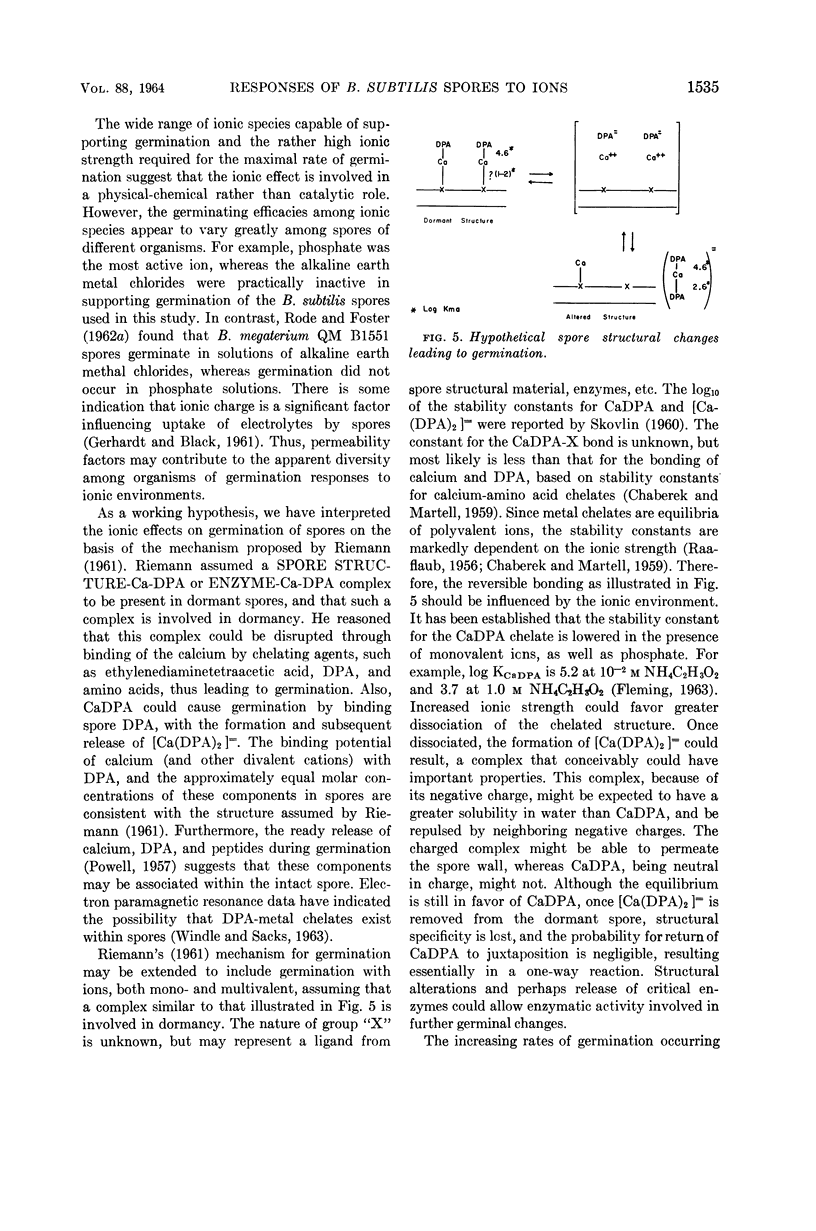
Abstract
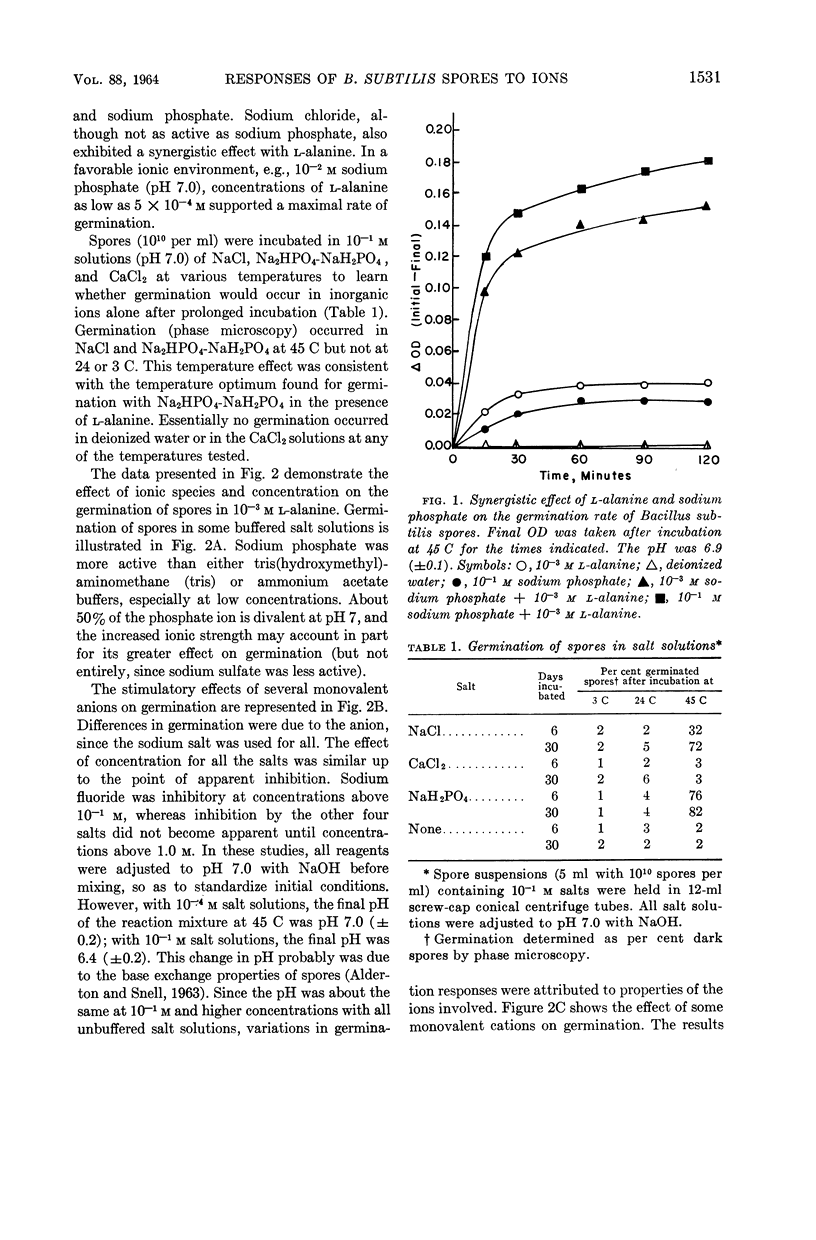
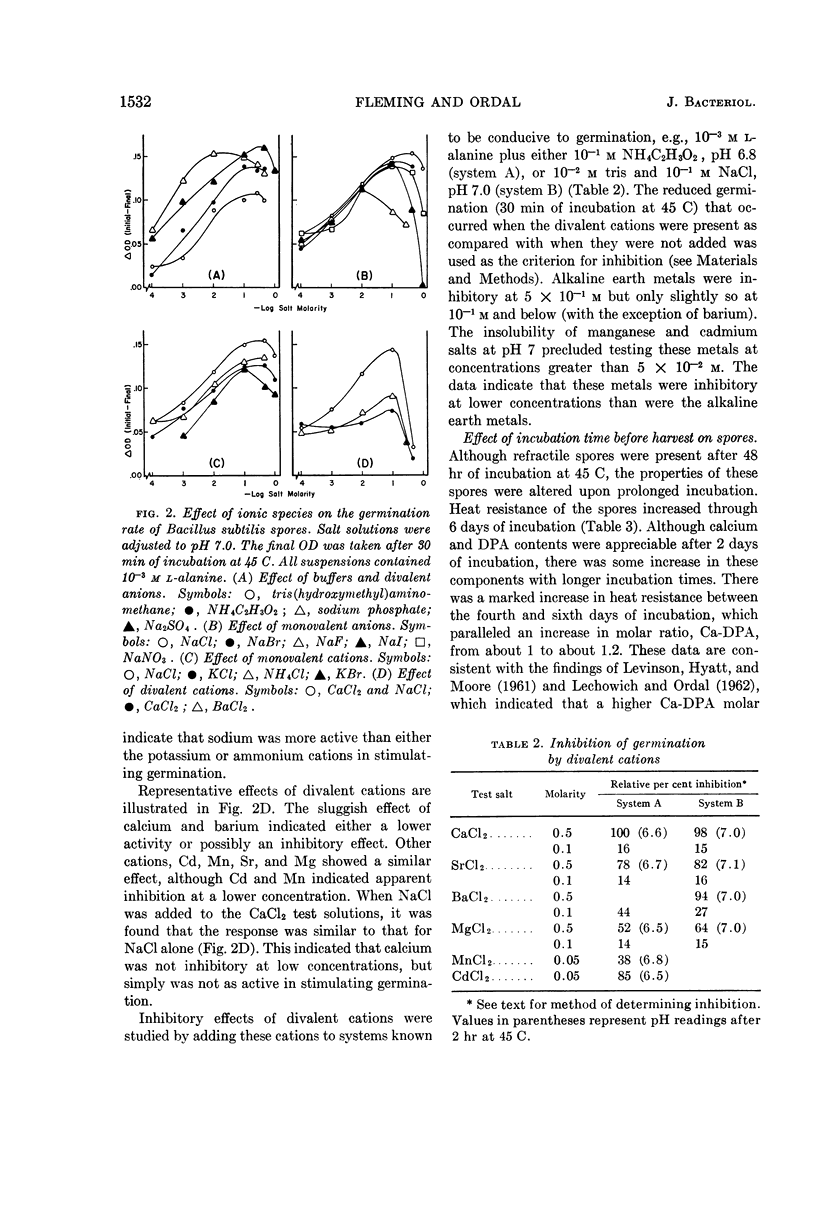
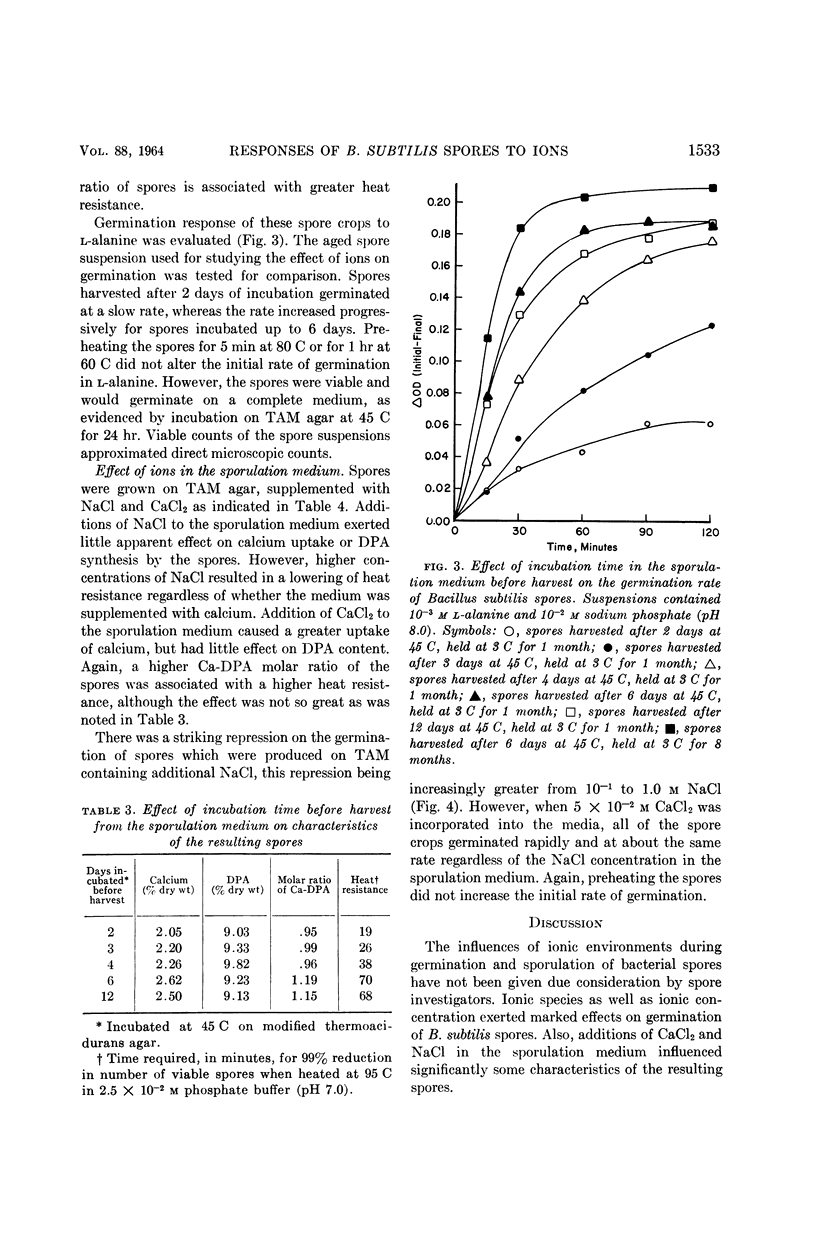
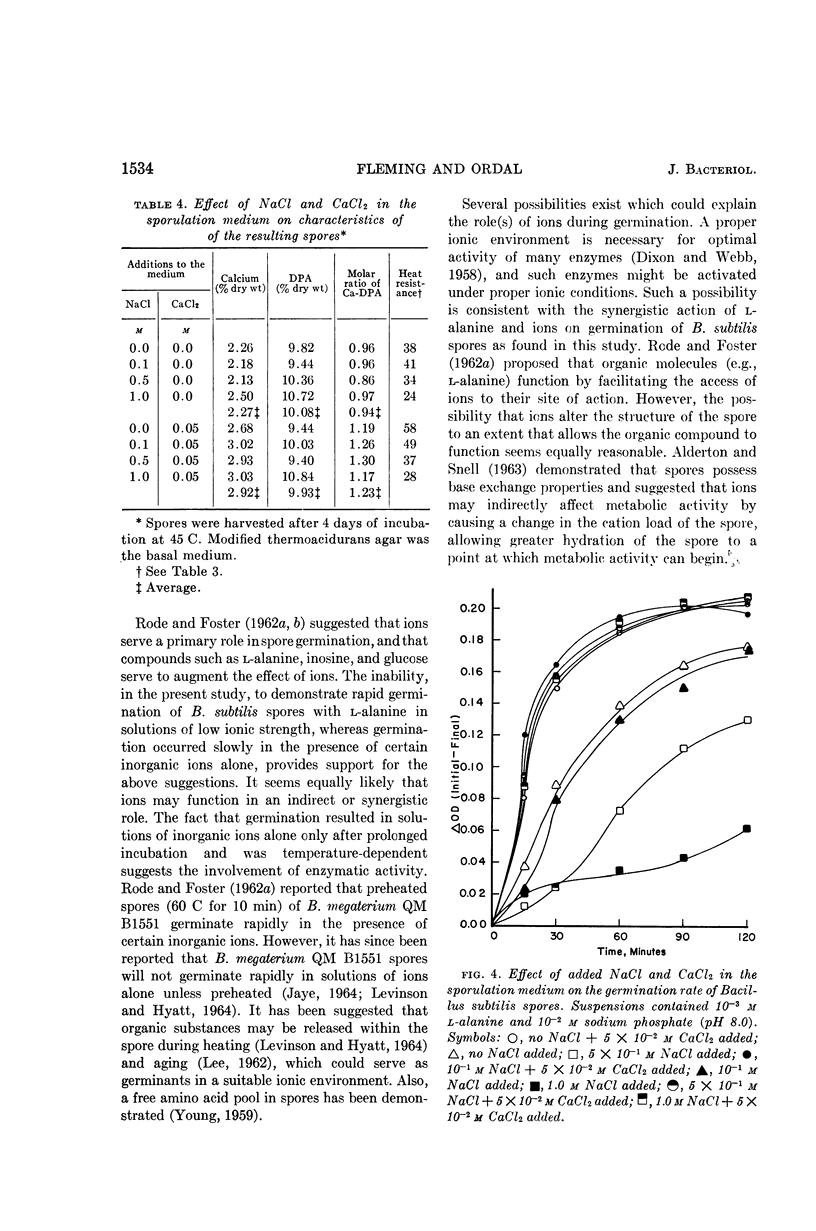
Fleming, H. P. (University of Illinois, Urbana), and Z. John Ordal. Responses of Bacillus subtilis spores to ionic environments during sporulation and germination. J. Bacteriol. 88:1529–1537. 1964.—The ionic environments of germination and sporulation menstrua had a prominent influence on characteristics of Bacillus subtilis spores. There was a synergistic effect for l-alanine and inorganic ions on spore germination. The maximal rate of germination in solutions of l-alanine was dependent on ionic concentration and species. Germination was negligible in l-alanine at low ionic strength but increased as the ionic strength was increased up to about 10−1m with a variety of salts. Phosphate was the most active ion tested, and divalent