for RhoA, a small GTPase that when activated can increase membrane tubulation and, in the presence of SKIP and the T3SS2 effector SseJ, promotes host membrane tubulation (Harrison et al., 2004; Lossi et al., 2008; Ohlson et al., 2008). In epithelial cells infected with mutants lacking SseJ cholesterol accumulation is increased compared with cells infected with wild-type bacteria, and this is associated with a decrease in intracellular replication (Ruiz-Albert et al., 2002; Nawabi et al., 2008). Intriguingly, in cells with abnormally high levels of cholesterol, rab9 is sequestered causing defects in membrane trafficking (Ganley and Pfeffer, 2006), suggesting a possible link exists between SseJ and rab9.
Three T3SS2 effectors interfere with host cell ubiquitin pathways (Quezada et al., 2009). SspH 1 and SspH 2 are members of a family of ubiquitin E3 ligases found in pathogenic bacteria including Shigella and Yersinia (Miao et al., 1999; Quezada et al., 2009). The function of these two close homologues remains unknown, although SspH 2 colocalizes with actin around the SCV (Miao et al., 2003) and it also has a targeting signal for localization at tight junctions in polarized epithelial cells (Quezada et al., 2009). SseL is a deubiquitinase that, like AvrA, can modulate NF-κB activation downstream of IκBα kinases although whether it causes suppression or activation of the pathway remains unclear (Coombes et al., 2007; Rytkonen et al., 2007; Le Negrate et al., 2008).
Virulence plasmid
Two genes, spvB and spvC, encode the principal factors for plasmid-mediated virulence of serovar Typhimurium (Matsui et al., 2001). Both are translocated via the T3SS2 into host cells (Browne et al., 2008; Mazurkiewicz et al., 2008). SpvB ADP-ribosylates actin, destabilizes the cytoskeleton and is associated with host cell cytotoxicity (Lesnick et al., 2001; Tezcan-Merdol et al., 2001; Kurita et al., 2003; Browne et al., 2008). SpvC has phosphothreonine lyase activity and can inactivate the mitogen-activated protein kinases Erk1/2, JNK and p38 in mammalian cells (Li et al., 2007; Mazurkiewicz et al., 2008).
Superoxide dismutase
Many host cells produce reactive oxygen species, largely through the activity of the phagosome NADPH oxidase (NOX2) that are required for killing of intracellular pathogens. To counteract this activity Salmonella uses a superoxide dismutase, SodCI, to confer protection from extracellular reactive oxygen species. SodCI is ‘tethered’ within the periplasm and protease resistance may be a critical property that allows this enzyme to function in the harsh environment of the phagosome (Krishnakumar et al., 2007; Pacello et al., 2008).
Ion acquisition
In the eukaryotic host, iron availability is limited due to the activity of iron-binding proteins such as transferrin and Nramp1 (natural resistance-associated macrophage protein one or Slc11A1), a divalent metal-proton symporter found in macrophages, neutrophils and DCs (Nairz et al., 2009). To overcome this limitation Salmonella produce two siderophores, enterobactin and salmochelin, in response to iron deprivation (for review, see Muller et al., 2009). Salmochelin is a glucosylated derivative of enterobactin and this modification may be important for resistance to lipocalin-2, an antimicrobial protein that prevents bacterial iron acquisition in the inflamed intestinal epithelium (Raffatellu et al., 2009). A recent study found that SCVs in macrophages contain enough iron to affect activity of metal-responsive promoters independently of Nramp1 (Taylor et al., 2009); however, it is possible that the requirement for iron transporters could change under different conditions, for example when cells are treated with IFN-γ (Nairz et al., 2007; Nairz et al., 2008). Comparison of mutant Typhimurium strains lacking the iron transporters encoded by feoB or sitABCD revealed that both are required for survival in Nramp1(−/−) mice and replication in macrophages and that the Nramp1 homologue MntH, which prefers Mn(II) over Fe(II), is also required for optimal virulence (Janakiraman and Slauch, 2000; Boyer et al., 2002; Zaharik et al., 2004).
Salmonella has three distinct systems for uptake of Mg2+: CorA, MgtA and MgtB, each of which is essential for virulence (Blanc-Potard and Groisman, 1997; Papp-Wallace et al., 2008). In addition, MgtC, encoded on the same operon as MgtB, while not a Mg2+ transporter is required for intramacrophage survival and growth in magnesium-depleted medium (Gunzel et al., 2006; Alix and Blanc-Potard, 2008).
