Abstract
The lipoamino acids and endovanilloids have multiple roles in nociception, pain, and inflammation, yet their biological reactivity has not been fully characterized. Cyclooxygenases (COXs) and lipoxygenases (LOs) oxygenate polyunsaturated fatty acids to generate signaling molecules. The ability of COXs and LOs to oxygenate arachidonyl-derived lipoamino acids and vanilloids was investigated. COX-1 and COX-2 were able to minimally metabolize many of these species. However, the lipoamino acids were efficiently oxygenated by 12S- and 15S-LOs. The kinetics and products of oxygenation by LOs were characterized. Whereas 15S-LOs retained positional specificity of oxygenation with these novel substrates, platelet-type 12S-LO acted as a 12/15-LO. Fatty acid oxygenases may play an important role in the metabolic inactivation of lipoaminoacids or vanilloids or may convert them to bioactive derivatives.
Keywords: Lipoxygenase, cyclooxygenase, lipoamino acids, endovanilloids, oxidative metabolism
Introduction
Cyclooxygenases (COXs) and lipoxygenases (LOs) are key enzymes involved in the metabolism of arachidonic acid and its derivatives. These enzymes are involved in the synthesis of prostaglandins, thromboxanes, and lipid hydroperoxides, molecules with important functions in cell differentiation, inflammation, and angiogenesis [1-3]. COXs and LOs also have been implicated in the termination of endocannabinoid signaling via degradation of arachidonyl ethanolamide (AEA) and 2-arachidonyl glycerol (2-AG) [4].
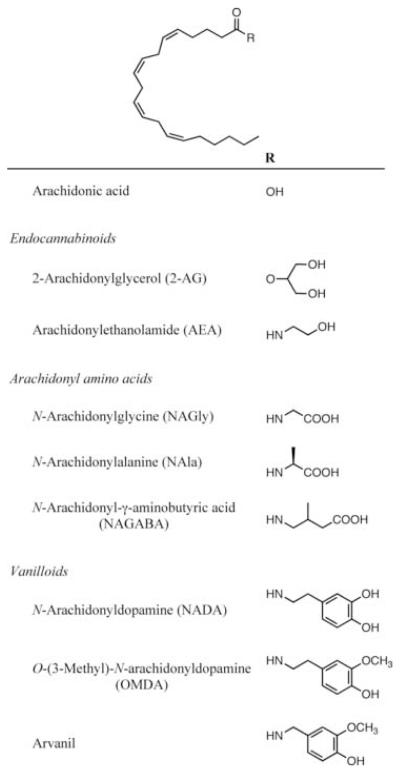
COX primarily catalyzes the bis-dioxygenation of arachidonic acid to yield the hydroperoxyendoperoxide prostaglandin G2 (PGG2), which is subsequently reduced to the hydroxyendoperoxide prostaglandin H2 (PGH2) [5].The two isoforms of COX, designated COX-1 and COX-2, catalyze the same reaction of arachidonic acid but differ in their substrate specificities. Of particular interest is the ability of COX-2, but not COX-1, to metabolize a variety of ester and amide derivatives of arachidonate including AEA, 2-AG, and the lipoamino acid, N-arachidonyl glycine (NAGly) (Fig. 1) [4, 6, 7].
Figure 1.
Chemical structures of arachidonyl derivatives.
LOs catalyze the regio- and stereospecific incorporation of a single molecule of dioxygen into polyunsaturated fatty acids, generating lipid hydroperoxides that can be further reduced to the corresponding hydroxy species [8]. LOs are distinguished by the position and stereochemistry of oxygen addition to arachidonic acid [9]. The substrate specificities for LO classes vary significantly [8]. To date, two isoforms of mammalian 12S- and 15S-LOs have been characterized. The platelet-type 12-LO (pl12-LO) primarily metabolizes C20 fatty acids; it can also oxygenate 6, 9,12-octadecatrienoic and 4, 7, 10, 13, 16, 19-docosahexaenoic acids, albeit less efficiently than arachidonic acid [10-12]. In contrast, the leukocyte 12-LO (lk12-LO) is more promiscuous and shares significant sequence similarity and substrate specificity with 15-LO-1. Though two 12S-LO genes have been characterized in humans, these correspond to the pl12-LO and a pseudogene thought to arise from gene duplication [13]. It is predicted that 15-LO-1 is the human homologue of lk12-LO [9]. 15-LO-1 and lk12-LO metabolize a range of C18, C20, and C22 fatty acids [11, 14, 15]. Notably, 15-LO-1 and lk12-LO are able to oxygenate polyunsaturated fatty acyl groups of phospholipids, as well as the ester and amide derivatives 2-AG and AEA [16-21]. In contrast, pl12-LO metabolizes these substrates much less efficiently, if at all. 15-LO-2, homologous to murine 8S-LO, only shares about 40% sequence identity with 12S-LOs and 15-LO-1 [22, 23]. Extensive studies on substrate specificity of 15-LO-2 are not available, but it does metabolize arachidonic acid, a C20 fatty acid, more efficiently than linoleic acid, a C18 substrate [22]. 15-LO-2 can utilize 2-AG and AEA to a similar extent to arachidonic acid [21].
Recently, several amide conjugates of arachidonic acid have been discovered in vivo that exhibit interesting biological activities. The lipoamino acids N-arachidonyl glycine (NAGly) N-arachidonyl alanine (NAla), and N-arachidonyl-γ-aminobutyric acid (NAGABA) [Fig. 1] have been isolated from mammlian tissues, and NAGly and NAGABA suppress tonic pain inhibition via cannabinoid receptor-independent pathways [24]. A number of fatty acid-derived vanilloids have been synthesized, and some have been isolated from tissues [25-27]. The vanilloids are structurally similar to capsaicin, a potent activator of the vanilloid receptor VR1 [28]. Though initially characterized as a cannabinoid receptor CB1 ligand [26], N-arachidonyl dopamine (NADA) interacts with the vanilloid receptor VR1, and, to some extent, fatty acid amide hydrolase to exert multiple biological effects including vasorelaxation and nociception [26, 27, 29]. With the exception of NAGly, the enzymatic peroxidation of these lipids has not been studied; therefore, we have investigated the ability of lipoamino acids to act as substrates for COXs and LOs. We demonstrate that these are indeed metabolized by COX-2, 15-LO-1, 15-LO-2, lk12-LO, and, notably, pl12-LO. We further show that NADA acts as a competitive inhibitor of pl12-LO in vitro. These investigations highlight potential interactions of neuroactive fatty acid amides with enzymes responsible for lipid peroxidation.
