Abstract
Women are more likely than men to relapse after initiating abstinence from cigarette smoking. The reasons for this phenomenon are unclear but may relate to negative mood, cigarette craving, or other symptoms of nicotine withdrawal. We addressed this issue in a study of 26 female and 38 male smokers. The Profile of Mood States, Shiffman–Jarvik Withdrawal Scale, and Urge to Smoke Scale were administered twice in each of two test sessions on different days. One session began within 1 hr after smoking ad libitum and the other followed overnight abstinence (>13 hr). On each test day, the two assessment blocks were separated by a 5–10-min break, during which each participant smoked one cigarette. In the first test block, both men and women reported higher scores after >13 hr abstinence than after <1 hr abstinence on the tension–anxiety and anger–hostility subscales of the Profile of Mood States, and for the craving and psychological symptoms of the Shiffman–Jarvik Withdrawal Scale. Scores of female subjects showed significantly larger differences between sessions on the tension–anxiety subscale and a trend toward significance (p=.050) on the anger–hostility subscale of Profile of Mood States than those of males. Moreover, on the tension–anxiety subscale, women also reported a greater reduction than men from smoking one cigarette after overnight abstinence. The findings indicate that overnight abstinence produces more negative mood symptoms and cigarette craving in female smokers than in males, and that resumption of smoking produces greater relief from these symptoms in female smokers. These differences may contribute to the greater likelihood of relapse when women try to quit smoking.
Introduction
Most studies of gender effects on cigarette smoking cessation have demonstrated that tobacco-dependent women are less likely to initiate abstinence and more likely to relapse than men (Bohadana, Nilsson, Rasmussen, & Martinet, 2003; Pogun, 2001; Pomerleau et al., 2005; Ward, Klesges, Zbikowski, Bliss, & Garvey, 1997; Wetter et al., 1999), although such a gender-based difference has not been observed universally (Gonzales et al., 2002; Gritz et al., 1998; Killen, Fortmann, Varady, & Kraemer, 2002). It has been proposed that negative reinforcement plays an important role in nicotine dependence, and that smokers use cigarettes to avoid nicotine withdrawal (Baker, Brandon, & Chassin, 2004; Eissenberg, 2004; Kenford et al., 2002; Piasecki, 2006; Piper et al., 2004). This view is supported by observations that: (a) dependent smokers experience nicotine withdrawal within a few hours of initiating abstinence from cigarette smoking (Kenford et al., 2002; Schuh & Stitzer, 1995); (b) cigarette smoking relieves nicotine withdrawal (Gentry, Hammersley, Hale, Nuwer, & Meliska, 2000; Powell, Pickering, Dawkins, West, & Powell, 2004); (c) the severity of nicotine withdrawal predicts the likelihood of relapse in smokers who try to quit (al’Absi, Hatsukami, Davis, & Wittmers, 2004; Hall et al., 1996; Piasecki, Fiore, McCarthy, & Baker, 2003; Swan, Ward, & Jack, 1996); and (d) the degree to which withdrawal is reduced after resumption of smoking predicts the likelihood of relapse (Perkins et al., 2002a; Piasecki et al., 2003).
Considering these findings, one might expect female smokers to experience more severe nicotine withdrawal than males upon initiating smoking cessation, and to enjoy a greater relief from withdrawal than males after resuming cigarette smoking. The core symptoms of nicotine withdrawal include depression, anxiety, tension, craving for cigarette smoking, and difficulty concentrating (American Psychiatric Association, 1994). Many studies found that female smokers exhibit more severe signs of depression, anxiety, anger, and craving for cigarettes than males after more than a few hours of abstinence from smoking (Abdullah, Lam, Chan, & Hedley, 2006; al’Absi, Amundrud, & Wittmers, 2002; Hogle & Curtin 2006; Jacobsen et al., 2005; Pogun, 2001; Strong, Kahler, Ramsey, Abrantes, & Brown, 2004); other studies, however, did not find such differences (Pomerleau et al., 2005; Svikis, Hatsukami, Hughes, Carroll, & Pickens, 1986). The reasons for these discrepancies are not clear but may include differences in the duration of abstinence and/or in the severity of nicotine dependence among participants, and reliance upon relatively modest sample sizes. Only a few studies reported gender differences in relief of nicotine withdrawal from smoking, and the findings were mixed. In one report, male and female smokers who were abstinent from smoking overnight showed no differences in relief of nicotine withdrawal after smoking a cigarette by taking puffs according to experimental rules (Perkins, Jacobs, Sanders, & Caggiula, 2002b), while another reported that after more than three days of abstinence from smoking, male smokers enjoyed a greater hedonic effect of smoking than female smokers (Pomerleau et al., 2005). Women also reportedly had a greater decrease in “desire to smoke” than men after smoking two cigarettes of their preferred brand, even though they took smaller and shorter puffs than men (Eissenberg, Adams, Riggins, & Likness, 1999).
