Abstract
Context
Cytomegalovirus (CMV) infection is associated with adverse clinical outcomes in immunosuppressed persons, but the incidence and association of CMV reactivation with adverse outcomes in persons lacking evidence of immunosuppression (“immunocompetent”) with critical illness have not been well-defined.
Objective
To determine the association of CMV reactivation with intensive care unit (ICU) and hospital length of stay in critically-ill immunocompetent persons.
Methods
We prospectively assessed CMV plasma DNAemia by real-time PCR twice weekly and clinical outcomes in a cohort of CMV seropositive, immunocompetent adults admitted to an ICU. Clinical parameters were assessed by personnel blinded to CMV PCR results. Risk factors for CMV reactivation and association with hospital and ICU length of stay (LOS) were assessed by multivariable logistic regression and proportional odds models.
Setting
Six ICU’s at two separate hospitals at a large tertiary care academic medical center between 2004–2006.
Participants
A total of 120 critically-ill, CMV seropositive adults lacking evidence of immunosuppression.
Main Outcome Measures
Association of CMV reactivation with prolonged hospital length of stay or death.
Results
The primary composite endpoint of continued hospitalization (n=35) or death (n=10) at 30 days occurred in 45 (35%) of the 120 patients. CMV viremia at any level or > 1,000 copies/ml occurred in 33% (39 of 120, 95% confidence interval [CI] 24%–41%) and 20% (24 of 120, 95% CI 13%–28%), at a median of 12 days (range 3–57) and 26 days (range 9–56), respectively. By logistic regression, CMV infection at any level (adjusted OR: 4.3 [1.6–11.9], p = 0.005), >1,000 copies/ml (adjusted OR 13.9 [3.2–60], p < 0.001), or average CMV area under the curve [AUC] (adjusted OR 2.1 [1.3–3.2], p < 0.001), was independently associated with hospitalization or death by 30 days. In multivariable partial proportional odds models, both CMV seven-day moving average (OR 5.1 (2.9–9.1) p < 0.0001) and CMV AUC (OR 3.2 (2.1–4.7), p < 0.0001) were independently associated with a hospital LOS ≥14 days.
Conclusions
These preliminary findings suggest that reactivation of CMV occurs frequently in critically-ill immunocompetent patients and is associated with prolonged hospitalization or death. A controlled trial of CMV prophylaxis in this setting is warranted.
Introduction
Cytomegalovirus (CMV) has long been recognized as an important viral pathogen in immunocompromised hosts. In addition to direct effects of CMV due to viral replication and resultant tissue injury, a range of indirect effects have been attributed to CMV in immunocompromised patients, including increased risk of secondary bacterial and fungal infections,1–5 predisposition to specific malignancies such as EBV-associated post-transplant lymphoproliferative disorder,6 cardiovascular disease,7, 8 and mortality.2–5, 9, 10 A causal role of CMV in mediating these indirect effects is supported by studies of antiviral prophylaxis in immunosuppressed patients demonstrating reductions in secondary bacterial and fungal infections,2–5 hospitalization,11 and mortality.2–5
The role of CMV infection in immunocompetent patients with critical illness has been investigated in several prior studies.12–20 Although these studies used various virologic and statistical methods and designs, most demonstrated that CMV infection occurs commonly in critically-ill patients and is associated with one or more adverse clinical outcomes.12–20 However, these prior studies had one or more significant limitations, including relatively small sample size, inclusion of only selected types of ICU patients, lack of quantitative methods for CMV detection, non-blinded assessment of clinical endpoints, and/or failure to include comprehensive and rigorous statistical analyses. To address some of these limitations, we prospectively assessed CMV plasma DNAemia by real-time PCR and clinical outcomes in a broad cohort of consecutive CMV seropositive, immunocompetent adults admitted to an intensive care unit (ICU), with the goal of defining the incidence, risk factors, timing, and association of CMV reactivation with clinically-significant outcomes.
Methods
Study Design
This prospective study was conducted at six intensive care units (ICU) at two separate hospitals at a large university-affiliated academic medical center between 2004 and 2006. The study was approved by the human subjects division at the University of Washington and written informed consent was obtained from study participants. Daily screening of new medical-surgical admissions to each ICU (Burn [BICU], Cardiac Care [CICU], Medical [MICU], and Trauma [TICU]) was performed by study personnel, and patients who met other inclusion criteria underwent screening with CMV serology within 24 hours. All patients meeting study inclusion criteria were offered participation regardless of race or ethnic status. Participants’ race/ethnic status was recorded as listed in the admitting/registration information, and was collected in compliance with reporting requirements for National Institutes of Health-funded clinical studies. Only patients who were newly admitted to the ICU from home or baseline residential setting were included (i.e., patients who were transferred to the ICU from within the hospital were excluded). Those who were CMV seronegative were excluded from further study. CMV seropositive patients who met all other inclusion criteria were enrolled and underwent prospective clinical assessments using standardized data collection forms. In addition, plasma samples were collected thrice weekly and stored at −20°C for subsequent CMV PCR analysis. All clinical information was collected prospectively by study personnel who were blinded to the CMV PCR results (which were performed after all clinical data had been compiled). Patients were followed prospectively until death or hospital discharge. Deaths occurring within 90 days after discharge from the hospital were assessed using state and national death registry data.
Inclusion and exclusion criteria
The inclusion criteria included: able to give informed consent (either from patient or next of kin), age ≥ 18 years, admission to the Burn intensive care unit (BICU) with ≥ 40% body surface burn or ≥ 20% body surface burn with inhalation injury or Trauma intensive care unit (TICU) with ISS score of >15 AND > 4U packed red blood cells within 24 hours or Medical intensive care unit (MICU) with suspected or documented sepsis or Cardiac intensive care unit (CICU) with a diagnosis of acute myocardial infarction, expected survival > 72 hours, and CMV seropositive. The exclusion criteria were: unable to give informed consent, age < 18 years, expected survival < 72 hours, use of antiviral agent cidofovir, foscarnet, ganciclovir, valacyclovir [HSV treatment doses of acyclovir, valacyclovir, or famciclovir permitted]) within the last 7 days, known or suspected HIV infection, and known or suspected underlying immune deficiency (transplant, congenital immunodeficiency, receipt of immunosuppressive medications [prednisone, azathioprine, tacrolimus, cyclosporin, sirolimus, cyclophosphamide] within 30 days).
