Abstract
Matriptase is a type II transmembrane serine protease. This protease is strongly expressed in simple epithelial cells such as enterocytes and kidney tubular cells in which the plasma membranes are separated into apical and basolateral domains. Although matriptase was found previously to occur exclusively on the basolateral membrane of enterocytes, the underlying mechanism of localization is unclear. In the present study, a full-length rat matriptase and a chimera consisting of the cytoplasmic and transmembrane regions of the protease and green fluorescent protein (designated as 1–86GFP) were found to localize exclusively to the basolateral membrane domain when expressed in Madin–Darby canine kidney epithelial cells. Mutagenesis analysis of 1–86GFP revealed that the matriptase cytoplasmic juxtamembrane amino acid residues (Lys45, Val47, and Arg50) play a role in mediating the localization in the cells. This study provides the first evidence that matriptase carries information for its localization in simple epithelia.
Keywords: Basolateral localization, Cytoplasmic juxtamembrane region, Madin–Darby canine kidney epithelial cells, Matriptase, Type II transmembrane serine protease
Introduction
Matriptase is a type II transmembrane serine protease that is strongly expressed in simple epithelial cells such as enterocytes and kidney tubular cells (Satomi et al. 2001; Oberst et al. 2003; List et al. 2007). The sites of expression and the ability to activate single-chain urokinase-type plasminogen activator (Lee et al. 2000; Takeuchi et al. 2000; Satomi et al. 2001) and pro-hepatocyte growth factor (Lee et al. 2000) via proteolytic cleavage and to digest extracellular matrix components (Satomi et al. 2001), lead to the proposal that matriptase plays important roles in the epithelial-cell turnover, including proliferation, migration, differentiation, and death.
The plasma membranes of simple epithelial cells are characterized by two structurally and functionally different domains: the apical and basolateral domains. We found previously that matriptase is processed post-translationally by cleavage between Gly149 and Ser150 and that the N-terminal fragment (NTF, which contains the cytoplasmic and transmembrane regions) (Met1–Gly149; Fig. 1a) is present on the basolateral membranes but not on the apical membranes of enterocytes in the jejunum of adult rats (Tsuzuki et al. 2005). These findings suggest that when matriptase is expressed in intact tissues, it can interact with the potential substrates that occur on the basolateral sides of epithelium.
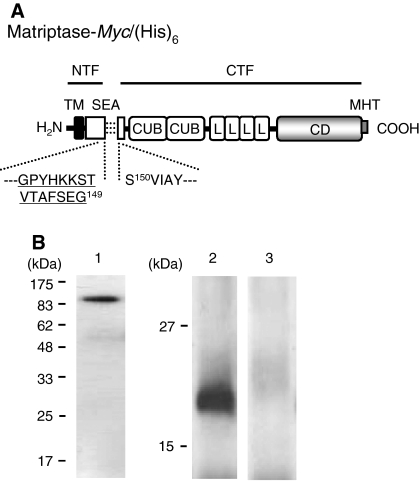
Fig. 1.
Processing of matriptase-Myc/(His)6 expressed in MDCK cells. a Schematic representation of matriptase-Myc/(His)6. Rat matriptase consists of 855 amino acids. The amino acid numbering starts from the putative N-terminus of the protein. Matriptase-Myc/(His)6 consists of all domains of rat matriptase with C-terminal Myc/(His)6-tag (MHT). The NTF (Met1–Gly149) and CTF (Ser150–Val855) parts are indicated with underlines, and the association is illustrated with three broken lines. The recognition site for Tmc172 is indicated by the amino acid sequence in the single-letter code underlined with amino acid numbering at the C-terminal residue (G149). The 5-amico-acid sequence of N-terminus of CTF (SVIAY) is also indicated with amino acid numbering at the N-terminal residue (S150). TM transmembrane domain; SEA sea-urchin sperm protein–enterokinase–agrin domain; CUB complement factor 1R-urchin embryonic growth factor-bone morphogenetic protein domain; L low-density lipoprotein receptor A modules domain; CD catalytic domain. b Western blot analysis of matriptase-Myc/(His)6. MDCK cells expressing matriptase-Myc/(His)6 cultured on a filter insert were extracted with Triton, and the recombinant matriptase was purified with Ni2+-charged resin and subjected to SDS–PAGE and Western transfer. The blot was probed with anti-Myc antibody (lane 1) or Tmc172 (lanes2 and 3). In lane 3, Tmc172 was preadsorbed with 200 μM antigenic peptide. The molecular sizes of protein markers (Tsuzuki et al. 2005) are indicated on the left in kilodaltons (kDa)
The underlying mechanism(s) by which matriptase localizes to the basolateral membrane domain of simple epithelial cells are unclear. It has been shown in a transient-expression system using human breast cancer BT549 cells that hepatocyte growth factor activator inhibitor type I (HAI-1), the cognate inhibitor of matriptase, is required for the proper intracellular trafficking of the protease (Oberst et al. 2005). Indeed, when expressed alone in this cell line, matriptase was retained in the endoplasmic reticulum and Golgi apparatus. In addition, HAI-1 is known to be present on the basolateral membrane domain of simple epithelia in intact tissues (Kataoka et al. 1999; Godiksen et al. 2008). These observations raise the possibility that the localization of matriptase in simple epithelia depends on HAI-1. On the other hand, matriptase was delivered to the extracellular environment when expressed alone in human embryonic kidney HEK293 cells (Désilets et al. 2008). Therefore, the possibility remains that the co-existence of HAI-1 is not necessarily required for the intracellular trafficking of matriptase in certain cell types.