Overall, the majority of evidence indicates that female smokers suffer greater nicotine withdrawal symptoms than male smokers when trying to quit, but it has been less clear whether females enjoy a greater relief of nicotine withdrawal after resuming cigarette smoking. Clarifying this point is important for determining the contribution of negative reinforcement to the greater risk of relapse for abstinent female vs. male smokers. In this study, we assessed indices of mood, craving for cigarettes, and other nicotine withdrawal signs in male and female smokers both before and after smoking a cigarette on two separate days (following either <1 hr abstinence or >13 hr overnight abstinence). In line with the hypothesized role of negative reinforcement and previous findings in the literature, we predicted that, after overnight abstinence from smoking, females would exhibit more severe nicotine withdrawal signs than males, including self-reports of negative mood and craving, and that they would enjoy greater relief from these symptoms than males after smoking a cigarette.
Method
Participants
Potential participants were recruited through flyers and newspaper ads in the greater Los Angeles area. They were excluded during an initial telephone interview if they indicated any one of the following conditions: smoking <15 or >40 cigarettes/day, smoking regularly <2 years, current use of medications that may affect cognition, any medical or psychiatric condition that could affect brain function, age <18 or >55 years, a history of head trauma, smoking >1 marijuana cigarette per week, using >10 standard drinks of alcohol per week (one standard drink consists of one 12-oz. beer, 6 oz. of wine, or one shot [1.5 oz.] of hard liquor [80 proof]), or regularly using substances of abuse other than alcohol or marijuana.
Participants received a detailed explanation of the study, signed a consent form that was approved by the UCLA Institutional Review Board, and were paid for their participation. During a baseline assessment, additional measures were obtained to determine eligibility. Carbon monoxide (CO) in expired air was taken as an objective measure of recent smoking (Micro Smokerlyzer II, Bedfont Scientific Instruments), with an inclusion criterion of ≥10 ppm at the time of screening. Participants also completed questionnaires assessing medical history, smoking history, including the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991), depressive symptoms (Beck Depression Inventory, BDI; Beck and Steer, 1987), and demographic information.
Procedure
Subsequent to baseline assessments, subjects participated in two test sessions, each on a different day. They reported to the laboratory in the afternoon after maintaining abstinence from smoking overnight (>13 hr abstinence) on one test day, and on another day when they had smoked ad libitum until 15–60 min before testing (<1 hr abstinence). The two test sessions were separated by 2–14 days, and their sequence was counterbalanced among the participants.
A female research assistant administrated the tests. In each test session, the participant first provided a breath sample for assay of CO and completed the following questionnaires related to mood, nicotine withdrawal, and cigarette craving: Profile of Mood States (McNair, Lott, & Droppleman, 1971), Shiffman–Jarvik Withdrawal Scales (Shiffman & Jarvik, 1976), and Urge To Smoke (Jarvik et al., 2000), and performed a battery of cognitive tests including Stroop Color-Word Naming Task, Emotional (smoking) Stroop Task, N-Back Working Memory Task, d2 Task, and Digit-Symbol Task for ~1 hr (data not presented in this report). Then he or she took a 5–10-min break outside the building and smoked one cigarette (usual brand). The participant then repeated the test battery. CO was measured and the Profile of Mood States, Shiffman–Jarvik Withdrawal Scale, and Urge to Smoke Scale were administered again at the end of the second test battery. Some data from the cognitive tests have been reported (Mendrek et al., 2006), and additional data from cognitive tests are to be reported elsewhere.
All testing sessions took place between 14:00 hr and 17:00 hr. The first measurement of expired CO ≤10 ppm on the day following >13 hr abstinence verified compliance with the requirement of abstinence from smoking. One participant was allowed to complete the session following overnight abstinence with an expired CO level of 13 ppm because this value was less than half of the value when this participant was tested after smoking ad libitum (<1 hr abstinence).
Measures
The Profile of Mood States and Shiffman–Jarvik Withdrawal Scale were used to assess mood states and nicotine withdrawal symptoms, as in previous reports (Hughes 2006; Jarvik et al., 2000; Knott, Bosman, Mahoney, Ilivitsky, & Quirt, 1999; Patten & Martin, 1996; Snively, Ahijevych, Bernhard, & Wewers, 2000). The Profile of Mood States consists of six subscales, collectively including 65 five-point items that describe mood, and these items are used to calculate a score for total mood disturbance. The range of possible scores is −32 to +200 for Total Mood Disturbance, calculated by subtracting the score for the vigor–activity subscale from the sum of scores for the remaining five subscales). The possible scores for the six subscales are as follows: tension–anxiety (0–36), depression–dejection (0–60), anger–hostility (0–48), vigor–activity (0–32), fatigue–inertia (0–28), and confusion–bewilderment (0–28). For the Total Mood Disturbance and all subscales, except for vigor–activity, a higher score indicates a more negative mood state.