Materials and Methods
Reagents
Arachidonic acid was purchased from Nu Chek Prep (Elysian, MN). OMethyl-N-arachidonyl dopamine (OMDA) was synthesized by standard carbodiimide coupling. All other arachidonyl derivatives were purchased from Cayman Chemical (Ann Arbor, MI). Ram seminal vesicles were purchased from Oxford Biomedical Research (Oxford, MI), and oCOX-1 was purified as previously described [30]. Expression of mCOX-2 was performed with baculovirus reagents from BD Biosciences (San Diego, CA). Human 15-LO-2 (h15-LO-2) was expressed in bacteria. Purification procedures for mCOX-2, h15-LO-2, and human platelet 12-LO (pl12-LO) were described previously [7, 18, 31]. All of the expressed proteins were over 95% pure by SDS-PAGE staining and analysis, except for h15-LO-2, which was 70% pure. Rabbit reticulocyte 15-LO-1 (r15-LO-1) and human 5-LO (h5-LO) were purchased as cell lysates from Calbiochem (La Jolla, CA) and Cayman Chemical (Ann Arbor, MI), respectively. All other chemicals were obtained from Sigma/Aldrich (St. Louis, MO).
COX Activity Assay
Quantification of cyclooxygenase activity was performed in a thermostatted cuvette at 37° C and monitored using a polarographic electrode with a YS5300 oxygen monitor (Yellow Springs Instrument Co. Inc., Yellow Springs, OH). All substrates were solubilized in dimethyl sulfoxide (DMSO). Activity assays were performed in 100 mM Tris-HCl buffer containing 500 μM phenol, with hematin-reconstituted protein (50 nM). Maximal reaction velocity data were obtained from the linear portion of the oxygen uptake curves, and normalized to the metabolism rate of arachidonic acid.
LO Activity Assay
LO activity was detected by monitoring the absorbance of the conjugated diene product at 236 nm. UV assays were monitored using a Hewlett Packard 8453 diode array spectrophotometer equipped with a thermostatted cuvette at 25° C, with stirring at 180 rpm. The enzyme reactions included reaction buffer [50 mM Tris-HCl (pH 7.4) with 0.03% Tween-20] and substrate, and were initiated by the addition of enzyme (r15-LO-1 - 3.7 μg/ml, h15-LO-2 - 170 μg/ml, and pl12-LO - 2 μg/ml). Compounds were dissolved in acetonitrile (ACN) containing 1% acetic acid before addition to the reaction buffer; ACN was kept below 1% reaction volume (2 ml). For the initial metabolism screen, compounds were diluted to a final concentration of 25 μM. To determine Michaelis-Menten kinetic parameters the concentration of substrate was varied (1-50 μM). Maximal reaction velocity data were obtained from the linear portion of the absorbance curves, and the data were analyzed by nonlinear regression with Prism 4.0 (GraphPad Software, San Diego, CA).
LO Product Identification
HpETE-Gly and -GABA regiochemistry was established by mass spectrometry [32]. Incubations of 10 μg of purified LO and 10 μg of NAGly or NAGABA (37° C, 10 min) in 50 mM Tris-HCl (pH 7.4) with 0.03% Tween-20 were conducted then terminated with an equal volume of methanol. Samples were centrifuged at 14,000 rpm for 10 min at 4 °C and then passed through a 0.22 μm Costar Spin-X filter. Reactions were adjusted to pH 3 and extracted two times with five volumes of ethyl acetate. Aliquots were removed and dried under a stream of argon. Samples were prepared for mass spectral analysis by reconstitution in 1:1 MeOH:H2O containing 70 μM silver acetate. Mass spectral analysis was performed by directly infusing the samples (flow rate = 20 μl/min) into a Finnigan TSQ 7000 triple quadrupole mass spectrometer equipped with an electrospray ionization source and operated in the positive ion mode. The TSQ 7000 was set to the following parameters: Capillary Temperature = 200 °C; Capillary Voltage = 25.3 V; Tube Lens Voltage = 97.4 V; Spray Voltage = 5 kV; Sheath Gas = 75 psi; Auxiliary Gas = 10 (no units). Silver ion coordination resulted in the observation of two [M + Ag]+ ions, due to the natural isotopic abundance of silver [107Ag (52%) and 109Ag (48%)]. Collision induced dissociation of the precursor ion [M + 107Ag]+ for NAGly (m/z = 500) and NAGABA (m/z = 528) were accomplished with argon as collision gas at 1.8 mtorr and an ion current of -15 eV in the second quadrupole. Data collected for selected reaction monitoring experiments were processed with Xcalibur (Finnigan, San Jose, CA).
HPLC-UV Analysis of LO products
Samples were prepared as described above for LO product identification by MS and resuspended in 1:1 acetonitrile:H2O with 0.1% acetic acid. High performance liquid chromatography (HPLC) was conducted on a Waters Alliance 2690 Separation Module using a Supelco C18 column (15.0 × 0.21 cm, 3 μm) heated at 40°C. Isocratic separation was achieved with a mobile phase of 70% water in acetonitrile with 0.1% acetic acid at a flow rate of 0.2 mL/min. Analytes were detected at 204 and 236 nm by a Waters 2487 Dual Wavelength Absorbance Detector.
LO Inhibition Assay
Reactions were performed under conditions similar to those described for the LO activity assay. The substrate concentration was kept constant at 20 μM from a stock of substrate in ACN with 1% acetic acid. Substrate and inhibitor (1 nM - 10 μM) were combined in reaction buffer before the initiation of the reaction by addition of enzyme. Reaction velocities were determined from the linear portion of the absorbance curves, normalized to ACN control, and plotted against inhibitor concentration. IC50 values were determined with Prism 4.0 using non-linear regression to a sigmoidal dose response curve.
Multiple Sequence Alignment
Multiple sequence alignment and analyses were performed using the programs AMPS and AMAS [33, 34].