Definitions
Major infections included pneumonia or bacteremia. Pneumonia was diagnosed on the basis of radiographic pulmonary infiltrates and a quantitative bacterial culture of BAL demonstrating ≥ 104 cfu/ml as previously defined.21 An episode of clinically-significant bacteremia was defined as signs or symptoms of infection (fever, leukocytosis) and isolation of a bacterial pathogen from at least 1 blood culture. Bacteremia (single positive blood cultures) due to coagulase negative Staphylococci and other known common blood culture contaminants including diphtheroids and Bacillus spp. was excluded. The APACHE II and Injury Severity Scores were calculated within 24 hours of admission to the ICU as previously described.22, 23 The term “immunocompetent” was used to describe patients lacking evidence of immunosuppression.
CMV assays
Antibodies to CMV indicating prior CMV infection were assessed using a commercial enzyme immunoassay kit for detection of total antibodies to CMV (“Abbott CMV Total AB EIA”, Abbott Laboratories, Abbott Park, IL). The assay was performed and interpreted according to manufacturer recommendations. CMV DNA was quantified in stored plasma samples using a previously described real-time PCR assay.24 DNA extraction was performed on 200 μl of plasma using a QIAamp DNA blood kit (Qiagen, Inc., Valencia, Calif.). Then, 100 μl of Tris (10 mM, pH 8.0) was used to elute the DNA, and 10 μl of the DNA was used for each PCR. The PCR conditions were 50°C for 2 min and 95°C for 2 min, followed by 45 cycles of 95°C for 20 s and 60°C for 1 min. Each 50 μl of PCR mixture contained a 400 nM concentration of primers, 5 μl of 10× buffer II (Perkin-Elmer Cetus), 10 mM MgCl2, 17.5 nM TaqStart antibody (Clontech), 1.25 U of AmpliTaq (Perkin-Elmer Cetus), 0.05 U of uracil-DNA-glycosylase, 8% glycerol, and 60 nM 6-carboxy-x-rhodamine. To ensure that negative results were not due to nonspecific inhibition of the PCR assay, each PCR also contained internal positive control EXO DNA (5,000 copies/reaction), primers, and probes. All negative CMV PCR results required detection of EXO DNA. One positive control with 5,000 copies of CMV DNA was co-processed with specimens to ensure DNA recovery. To monitor for false-positive results, specimens were processed in parallel with aliquots of 1× phosphate-buffered saline. PCRs without DNA also were included in each PCR run. PCRs were run in duplicate, with results deemed positive if both reactions were positive; results that were positive-negative were deemed indeterminate and repeated. Quantitative PCR levels were reported as copies per milliliter of plasma.
Statistical analysis
Patient characteristics were summarized using percentages or median and range values. Cumulative incidence estimates for CMV viremia considered death or discharge from hospital as competing risk events. In a landmark analysis of patients still hospitalized by 30 days after admission, probability of discharge after day 30 was calculated for subjects who had reactivated CMV prior to day 30 and those who had not using cumulative incidence estimates with death considered a competing risk event. Log-rank tests were used to compare the hazards of discharge between groups. Proportion of days transfused or ventilated were calculated by summing the number of days the patient was transfused or ventilated by the total number of days followed, up to a maximum of 30 days for the composite endpoint analysis.
Logistic regression models were used to identify risk factors for CMV reactivation and for the composite endpoint of continued hospitalization or death by day 30. The odds ratios (OR) and 95% confidence intervals (CI) were reported. Potential risk factors for CMV reactivation included age, race, gender, unit, baseline APACHE score, baseline transfusion receipt, baseline ventilator use. Potential risk factors for the composite endpoint included the above as well as major infection, CMV viral load measurements and the proportion of hospitalized days spent transfused or ventilated. Risk factors that were univariately significant at p<0.10 were considered for entry into multivariable models which were limited to three factors due to the number of events.
A landmark analysis of patients who remained hospitalized by day 30 was performed. This specific time-point was chosen because all patients had equal follow-up assessments of CMV reactivation, virtually all patients who ever reactivated CMV had done so by 30 days and because it took into consideration a biologically-relevant time-lag for CMV effects.
The primary interest was the association between viral load and length of stay (LOS), thus, we categorized patients as remaining hospitalized longer than each of four time points: 14, 28, 42 and 56 days after admission. Since the covariate effects on the outcome of continued hospitalization longer than 14 days could be different than those on continued hospitalization longer than, for example, 42 days, we used partial proportional odds models to estimate odds of increased length of stay past each consecutive time point. The proportional odds model25, 26 constrains the odds ratios for explanatory variables to be the same across outcome time points, whereas the partial proportional odds model allows the impact of some factors to vary across outcome time points while other factors maintain a constant effect 27 We selected the partial proportional odds model as a means to evaluate the impact of CMV viral load on length of stay.
We modeled CMV viral load in two ways: the average area under the curve to reflect all follow-up, and the seven-day moving average to reflect a shorter window of follow-up. With longitudinal measurements for each patient, we used these methods to smooth the viral load peaks and nadirs. The average area under the curve (AUC) of CMV was calculated for each day of follow-up by summing each patient’s CMV PCR measurements and dividing by the number of days followed thus far. The seven-day moving average was calculated for each day of follow-up by summing the CMV PCR measurements over the previous seven days and calculating the average value. For example, on day 7, the seven-day moving average would be the average of viral load measurements on days 1 through 7; the moving average on day 8 would average the measurements on days 2 through 8; on day 9, it would average the measurements on days 3 through 9; and so on. The average AUC, on the other hand, accumulates over all days followed: on day 7, the average AUC would be the average of viral load measurements on days 1 through 7; but on day 8, average AUC would be the average of the measurements on days 1 through 8; on day 9 it would be the average of viral loads on days 1 through 9; etc. Therefore, each patient’s viral load measurements were cumulated to reflect short-term and long-term averages while still contributing multiple data points.
Since each subject contributed observations from multiple time points to the analysis, we used generalized estimating equations (GEE) with robust sandwich variance estimates to appropriately account for intra-subject correlations.25
Multivariable models were limited to three factors due to number of events or subjects. All reported P values are 2 sided and p<0.05 was considered significant. SAS version 9.1 (SAS Institute, Cary, NC) was used for all analyses and figures were created with GraphPad Prism version 4.03 for Windows (GraphPad Software, San Diego, CA).