The Shiffman–Jarvik Withdrawal Scale consists of 25 seven-point items, which are used to determine the scores for five subscales (i.e., craving, psychological, physical, stimulation/sedative, and appetite symptoms) and a total withdrawal score. The maximum score possible on each of the five Shiffman–Jarvik Withdrawal Scale subscales is 7, and the highest possible total score is 35. In each case, a higher score indicates more severe withdrawal. The Urge to Smoke Scale was used to assess craving for cigarette smoking. The highest possible score is 7, and higher scores indicate greater craving.
Data analysis
The CO measures and scores for the Profile of Mood States (total mood disturbance and each subscale), Shiffman–Jarvik Withdrawal Scale, and Urge to Smoke Scale were analyzed separately using general linear modeling for repeated measures in SPSS (Norusis, 1993). These scores were dependent variables, test sessions and blocks were within-subject variables, and gender was a between-subject variable. Statistical analyses were performed to assess the main effect of gender, and two- and three-way interactions among gender, test session (>13 hr vs. <1 hr abstinence), and test block (pre- vs. post-smoking). When a significant (p<.05) interaction regarding a self-report was detected, a two-sample t-test was used to test for gender effects in the difference between the presmoking scores in the >13 hr abstinence session vs. corresponding scores in the <1 hr abstinence session; and on changes from the presmoking to the postsmoking scores in the >13 hr abstinence session. These two-sample tests help identify factors contributing to the interactions. Given the sample size, 75% power was available to detect an effect of gender (with α=.05, and two-tailed analysis) given a true effect size of .68. Therefore, power was only adequate for detecting medium to large underlying effects of gender (Cohen, 1992).
Results
Subjects
The male and female groups did not differ significantly in age, years of education, years of smoking, cigarettes smoked per day, and scores on the Fagerström Test for Nicotine Dependence and Beck Depression Inventory (Table 1).
Table 1.
Demographic information on male and female smokers
| Sex | n | Age (years), mean (SD) | Education (years), mean (SD) | Smoking history (years), mean (SD) | Cigarettes/day, mean (SD) | Fagerström score, mean (SD) | Beck Depression Inventory score, mean (SD) |
|---|---|---|---|---|---|---|---|
| Male | 38 | 36.8 (9.8) | 14.4 (2.2) | 16.6 (9.5) | 20.9 (6.5) | 5.4 (1.9) | 2.7 (3.5) |
| Female | 26 | 35.3 (11.6) | 14.3 (2.4) | 17.7 (10.8) | 20.5 (6.0) | 5.0 (3.4) | 1.5 (3.0) |
CO in expired air
CO showed main effects of session and block, and a two-way interaction of session by block (Tables 2 and 3). For both groups, CO was lower after >13 hr abstinence than after <1 hr abstinence, and in the first than in the second test block on both test days. CO also showed a greater increase related to smoking after >13 hr abstinence than after <1 hr abstinence. These measures confirmed that the participants adhered to abstinence from smoking as instructed. CO measures did not show gender differences.
Table 2.
Shiffman–Jarvik Withdrawal Scale, Urge to Smoke, and CO measures on male and female smokers in the two test sessions
| < 1 h Abstinence |
> 13 h Abstinence |
|||
|---|---|---|---|---|
| Presmoking | Postsmoking | Presmoking | Postsmoking | |
| Males | ||||
| Craving | 4.3 (1.2) | 3.9 (1.1) | 5.9 (1.0) | 4.2 (1.0) |
| Psychological | 2.6 (0.6) | 2.9 (0.5) | 3.5 (1.0) | 3.0 (0.7) |
| Physiological | 1.7 (.9) | 1.6 (0.7) | 2.1 (1.1) | 1.8 (.9) |
| Stimulus | 2.1 (1.2) | 2.9 (1.2) | 2.6 (1.2) | 3.1 (1.4) |
| Appetite | 3.8 (1.1) | 4.0 (1.1) | 4.2 (1.0) | 4.3 (.9) |
| Urge to Smoke scale | 3.4 (1.5) | 1.9 (1.4) | 5.6 (1.3) | 2.3 (1.3) |
| CO | 22.1 (10.1) | 24.59 (11.9) | 7.0 (4.9) | 13.6 (7.0) |
| Females | ||||
| Craving | 3.7 (.9) | 3.9 (1.2) | 6.2 (1.0) | 4.3 (1.2) |
| Psychological | 2.5 (.6) | 2.8 (0.8) | 4.3 (1.4) | 2.9 (1.1) |
| Physiological | 1.5 (0.6) | 1.4 (0.6) | 2.5 (1.4) | 1.7 (.9) |
| Stimulation | 2.5 (1.3) | 3.1 (1.3) | 2.6 (1.4) | 3.4 (1.9) |
| Appetite | 3.4 (1.0) | 4.1 (1.1) | 4.1 (1.3) | 4.2 (1.2) |
| Urge to Smoke scale | 2.9 (1.5) | 1.6 (.8) | 5.5 (1.7) | 2.3 (1.4) |
| CO | 21.6 (8.9) | 23.3 (7.4) | 6 (4.2) | 12.9 (4.6) |
Note. Values are means and standard deviations (in parentheses).