cations were the least active in supporting germination in l-alanine. Germination progressed slowly at 45 C in sodium chloride or sodium phosphate alone but not in CaCl2 alone. Germination rates in l-alanine were retarded at high ionic strengths (μ in the range of 0.1 to 1.0). Inhibitory effects of high concentrations of certain divalent cations on germination were related to the binding abilities of these metals. High concentrations of NaCl (10−1 to 1.0 m) in the sporulation medium resulted in lowered heat resistance and germination rate of the resulting spores. The addition of calcium (5 × 10−2m CaCl2) to the sporulation medium relieved the repression of NaCl on germination and caused the spores to have a greater heat resistance. Calcium and dipicolinic acid (DPA) contents of the spores were unaffected by NaCl in the sporulation medium. The calcium, but not the DPA, content of spores increased as a result of supplementing the sporulation medium with calcium. Possible roles of ions in the germination of spores are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMAHA M., ORDAL Z. J. Effect of divalent cations in the sporulation medium on the thermal death rate of Bacillus coagulans var. thermoacidurans. J Bacteriol. 1957 Nov;74(5):596–604. doi: 10.1128/jb.74.5.596-604.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-BISI H. M., ORDAL Z. J. The effect of certain sporulation conditions on the thermal death rate of Bacillus coagulans var. thermoacidurans. J Bacteriol. 1956 Jan;71(1):1–9. doi: 10.1128/jb.71.1.1-9.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEMING H. P., ORDAL Z. J. SPECTROPHOTOMETRIC METHOD FOR THE DETERMINATION OF CALCIUM IN BACTERIAL SPORES. Can J Microbiol. 1964 Feb;10:95–98. doi: 10.1139/m64-015. [DOI] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HYATT M. T., LEVINSON H. S. Interaction of heat, glucose, L-alanine, and potassium nitrate in spore germination of Bacillus megaterium. J Bacteriol. 