Results
Study population
A total of 221 patients were initially screened for inclusion in the study, and 101 were excluded on the basis of: a negative CMV serology (n = 78), death or discharge within 72 hours of admission (n = 8), inability to obtain informed consent (n = 9), or other miscellaneous reason(s) (n = 6), leaving 120 patients who comprised the study population. The characteristics of the study population stratified by ICU are shown in Table 1. Forty patients were enrolled in each the MICU and TICU and 20 patients each in the BICU and CICU. The primary composite endpoint of continued hospitalization or death by 30 days occurred in 45 of 120 patients (38%).
Table 1.
Characteristics of the study population
| Characteristic | ALL n = 120 | BICU n = 20 | CICU n = 20 | MICU n = 40 | TICU n = 40 |
|---|---|---|---|---|---|
| Age in years, median (range) | 52 (18–90) | 46 (19–80) | 60 (42–90) | 54 (19–80) | 42 (18–87) |
| Male Gender, n (%) | 73 (61) | 14 (70) | 13 (65) | 23 (58) | 23 (58) |
| Caucasian Race, n (%) | 94 (78) | 18 (90) | 15 (75) | 28 (70) | 33 (83) |
| APACHE II score, median (range) | 21 (7–36) | 20 (11–33) | 16 (7–34) | 28 (10–36) | 20 (11–30) |
| Transfusion within 24hr of admission, n (%) | 5 (4) | 0 (0) | 0 (0) | 2 (5) | 3 (8) |
| Ventilator use at admission, n (%) | 93 (78) | 17 (85) | 9 (45) | 29 (73) | 38 (95) |
| Major Infection, n (%) | 41 (34) | 15 (75) | 1 (5) | 11 (28) | 14 (35) |
| Hospital length of stay in days, median (range) | 17 (2–181) | 55 (8–181) | 7 (2–41) | 13 (4–94) | 18 (6–86) |
| ICU length of stay in days, median (range) | 10 (1–126) | 43 (8–126) | 5 (1–18) | 9 (3–55) | 10 (3–56) |
| Deceased by day 30 post-enrollment, n (%) | 10 (8) | 2 (10) | 5 (25) | 2 (5) | 1 (3) |
| Hospitalized at day 30 post-enrollment, n (%) | 35 (29) | 17 (85) | 1 (5) | 6 (15) | 11 (28) |
| In ICU at day 30 post-enrollment, n (%) | 20 (17) | 13 (65) | 0 (0) | 2 (5) | 5 (13) |
Incidence and quantitation of CMV reactivation (viremia)
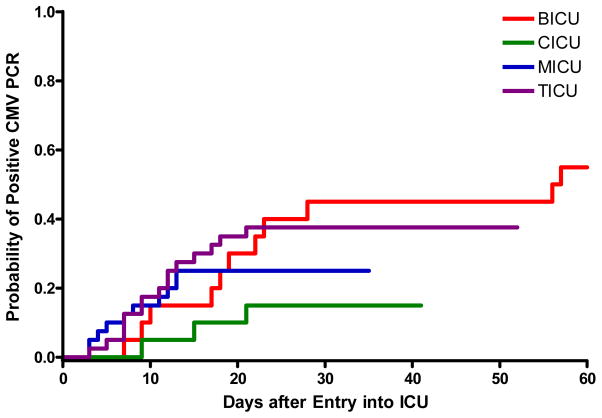
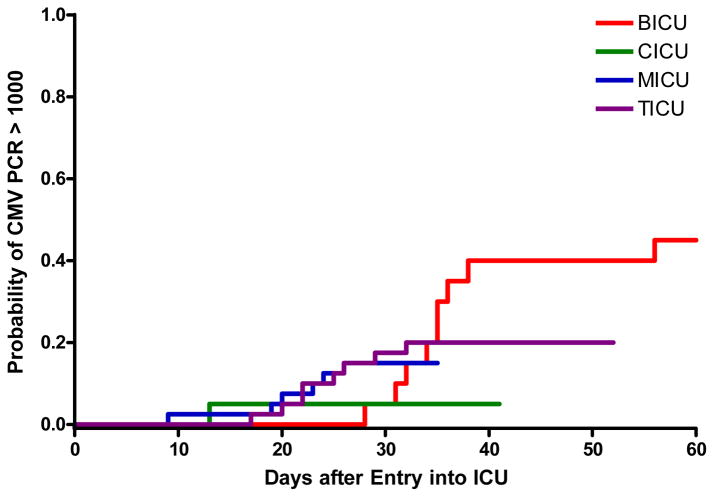
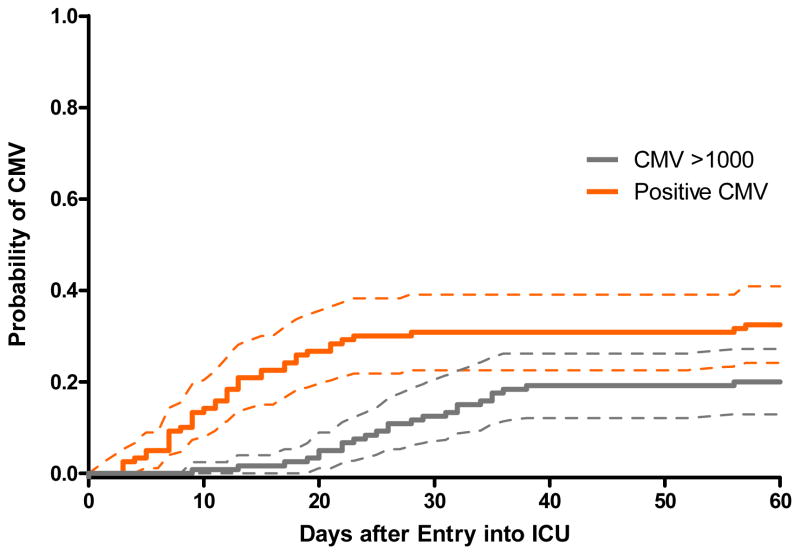
The incidence of CMV viremia stratified by ICU is shown in Table 2. A total of 1,954 samples were tested from the 120 enrolled patients, with a median and range of 11 (1–89) samples tested per patient. The cumulative incidence of CMV viremia at any level (panel A) and >1,000 copies/ml (panel B) stratified by ICU, and for the entire cohort (panel C) is shown in Figure 1. The cumulative incidence estimate of CMV viremia at any level was 33% (39 of 120, 95% CI 24%–41%). Among patients in whom viremia ever developed, 37 of 39 (95%) did so within the first 30 days after admission to the ICU, and half within the first 12 days (range 3–57 days to first detectable viremia). The cumulative incidence estimate of CMV viremia > 1,000 copies/ml was 20% (24 of 120, 95% CI 13%–28%), occurring at a median of 26 days (range 9–56). The 95% CI for the cumulative incidence estimates of CMV viremia at 30 days at either any level or > 1,000 copies/ml for the BICU, CICU, MICU, and TICU were: 0.23–0.67, 0–0.31, 0.12–0.38, 0.22–0.53 and 0–0.23, 0–0.15, 0.04–0.26, and 0.08–0.32, respectively.