Table 3.
Statistical results of Profile of Mood States, Shiffman–Jarvik Withdrawal Scale, Urge to Smoke Scale, and CO measures on male and female smokers in the two test sessions
| Main effect |
Two-way interaction |
||||||
|---|---|---|---|---|---|---|---|
| Session | Block | Gender | Session × block | Session × gender | Block × gender | Three-way interaction | |
| Profile of Mood States | |||||||
| Tension–anxiety | *** | *** | *** | * | ** | ||
| Anger–hostility | *** | ** | ** | ||||
| Depression–dejection | * | * | ** | ||||
| Vigor–activity | * | * | * | * | |||
| Fatigue–inertia | |||||||
| Confusion–bewilderment | *** | ** | * | ||||
| Total mode disturbance | *** | ** | * | ||||
| Shiffman–Jarvik Withdrawal Scale | |||||||
| Craving | *** | *** | *** | ||||
| Psychological | *** | ** | *** | * | * | ||
| Physical | *** | ** | ** | * | |||
| Stimulation/sedative | * | *** | |||||
| Appetite | ** | ||||||
| Urge To Smoke Scale | *** | *** | *** | ||||
| CO | *** | *** | *** | ||||
Note. p<.05,
p<.01,
p<.001.
Profile of Mood States
Scores for the tension–anxiety subscale showed three-way interactions of gender by session by block, F(1,60)=7.47, p=.008 (Figure 1, Tables 3 and 4). Compared with scores on the day of ad libitum smoking, participants of both genders had higher scores in the presmoking test block after overnight abstinence (Tables 3 and 4); and two-sample t-tests showed that females reported greater differences between sessions than males, t(60)=2.39, p=.02. Females also reported a greater decrease in these scores than males after smoking a cigarette between test blocks following overnight abstinence, t(60)=−2.4, p=.01. Scores for the anger–hostility subscale of Profile of Mood States showed a trend toward a significant three-way interactions of gender by session by block, F(1,60)=2.5, p=.12. Scores for the vigor–activity subscale showed a two-way interaction of session by gender, F(1,60)=4.8, p=.03 (Tables 3 and 4).
Figure 1.
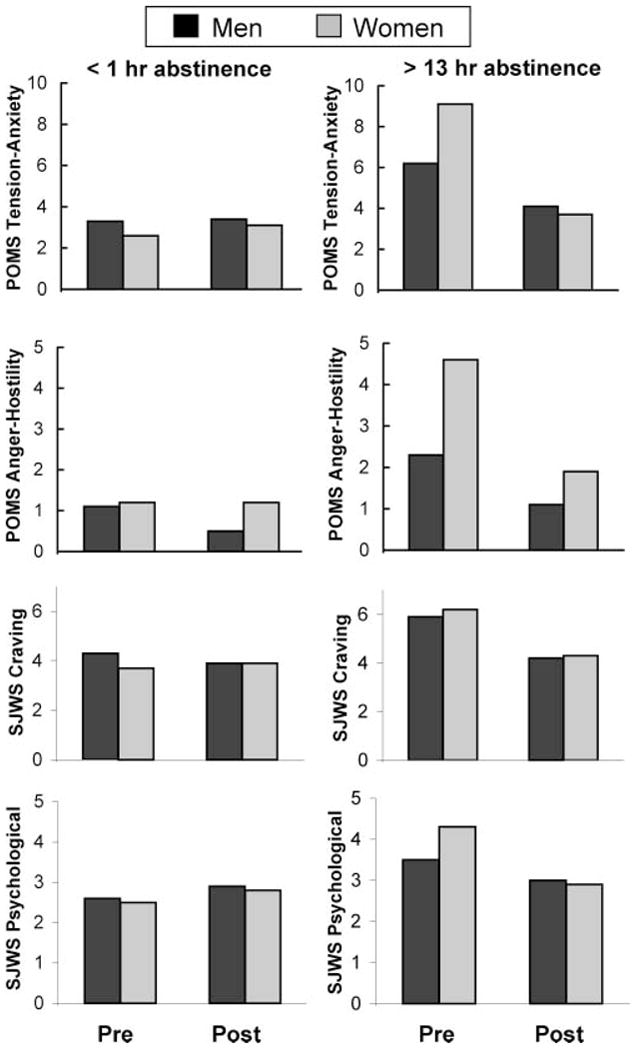
Three-way interaction (gender × session × block) of scores for Profile of Mood States subscales of tension–anxiety, F(1,60)=7.47, p=.008, and anger–hostility, F(1,60)=2.5, p=.12; and scores for Shiffman–Jarvik Withdrawal Scale subscales of craving for smoking, F(1,59)=3.40, p=.07, and psychological symptoms, F(1,59)=6.89, p=.011. These interactions indicated that female smokers exhibited greater changes in mood than male smokers after overnight abstinence and resumption of smoking. Abbreviation: pre: pre-smoking test; post: post-smoking test.
Table 4.