Two other metal ions implicated in intracellular survival are K+ and Zn2+. The ZnuABC high-affinity Zn2+ uptake system is required for growth of S. Typhimurium in low-zinc conditions and intracellularly in some cultured cells and ZnuABC mutants are defective for virulence in both susceptible and resistant mouse strains (Ammendola et al., 2007). The Trk system is a multiunit protein complex that functions as a low-affinity K+ transporter and may function in resistance to antimicrobial peptides (Parra-Lopez et al., 1994).
Conclusions
In the last 20 years remarkable progress has been made in our understanding of how Salmonella interact with eukaryotic host cells. Nevertheless, many of the most interesting questions remain unanswered. Development of more sophisticated in vitro systems such as co-cultures or 3D cultures that more closely recreate the in vivo environment is one promising area.
Acknowledgments
We apologize to all those whose work is not cited, or has not been adequately acknowledged, due to space limitations. Research in O.S.-M.'s laboratory is supported by the Intramural Research Program (DIR) of the NIH, NIAID.
References
- Alix E, Blanc-Potard AB. Peptide-assisted degradation of the Salmonella MgtC virulence factor. EMBO J. 2008;27:546–557. doi: 10.1038/sj.emboj.7601983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. doi: 10.1128/IAI.00559-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J Cell Biol. 2002;156:511–518. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuzon CR, Salcedo SP, Holden DW. Growth and killing of a Salmonella enterica serovar Typhimurium sifA mutant strain in the cytosol of different host cell lines. Microbiology. 2002;148:2705–2715. doi: 10.1099/00221287-148-9-2705. [DOI] [PubMed] [Google Scholar]
- Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer E, Bergevin I, Malo D, Gros P, Cellier MF. Acquisition of Mn(II) in addition to Fe(II) is required for full virulence of Salmonella enterica serovar Typhimurium. Infect Immun. 2002;70:6032–6042. doi: 10.1128/IAI.70.11.6032-6042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawn LC, Hayward RD, Koronakis V. Salmonella SPI1 effector SipA persists after entry and cooperates with a SPI2 effector to regulate phagosome maturation and intracellular replication. Cell Host Microbe. 2007;1:63–75. doi: 10.1016/j.chom.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SH, Hasegawa P, Okamoto S, Fierer J, Guiney DG. Identification of Salmonella SPI-2 secretion system components required for SpvB-mediated cytotoxicity in macrophages and virulence in mice. FEMS Immunol Med Microbiol. 2008;52:194–201. doi: 10.1111/j.1574-695X.2007.00364.x. [DOI] [PubMed] [Google Scholar]
- Brumell JH, Tang P, Zaharik ML, Finlay BB. Disruption of the Salmonella-containing vacuole leads to increased replication of Salmonella enterica serovar typhimurium in the cytosol of epithelial cells. Infect Immun. 2002;70:3264–3270. doi: 10.1128/IAI.70.6.3264-3270.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes BK, Lowden MJ, Bishop JL, Wickham ME, Brown NF, Duong N, et al. SseL is a salmonella-specific translocated effector integrated into the SsrB-controlled salmonella pathogenicity island 2 type III secretion system. Infect Immun. 2007;75:574–580. doi: 10.1128/IAI.00985-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiwick J, Salcedo SP, Boucrot E, Gilliland SM, Henry T, Petermann N, et al. The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect Immun. 2006;74:6965–6972. doi: 10.1128/IAI.00648-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drecktrah D, Knodler LA, Galbraith K, Steele-Mortimer O. The Salmonella SPI1 effector SopB stimulates nitric oxide production long after invasion. Cell Microbiol. 2005;7:105–113. doi: 10.1111/j.1462-5822.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- Drecktrah D, Levine-Wilkinson S, Dam T, Winfree S, Knodler LA, Schroer TA, Steele-Mortimer O. Dynamic behavior of Salmonella-induced membrane tubules in epithelial cells. Traffic. 2008;9:2117–2129. doi: 10.1111/j.1600-0854.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- Fu Y, Galan JE. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature. 1999;401:293–297. doi: 10.1038/45829. [DOI] [PubMed] [Google Scholar]