Table 2.
CMV reactivation as assessed by PCR
| CMV Variable | ALL n=120 | BICU n=20 | CICU n=20 | MICU n=40 | TICU n=40 |
|---|---|---|---|---|---|
| CMV viremia at any level, n (%) | 39 (33) | 11 (55) | 3 (15) | 10 (25) | 15 (38) |
| CMV viremia >1000 copies/ml, n (%) | 24 (20) | 9 (45) | 1 (5) | 6 (15) | 8 (20) |
| CMV viremia >10,000 copies/ml, n (%) | 11 (9) | 4 (20) | 0 (0) | 4 (10) | 3 (8) |
| Maximum CMV load (log10 PCR copies), median (range) | 3.3 (1.8–5.5) | 3.9 (2.5–5.5) | 2.4 (1.8–3.7) | 3.4 (2.3–4.8) | 3.1 (2.1–4.5) |
| Days to first detectable CMV viremia, median (range) | 12 (3–57) | 19 (7–57) | 15 (9–21) | 8 (3–13) | 11 (3–21) |
| Duration of shedding in days, median (range) | 17 (2–45) | 20 (4–45) | 4 (2–17) | 18 (4–38) | 14 (2–32) |
Figure 1.
Figure 1A. Cumulative incidence of CMV viremia at any level stratified by ICU
Figure 1B. Cumulative incidence of CMV viremia at >1,000 copies/ml stratified by ICU
Figure 1C. Cumulative incidence (and 95% confidence intervals) of CMV viremia at any level or >1,000 copies/ml (all patients, n=120)
Risk factors for CMV reactivation
Multivariable logistic regression analysis of factors associated with CMV viremia at any level is shown in Table 3. In multivariable models, male gender was associated with an increased risk for CMV reactivation. The APACHE II score at admission was not associated with an increased risk of subsequent CMV reactivation. The results were similar when a CMV viremia endpoint of >1,000 copies/ml was used, except that the baseline variables of ventilator use (adjusted OR 8.5 [1.1–66.5], p = 0.04) and receipt of a transfusion (adjusted OR 6.7 [1.1–42.7], p = 0.05) were associated with an increased risk for CMV reactivation at that level (data not shown).
Table 3.
Risk factors for CMV reactivation. Odds ratios (OR) and 95% confidence intervals (CI) estimated by logistic regression models
| Comparison | Odds Ratio(95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Baseline Characteristic | |||||
| Age | 10-year increments | 1.1 (0.9–1.4) | 0.43 | -- | -- |
| Unit | TICU | 1.0 | 1.0 | ||
| BICU | 2.0 (0.7–6.1) | 0.20 | 1.8 (0.6–5.7) | 0.30 | |
| CICU | 0.3 (0.1–1.2) | 0.08 | 0.2 (0.1–1.0) | 0.06 | |
| MICU | 0.6 (0.2–1.5) | 0.23 | 0.5 (0.2–1.4) | 0.20 | |
| Race | Caucasian | 1.0 | |||
| Other | 0.9 (0.4–2.3) | 0.83 | -- | -- | |
| Gender | Female | 1.0 | 1.0 | ||
| Male | 3.6 (1.5–8.8) | <0.01 | 3.8 (1.5–9.5) | <0.01 | |
| APACHE II score | <16 | 1.0 | |||
| ≥16, <21 | 2.1 (0.5–8.6) | 0.29 | -- | -- | |
| ≥21, <27.5 | 0.8 (0.2–3.9) | 0.83 | -- | -- | |
| ≥ 27.5 | 2.8 (0.7–11.1) | 0.15 | -- | -- | |
| Transfusion | No | 1.0 | |||
| Yes | 9.1 (1.0–84.7) | 0.05 | -- | -- | |
| Ventilator | No | 1.0 | |||
| Yes | 2.5 (0.9–7.3) | 0.09 | -- | -- | |
Risk factors for death or continued hospitalization by 30 days
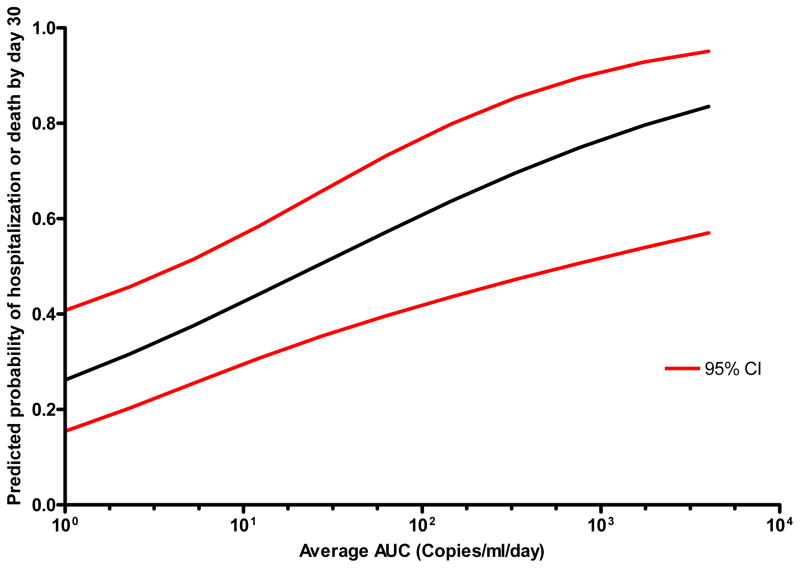
Table 4 shows the raw data for: discharge, death, continued hospitalization, and CMV reactivation status of the cohort by day 7, 10, 15, 20, and 30 after admission to the intensive care unit. Table 5 shows the logistic regression univariable and multivariable analysis of factors associated with the composite endpoint of death or continued hospitalization by 30 days after admission to the ICU. Even when adjusted for other significant baseline or time-dependent variables, CMV reactivation assessed in any one of four ways (viremia at any level, >1,000 copies/ml, maximum viremia in log10 copies/ml, or average AUC) was independently associated with death or continued hospitalization by 30 days. Furthermore, there was a quantitative association, such that the greater the amount of CMV reactivation the greater the risk for continued hospitalization or death by 30 days. A similar association between CMV reactivation and death or continued hospitalization by the earlier time-point of 15 days was evident (adjusted HR [95% confidence interval] for viremia at any level; 6.1 [1.7–21.7], p < 0.01, maximum viremia in log10 copies/ml; 2.1 [1.2–3.7], p < 0.01, or average AUC; 2.6 [1.1–6.2], p = 0.03). Figure 2 shows the predicted probability (with 95% CIs) of death or continued hospitalization by 30 days as a function of the average CMV AUC based on a logistic regression model. Each log increase in the average CMV AUC was associated with