Profile of Mood States scales in the two test sessions
| <1 hr abstinence |
>13 hr abstinence |
|||
|---|---|---|---|---|
| Presmoking | Postsmoking | Presmoking | Postsmoking | |
| Males | ||||
| Tension–anxiety | 3.3 (2.5) | 3.4 (3.3) | 6.2 (5.7) | 4.1 (3.3) |
| Depression–dejection | 1.5 (2.4) | 1.1 (3.0) | 2.7 (5.1) | 1.1 (3.1) |
| Anger–hostility | 1.1 (2.1) | 0.5 (1.4) | 2.3 (3.6) | 1.1 (2.1) |
| Vigor–activity | 16.1 (7.8) | 14.5 (7.1) | 15.0 (7.7) | 14.3 (8.1) |
| Fatigue–inertia | 3.5 (5.6) | 4.4 (6.0) | 4.6 (6.5) | 4.8 (6.6) |
| Confusion–bewilderment | 2.7 (2.5) | 2.6 (2.4) | 3.7 (2.7) | 3.0 (2.3) |
| Total mood disturbance* | −7.1 (12.7) | −5.8 (14.1) | 2.1 (24.0) | −1.7 (15.9) |
| Females | ||||
| Tension–anxiety | 2.6 (2.7) | 3.1 (3.2) | 9.1 (6.3) | 3.7 (3.5) |
| Depression–dejection | 0.8 (1.7) | 1.3 (2.7) | 2.6 (3.8) | 1.3 (2.4) |
| Anger–hostility | 1.2 (2.4) | 1.2 (2.2) | 4.6 (5.2) | 1.9 (4.6) |
| Vigor–activity | 16.2 (7.8) | 13.3 (9.4) | 12.4 (8.5) | 11.8 (8.5) |
| Fatigue–inertia | 4.0 (5.0) | 3.7 (3.9) | 4.2 (5.1) | 3.9 (4.7) |
| Confusion–bewilderment | 2.8 (2.5) | 3.8 (4.0) | 4.8 (3.4) | 5.0 (4.9) |
| Total mood disturbance | −5.6 (13.0) | −2.23 (17.9) | 11.6 (23.6) | 5.16 (20.6) |
Note. Values are means and standard deviations (in parentheses).
The total mood disturbance score was calculated by subtracting the vigor–activity score from the sum of scores of tension–anxiety, depression–dejection, anger–hostility, fatigue–inertia, and confusion–bewilderment subscales.
Shiffman–Jarvik Withdrawal Scale and Urge to Smoke Scale
Scores for the psychological subscale showed a three-way interaction of gender by session by block, F(1,59)=6.89, p=.011, and a trend toward this direction for the craving subscale, F(1,59)=3.40, p=.07 (Figure 1, Tables 2 and 3). Both groups had significantly higher corresponding scores on these measures in the presmoking test block following overnight abstinence than in the session following ad libitum smoking (Tables 2 and 3); two-sample t-tests showed that females reported significantly greater differences in these scores than males [psychological subscale; t(60)=−2.4, p=.02; craving subscale, t(60)=−2.3, p=.02.] Moreover, females also reported a significantly greater decrease in score for psychological symptoms than males in the postsmoking test relative to the presmoking test after overnight abstinence t(59)=−2.6, p=.01. The mean Urge to Smoke Scale score revealed a significant main effect of session, F(1,52)=51.42, p=.001; there were no significant two-way (session by gender) or three-way (session by block by gender) interactions.
Discussion
This study yielded two main findings. The first one is that female smokers suffered more severe symptoms of negative mood than males after overnight abstinence, as reflected in self-reports of greater tension–anxiety subscales of Profile of Mood States and psychological symptoms of Shiffman–Jarvik Withdrawal Scale. The second is that female smokers, abstinent overnight, enjoyed a greater relief from these symptoms than their male counterparts after smoking of a cigarette of their preferred brand.
The observation that female smokers experienced more severe symptoms of negative mood than male smokers after overnight abstinence from cigarette smoking is consistent with previous reports on smokers who may not have been interested in quitting (Abdullah et al., 2006; al’Absi et al., 2002; Hogle & Curtin 2006; Jacobsen et al., 2005; Pogun, 2001; Strong et al., 2004) and also on smokers who were trying to quit (Piasecki, 2006; Piasecki et al., 2003; Wetter et al., 1999). Among smokers who attempted to quit cigarette smoking, females reported more severe withdrawal symptoms than males after the initiation of smoking cessation, and they showed higher rates of relapse than males (Piasecki, 2006; Piasecki et al., 2003; Wetter et al., 1999). Severity of negative mood is correlated with impulsivity (Leith & Baumeister, 1996; Tice, Bratslavsky, & Baumeister, 2001), craving for cigarette smoking (Niaura, Shadel, Britt, & Abrams, 2002; Zinser, Baker, Sherman, & Cannon, 1992), intolerance to stress (Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005; Vanderkaay & Patterson, 2006), and weaker coping skills for aversive events (Drobes, Meier, & Tiffany, 1994). In turn, craving for smoking, impulsivity, stress intolerance, and weakness of coping skills are correlated with likelihood of relapse to smoking (Barrios and Niehaus, 1985; Brown et al., 2005; Doran, Spring, McChargue, Pergadia, & Richmond, 2004; Gwaltney, Shiffman, Balabanis, & Paty, 2005).