The present study aimed to address whether matriptase carries information within the molecule for its localization to the basolateral membrane domain of simple epithelia. For this, we stably expressed rat matriptase and its mutants in Madin–Darby canine kidney (MDCK) epithelial cells and investigated their distribution. This cell line is thought to have originated from distal renal tubular epithelial cells, which also express matriptase (Oberst et al. 2003; List et al. 2007), and has been widely used as a model of simple epithelial cells (Bonifacino and Traub 2003). In the present study, we show that a recombinant matriptase occurs exclusively to the basolateral membrane domain when expressed in MDCK cells and provide evidence that the cytoplasmic juxtamembrane region plays an important role in mediating its localization in the cell line.
Materials and methods
Antibodies
The procedure for the production of a rabbit polyclonal antibody raised against a 15-amino-acid peptide corresponding to the C-terminus of matriptase NTF (Gly135–Gly149; Fig. 1a) was described previously (Tsuzuki et al. 2005). The antibody has been designated as Tmc172. A mouse anti-Myc-epitope-tag antibody (described hereafter as anti-Myc antibody) and goat anti-mouse and anti-rabbit IgG antibodies conjugated with AlexaFluor-488 were purchased from Invitrogen (Carlsbad, CA, USA). A mouse anti-Na,K-ATPase (α-subunit) antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA).
Construction of expression plasmids
The procedure for the construction of a plasmid using pcDNA3.1(+) (Invitrogen) for expression of full-length rat matriptase (Met1–Val855) in which a Myc-epitope/hexahistidine [Myc/(His)6] tag is fused at the C-terminus has been described previously (Satomi et al. 2001). In the present study, the expression plasmid and the expression product were designated pcDNAmatriptase-Myc/(His)6 and matriptase-Myc/(His)6 (Fig. 1a), respectively. A plasmid for 1–86GFP that contains the cytoplasmic and transmembrane regions of matriptase (Met1–Ile86), green fluorescent protein (GFP), and Myc/(His)6-tag (pcDNA1–86GFP) was created as follows: PIND-GFP (Invitrogen) was digested with PmeI, and the resulting 761-bp fragment containing GFP coding sequence was ligated into pcDNAmatriptase-Myc/(His)6 that had been digested with BamHI and XbaI, blunt-ended, and dephosphorylated. Plasmids for deletion mutant of 1–86GFP were created by PCR using pcDNA1–86GFP as the template. Plasmids for site-directed mutants in the context of 1–86GFP were created using LA PCR in vitro mutagenesis kit (Takara Bio, Ohtsu, Japan) with pcDNA1–86GFP as the template. The sequences of all the expression plasmids constructed in the present study were determined in both directions as described previously (Satomi et al. 2001).
Cell culture, transfection, and establishment of stably expressing transfectants
MDCK cells were purchased from RIKEN Bioresource Center (Ibaraki, Japan) and were maintained in minimal essential medium supplemented with 10% fetal bovine serum. Cells were transfected using LipofectAMINE2000 (Invitrogen) as described previously (Kojima et al. 2008). The cells grown in media containing 0.5 mg/mL G418 (Invitrogen) were pooled, propagated, and used for experiments.