a 14% increase in the probability of death or continued hospitalization by 30 days. A similar analysis but using as the composite endpoint death or ICU (rather than total) hospitalization by 30 days yielded similar results: each of the CMV variables remained associated with death or ICU hospitalization by 30 days, with adjusted odds ratios and 95% CIs for CMV viremia at any level (5.7 [2.1–15.6], p < 0.001), >1,000 copies/ml (4.6 [1.2–17.4], p = 0.02), each log10 maximum CMV (1.7 [1.2–2.4], p = 0.002), and average AUC of CMV (2.0 [1.3–3.1], p = 0.003), respectively. As reported in prior studies, we confirmed that development of a major infection (nosocomial bacteremia or pneumonia) was associated with an increased hospital LOS (adjusted OR 3.0, 95% CI, 1.1–8.4, p = 0.04). The association between CMV and death or continued hospitalization by 30 days after admission to the ICU remained significant when the analysis was restricted to the MICU and TICU cohorts only, with adjusted odds ratios and 95% CIs for CMV viremia at any level (7.3 [2.3–22.9], p < 0.0001), >1,000 copies/ml (32.4 [5.8–18.3], p < 0.0001), each log10 maximum CMV (2.1 [1.5–3.0], p < 0.0001), and average AUC of CMV (2.7 [1.6–4.3], p < 0.0001), respectively.
Table 4.
Raw data for status of hospitalization, mortality, and CMV reactivation by day after admission
| Index Day |
|||||
|---|---|---|---|---|---|
| Day 7 | Day 10 | Day 15 | Day 20 | Day 30 | |
| Discharged before | 20 | 33 | 46 | 61 | 75 |
| Died before | 5 | 7 | 9 | 9 | 10 |
| Continued hospitalization on | 95 | 80 | 65 | 50 | 35 |
| Among patients still hospitalized on index day | |||||
| Never CMV reactivated | 56 | 42 | 31 | 20 | 12 |
| Reactivated before | 11 | 16 | 22 | 23 | 21 |
| Reactivated after | 28 | 22 | 12 | 7 | 2 |
| CMV 7-day moving average at index day in log copies/ml (median, range) | 0.5 (0.2–2.4) | 0.7 (0.2–2.5) | 0.6 (0–3.3) | 1.2 (0–3.4) | 2.4 (0–4.1) |
| P-value* | <0.0001 | <0.0001 | 0.0001 | <0.0001 | |
| CMV average AUC at index day in log copies/ml (median, range) | 1.3 (0.2–2.4) | 1.4 (0.6–2.6) | 1.3 (0.3–3.1) | 1.6 (0.3–3.6) | 2.3 (0.9–3.5) |
| P-value# | 0.001 | <0.0001 | 0.002 | <0.0001 | |
P-value compares CMV 7-day moving average at index day with 7-day moving average at day 30 adjusted for intra-subject correlation using GEE.
P-value compares average CMV AUC at index day with average AUC at day 30 adjusted for intra-subject correlation using GEE.
Table 5.
Risk factors for continued hospitalization or death at 30 days after admission to ICU. Odds ratios (OR) and 95% confidence intervals (CI) estimated by logistic regression models
| Comparison | Odds Ratio (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Baseline Variable | |||||
| Age | 10-year increments | 1.1 (0.9–1.4) | 0.31 | -- | -- |
| Unit | 1.0 | 1.0 | |||
| TICU | 44.3 (5.3–370) | <0.001 | 90 (8.3–980)# | <0.001 | |
| BICU | 1.0 (0.3–3.2) | 1.00 | 2.6 (0.6–10.2) # | 0.18 | |
| CICU | 0.8 (0.3–2.1) | 0.62 | 0.8 (0.3–2.2) # | 0.65 | |
| MICU | |||||
| Race | 1.0 (ref) | ||||
| Caucasian | 0.4 (0.2–1.2) | 0.09 | -- | -- | |
| Other | |||||
| Gender | 1.0 | ||||
| Female | 1.1 (0.5–2.3) | 0.88 | -- | -- | |
| Male | |||||
| APACHE quartile | 1.0 | ||||
| <16 | 1.0 (0.3–3.8) | 0.98 | -- | -- | |
| ≥16, <21 | 1.0 (0.3–3.8) | 0.98 | -- | -- | |
| ≥21, <27.5 | 1.6 (0.5–5.8) | 0.46 | -- | -- | |
| ≥ 27.5 | |||||
| Transfusion | 1.0 | ||||
| No | 2.4 (0.4–15.1) | 0.34 | -- | -- | |
| Yes | |||||
| Ventilator | 1.0 | 1.0 | |||
| No | 4.5 (1.5–14.1) | 0.009 | 10.2 (1.9–55.5)~ | 0.007 | |
| Yes | |||||
| Hospital Stay Variable | |||||
| Major Infection | No | 1.0 | 1.0 | ||
| Yes | 4.8 (2.1–10.7) | <0.001 | 3.0 (1.1–8.4)* | 0.04 | |
| CMV viremia at any level | No | 1.0 | 1.0 | ||
| Yes | 4.6 (2.0–10.3) | <0.001 | 4.3 (1.6–11.9)* | 0.005 | |
| CMV viremia >1000 copies/ml | No | 1.0 | 1.0 | ||
| Yes | 7.8 (2.0–29.7) | 0.003 | 13.9 (3.2–60.9)* | <0.001 | |
| Maximum CMV load | Log10 | 1.8 (1.4–2.3) | <0.0001 | 1.8 (1.3–2.4)* | <0.001 |
| Average CMV AUC | Log10/day | 1.8 (1.3–2.7) | 0.001 | 2.1 (1.4–3.2)* | <0.001 |
| Proportion of transfusion days | 10% increments | 1.2 (0.8–1.7) | 0.43 | -- | -- |
| Proportion of ventilator days | 10% increments | 1.2 (1.0–1.3) | 0.01 | 1.3 (1.1–1.7)* | 0.01 |
Adjusted for baseline ventilator.