Smoking one cigarette provided greater relief from nicotine withdrawal symptoms to female than to male smokers is consistent with a previous report that female smokers exhibited a greater reduction in craving and other nicotine withdrawal signs than males after smoking two cigarettes (Eissenberg et al., 1999). One study, however, reported that after more than three days of smoking abstinence, male smokers enjoyed a greater hedonic effect of smoking than female smokers (Pomerleau et al., 2005). This difference may reflect the substantially longer abstinence time in that study than in the present one. In another report, abstinent (overnight) female and male smokers did not differ in relief from nicotine withdrawal after controlled smoking of a cigarette provided by investigators (Perkins et al., 2002b). Here again, the methods differed from ours, and the experimentally controlled smoking of cigarettes that are not of the participant’s preferred brand can provide a different experience from uncontrolled smoking a cigarette chosen by the subject. Among smokers who attempted to quit, the extent of withdrawal reduction after cigarette smoking or nicotine administration was positively correlated with relapse (i.e., greater reduction predicted greater chance of relapse; Perkins et al., 2002a; Piasecki et al., 2003). The greater relief of nicotine withdrawal symptoms experienced by female smokers in the present study, therefore, may relate to the higher relapse rate of female smokers than male smokers.
In several previous studies, abstinent female smokers reported less reduction of withdrawal than their male counterparts after nicotine administration by routes other than smoking (e.g., via the patch or nasal spray) (Evans, Blank, Sams, Weaver, & Eissenberg, 2006; Perkins, Donny, & Caggiula, 1999; Robinson et al., 2007; Wetter et al., 1999). Females may be less sensitive than males to nicotine administered by routes other than smoking, but may be more sensitive to other smoking-related stimuli, such as visual, motor, and somatosensory components of the experience (Bohadana et al., 2003; Perkins, 2001; Pogun, 2001). Accordingly, substantial relief from negative mood symptoms in female smokers after smoking a cigarette of their preferred brand may enhance the expectancy of a positive outcome from cigarette smoking, and reinforce their dependence on smoking to avoid negative mood symptoms and cigarette craving associated with withdrawal. This may be especially true when abstinent smokers face aversive events or other stressors (Baker et al., 2004; Piper et al., 2004; Eissenberg, 2004; Kenford et al., 2002; Piasecki et al., 2002; Shiffman, Sweeney, & Dresler, 2005). In this regard, adding therapeutic manipulations to mimic the rewarding effects of physical stimuli other than nicotine itself but conditioned to cigarette smoking (e.g., de-nicotinized cigarettes) with available pharmacological therapies (e.g., nicotine replacement therapy, bupropion HCl, varenicline) may be particularly helpful for women initiating smoking cessation.
There are several limitations of the study. First, as the sample size is relatively small, additional study with a larger sample size is warranted. Second, as plasma nicotine concentration was not assayed, we cannot exclude the possibility that female smokers enjoyed a greater relief from nicotine withdrawal because within-session smoking provided them with more nicotine. Even though female smokers usually take lighter puffs than male smokers (Eissenberg et al., 1999; Pogun, 2001), they may still have higher levels of nicotine in plasma, considering that females have smaller plasma volumes on average than males. Third, the assessment of mood was solely based on self-report measures, which may suffer from low reliability and validity (Patten & Martin, 1996). The Profile of Mood States and the craving subscale of Shiffman–Jarvik Withdrawal Scale, however, showed high reliability and consistency in previous studies (Hughes, 2006; Patten & Martin, 1996). Objective physiological measures, which are related to negative affective states, should be incorporated into the design of future studies (e.g., electrodermal recording, startle reflex).
Cognitive social learning theory proposes that changes of human behavior are motivated by expectancy of positive outcome resulting from the change (Bandura & Adams, 1977; Marlatt, 1985). According to this theory, the likelihood of relapse for smokers who try to quit smoking is positively correlated with their expectancy of positive outcomes from cigarette smoking (i.e., withdrawal relief). Our findings indicate that, relative to their male counterparts, female smokers experienced more negative mood symptoms after overnight abstinence, as well as greater relief of these symptoms after cigarette smoking, a condition that promotes a greater expectancy of positive outcome from smoking. Thus, this study suggested that such negative reinforcement processes underlie at least a portion of the greater likelihood of relapse of female smokers than males after the initiation of smoking cessation.