Purification of matriptase-Myc/(His)6 from MDCK cells cultured on filter inserts
MDCK cells expressing matriptase-Myc/(His)6 were seeded on Transwell™ with polycarbonate filter inserts (24 mm diameter, 0.4 μm pore size; Costar Corning, Acton, MA, USA) at a density of 1 × 105 cells per well. When the transepithelial resistance of the monolayer exceeded 700 W/cm2, the cells were washed three times with phosphate-buffered saline (PBS, 8 mM Na2HPO4, 1.5 mM KH2PO4, 136 mM NaCl, 2.7 mM KCl, pH 7.4). Then, the polycarbonate filter was excised, transferred to a 35 mm plastic Petri dish (cell-binding side up), exposed to 0.5 mL of PBS containing 1% Triton X-100 and Complete™ (a protease inhibitor cocktail, Roche Diagnostics, Mannheim, Germany), and incubated for 5 min on ice. Cells in each dish were scraped along with the Triton solution, transferred to a 1.5 mL microcentrifuge tube, and incubated for 1 h on ice with occasional agitation. After incubation, the mixture was centrifuged at 10,000g for 20 min at 4 °C. The resulting supernatant (Triton extract) was incubated for 30 min with 30 μL of a slurry of Ni2+-charged resin (HisLink™, Promega, Madison, WI, USA) at 22 °C with rocking. After centrifugation at 5,000g for 5 s at 22 °C, the precipitated slurry was washed three times with PBS containing 50 mM imidazole and 0.1% Triton X-100, and boiled for 3 min with 30 μL of 2 × Laemmli protein sample buffer (Laemmli buffer) [1 × Laemmli buffer: 0.05 M Tris–HCl, pH 6.8, 10% glycerol, 2% sodium dodecyl sulfate (SDS), 0.005% bromophenol blue] (Laemmli 1970). The eluate was collected and stored at −20 °C.
Domain-selective biotinylation
MDCK cells expressing matriptase-Myc/(His)6 and 1–86GFP (and its mutants) were seeded on Transwell™ (24 mm diameter, 0.4 μm pore size) as described above. When the transepithelial resistance of the monolayer exceeded 700 W/cm2, the cells were washed with PBS, exposed to fresh medium [1.0 mL for the upper (apical) chambers, 2.0 mL for the bottom (basolateral) chambers], and incubated for 24 h. After removal of media, the apical and basolateral chambers were washed three times with 2 and 4 mL of ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (CM-PBS), respectively. The apical and basolateral chambers were exposed to 0.5 and 1.0 mL of CM-PBS containing 0.5 mg/mL biotin derivative (sulfo-NHS-SS-biotin, Pierce Chemical, Rockford, IL, USA), respectively, and incubated for 30 min on ice in a cold room. Biotinylation with freshly prepared biotin solution was repeated once. To quench the biotinylation reaction, 50 and 100 μL of CM-PBS containing 0.2 M glycine were added to the apical and basolateral chambers, respectively, and the chamber was incubated for an additional 3 min on ice. The solution was removed and the chamber was washed three times with PBS, then each polycarbonate filter was excised, transferred to a 35 mm plastic Petri dish, exposed to 0.5 mL of PBS containing 1% Triton X-100 and Complete™. Triton extracts were obtained as described above and were incubated for 30 min with 30 μL of a slurry of immobilized avidin (Neutravidin™ agarose, Pierce Chemical) at 22 °C with rocking. After centrifugation at 5,000g for 5 s at 22 °C, the precipitated slurry was washed three times with PBS containing 0.1% Triton X-100 (PBST) and boiled for 3 min with 30 μL of 2 × Laemmli buffer. The eluate was collected and stored as described above.
SDS-polyacrylamide gel electrophoresis and Western blotting
Samples were thawed and dithiothreitol was added to each sample at a final concentration of 12 mM. The samples were boiled for 3 min. Each sample was subjected to SDS-polyacrylamide gel electrophoresis (SDS–PAGE; 16% gel for analysis of NTF; 12% gel for that of the others). Electrical transfer of proteins on to a polyvinylidene difluoride (PVDF) membrane, visualization of proteins of interest by means of Western blotting, and densitometric quantification of signals on X-ray films were performed as described previously (Tsuzuki et al. 2005).