Adjusted for unit.
Adjusted for unit and baseline ventilator.
Figure 2. Predicted probability of death or continued hospitalization by day 30 as a function of average CMV AUC, adjusted for unit and baseline ventilator use.
Note: The predicted probabilities of death or continued hospitalization were estimated from a logistic regression model of average CMV AUC adjusted for unit and baseline ventilator use.
Risk factors for increased length of hospitalization
We used the variable “seven-day CMV moving average” to model the short-term effects of higher CMV viral load on the odds of staying longer in the hospital. In addition, we used the “average CMV AUC” to model the long-term effects; i.e., the lasting effects of previous high viral loads on length of hospitalization. Table 5 shows that overall, a higher CMV moving average over the previous seven days or average CMV AUC was associated with an increased hospital LOS. For example, for each log-10 copy/ml increase in viral load over the previous seven days, there was a 5.1-fold increased odds of being hospitalized for more than 14 days; similarly, for each log-10 increase in viral load seven-day moving average, there was a 2.8-fold increased odds of being hospitalized for more than 28 days. This association did not remain significant for the more extreme LOS (i.e., for LOS greater than either 42 or 56 days). The average CMV AUC was also associated with an increased odds of continued hospitalization, regardless of when during the hospital stay this parameter was assessed (Table 5).
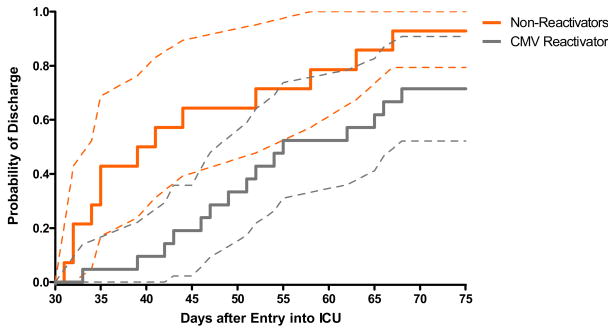
To assess the impact of CMV reactivation on length of stay in a group who was uniformly monitored for CMV reactivation, we performed a landmark analysis and assessed the cumulative incidence of time to discharge among the 35 patients who were still hospitalized by day 30 after admission. Patients were categorized as CMV reactivators if they tested positive by PCR prior to day 30. Figure 3 shows that the hazard of discharge is significantly greater in non-reactivators as compared to reactivators (p-value = 0.03 by log-rank test). The median length of stay after day 30 in reactivators (n=21) was 24 days (range 3–64) compared to 10 days (range1–151) in non-reactivators (n=14).
Figure 3. Cumulative incidence (with 95% confidence intervals) of discharge from hospital after 30 days according to CMV reactivation status.
Note: Figure 3 shows probability of discharge after day 30 among 35 patients still hospitalized by day 30. Patients were categorized as CMV reactivators if they tested positive by PCR prior to day 30. Log-rank test p-value=0.03.
Discussion
Using a prospective, blinded study design and rigorous statistical analyses in a broad range of immunocompetent patients with critical illness, we demonstrated that reactivation of CMV occurs frequently and is independently and quantitatively associated with a clinically-relevant endpoint of continued hospitalization or death by 30 days after admission to the ICU. Thus, we have identified a novel and potentially modifiable risk factor for death or prolonged hospitalization in critically-ill patients.
Given the number, complexity, potential bi-directional relationships between CMV and other variables analyzed, and the time-varying nature of the endpoints, we used a variety of statistical methods to comprehensively assess the relationship between CMV and adverse clinical outcomes. These included use of partial proportional odds models, use of a novel parameter of seven-day moving average of CMV viral load throughout the hospital stay, and use of a composite endpoint of death or continued hospitalization by 30 days. In particular, use of the composite endpoint was objective, clinically relevant and one that could be used as a primary endpoint in subsequent interventional studies of CMV prevention in this setting. Furthermore, the composite endpoint (rather than use of only length of stay alone) was used to reduce the potential impact that early deaths might have on assessment of the relationship between CMV reactivation and LOS. Similarly, use of the partial proportional odds models allowed us to control for the observed relationship between length of stay and onset of CMV reactivation, thereby allowing the relationship of CMV reactivation and subsequent LOS to be assessed throughout the hospital stay. In addition, given the concern that longer LOS would lead to a greater opportunity to detect CMV reactivation (and thus potentially lead to a spurious association between CMV reactivation and LOS), we performed a landmark analysis among those who were hospitalized for at least 30 days (a time-point by which 95% of those who ultimately ever reactivated CMV had done so, and also a subset who all had a uniform duration of monitoring for CMV). And, as in the previous analyses, CMV reactivation was associated with longer durations of subsequent hospitalization compared to those who did not reactivate by day 30 (Figure 3). The association between CMV reactivation and prolonged hospitalization or death remained robust throughout all of the analyses. Thus, our data are consistent with the possibility that CMV reactivation is causally related to prolongation of hospital stay in this clinical setting and this contention is also supported by animal studies.28 However, we are careful to emphasize that an observational study design cannot establish causality, and that the data presented here are also consistent with the possibility that CMV reactivation is simply a marker (rather than determinant) for prolonged hospital stay. Importantly, we did not find an association between severity of illness (as assessed by the APACHE score) and risk of CMV reactivation, thereby diminishing the likelihood that CMV reactivation was simply a surrogate marker of illness severity.
The only definitive means of differentiating between a role of CMV as a cause versus marker for adverse clinical outcomes is by means of a randomized controlled trial of antiviral prophylaxis in this clinical setting. Given the major importance of the clinical problem, the availability of generally safe and well-tolerated antiviral agents with activity against CMV, combined with the data regarding CMV incidence and endpoint estimates generated in this study, we feel that a randomized placebo-controlled trial of antiviral prophylaxis is both warranted and feasible, and should be a priority among studies to improve the outcomes of patients with critical illness.