Results
Oxygenation of arachidonyl derivatives by COX
NAGly is selectively oxygenated by mCOX-2 but not oCOX-1 [7]. The ability of COXs to utilize other lipoamino acids, NAla and NAGABA, was examined. Both NAla and NAGABA were oxygenated by mCOX-2, though at modest rates compared to arachidonic acid (Table 1). oCOX-1 metabolized NAla and NAGABA but the extent of conversion was lower than for mCOX-2. Furthermore, mCOX-2 utilized NADA and its derivatives, OMDA and arvanil, as substrates, though these were less than 10% as active as arachidonic acid. Metabolism of the vanilloids by oCOX-1 was negligible.
Table 1.
Percent activity of compounds relative to arachidonate
| Lipoamino acids |
Vanilloids |
||||||
|---|---|---|---|---|---|---|---|
| AA | NAGly | NAla | NAGABA | NADA | OMDA | Arvanil | |
| pl12-LO | 100. ± 4 | 132 ± 4 | 122 ± 2 | 130. ± 3 | NDa | ND | ND |
| lk12-LO | 100. ± 6 | 105 ± 4 | 54 ± 5 | 78 ± 2 | 8 ± 3 | ND | ND |
| r15-LO-1 | 100. ± 9 | 94 ± 12 | 79 ± 7 | 67 ± 7 | 27 ± 9 | ND | 23 ± 7 |
| h15-LO-2 | 100. ± 16 | 99 ± 4 | 96 ± 19 | 83 ± 3 | ND | ND | ND |
| oCOX-1 | 100. ± 5 | ND | 10.8 ± 0.3 | 9 ± 1 | 1.3 ± 0.3 | ND | 1.9 ± 0 |
| mCOX-2 | 100. ± 6 | 40. ± 2 | 31 ± 2 | 20. ± 1 | 7 ± 5 | 4 ± 3 | 5.6 ± 0.9 |
Rates of metabolism by LOs were determined by monitoring formation of the conjugated diene system via absorbance at 236 nm. Rates of metabolism by COXs were determined by oxygen uptake. Conditions are outlined in “Materials and Methods”. Rates of lipoamino acid and vanilloid metabolism were normalized to arachidonic acid.
ND, not detectable.
Oxygenation of arachidonyl derivatives by LOs
The metabolism of the lipoamino acids and the vanilloids by LOs was characterized by monitoring conjugated diene formation via UV absorption at 236 nm. At a substrate concentration of 25 μM, NAGly, NAla, and NAGABA were oxygenated by LOs at rates comparable to arachidonic acid (Table 1). The most notable difference was metabolism of NALA by lk12-LO, which was oxygenated at a rate less than 60% of arachidonic acid. In contrast, the vanilloids served as poor substrates for mammalian LOs, if they were utilized at all (Table 1). OMDA was not metabolized by any of the LOs examined. NADA and arvanil were oxygenated by r15-LO-1 at rates approximately one-quarter that of arachidonic acid. Minimal metabolism of NADA (<10% compared to arachidonic acid) was observed with lk12-LO; however, arvanil was not oxygenated by lk12-LO. h15-LO-2 and pl12-LO exhibited no appreciable activity toward any of the vanilloids.
Kinetics of lipoamino acid oxygenation by LOs
Mammalian LO enzymes were incubated with varying concentrations of substrate in order to determine vmax and Km. The kinetic parameters determined for each enzyme with arachidonic acid were in agreement with values reported in the literature [2, 35-37]. Notably pl12-LO exhibited very similar kinetic parameters for the lipaomino acids and arachidonate. For lk12-LO, Km was the same for the lipoamino acids and arachidonic acid, but modest reductions in the maximal rate of reaction were observed (Table 2). The most prevalent difference was seen with NALA. Alterations in vmax resulted in a 50% decrease in efficiency for lipoamino acid metabolism as compared to arachidonic acid. Similarly, r15-LO-1, which shares significant sequence identity with lk12-LO, exhibited approximately 50% decrease in efficiency for the lipoamino acids in comparison with arachidonic acid. However, reductions in efficiency for r15-LO-1 were attributable to increases in Km. Kinetics of oxygenation of the lipoamino acids by h15-LO-2 were similar to arachidonic acid in all cases.
Table 2.
Kinetic parameters of metabolism of arachidonate and arachidonyl amino acids
| vmax (μM/s) | Km (μM) | vmax/Km (s-1) | |
|---|---|---|---|
| pl12-LO | |||
| Arachidonic acid | 13.3 ± 0.3 | 9.5 ± 0.7 | 1.4 ± 0.7 |
| N-Arachidonyl glycine | 20.4 ± 0.5 | 10.1 ± 0.8 | 2.0 ± 0.8 |