N-terminal sequencing
MDCK cells expressing matriptase-Myc/(His)6 were cultured in a 100-mm dish. After reaching confluent, cells were washed twice with PBS and extracted with Triton X-100 as described above. Matriptase-Myc/(His)6 was purified and subjected to SDS–PAGE (12% gel) under reducing conditions as described above. Following SDS–PAGE, the proteins were transferred on to a PVDF membrane and stained with Coomassie brilliant blue. The portion of the membrane where a protein band of 93 kDa was detected was excised and subjected to N-terminal sequencing with an automated protein sequencer (Procise 494 cLC; Applied Biosystems, Tokyo blanch; Tsuzuki et al. 2003). About 3 pmol of the 93-kDa protein was recovered.
Confocal laser scanning microscopy
Immunofluorescence microscopic analysis of pcDNAmatriptase-Myc/(His)6 and Na,K-ATPase using Confocal laser scanning microscopy (CLSM) was performed as follows: The stably transfected MDCK cells were seeded on Transwell™ (6 mm diameter, 0.4 μm pore size). When the resistance of the monolayer exceeded 700 Ω/cm2, the chambers were washed three times with PBS and then fixed for 30 min at 22 °C in PBS containing 4% paraformaldehyde. After the chambers were washed three times with PBS, they were incubated for 30 min at 22 °C with PBST for permeabilization. After washing three times with PBS, they were incubated with PBST containing 3% goat serum for 20 min at 22 °C, then incubated with anti-Myc antibody (1:1,000 dilution), Tmc172 (1:200 dilution), or anti-Na,K-ATPase antibody (1:400 dilution) in PBST containing 3% goat serum for 6 h at 22 °C. After washing three times with PBS, the inserts were incubated with anti-rabbit or anti-mouse IgG antibodies conjugated with fluorescence dye at a dilution of 1:400 for 60 min at 22 °C. After washing three times with PBS, the filter inserts were sliced from the chambers and mounted on glass slides. After the addition of PBS containing 50% glycerol and a coverglass, the slides were examined by CLSM (Fluoview FV300, Olympus, Tokyo, Japan). MDCK cells expressing GFP-fused matriptase variants were analyzed by CLSM using the method described above except that blocking and incubation with antibodies were not performed.
Results and discussion
Processing of matriptase-Myc/(His)6 expressed in MDCK cells
In the present study, we stably expressed full-length rat matriptase [designated as matriptase-Myc/(His)6, Fig. 1a] in MDCK cells. In this recombinant matriptase variant, an Myc-epitope/hexahistidine tag was fused to the C-terminus for convenient detection with anti-Myc antibody and for purification with Ni2+-charged resin. First we assessed whether matriptase-Myc/(His)6 is processed post-translationally via cleavage after Gly149 in the cells cultured on filter inserts. Matriptase-Myc/(His)6 was purified from the Triton extract of cells and analyzed by SDS–PAGE and Western blotting. When the blot was probed with anti-Myc antibody, a 93-kDa band was visualized (Fig. 1b, lane 1). The N-terminal sequence was determined to be SVIAY, indicating that the 93-kDa band is produced from the C-terminal fragment (CTF, which constitutes most of the extracellular part) of matriptase (refer to Fig. 1a).
We have shown previously that an anti-rat matriptase NTF antibody (Tmc172) probes a 22-kDa band from Triton extracts of normal rat enterocytes (Tsuzuki et al. 2005). A 22-kDa fragment was detected with Tmc172 in the Triton extract of matriptase-Myc/(His)6-expressing cells (Fig. 1b, lane 2). The 22-kDa band was not produced when the blot was incubated with Tmc172 that had been preadsorbed with 200 μM antigenic peptide (Fig. 1b, lane 3). This indicates that the 22-kDa band is produced from the NTF part of matriptase-Myc/(His)6. All results shown in Fig. 1b indicate the occurrence of post-translational cleavage after Gly149 and the association between CTF and NTF in the transfected MDCK cells.