- Ganley IG, Pfeffer SR. Cholesterol accumulation sequesters Rab9 and disrupts late endosome function in NPC1-deficient cells. J Biol Chem. 2006;281:17890–17899. doi: 10.1074/jbc.M601679200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganley IG, Carroll K, Bittova L, Pfeffer S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol Biol Cell. 2004;15:5420–5430. doi: 10.1091/mbc.E04-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach RG, Jackel D, Stecher B, Wagner C, Lupas A, Hardt WD, Hensel M. Salmonella Pathogenicity Island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell Microbiol. 2007;9:1834–1850. doi: 10.1111/j.1462-5822.2007.00919.x. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- Gunzel D, Kucharski LM, Kehres DG, Romero MF, Maguire ME. The MgtC virulence factor of Salmonella enterica serovar Typhimurium activates Na(+),K(+)-ATPase. J Bacteriol. 2006;188:5586–5594. doi: 10.1128/JB.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Lasaro MA, Sirard JC, Kraehenbuhl JP, Schifferli DM. Adhesin-dependent binding and uptake of Salmonella enterica serovar Typhimurium by dendritic cells. Microbiology. 2007;153:1059–1069. doi: 10.1099/mic.0.2006/000331-0. [DOI] [PubMed] [Google Scholar]
- Harrison RE, Brumell JH, Khandani A, Bucci C, Scott CC, Jiang X, et al. Salmonella impairs RILP recruitment to Rab7 during maturation of invasion vacuoles. Mol Biol Cell. 2004;15:3146–3154. doi: 10.1091/mbc.E04-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry T, Couillault C, Rockenfeller P, Boucrot E, Dumont A, Schroeder N, et al. The Salmonella effector protein PipB2 is a linker for kinesin-1. Proc Natl Acad Sci USA. 2006;103:13497–13502. doi: 10.1073/pnas.0605443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel M, Shea JE, Waterman SR, Mundy R, Nikolaus T, Banks G, et al. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- Hernandez LD, Hueffer K, Wenk MR, Galan JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science. 2004;304:1805–1807. doi: 10.1126/science.1098188. [DOI] [PubMed] [Google Scholar]
- Humphries AD, Townsend SM, Kingsley RA, Nicholson TL, Tsolis RM, Baumler AJ. Role of fimbriae as antigens and intestinal colonization factors of Salmonella serovars. FEMS Microbiol Lett. 2001;201:121–125. doi: 10.1111/j.1574-6968.2001.tb10744.x. [DOI] [PubMed] [Google Scholar]
- Humphreys D, Hume PJ, Koronakis V. The Salmonella effector SptP dephosphorylates host AAA+ ATPase VCP to promote development of its intracellular replicative niche. Cell Host Microbe. 2009;5:225–233. doi: 10.1016/j.chom.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LK, Nawabi P, Hentea C, Roark EA, Haldar K. The Salmonella virulence protein SifA is a G protein antagonist. Proc Natl Acad Sci USA. 2008;105:14141–14146. doi: 10.1073/pnas.0801872105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiraman A, Slauch JM. The putative iron transport system SitABCD encoded on SPI1 is required for full virulence of Salmonella typhimurium. Mol Microbiol. 2000;35:1146–1155. doi: 10.1046/j.1365-2958.2000.01783.x. [DOI] [PubMed] [Google Scholar]
- Jones RM, Wu H, Wentworth C, Luo L, Collier-Hyams L, Neish AS. Salmonella AvrA coordinates suppression of host immune and apoptotic defenses via JNK pathway blockade. Cell Host Microbe. 2008;3:233–244. doi: 10.1016/j.chom.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Kage H, Takaya A, Ohya M, Yamamoto T. Coordinated regulation of expression of Salmonella pathogenicity island 1 and flagellar type III secretion systems by ATP-dependent ClpXP protease. J Bacteriol. 2008;190:2470–2478. doi: 10.1128/JB.01385-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Steele-Mortimer O. The Salmonella effector PipB2 affects late endosome/lysosome distribution to mediate Sif extension. Mol Biol Cell. 2005;16:4108–4123. doi: 10.1091/mbc.E05-04-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler LA, Finlay BB, Steele-Mortimer O. The Salmonella effector protein SopB protects epithelial cells from apoptosis by sustained activation of Akt. J Biol Chem. 2005;280:9058–9064. doi: 10.1074/jbc.M412588200. [DOI] [PubMed] [Google Scholar]