| N-Arachidonyl alanine | 20.7 ± 0.4 | 15.7 ± 0.8 | 1.3 ± 0.8 |
| N-Arachidonyl-γ-aminobutyric acid | 20.2 ± 0.3 | 10.9 ± 0.5 | 1.9 ± 0.5 |
| lk12-LO | |||
| Arachidonic acid | 13.1 ± 0.7 | 7.8 ± 1.3 | 2 ± 1 |
| N-Arachidonyl glycine | 9.9 ± 0.3 | 8.0 ± 0.7 | 1 ± 1 |
| N-Arachidonyl alanine | 7.6 ± 0.7 | 9 ± 2 | 1 ± 2 |
| N-Arachidonyl-γ-aminobutyric acid | 10.4 ± 0.3 | 7.8 ± 0.7 | 1.3 ± 0.8 |
| r15-LO-1 | |||
| Arachidonic acid | 8.6 ± 0.4 | 20 ± 3 | 0.4 ± 3 |
| N-Arachidonyl glycine | 11.2 ± 0.9 | 49 ± 10 | 0.23 ± 11 |
| N-Arachidonyl alanine | 17 ± 2 | 98 ± 25 | 0.17 ± 25 |
| N-Arachidonyl-γ-aminobutyric acid | 9 ± 1 | 48 ± 14 | 0.2 ± 14 |
| h15-LO-2 | |||
| Arachidonic acid | 15.6 ± 0.5 | 8 ± 1 | 2 ± 1 |
| N-Arachidonyl glycine | 23.1 ± 0.3 | 11.0 ± 0.4 | 2.1 ± 0.5 |
| N-Arachidonyl alanine | 12 ± 2 | 6 ± 3 | 2 ± 3 |
| N-Arachidonyl-γ-aminobutyric acid | 14.5 ± 0.9 | 8 ± 2 | 2 ± 2 |
Lipoamino acid oxygenated product identification
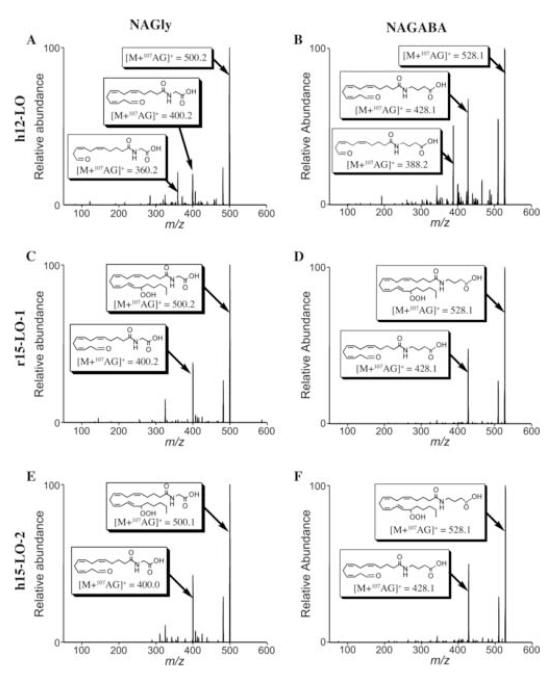
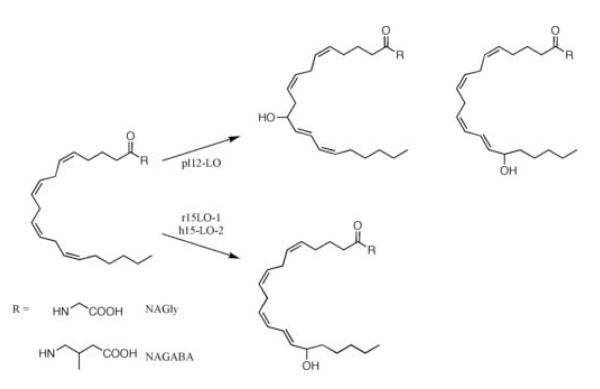
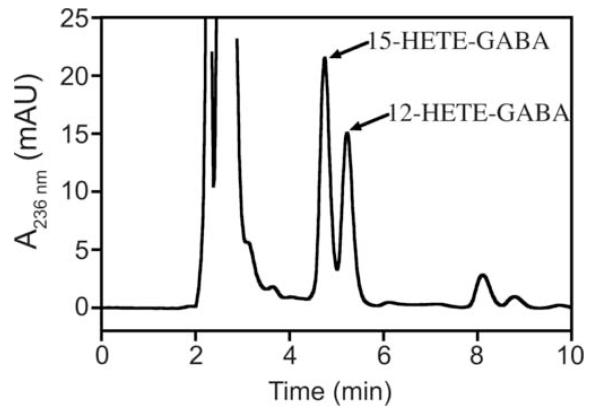
The products of NAGly and NAGABA metabolism by pl12-LO and 15-LOs were characterized using mass spectrometry. Collisional induced dissociation of [M+107Ag]+ produces Hock fragmentation of lipid hydroperoxides that are coordinated by silver cation, which was used to determine regiochemistry of substrate oxygenation [32]. Surprisingly, three major ions were observed from the reaction of pl12-LO with NAGly or NAGABA (Fig. 2A, B). The predominant ion corresponded to addition of two oxygen atoms to substrate, representing HpETE-Gly (m/z = 500) or HpETE-GABA (m/z = 528). The other major ions corresponded to losses of 100 and 140, which indicate peroxidation at C-15 and C-12 respectively (Fig. 3). Analysis by HPLC-UV revealed that the 12- and 15-hydroperoxy products were formed in approximately equal quantities, both with NAGly and NAGABA (Fig. 4).
Figure 2. Regiochemistry of oxygenated NAGly and NAGABA products.
Representative collision-induced dissociation mass spectra of NAGly and NAGABA metabolites of LO oxygenation. Reaction conditions are outlined in “Materials and Methods.” Chemical structures indicate the proposed assignments for the most abundant [M + 107Ag]+ ions. Contents of panels are as follows: A) pl12-LO with NAGly, B) pl12-LO with NAGABA, C) r15-LO-1 with NAGly, D) r15-LO-1 with NAGABA, E) h15-LO-2 with NAGly, and F) h15-LO-2 with NAGABA.
Figure 3.
Products of oxygenation of lipoamino acids by LOs.
Figure 4. UV profile of pl12-LO metabolites of NAGABA.
Representative chromatogram with UV detection at 236 nm. Products of NAGABA oxygenation by pl12-LO were separated on HPLC as described in “Materials and Methods”. The 12- and 15-HpETE-GABA products are formed in approximately 1:1 ratio.
The reactions of r15-LO-1 and h15-LO-2 with NAGly and NAGABA gave similar results (Fig. 2C-F). For both substrates, the major ion corresponded to addition of two oxygen atoms, suggesting the formation of HpETE-Gly or −GABA. The product of fragmentation resulted from a mass loss of 100, indicating peroxidation predominantly at C-15 (Fig. 3).
LO inhibition by NADA
Catechols and compounds containing phenolic groups have been reported to inhibit pl12-LO activity [38-41]. Given the structure of the vanilloids and their minimal oxygenation by pl12-LO, the ability of these compounds to inhibit pl12-LO was determined. NADA inhibited arachidonic acid metabolism with an IC50 of 150 ± 5 nM. However, metabolism was attenuated less than 40% by OMDA and less than 30% by arvanil.
Discussion
The lipoamino acids and endovanilloids exhibit interesting biological activities [42, 43]. A few targets of the lipoamino acids and endovanilloids have been identified, but for the most part, their mechanisms of action and regulation remain unclear. The current studies were initiated to characterize the interaction of novel arachidonyl derivatives with important lipid-oxidizing enzymes, COXs and LOs.