The mechanism(s) underlying the observed association between CMV and adverse clinical outcomes are not defined in the present study. One possibility is direct CMV pathogenicity and this has previously been reported in the setting of otherwise immunocompetent patients with critical illness, but appears to be uncommon.16 Another possibility is that one or more CMV indirect effects are responsible for the observed association between CMV reactivation and adverse clinical outcomes. CMV-mediated immunosuppression leading to an increased risk for secondary infections 2–5 and CMV-mediated lung injury 28, 29 are the most plausible mechanisms in this clinical setting. In support of these possibilities are in vitro and animal model experimental data,28, 30, 31 clinical observational studies1, 9, 32 and the demonstration that antiviral therapy reduces these effects in animal models28 and in controlled clinical trials in certain patient populations.2–5 Larger prospective studies that include laboratory investigations will be necessary to define the mechanism(s) underlying the association of CMV reactivation with adverse clinical outcomes in patients with critical illness.
There were several strengths of the present study, including the prospective, blinded study design, inclusion of a broad range of critically-ill patients, use of quantitative CMV assessments, and the use of comprehensive statistical analyses with an adequate number and frequency of clinically-relevant endpoints. This is the largest study conducted to date and the results are statistically robust. It is reassuring that factors previously reported to be associated with increased LOS (bacteremia, pneumonia) were confirmed to be associated with LOS in the present study.33–35 We also acknowledge potential limitations. Monitoring for CMV reactivation was not performed in discharged patients, and while we think it is unlikely, it is possible that some discharged patients may have first reactivated CMV after hospital discharge. Although this would not have altered the statistical assessment of the association between CMV and LOS, it would make it more difficult to conclude that CMV was having a biologically significant impact in this clinical setting. There is also the potential concern that the association between CMV reactivation and prolonged hospital stay could, in part, be related to a greater opportunity to detect CMV reactivation in those with longer hospital stays (i.e., “circular reasoning”). However, the known biological time-lag of CMV effects in other settings, the quantitative nature of the association demonstrated in the present study, and the consistent finding of the association between CMV reactivation and prolonged LOS in the landmark analysis and partial proportional odds models (both of which directly addressed the time-dependent nature of CMV reactivation) all support the contention that CMV reactivation was associated with prolongation of hospital stay rather than a spurious finding. We are careful to emphasize that our study design (or any observational study design) cannot prove causality between CMV and adverse clinical outcomes in this setting. Rather, we consider these results to be hypothesis-generating and provide useful background data, which when combined with prior investigations, provide the rationale for performing definitive interventional studies. Even though a strong association between CMV reactivation and prolonged length of stay was identified, the mechanism(s) underlying this association could not be defined in this study. And, not all variables previously reported to be associated with an increased LOS were examined in the present study.
In summary, we have demonstrated an independent and quantitative association between CMV viral load and prolonged length of stay in a broad range of immunocompetent patients with critical illness. These findings, combined with data from prior investigations, provide a strong rationale for a randomized controlled trial of antiviral prophylaxis in this clinical setting.
Table 6.
Partial proportional odds model results for the association of seven-day CMV moving average or average CMV AUC with hospital length of stay, adjusted for unit
| LOS in Days |
||||
|---|---|---|---|---|
| ≥ 14 | ≥ 28 | ≥ 42 | ≥ 56 | |
| OR (95% CI) p-value |
OR (95% CI) p-value |
OR (95% CI) p-value |
OR (95% CI) p-value |
|
| CMV Seven-Day Moving Average, per log-10 copies/ml of viral load | 5.1 (2.9–9.1) p<0.0001 |
2.8 (1.5–5.4) p<0.01 |
1.7 (0.8–3.5) p=0.18 |
1.1 (0.5–2.5) p=0.78 |
| Average AUC of CMV, per log-10 copies/ml of viral load | 3.2 (2.1–4.7) p<0.0001 |
3.47 (2.2–5.7) p<0.0001 |
3.2 (1.9–5.4) p<0.0001 |
3.1 (1.7–5.6) p<0.0001 |
Acknowledgments
This investigator-initiated study was supported in part by Roche Laboratories, Inc. A. Limaye has received research funding and speaking and/or consulting fees from Roche Laboratories, Genentech Inc. and research funding from Viropharma, Inc. M. Boeckh has received research funding, speaking and consulting fees from Roche Laboratories and Viropharma, Inc, research funding and consulting fees from Novartis, research funding from Vical, Inc. and consulting fees from AiCuris AG, Chimerix Inc., and Alpha Vax Inc.. No other disclosures or compensation for the other authors. The funding sources for the study had no role in the study design, collection, analysis, or interpretation of data, writing of the report, or in the decision to submit the study for publication. L. Corey has received grant support from GlaxoSmithKline and Novartis, two companies that make antiviral drugs for the treatment of herpesviruses. However, he receives no salary support from these studies.
We are grateful to Lucretia Granger, B.Sc. for coordinating the study and to Elaine Brooks for assistance with manuscript preparation.
Footnotes
Presented in part at the American Thoracic Society International Conference (Abstract #406), San Francisco, CA, May 2007
Contributions
A. Limaye had full access to the data. The study was designed jointly by A. Limaye and M. Boeckh. A. Limaye was responsible for patient recruitment and data collection. E. Bulger, N. Gibran, and M. Neff provided critical input for the study design, patient selection, and recruitment. M.H. Huang and T.K. Santo performed the CMV PCR assays and L. Corey provided resources for PCR testing. A. Limaye, K. Kirby, M. Boeckh, and W. Leisenring analyzed the data with critical input from G.D. Rubenfeld. The paper was drafted by A. Limaye and M. Boeckh with input from all other authors. All authors reviewed and approved the final version.