- Knodler LA, Winfree S, Drecktrah D, Ireland R, Steele-Mortimer O. Ubiquitination of the bacterial inositol phosphatase, SopB, regulates its biological activity at the plasma membrane: Cell Microbiol. (epub ahead of print). doi: 10.1111/j.1462-5822.2009.01356x. [DOI] [PMC free article] [PubMed]
- Krishnakumar R, Kim B, Mollo EA, Imlay JA, Slauch JM. Structural properties of periplasmic SodCI that correlate with virulence in Salmonella enterica serovar Typhimurium. J Bacteriol. 2007;189:4343–4352. doi: 10.1128/JB.00010-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle V, Hensel M. SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell Microbiol. 2002;4:813–824. doi: 10.1046/j.1462-5822.2002.00234.x. [DOI] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S. Pathogen recognition in the innate immune response. Biochem J. 2009;420:1–16. doi: 10.1042/BJ20090272. [DOI] [PubMed] [Google Scholar]
- Kurita A, Gotoh H, Eguchi M, Okada N, Matsuura S, Matsui H, et al. Intracellular expression of the Salmonella plasmid virulence protein, SpvB, causes apoptotic cell death in eukaryotic cells. Microb Pathog. 2003;35:43–48. doi: 10.1016/s0882-4010(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Latasa C, Roux A, Toledo-Arana A, Ghigo JM, Gamazo C, Penades JR, Lasa I. BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enterica serovar Enteritidis. Mol Microbiol. 2005;58:1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- Le Negrate G, Faustin B, Welsh K, Loeffler M, Krajewska M, Hasegawa P, et al. Salmonella secreted factor L deubiquitinase of Salmonella typhimurium inhibits NF-kappaB, suppresses IkappaBalpha ubiquitination and modulates innate immune responses. J Immunol. 2008;180:5045–5056. doi: 10.4049/jimmunol.180.7.5045. [DOI] [PubMed] [Google Scholar]
- Lesnick ML, Reiner NE, Fierer J, Guiney DG. The Salmonella spvB virulence gene encodes an enzyme that ADP-ribosylates actin and destabilizes the cytoskeleton of eukaryotic cells. Mol Microbiol. 2001;39:1464–1470. doi: 10.1046/j.1365-2958.2001.02360.x. [DOI] [PubMed] [Google Scholar]
- Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, et al. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- Lossi NS, Rolhion N, Magee AI, Boyle C, Holden DW. The Salmonella SPI-2 effector SseJ exhibits eukaryotic activator-dependent phospholipase A and glycerophospholipid: cholesterol acyltransferase activity. Microbiology. 2008;154:2680–2688. doi: 10.1099/mic.0.2008/019075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhie EJ, Brawn LC, Hume PJ, Humphreys D, Koronakis V. Salmonella takes control: effector-driven manipulation of the host. Curr Opin Microbiol. 2009;12:117–124. doi: 10.1016/j.mib.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main-Hester KL, Colpitts KM, Thomas GA, Fang FC, Libby SJ. Coordinate regulation of Salmonella pathogenicity island 1 (SPI1) and SPI4 in Salmonella enterica serovar Typhimurium. Infect Immun. 2008;76:1024–1035. doi: 10.1128/IAI.01224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo GV, Espina M, Smith AC, Terebiznik MR, Aleman A, Finlay BB, et al. SopB promotes phosphatidylinositol 3-phosphate formation on Salmonella vacuoles by recruiting Rab5 and Vps34. J Cell Biol. 2008;182:741–752. doi: 10.1083/jcb.200804131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H, Bacot CM, Garlington WA, Doyle TJ, Roberts S, Gulig PA. Virulence plasmid-borne spvB and spvC genes can replace the 90-kilobase plasmid in conferring virulence to Salmonella enterica serovar Typhimurium in subcutaneously inoculated mice. J Bacteriol. 2001;183:4652–4658. doi: 10.1128/JB.183.15.4652-4658.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurkiewicz P, Thomas J, Thompson JA, Liu M, Arbibe L, Sansonetti P, Holden DW. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol Microbiol. 2008;67:1371–1383. doi: 10.1111/j.1365-2958.2008.06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Scherer CA, Tsolis RM, Kingsley RA, Adams LG, Baumler AJ, Miller SI. Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems. Mol Microbiol. 1999;34:850–864. doi: 10.1046/j.1365-2958.1999.01651.x. [DOI] [PubMed] [Google Scholar]