COX-2 metabolizes ester and amide derivatives of arachidonic acid, whereas these are poor substrates for COX-1 [4, 6, 7, 44]. In agreement with a previous report, COX-2 was able to metabolize NAGly at a rate approximately 40% of that for arachidonic acid [7]. Substitution of a methyl group α to the amide (NAla) reduces substrate metabolism by approximately 10%, whereas insertion of two additional carbons between the carboxylate and the amide (NAGABA) reduces the oxygenation by 20%. Previous mutagenesis studies have demonstrated that Arg-120, Glu-524, and Arg-513 play critical roles in the binding of AEA, 2-AG, and NAGly [7, 44, 45]. Based upon these findings and the crystal structure of the productive conformation of arachidonate bound to COX-1, models of binding of these substrates to COX-2 have been developed. The model of NAGly binding to COX-2 predicts that the amide carbonyl oxygen will project between Arg-120 and Tyr-355, while the carboxylate forms an ionic bond with the guanidinium of Arg-513 [7]. This proposed binding mode might also explain the alteration in substrate specificity induced by subtle structural changes. Extension of the chain length between the amide and the carboxylate or even a small substitution α to the amide would likely restrict the conformation of the chain and possibly perturb ligand-protein interactions. Similarly, the steric bulk of the vanilloids is prohibitive to metabolism by COX. It is likely that the vanilloids cannot participate in the stabilizing interactions predicted for NAGly with COX-2 and therefore must adopt an alternative conformation that is unfavorable for catalysis, if they bind at all.
The 12S- and 15-LOs can efficiently utilize the lipoamino acids as substrates. Typically lk12-LO and r15-LO-1 are tolerant to changes in substrate structure and are even able to metabolize polyunsaturated fatty acyl chains of phospholipids [16, 17]. However, lk12-LO and r15-LO-1 exhibit reductions in metabolism of the lipoamino acids, as compared to arachidonic acid. In contrast, pl12-LO and h15-LO-2 are able to utilize arachidonyl amino acids with equal or better efficiency than arachidonate. Metabolism of lipoamino acids by pl12-LO is particularly striking in the context of this enzyme’s substrate specificity. Although human platelets and purified pl12-LO will oxygenate the endocannabinoid arachidonyl ethanolamide, it is utilized much less efficiently than arachidonic acid [19, 20].
The fidelity for oxygen insertion can vary for LO isoforms. Although 15-LO-1 generates 15-HETE as its predominant product, it will oxygenate arachidonate at C-12, producing 10-20% 12-HETE [46]. In contrast, oxygen insertion is more tightly controlled by 15-LO-2 and pl12-LO, which produce a single HETE isomer almost exclusively [22, 47]. Modification of the carboxylate group of arachidonic acid might alter substrate binding to LOs and thus the regiochemistry of oxygen addition. The product regiochemistry was characterized by MS/MS for HETE-Gly and –GABA (Fig. 2 and 3). The positional specificity of oxygenation is retained for r15-LO-1 and h15-LO-2, indicating that binding orientation of the arachidonyl chain is not significantly altered by modification of the carboxylic acid. However, pl12-LO acts upon the arachidonyl amino acids as a 12/15-LO.
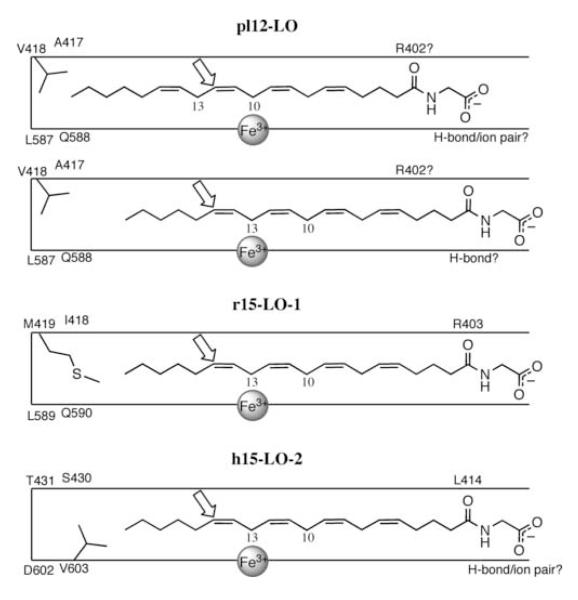
Based upon mutagenesis and the crystal structure of r15-LO-1, a “tail first” orientation for substrate binding has been proposed for pl12-LO, r15-LO-1 and h15-LO-2 (Fig. 5)[48, 49]. In this model, the methyl end of the substrate binds in a long hydrophobic channel with the carboxylic acid interacting with a charged residue, proposed to be Arg-403 in r15-LO-1 [49, 50]. The volume of the active site at the methyl end of the substrate defines how far the substrate can penetrate and thus, the position of oxygenation. In 15-LO-1, substitution of valine for Met-419 enlarges the pocket and shifts the product profile toward 12-HETE. Mutation of additional residues (Q417K/I418A) in conjunction with M419V increases the active site volume further and creates a functional 12-LO [49]. Though the model of substrate binding is similar for 15-LO-2, two distinct Asp-602 and Val-603 are key determinants of positional specificity for this enzyme (Fig. 5)[51]. This model of substrate binding would predict retention of positional specificity for both 12S- and 15S-LOs, regardless of modification of the carboxylic acid moiety of the substrate. While this is the case for 15-LO-1 and 15-LO-2, it does not account for the dual specificity of pl12-LO.
Figure 5. Proposed orientation of NAGly in pl12-LO, 15-LO-1, and 15-LO-2 active sites.
Based upon previous studies and the regiochemistry of oxygenation by LOs, orientation of NAGly in the active site of LOs is represented schematically. Two binding orientations are proposed for pl12-LO to account for the dual specificity it exhibits with NAGly and NAGABA.
The importance of Arg-403 for substrate binding remains unclear. Mutation of this residue to leucine in r15-LO-1 results in a significant loss of activity toward arachidonic and linolenic acids but not their methyl esters [50]. However, substitution of lysine at this position is only able to partially rescue activity. Furthermore, h15-LO-2 has a leucine at the corresponding position, yet it is still capable of metabolizing arachidonic acid. This study raises further questions regarding critical determinants for binding of the substrate’s head group by 12S- and 15S-LOs. Whereas r15-LO-1 exhibits a loss of activity with the lipoamino acids, h15-LO-2 is able to metabolize these substrates as efficiently as arachidonic acid, possibly indicative of a stabilizing interaction beyond the defined binding site of arachidonic acid. The dual specificity exhibited by pl12-LO with the arachidonyl amino acids further support this thought. Assuming that Arg-402 (analogous to Arg-403 of r15-LO-1) coordinates the carboxylic acid of arachidonic acid, then the same placement of NAGly would allow for hydrogen bonding between the substrate amide and Arg-402, hydrogen abstraction at C-10 and subsequent oxygenation at C-12, giving rise to 12-HETE-Gly (Fig. 5). However, to generate 15-HETE-Gly, NAGly must slide out of the active site to position C-13 at the iron center for hydrogen abstration. For this to occur, the interaction of the amide with Arg-402 must be disrupted. Yet arachidonyl amino acids are efficient substrates for pl12-LO, and pl12-LO generates a significant amount of the 15-isomer. There is likely an additional stabilizing interaction at the head group that has yet to be identified.