Acknowledgments
Supported by NIH grants DA014093 (EDL), DA22364 (JX), DA015059 (ALB), UC Tobacco-Related Disease Research Program awards 10RT-0091 (EDL) and 11RT-0024 (ALB), VA Merit Review Type I Award (ALB), and Philip Morris USA (EDL). Dr. Xu was supported by a training grant (DA 07272), and clinical support was provided by the UCLA GCRC (RR 00865). The authors have no other conflicts of interest to report.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Jiansong Xu, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
Allen Azizian, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
John Monterosso, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
Catherine P. Domier, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
Arthur L. Brody, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
Edythe D. London, Department of Psychiatry and Biobehavioral Sciences, Department of Molecular and Medical Pharmacology, Brain Research Institute, and Biomedical Engineering Interdepartmental Faculty, University of California Los Angeles, Los Angeles, CA
Timothy W. Fong, Department of Psychiatry and Biobehavioral Sciences, University of California Los Angeles, Los Angeles, CA
References
- Abdullah AS, Lam TH, Chan SS, Hedley AJ. Smoking cessation among Chinese young smokers: Does gender and age difference matters and what are the predictors? Addictive Behaviors. 2006;31:913–921. doi: 10.1016/j.addbeh.2005.08.009. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology Biochemistry and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- American Psychiatric A. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Bandura A, Adams NE. Analysis of self-efficacy theory of behavioral change. Cognitive Therapy and Research. 1977;1:287–308. [Google Scholar]
- Barrios FX, Niehaus JC. The influence of smoker status, smoking history, sex, and situational variables on smokers’ self-efficacy. Addictive Behaviors. 1985;10:425–429. doi: 10.1016/0306-4603(85)90040-1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Manual for the Revised Beck Depression Inventory. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine & Tobacco Research. 2003;5:111–116. doi: 10.1080/1462220021000060482. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological Bulletin. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Doran N, Spring B, McChargue D, Pergadia M, Richmond M. Impulsivity and smoking relapse. Nicotine & Tobacco Research. 2004;6:641–647. doi: 10.1080/14622200410001727939. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Meier EA, Tiffany ST. Assessment of the effects of urges and negative affect on smokers’ coping skills. Behaviour Research and Therapy. 1994;32:165–174. doi: 10.1016/0005-7967(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T. Measuring the emergence of tobacco dependence: The contribution of negative reinforcement models. Addiction. 2004;99(Suppl 1):5–29. doi: 10.1111/j.1360-0443.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Adams C, Riggins EC, 3rd, Likness M. Smokers’ sex and the effects of tobacco cigarettes: Subject-rated and physiological measures. Nicotine & Tobacco Research. 1999;1:317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- Evans SE, Blank M, Sams C, Weaver MF, Eissenberg T. Transdermal nicotine-induced tobacco abstinence symptom suppression: Nicotine dose and smokers’ gender. Experimental and Clinical Psychopharmacology. 2006;14:121–135. doi: 10.1037/1064-1297.14.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry MV, Hammersley JJ, Hale CR, Nuwer PK, Meliska CJ. Nicotine patches improve mood and response speed in a lexical decision task. Addictive Behaviors. 2000;25:549–557. doi: 10.1016/s0306-4603(00)00072-1. [DOI] [PubMed] [Google Scholar]
- Gonzales D, Bjornson W, Durcan MJ, White JD, Johnston JA, Buist AS, Sachs DP, Rigotti NA, Niaura R, Hays JT, Hurt RD. Effects of gender on relapse prevention in smokers treated with bupropion SR. American Journal of Preventive Medicine. 2002;22:234–239. doi: 10.1016/s0749-3797(02)00419-1. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Thompson B, Emmons K, Ockene JK, McLerran DF, Nielsen IR. Gender differences among smokers and quitters in the Working Well Trial. Preventive Medicine. 1998;27:553–561. doi: 10.1006/pmed.1998.0325. [DOI] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, Paty JA. Dynamic self-efficacy and outcome expectancies: Prediction of smoking lapse and relapse. Journal of Abnormal Psychology. 2005;114:661–675. doi: 10.1037/0021-843X.114.4.661. [DOI] [PubMed] [Google Scholar]
- Hall SM, Muñoz RF, Reus VI, Sees KL, Duncan C, Humfleet GL, Hartz DT. Mood management and nicotine gum in smoking treatment: A therapeutic contact and placebo-controlled study. Journal of Consulting and Clinical Psychology. 1996;64:1003–1009. doi: 10.1037//0022-006x.64.5.1003. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Clinical significance of tobacco withdrawal. Nicotine & Tobacco Research. 2006;8:153–156. doi: 10.1080/14622200500494856. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology, Biochemistry, and Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70:216–227. [PubMed] [Google Scholar]
- Killen JD, Fortmann SP, Varady A, Kraemer HC. Do men outperform women in smoking cessation trials? Maybe, but not by much. Experimental and Clinical Psychopharmacology. 2002;10:295–301. doi: 10.1037//1064-1297.10.3.295. [DOI] [PubMed] [Google Scholar]