- Miao EA, Brittnacher M, Haraga A, Jeng RL, Welch MD, Miller SI. Salmonella effectors translocated across the vacuolar membrane interact with the actin cytoskeleton. Mol Microbiol. 2003;48:401–415. doi: 10.1046/j.1365-2958.2003.t01-1-03456.x. [DOI] [PubMed] [Google Scholar]
- Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- Miao EA, Andersen-Nissen E, Warren SE, Aderem A. TLR5 and Ipaf: dual sensors of bacterial flagellin in the innate immune system. Semin Immunopathol. 2007;29:275–288. doi: 10.1007/s00281-007-0078-z. [DOI] [PubMed] [Google Scholar]
- Morgan E, Bowen AJ, Carnell SC, Wallis TS, Stevens MP. SiiE is secreted by the Salmonella enterica serovar Typhimurium pathogenicity island 4-encoded secretion system and contributes to intestinal colonization in cattle. Infect Immun. 2007;75:1524–1533. doi: 10.1128/IAI.01438-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller SI, Valdebenito M, Hantke K. Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals. 2009;22:691–695. doi: 10.1007/s10534-009-9217-4. [DOI] [PubMed] [Google Scholar]
- Nairz M, Theurl I, Ludwiczek S, Theurl M, Mair SM, Fritsche G, Weiss G. The co-ordinated regulation of iron homeostasis in murine macrophages limits the availability of iron for intracellular Salmonella typhimurium. Cell Microbiol. 2007;9:2126–2140. doi: 10.1111/j.1462-5822.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Brunner P, Talasz H, Hantke K, Weiss G. Interferon-gamma limits the availability of iron for intramacrophage Salmonella typhimurium. Eur J Immunol. 2008;38:1923–1936. doi: 10.1002/eji.200738056. [DOI] [PubMed] [Google Scholar]
- Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G. Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol. 2009;11:1365–1381. doi: 10.1111/j.1462-5822.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawabi P, Catron DM, Haldar K. Esterification of cholesterol by a type III secretion effector during intracellular Salmonella infection. Mol Microbiol. 2008;68:173–185. doi: 10.1111/j.1365-2958.2008.06142.x. [DOI] [PubMed] [Google Scholar]
- Ohlson MB, Huang Z, Alto NM, Blanc MP, Dixon JE, Chai J, Miller SI. Structure and function of Salmonella SifA indicate that its interactions with SKIP, SseJ, and RhoA family GTPases induce endosomal tubulation. Cell Host Microbe. 2008;4:434–446. doi: 10.1016/j.chom.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacello F, Ceci P, Ammendola S, Pasquali P, Chiancone E, Battistoni A. Periplasmic Cu,Zn superoxide dismutase and cytoplasmic Dps concur in protecting Salmonella enterica serovar Typhimurium from extracellular reactive oxygen species. Biochim Biophys Acta. 2008;1780:226–232. doi: 10.1016/j.bbagen.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Papp-Wallace KM, Nartea M, Kehres DG, Porwollik S, McClelland M, Libby SJ, et al. The CorA Mg2+ channel is required for the virulence of Salmonella enterica serovar typhimurium. J Bacteriol. 2008;190:6517–6523. doi: 10.1128/JB.00772-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Lopez C, Lin R, Aspedon A, Groisman EA. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 1994;13:3964–3972. doi: 10.1002/j.1460-2075.1994.tb06712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Hueffer K, Lam TT, Galan JE. Diversification of a Salmonella virulence protein function by ubiquitin-dependent differential localization. Cell. 2009;137:283–294. doi: 10.1016/j.cell.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada CM, Hicks SW, Galan JE, Stebbins CE. A family of Salmonella virulence factors functions as a distinct class of autoregulated E3 ubiquitin ligases. Proc Natl Acad Sci USA. 2009;106:4864–4869. doi: 10.1073/pnas.0811058106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Wilson RP, Chessa D, Andrews-Polymenis H, Tran QT, Lawhon S, et al. SipA, SopA, SopB, SopD, and SopE2 contribute to Salmonella enterica serotype typhimurium invasion of epithelial cells. Infect Immun. 