It is interesting to consider these results in the context of the recent structure of the 8RLO derived from a fusion protein with an allene-oxide synthase in the soft coral Plexaura homomalla [52]. Although the 8R-LO shares less than 35% sequence identity with mammalian 12- and 15-LOs, the backbones of 8R-LO and r15-LO-1 can be superimposed within 1 Å RMSD. Furthermore it is proposed that arachidonic acid binds in the same orientation in 8R-LO and 12SLO, with C-10 positioned for hydrogen abstraction by the iron center; in this comparison, the positional and stereochemical specificities are controlled by Gly-427 in 8R-LO and Ala-404 in 12S-LO, with the methyl group blocking access of O2 to C-8 in 12S-LO [53]. Based upon modeling with 8R-LO, the carboxylate of arachidonic acid is proposed to interact with Arg-183 [52]. The corresponding region in the r15-LO-1 structure is disordered, though this is very possibly an artifact of crystal packing rather than an element of intrinsic protein structure. Multiple sequence alignment of the coral 8R-LO with mammalian LOs indicates that there is not a corresponding arginine in either the 12- or 15-LOs. However, human and murine 12S-LOs possess a conserved lysine in this region (residue 179 in pl12-LO), which is not present in 15-LOs. There are two asparagine residues and a glutamine residue in this region of h15-LO-2, which might be able to contribute hydrogen bonds to substrates. Further study is required to characterize the importance of these residues in 12S-LOs and 15-LO-2.
The ability of COX-2 and LOs to oxygenate lipoamino acids potentially has important implications in vivo. COXs and LOs are expressed in tissues where these lipids can be found and are involved in many of the same signaling pathways as the lipoamino acids and vanilloids. While NAGly and NAGABA do exhibit anti-inflammatory and anti-nociceptive effects in mammals [24], it is unclear if these effects are due to the parent lipid or some metabolite. Further studies are required to determine if the lipoamino acids are oxygenated in vivo and what functions the oxygenated products might serve.
Previous findings indicate that oxidation of the arachidonyl moiety of NADA is not a major pathway of inactivation for the endovanniloid [27]. As with the COXs, the LOs were unable to efficiently oxygenate the vanilloids, if they were able to metabolize them at all. However, in agreement with several studies reporting on the ability of catechols to inhibit pl12-LO [8, 39-41], NADA attenuated arachidonic acid metabolism. OMDA and Arvanil were less efficient at inhibiting pl12-LO activity, indicating a structural requirement for the 3-hydroxyl on the aromatic ring. The ability of NADA to inhibit pl12-LO may represent a point of crosstalk between the vanilloid and LO pathways. HpETEs, including 12-HpETE, are able to induce endothelium-dependent vasoconstriction that cannot be blocked by the COX inhibitors aspirin or indomethacin [54, 55]. NADA is a potent vasorelaxant via mechanisms, which are also largely dependent upon the endothelium [56]. The vasorelaxation properties of NADA have been attributed to its action at cannabinoid and vanilloid receptors. This does not preclude additional mechanisms such as inhibition of HpETE production, but further investigation is required to determine if modulation of pl12-LO activity by NADA occurs in vivo.
Several questions remain regarding the in vivo interactions of lipoamino acids and endovanilloids with lipid-oxidizing enzymes. However, this study demonstrates that COXs and LOs are able to metabolize the lipoamino acids, and further that the activity of LOs can be modulated by vanilloids. Lipid oxidation may represent a pathway for inactivation of lipoamino acids or for formation of a novel class of eicosanoids.
Acknowledgements
The authors wish to thank K. R. Kozak and J. S. Moody for the purification of h15-LO-2 and pl12-LO, respectively, and C. Schneider for helpful discussion and review of the manuscript. This research was supported in part by the Volkswagenstiftung and by the National Institutes of Health Research Grants CA89450 and GM15431 and training grants to AV (ES00267) and MVT (GM65806 and ES07028). MVT is the recipient of a Ruth L. Kirschstein National Research Service Award (DA02014) from the National Institutes of Health/National Institute of Drug Abuse and a fellowship from the Vanderbilt Institute of Chemical Biology.