- Knott V, Bosman M, Mahoney C, Ilivitsky V, Quirt K. Transdermal nicotine: Single dose effects on mood, EEG, performance, and event-related potentials. Pharmacology, Biochemistry, and Behavior. 1999;63:253–261. doi: 10.1016/s0091-3057(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Leith KP, Baumeister RF. Why do bad moods increase self-defeating behavior? Emotion, risk taking, and self-regulation. Journal of Personality and Social Psychology. 1996;71:1250–1267. doi: 10.1037//0022-3514.71.6.1250. [DOI] [PubMed] [Google Scholar]
- Marlatt GA. Relapse prevention: Theoretical rationale and overview of the model. In: Marlatt GA, Gordon JR, editors. Relapse prevention: Maintenance strategies in the treatment of addictive behaviors. New York: Guilford; 1985. pp. 3–70. [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of Mood States (Manual) San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: Comparison to non-smokers and effects of abstinence. Addictive Behaviors. 2006;31:833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addictive Behaviors. 2002;27:241–250. doi: 10.1016/s0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE. Measuring tobacco withdrawal: A review of self-report questionnaires. Journal of Substance Abuse. 1996;8:93–113. doi: 10.1016/s0899-3289(96)90115-7. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Broge M, Gerlach D, Sanders M, Grobe JE, Cherry C, Wilson AS. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychology. 2002a;21:332–339. doi: 10.1037//0278-6133.21.4.332. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nicotine & Tobacco Research. 1999;1:301–315. doi: 10.1080/14622299050011431. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Jacobs L, Sanders M, Caggiula AR. Sex differences in the subjective and reinforcing effects of cigarette nicotine dose. Psychopharmacology (Berlin) 2002b;163:194–201. doi: 10.1007/s00213-002-1168-1. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clinical Psychology Review. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Have we lost our way? The need for dynamic formulations of smoking relapse proneness. Addiction. 2002;97:1093–108. doi: 10.1046/j.1360-0443.2002.00216.x. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Fiore MC, McCarthy DE, Baker TB. Smoking withdrawal dynamics: II. Improved tests of withdrawal-relapse relations. Journal of Abnormal Psychology. 2003;112:14–27. [PubMed] [Google Scholar]
- Piper ME, Piasecki TM, Federman EB, Bolt DM, Smith SS, Fiore MC, Baker TB. A multiple motives approach to tobacco dependence: The Wisconsin Inventory of Smoking Dependence Motives (WISDM-68) Journal of Consulting and Clinical Psychology. 2004;72:139–154. doi: 10.1037/0022-006X.72.2.139. [DOI] [PubMed] [Google Scholar]
- Pogun S. Sex differences in brain and behavior: Emphasis on nicotine, nitric oxide and place learning. International Journal of Psychophysiology. 2001;42:195–208. doi: 10.1016/s0167-8760(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS, Mehringer AM, Snedecor SM, Ninowski R, Sen A. Nicotine dependence, depression, and gender: Characterizing phenotypes based on withdrawal discomfort, response to smoking, and ability to abstain. Nicotine & Tobacco Research. 2005;7:91–102. doi: 10.1080/14622200412331328466. [DOI] [PubMed] [Google Scholar]
- Powell JH, Pickering AD, Dawkins L, West R, Powell JF. Cognitive and psychological correlates of smoking abstinence, and predictors of successful cessation. Addictive Behaviors. 2004;29:1407–1426. doi: 10.1016/j.addbeh.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addictive Behaviors. 2007;32:543–561. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte RH, Breteler MH. Withdrawal symptoms and previous attempts to quit smoking: Associations with self-efficacy. Substance Use and Misuse. 1997;32:133–148. doi: 10.3109/10826089709027303. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology (Berlin) 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Dresler CM. Nicotine patch and lozenge are effective for women. Nicotine & Tobacco Research. 2005;7:119–127. doi: 10.1080/14622200412331328439. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 1976;50:35–9. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: Results from single smoking sessions and the natural environment. Psychoneuroendocrinology. 2000;25:677–691. doi: 10.1016/s0306-4530(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Strong DR, Kahler CW, Ramsey SE, Abrantes A, Brown RA. Nicotine withdrawal among adolescents with acute psychopathology: An item response analysis. Nicotine & Tobacco Research. 2004;6:547–557. doi: 10.1080/14622200410001696484. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Hatsukami DK, Hughes JR, Carroll KM, Pickens RW. Sex differences in tobacco withdrawal syndrome. Addictive Behaviors. 1986;11:459–462. doi: 10.1016/0306-4603(86)90028-6. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of 28-day relapse in smokers. Addictive Behaviors. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: If you feel bad, do it! Journal of Personality and Social Psychology. 2001;80:53–67. [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: Effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biological Psychology. 2006;71:191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Ward KD, Klesges RC, Zbikowski SM, Bliss RE, Garvey AJ. Gender differences in the outcome of an unaided smoking cessation attempt. Addictive Behaviors. 1997;22:521–533. doi: 10.1016/s0306-4603(96)00063-9. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relation between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. Journal of Abnormal Psychology. 1992;101:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]