Distribution of matriptase-Myc/(His)6 in MDCK cells
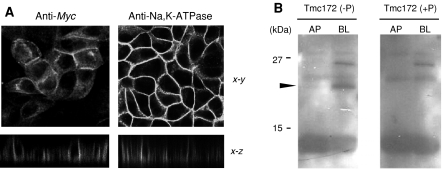
Then we analyzed the localization of matriptase-Myc/(His)6 by immunofluorescence microscopy using CLSM. When anti-Myc antibody was used as a probe, heterogeneous expression in MDCK cells of matriptase-Myc/(His)6 (CTF) was shown (Fig. 2a). The fluorescence was evident on the basolateral membranes but not on the apical membranes (Fig. 2a). This is consistent with the observation that when a full-length human matriptase cDNA was transfected in MDCK cells, mature matriptase with the protease domain occurred on the basolateral membranes but not on the apical membranes (Godiksen et al. 2008). An antibody raised against Na,K-ATPase (a basolateral membrane marker) gave fluorescence on the basolateral membranes (Fig. 2a). Tmc172 gave non-specific staining (data not shown).
Fig. 2.
Localization of matriptase-Myc/(His)6 in MDCK cells. a Analysis of localization of CTF using immunofluorescence with CLSM. MDCK cells stably expressing matriptase-Myc/(His)6 were probed with anti-Myc antibody (anti-Myc) or an anti-Na,K-ATPase antibody. In each experiment, incubation with goat anti-mouse IgG antibody conjugated with fluorescence dye was applied. The x–y and x–z on the right show the x–y scans parallel to the plane of the filter near the apex of the cells and scans along the x–z dimension, respectively. b Analysis of distribution of NTF using domain-selective biotinylation. MDCK cells expressing matriptase-Myc/(His)6 were labeled at the apical (AP) or basolateral (BL) sides. Samples were analyzed by SDS–PAGE and Western blotting with Tmc172 [Tmc172 (−P)]. A 24-kDa band representing biotinylated NTF is indicated by arrowhead on the left. Tmc172 (+P) indicates that the blot was incubated with Tmc172 that had been adsorbed with 200 μM antigenic peptide. The molecular sizes of protein markers are indicated on the left in kilodaltons (kDa)
We analyzed the distribution of NTF by domain-selective biotinylation and Western blotting with Tmc172. A 24-kDa band was produced from samples labeled at the basolateral side but less from those labeled at the apical side [Fig. 2b, Tmc172 (−P)]. The 24-kDa band was not visualized when the blot was incubated with Tmc172 preadsorbed with the antigenic peptide [Fig. 2b, Tmc172 (+P)], indicating that the band was produced from biotinylated NTF. Note that the NTF, when biotinylated, migrates more slowly than expected on SDS–PAGE. This may be because of the addition of the sulfo-NHS-SS-biotin spacer. Results shown in Figs. 1b and 2 indicate that MDCK cells can be used for dissecting the mechanisms of localization of matriptase in simple epithelial cells.
Distribution of 1–86GFP and its mutants in MDCK cells
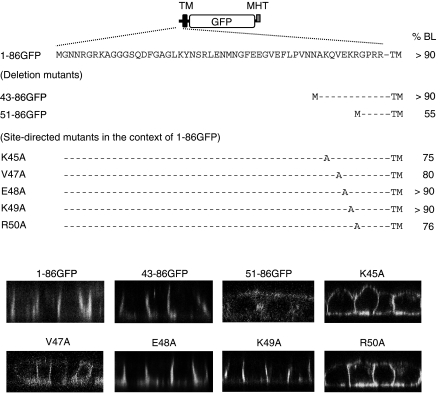
In general, basolaterally delivered transmembrane proteins contain sorting motifs in the cytoplasmic region (Bonifacino and Traub 2003). For this reason, we constructed a plasmid encoding a chimera protein consisting of the cytoplasmic and transmembrane regions of rat matriptase (Met1–Ile86), GFP, and Myc/(His)6-tag (1–86GFP, Fig. 3). Domain-selective biotinylation and CLSM showed that 1–86GFP localizes exclusively to the basolateral sides of MDCK cells (Fig. 3). This suggests that the cytoplasmic region of matriptase plays a role in mediating its localization in MDCK cells. However, there are no amino acid sequences typical of those required for basolateral sorting such as leucine- and tyrosine-based motifs (Bonifacino and Traub 2003). Indeed, a deletion mutant of 1–86GFP (designated 43–86GFP) localized exclusively to the basolateral sides (Fig. 3). Furthermore, site-directed mutants in the context of 1–86GFP in which Tyr22, Ser24 or Glu33/Glu34 was changed to alanine residue(s) and a mutant where Phe38 and Leu39 were deleted also localized to the basolateral sides (data not shown). A deletion mutant (51–86GFP) exhibited a non-polarized distribution (Fig. 3). These results indicate that critical amino acid residue(s) for mediating the localization of 1–86GFP exist between Lys45 and Arg50.