References
- 1.George MJ, Snydman DR, Werner BG, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med. 1997 Aug;103(2):106–113. doi: 10.1016/s0002-9343(97)80021-6. [DOI] [PubMed] [Google Scholar]
- 2.Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet. 2005 Jun 18–24;365(9477):2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 3.Kalil AC, Levitsky J, Lyden E, Stoner J, Freifeld AG. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005 Dec 20;143(12):870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Small LN, Lau J, Snydman DR. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006 Oct 1;43(7):869–880. doi: 10.1086/507337. [DOI] [PubMed] [Google Scholar]
- 5.Strippoli GF, Hodson EM, Jones C, Craig JC. Preemptive treatment for cytomegalovirus viremia to prevent cytomegalovirus disease in solid organ transplant recipients. Transplantation. 2006 Jan 27;81(2):139–145. doi: 10.1097/01.tp.0000183970.71366.da. [DOI] [PubMed] [Google Scholar]
- 6.Manez R, Breinig MC, Linden P, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997 Dec;176(6):1462–1467. doi: 10.1086/514142. [DOI] [PubMed] [Google Scholar]
- 7.Kalil RS, Hudson SL, Gaston RS. Determinants of cardiovascular mortality after renal transplantation: a role for cytomegalovirus? Am J Transplant. 2003 Jan;3(1):79–81. doi: 10.1034/j.1600-6143.2003.30114.x. [DOI] [PubMed] [Google Scholar]
- 8.Valantine HA, Gao SZ, Menon SG, et al. Impact of prophylactic immediate posttransplant ganciclovir on development of transplant atherosclerosis: a post hoc analysis of a randomized, placebo-controlled study. Circulation. 1999 Jul 6;100(1):61–66. doi: 10.1161/01.cir.100.1.61. [DOI] [PubMed] [Google Scholar]
- 9.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006 Jun 27;81(12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 10.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004 Jul;66(1):329–337. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 11.Lowance D, Neumayer HH, Legendre CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N Engl J Med. 1999 May 13;340(19):1462–1470. doi: 10.1056/NEJM199905133401903. [DOI] [PubMed] [Google Scholar]
- 12.Bale JF, Jr, Kealey GP, Massanari RM, Strauss RG. The epidemiology of cytomegalovirus infection among patients with burns. Infect Control Hosp Epidemiol. 1990 Jan;11(1):17–22. doi: 10.1086/646073. [DOI] [PubMed] [Google Scholar]
- 13.Cook CH, Martin LC, Yenchar JK, et al. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit Care Med. 2003 Jul;31(7):1923–1929. doi: 10.1097/01.CCM.0000070222.11325.C4. [DOI] [PubMed] [Google Scholar]
- 14.Cook CH, Yenchar JK, Kraner TO, Davies EA, Ferguson RM. Occult herpes family viruses may increase mortality in critically ill surgical patients. Am J Surg. 1998 Oct;176(4):357–360. doi: 10.1016/s0002-9610(98)00205-0. [DOI] [PubMed] [Google Scholar]
- 15.Domart Y, Trouillet JL, Fagon JY, Chastre J, Brun-Vezinet F, Gibert C. Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest. 1990 Jan;97(1):18–22. doi: 10.1378/chest.97.1.18. [DOI] [PubMed] [Google Scholar]
- 16.Heininger A, Jahn G, Engel C, Notheisen T, Unertl K, Hamprecht K. Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med. 2001 Mar;29(3):541–547. doi: 10.1097/00003246-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Jaber S, Chanques G, Borry J, et al. Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest. 2005 Jan;127(1):233–241. doi: 10.1378/chest.127.1.233. [DOI] [PubMed] [Google Scholar]
- 18.Kealey GP, Bale JF, Strauss RG, Massanari RM. Cytomegalovirus infection in burn patients. J Burn Care Rehabil. 1987 Nov–Dec;8(6):543–545. [PubMed] [Google Scholar]
- 19.Kutza AS, Muhl E, Hackstein H, Kirchner H, Bein G. High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis. 1998 May;26(5):1076–1082. doi: 10.1086/520307. [DOI] [PubMed] [Google Scholar]
- 20.von Muller L, Klemm A, Weiss M, et al. Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis. 2006 Oct;12(10):1517–1522. doi: 10.3201/eid1210.060411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chastre J, Fagon JY, Bornet-Lecso M, et al. Evaluation of bronchoscopic techniques for the diagnosis of nosocomial pneumonia. Am J Respir Crit Care Med. 1995 Jul;152(1):231–240. doi: 10.1164/ajrccm.152.1.7599829. [DOI] [PubMed] [Google Scholar]
- 22.Baker SP, O’Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974 Mar;14(3):187–196. [PubMed] [Google Scholar]
- 23.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985 Oct;13(10):818–829. [PubMed] [Google Scholar]
- 24.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004 Mar;42(3):1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 26.McCullagh P. Regression models for ordinal data (with discussion) Journal of the Royal Statistical Society, Series B. 1980;42:109–142. [Google Scholar]
- 27.Peterson B, Harrell FE., Jr Partial proportional odds models for ordinal response variables. Applied Statistics. 1990;39:205–217. [Google Scholar]
- 28.Cook CH, Zhang Y, Sedmak DD, Martin LC, Jewell S, Ferguson RM. Pulmonary cytomegalovirus reactivation causes pathology in immunocompetent mice. Crit Care Med. 2006 Mar;34(3):842–849. doi: 10.1097/01.ccm.0000201876.11059.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998 Jun 11;338(24):1741–1751. doi: 10.1056/NEJM199806113382407. [DOI] [PubMed] [Google Scholar]
- 30.Cook CH, Zhang Y, McGuinness BJ, Lahm MC, Sedmak DD, Ferguson RM. Intra-abdominal bacterial infection reactivates latent pulmonary cytomegalovirus in immunocompetent mice. J Infect Dis. 2002 May 15;185(10):1395–1400. doi: 10.1086/340508. [DOI] [PubMed] [Google Scholar]
- 31.Cook CH, Trgovcich J, Zimmerman PD, Zhang Y, Sedmak DD. Lipopolysaccharide, tumor necrosis factor alpha, or interleukin-1beta triggers reactivation of latent cytomegalovirus in immunocompetent mice. J Virol. 2006 Sep;80(18):9151–9158. doi: 10.1128/JVI.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Price LS, Slifkin M, Ruthazer R, et al. The clinical impact of ganciclovir prophylaxis on the occurrence of bacteremia in orthotopic liver transplant recipients. Clin Infect Dis. 2004 Nov 1;39(9):1293–1299. doi: 10.1086/425002. [DOI] [PubMed] [Google Scholar]
- 33.Beyersmann J, Gastmeier P, Grundmann H, et al. Use of multistate models to assess prolongation of intensive care unit stay due to nosocomial infection. Infect Control Hosp Epidemiol. 2006 May;27(5):493–499. doi: 10.1086/503375. [DOI] [PubMed] [Google Scholar]
- 34.Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. Jama. 1994 May 25;271(20):1598–1601. doi: 10.1001/jama.271.20.1598. [DOI] [PubMed] [Google Scholar]
- 35.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005 Oct;33(10):2184–2193. doi: 10.1097/01.ccm.0000181731.53912.d9. [DOI] [PubMed] [Google Scholar]