2005;73:146–154. doi: 10.1128/IAI.73.1.146-154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE. Flagellin-deficient Legionella mutants evade caspase-1- and Naip5-mediated macrophage immunity. PLoS Pathog. 2006;2:e18. doi: 10.1371/journal.ppat.0020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Albert J, Yu XJ, Beuzon CR, Blakey AN, Galyov EE, Holden DW. Complementary activities of SseJ and SifA regulate dynamics of the Salmonella typhimurium vacuolar membrane. Mol Microbiol. 2002;44:645–661. doi: 10.1046/j.1365-2958.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- Rytkonen A, Poh J, Garmendia J, Boyle C, Thompson A, Liu M, et al. SseL, a Salmonella deubiquitinase required for macrophage killing and virulence. Proc Natl Acad Sci USA. 2007;104:3502–3507. doi: 10.1073/pnas.0610095104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo SP, Holden DW. SseG, a virulence protein that targets Salmonella to the Golgi network. EMBO J. 2003;22:5003–5014. doi: 10.1093/emboj/cdg517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo SP, Noursadeghi M, Cohen J, Holden DW. Intracellular replication of Salmonella typhimurium strains in specific subsets of splenic macrophages in vivo. Cell Microbiol. 2001;3:587–597. doi: 10.1046/j.1462-5822.2001.00137.x. [DOI] [PubMed] [Google Scholar]
- Sano G, Takada Y, Goto S, Maruyama K, Shindo Y, Oka K, et al. Flagella facilitate escape of Salmonella from oncotic macrophages. J Bacteriol. 2007;189:8224–8232. doi: 10.1128/JB.00898-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, et al. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect Immun. 2001;69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele-Mortimer O, Meresse S, Gorvel JP, Toh BH, Finlay BB. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1999;1:33–49. doi: 10.1046/j.1462-5822.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Sun J, Hobert ME, Rao AS, Neish AS, Madara JL. Bacterial activation of beta-catenin signaling in human epithelia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G220–G227. doi: 10.1152/ajpgi.00498.2003. [DOI] [PubMed] [Google Scholar]
- Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- Takaya A, Suzuki A, Kikuchi Y, Eguchi M, Isogai E, Tomoyasu T, Yamamoto T. Derepression of Salmonella pathogenicity island 1 genes within macrophages leads to rapid apoptosis via caspase-1- and caspase-3-dependent pathways. Cell Microbiol. 2005;7:79–90. doi: 10.1111/j.1462-5822.2004.00435.x. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Osman D, Cavet JS. Differential expression from two iron-responsive promoters in Salmonella enterica serovar Typhimurium reveals the presence of iron in macrophage-phagosomes. Microb Pathog. 2009;46:114–118. doi: 10.1016/j.micpath.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Terebiznik MR, Vieira OV, Marcus SL, Slade A, Yip CM, Trimble WS, et al. Elimination of host cell PtdIns(4,5)P(2) by bacterial SigD promotes membrane fission during invasion by Salmonella. Nat Cell Biol. 2002;4:766–773. doi: 10.1038/ncb854. [DOI] [PubMed] [Google Scholar]
- Tezcan-Merdol D, Nyman T, Lindberg U, Haag F, Koch-Nolte F, Rhen M. Actin is ADP-ribosylated by the Salmonella enterica virulence-associated protein SpvB. Mol Microbiol. 2001;39:606–619. doi: 10.1046/j.1365-2958.2001.02258.x. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Wu H, Jones R, Grant G, Babbin B, King TP, et al. Flagellin suppresses epithelial apoptosis and limits disease during enteric infection. Am J Pathol. 2006;169:1686–1700. doi: 10.2353/ajpath.2006.060345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Petrof EO, Boone D, Claud EC, Sun J. Salmonella effector AvrA regulation of colonic epithelial cell inflammation by deubiquitination. Am J Pathol. 2007;171:882–892. doi: 10.2353/ajpath.2007.070220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaharik ML, Cullen VL, Fung AM, Libby SJ, Kujat Choy SL, Coburn B, et al. The Salmonella enterica serovar typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Higashide WM, McCormick BA, Chen J, Zhou D. The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol Microbiol. 2006;62:786–793. doi: 10.1111/j.1365-2958.2006.05407.x. [DOI] [PubMed] [Google Scholar]