2Abbreviations used are
COX, cyclooxygenase; mCOX-2, murine COX-2; oCOX-1, ovine COX-1; LO, lipoxygenase; pl12-LO, human platelet-type 12-LO; lk12-LO, porcine leukocyte-type 12-LO; r15-LO-1, rabbit 15-LO-1 (reticulocyte-type); h15-LO-2, human 15-LO-2 (epidermis-type); 8R-LO, coral 8R-LO domain of allene oxide fusion protein; NAla, N-arachidonylalanine; NAGly, N-arachidonylglycine; NAGABA, N-arachidonyl-γ-aminobutryic acid; NADA, N-arachidonyldopamine; OMDA, O-(3-methyl)-N-arachidonyldopamine; HpETE, hydroperoxyeicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; PGG2, prostaglandin G2; PGH2, prostaglandin H2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68-69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- [2].Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- [3].Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- [4].Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- [5].Hamberg M, Samuelsson B. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1973;70:899–903. doi: 10.1073/pnas.70.3.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- [7].Prusakiewicz JJ, Kingsley PJ, Kozak KR, Marnett LJ. Selective oxygenation of N-arachidonylglycine by cyclooxygenase-2. Biochem Biophys Res Commun. 2002;296:612–617. doi: 10.1016/s0006-291x(02)00915-4. [DOI] [PubMed] [Google Scholar]
- [8].Yamamoto S. “Enzymatic” lipid peroxidation: reactions of mammalian lipoxygenases. Free Radic Biol Med. 1991;10:149–159. doi: 10.1016/0891-5849(91)90008-q. [DOI] [PubMed] [Google Scholar]
- [9].Kuhn H, Thiele BJ. The diversity of the lipoxygenase family. Many sequence data but little information on biological significance. FEBS Lett. 1999;449:7–11. doi: 10.1016/s0014-5793(99)00396-8. [DOI] [PubMed] [Google Scholar]
- [10].Nugteren DH. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975;380:299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- [11].Claeys M, Kivits GA, Christ-Hazelhof E, Nugteren DH. Metabolic profile of linoleic acid in porcine leukocytes through the lipoxygenase pathway. Biochim Biophys Acta. 1985;837:35–51. doi: 10.1016/0005-2760(85)90083-9. [DOI] [PubMed] [Google Scholar]
- [12].Hada T, Ueda N, Takahashi Y, Yamamoto S. Catalytic properties of human platelet 12-lipoxygenase as compared with the enzymes of other origins. Biochim Biophys Acta. 1991;1083:89–93. doi: 10.1016/0005-2760(91)90128-5. [DOI] [PubMed] [Google Scholar]
- [13].Funk CD, Funk LB, FitzGerald GA, Samuelsson B. Characterization of human 12-lipoxygenase genes. Proc Natl Acad Sci U S A. 1992;89:3962–3966. doi: 10.1073/pnas.89.9.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Narumiya S, Salmon JA, Cottee FH, Weatherley BC, Flower RJ. Arachidonic acid 15-lipoxygenase from rabbit peritoneal polymorphonuclear leukocytes. Partial purification and properties. J Biol Chem. 1981;256:9583–9592. [PubMed] [Google Scholar]
- [15].Kuhn H, Sprecher H, Brash AR. On singular or dual positional specificity of lipoxygenases. The number of chiral products varies with alignment of methylene groups at the active site of the enzyme. J Biol Chem. 1990;265:16300–16305. [PubMed] [Google Scholar]
- [16].Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem. 1990;265:18351–18361. [PubMed] [Google Scholar]
- [17].Takahashi Y, Glasgow WC, Suzuki H, Taketani Y, Yamamoto S, Anton M, Kuhn H, Brash AR. Investigation of the oxygenation of phospholipids by the porcine leukocyte and human platelet arachidonate 12-lipoxygenases. Eur J Biochem. 1993;218:165–171. doi: 10.1111/j.1432-1033.1993.tb18362.x. [DOI] [PubMed] [Google Scholar]
- [18].Moody JS, Kozak KR, Ji C, Marnett LJ. Selective oxygenation of the endocannabinoid 2-arachidonylglycerol by leukocyte-type 12-lipoxygenase. Biochemistry. 2001;40:861–866. doi: 10.1021/bi002303b. [DOI] [PubMed] [Google Scholar]
- [19].Ueda N, Yamamoto K, Yamamoto S, Tokunaga T, Shirakawa E, Shinkai H, Ogawa M, Sato T, Kudo I, Inoue K, Takizawa H, Nagano T, Hirobe M, Matsuki N, Saito H. Lipoxygenase-catalyzed oxygenation of arachidonylethanolamide, a cannabinoid receptor agonist. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1995;1254:127–134. doi: 10.1016/0005-2760(94)00170-4. [DOI] [PubMed] [Google Scholar]
- [20].Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human Platelets and Polymorphonuclear Leukocytes Synthesize Oxygenated Derivatives of Arachidonylethanolamide (Anandamide): Their Affinities for Cannabinoid Receptors and Pathways of Inactivation. Mol Pharmacol. 1998;54:180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- [21].Kozak KR, Gupta RA, Moody JS, Ji C, Boeglin WE, DuBois RN, Brash AR, Marnett LJ. 15-Lipoxygenase metabolism of 2-arachidonylglycerol. Generation of a peroxisome proliferator-activated receptor alpha agonist. J Biol Chem. 2002;277:23278–23286. doi: 10.1074/jbc.M201084200. [DOI] [PubMed] [Google Scholar]
- [22].Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jisaka M, Kim RB, Boeglin WE, Nanney LB, Brash AR. Molecular Cloning and Functional Expression of a Phorbol Ester-inducible 8S-Lipoxygenase from Mouse Skin. J. Biol. Chem. 1997;272:24410–24416. doi: 10.1074/jbc.272.39.24410. [DOI] [PubMed] [Google Scholar]
- [24].Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. Identification of a new class of molecules, the arachidonyl amino acids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- [25].Melck D, Bisogno T, De Petrocellis L, Chuang H, Julius D, Bifulco M, Di Marzo V. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem Biophys Res Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- [26].Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351(Pt 3):817–824. [PMC free article] [PubMed] [Google Scholar]
- [27].Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [29].De Petrocellis L, Chu CJ, Moriello AS, Kellner JC, Walker JM, Di Marzo V. Actions of two naturally occurring saturated N-acyldopamines on transient receptor potential vanilloid 1 (TRPV1) channels. Br J Pharmacol. 2004;143:251–256. doi: 10.1038/sj.bjp.0705924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marnett LJ, Siedlik PH, Ochs RC, Pagels WR, Das M, Honn KV, Warnock RH, Tainer BE, Eling TE. Mechanism of the stimulation of prostaglandin H synthase and prostacyclin synthase by the antithrombotic and antimetastatic agent, nafazatrom. Mol Pharmacol. 1984;26:328–335. [PubMed] [Google Scholar]