1961 Feb;81:204–211. doi: 10.1128/jb.81.2.204-211.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LECHOWICH R. V., ORDAL Z. J. The influence of the sporulation temperature on the heat resistance and chemical composition of bacterial spores. Can J Microbiol. 1962 Jun;8:287–295. doi: 10.1139/m62-040. [DOI] [PubMed] [Google Scholar]
- LEVINSON H. S., HYATT M. T. EFFECT OF SPORULATION MEDIUM ON HEAT RESISTANCE, CHEMICAL COMPOSITION, AND GERMINATION OF BACILLUS MEGATERIUM SPORES. J Bacteriol. 1964 Apr;87:876–886. doi: 10.1128/jb.87.4.876-886.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINSON H. S., SEVAG M. G. Stimulation of germination and respiration of the spores of Bacillus megatherium by manganese and monovalent anions. J Gen Physiol. 1953 May;36(5):617–629. doi: 10.1085/jgp.36.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAAFLAUB J. Applications of metal buffers and metal indicators in biochemistry. Methods Biochem Anal. 1956;3:301–325. doi: 10.1002/9780470110195.ch10. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Ionic and non-ionic compounds in the germination of spores of Bacillus megaterium Texas. Arch Mikrobiol. 1962;43:201–212. doi: 10.1007/BF00406436. [DOI] [PubMed] [Google Scholar]
- RODE L. J., FOSTER J. W. Ionic germination of spores of Bacillus megaterium QM B 1551. Arch Mikrobiol. 1962;43:183–200. doi: 10.1007/BF00406435. [DOI] [PubMed] [Google Scholar]
- WINDLE J. J., SACKS L. E. Electron paramagnetic resonance of managanese(II) and copper(II) in spores. Biochim Biophys Acta. 1963 Mar 19;66:173–179. doi: 10.1016/0006-3002(63)91183-1. [DOI] [PubMed] [Google Scholar]
- YOUNG I. E. A relationship between the free amino acid pool, dipicolinic acid, calcium from resting spores of Bacillus megaterium. Can J Microbiol. 1959 Apr;5(2):197–202. doi: 10.1139/m59-024. [DOI] [PubMed] [Google Scholar]