- [31].Rowlinson SW, Crews BC, Lanzo CA, Marnett LJ. The binding of arachidonic acid in the cyclooxygenase active site of mouse prostaglandin endoperoxide synthase-2 (COX-2). A putative L-shaped binding conformation utilizing the top channel region. J Biol Chem. 1999;274:23305–23310. doi: 10.1074/jbc.274.33.23305. [DOI] [PubMed] [Google Scholar]
- [32].Havrilla CM, Hachey DL, Porter NA. Coordination (Ag+) Ion Spray-Mass Spectrometry of Peroxidation Products of Cholesterol Linoleate and Cholesterol Arachidonate: High-Performance Liquid Chromatography-Mass Spectrometry Analysis of Peroxide Products from Polyunsaturated Lipid Autoxidation. J. Am. Chem. Soc. 2000;122:8042–8055. [Google Scholar]
- [33].Livingstone CD, Barton GJ. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. Comput Appl Biosci. 1993;9:745–756. doi: 10.1093/bioinformatics/9.6.745. [DOI] [PubMed] [Google Scholar]
- [34].Livingstone CD, Barton GJ. Identification of functional residues and secondary structure from protein multiple sequence alignment. Methods Enzymol. 1996;266:497–512. doi: 10.1016/s0076-6879(96)66031-5. [DOI] [PubMed] [Google Scholar]
- [35].Juranek I, Suzuki H, Yamamoto S. Affinities of various mammalian arachidonate lipoxygenases and cyclooxygenases for molecular oxygen as substrate. Biochim Biophys Acta. 1999;1436:509–518. doi: 10.1016/s0005-2760(98)00159-3. [DOI] [PubMed] [Google Scholar]
- [36].Chen XS, Brash AR, Funk CD. Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur J Biochem. 1993;214:845–852. doi: 10.1111/j.1432-1033.1993.tb17988.x. [DOI] [PubMed] [Google Scholar]
- [37].Kilty I, Logan A, Vickers PJ. Differential characteristics of human 15-lipoxygenase isozymes and a novel splice variant of 15S-lipoxygenase. Eur J Biochem. 1999;266:83–93. doi: 10.1046/j.1432-1327.1999.00818.x. [DOI] [PubMed] [Google Scholar]
- [38].Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, Inoue T, Ogino R, Tatsuoka T, Ishihara T, et al. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34:1503–1505. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- [39].Byczkowski JZ, Ramgoolie PJ, Kulkarni AP. Some aspects of activation and inhibition of rat brain lipoxygenase. Int J Biochem. 1992;24:1691–1695. doi: 10.1016/0020-711x(92)90114-g. [DOI] [PubMed] [Google Scholar]
- [40].Tseng CF, Iwakami S, Mikajiri A, Shibuya M, Hanaoka F, Ebizuka Y, Padmawinata K, Sankawa U. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-beta-phenethylamine and N-acyldopamine derivatives. Chem Pharm Bull (Tokyo) 1992;40:396–400. doi: 10.1248/cpb.40.396. [DOI] [PubMed] [Google Scholar]
- [41].Simpson J, Forrester R, Tisdale MJ, Billington DC, Rathbone DL. Effect of catechol derivatives on cell growth and lipoxygenase activity. Bioorg Med Chem Lett. 2003;13:2435–2439. doi: 10.1016/s0960-894x(03)00528-6. [DOI] [PubMed] [Google Scholar]
- [42].Bradshaw HB, Walker JM. The expanding field of cannabimimetic and related lipid mediators. Br J Pharmacol. 2005;144:459–465. doi: 10.1038/sj.bjp.0706093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- [44].Kozak KR, Prusakiewicz JJ, Rowlinson SW, Prudhomme DR, Marnett LJ. Amino acid determinants in cyclooxygenase-2 oxygenation of the endocannabinoid anandamide. Biochemistry. 2003;42:9041–9049. doi: 10.1021/bi034471k. [DOI] [PubMed] [Google Scholar]
- [45].Kozak KR, Prusakiewicz JJ, Rowlinson SW, Schneider C, Marnett LJ. Amino acid determinants in cyclooxygenase-2 oxygenation of the endocannabinoid 2-arachidonylglycerol. J Biol Chem. 2001;276:30072–30077. doi: 10.1074/jbc.M104467200. [DOI] [PubMed] [Google Scholar]
- [46].Bryant RW, Bailey JM, Schewe T, Rapoport SM. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15-S-hydroperoxy-eicosatetraenoic acid. J Biol Chem. 1982;257:6050–6055. [PubMed] [Google Scholar]
- [47].Burger F, Krieg P, Marks F, Furstenberger G. Positional- and stereo-selectivity of fatty acid oxygenation catalysed by mouse (12S)-lipoxygenase isoenzymes. Biochem J. 2000;348(Pt 2):329–335. [PMC free article] [PubMed] [Google Scholar]
- [48].Gillmor SA, Villasenor A, Fletterick R, Sigal E, Browner MF. The structure of mammalian 15-lipoxygenase reveals similarity to the lipases and the determinants of substrate specificity. Nat Struct Biol. 1997;4:1003–1009. doi: 10.1038/nsb1297-1003. [DOI] [PubMed] [Google Scholar]
- [49].Sloane DL, Leung R, Craik CS, Sigal E. A primary determinant for lipoxygenase positional specificity. Nature. 1991;354:149–152. doi: 10.1038/354149a0. [DOI] [PubMed] [Google Scholar]
- [50].Gan QF, Browner MF, Sloane DL, Sigal E. Defining the arachidonic acid binding site of human 15-lipoxygenase. Molecular modeling and mutagenesis. J Biol Chem. 1996;271:25412–25418. doi: 10.1074/jbc.271.41.25412. [DOI] [PubMed] [Google Scholar]
- [51].Jisaka M, Kim RB, Boeglin WE, Brash AR. Identification of amino acid determinants of the positional specificity of mouse 8S-lipoxygenase and human 15S-lipoxygenase-2. J Biol Chem. 2000;275:1287–1293. doi: 10.1074/jbc.275.2.1287. [DOI] [PubMed] [Google Scholar]
- [52].Oldham ML, Brash AR, Newcomer ME. Insights from the X-ray crystal structure of coral 8R-lipoxygenase: calcium activation via a C2-like domain and a structural basis of product chirality. J Biol Chem. 2005;280:39545–39552. doi: 10.1074/jbc.M506675200. [DOI] [PubMed] [Google Scholar]
- [53].Coffa G, Brash AR. A single active site residue directs oxygenation stereospecificity in lipoxygenases: stereocontrol is linked to the position of oxygenation. Proc Natl Acad Sci U S A. 2004;101:15579–15584. doi: 10.1073/pnas.0406727101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Asano M, Hidaka H. Contractile response of isolated rabbit aortic strips to unsaturated fatty acid peroxides. J Pharmacol Exp Ther. 1979;208:347–353. [PubMed] [Google Scholar]
- [55].Nishiyama M, Okamoto H, Watanabe T, Hori T, Sasaki T, Kirino T, Shimizu T. Endothelium is required for 12-hydroperoxyeicosatetraenoic acid-induced vasoconstriction. Eur J Pharmacol. 1998;341:57–63. doi: 10.1016/s0014-2999(97)01353-8. [DOI] [PubMed] [Google Scholar]
- [56].O’Sullivan SE, Kendall DA, Randall MD. Characterisation of the vasorelaxant properties of the novel endocannabinoid N-arachidonoyl-dopamine (NADA) Br J Pharmacol. 2004;141:803–812. doi: 10.1038/sj.bjp.0705643. [DOI] [PMC free article] [PubMed] [Google Scholar]