Fig. 3.
Analysis of structural elements and critical amino acid residues for the exclusive localization of matriptase to the basolateral membrane domain of MDCK cells. Schematic illustration of the structure of the 1–86GFP mutant is shown on the top. This mutant consists of the cytoplasmic and transmembrane (TM) regions of matriptase followed by GFP and Myc/(His)6 tag (MHT). The amino acid sequence of the cytoplasmic region of matriptase (Met1–Arg54) is depicted using the single-letter code. Amino acids converted to alanine residues by site-directed mutagenesis are indicated, while amino acids that were not converted are indicated by hyphens in the deletion and site-directed mutants. The ratios of basolateral delivery (%BL) were calculated from data obtained using domain-selective biotinylation and Western blotting with anti-Myc antibody; data are expressed as the means of 2–4 experiments. Biotinylated 1–86GFP (and its site-directed mutants), 43–86GFP, 51–86GFP migrated to positions corresponding to 40, 36, and 35 kDa, respectively, upon SDS–PAGE. CLSM data are also shown. Only scans along the x–z dimension are indicated
Cytoplasmic juxtamembrane amino acid residues important for the exclusive localization of 1–86GFP to the basolateral membrane domain of MDCK cells
To define the specific residues between Lys45 and Arg50 that mediate the localization of 1–86GFP, we replaced individual amino acids with an alanine residue in the context of this chimera (Fig. 3). Gln46 was not mutated because human matriptase has a lysine residue at this position. The results demonstrated that substitution of Lys45, Val47, and Arg50 but not Glu48 and Lys49 abolished exclusive basolateral accumulation (Fig. 3). These results demonstrated the importance of Lys45, Val47, and Arg50 for the localization of this chimera in MDCK cells.
The mechanisms by which these amino acid residues exert the effect are unclear. Lys45 and Val47 are part of sequence KQVE, resembling sequence RNVD in the 17-amino-acid juxtamembrane region (cytoplasmic region) of the polymeric immunoglobulin receptor (pIgR), in which the valine residue (Val660) is the critical residue for basolateral sorting (Fig. 4; Aroeti et al. 1993). Furthermore, basic amino acids within the 17-amino-acid region (His656 and Arg657) also contribute to basolateral sorting in a manner additive to Val660 (Reich et al. 1996). By analogy, Lys45/Arg50 and Val47 may play similar roles to those of pIgR His656/Arg657 and Val660, respectively. However, there is no direct evidence that these residues of pIgR are essential for interaction with adaptor molecules such as γ-adaptin, which are required for formation of the basolaterally directed vesicles found in the trans-Golgi-network (Futter et al. 1998). Indeed, the RQVD sequence in the cytosolic region of the transferrin receptor is not important for basolateral sorting and interaction with γ-adaptin (Odorizzi and Trowbridge 1997). One explanation for the mechanism is that the valine and positively charged amino acid residues are involved in their intracellular trafficking by mediating interaction with certain other cell-surface membrane proteins that carry information for basolateral sorting. It is, therefore, interesting to speculate that interaction with HAI-1 in intracellular environments, which is mediated via a mechanism involving Lys45, Val47, and Arg50, allows matriptase to localize exclusively to the basolateral membrane domain of simple epithelia.
Fig. 4.
Sequence comparison of the cytoplasmic region between rat matriptase (Lys45–Arg54) and rabbit pIgR (Arg653–Thr670). Sequences are shown in single-letter code. Lys45, Val47, and Arg50 (matriptase) and His656, Arg657, and Val660 (pIgR) are indicated by asterisk. KQVE (matriptase) and RNVD (pIgR) sequences are indicated by underline. The numbers of residues (aa) separating from N-terminus (NH2) and C-terminus (COOH) are indicated. TM transmembrane domain
In summary, we showed that the cytoplasmic juxtamembrane region of matriptase is involved in mediating its exclusive localization to the basolateral membrane domain of MDCK cells. At present, it cannot be concluded that the region plays a role as the basolateral-sorting signal such as leucine-based motif (Bonifacino and Traub 2003). To our knowledge, however, the present study provides the first evidence that matriptase carries information within the molecule for its localization in simple epithelia.
Acknowledgments
We thank Kenji Kojima and Seiya Mochida for valuable discussions. This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Japan Foundation for Applied Enzymology.
Abbreviations
- CLSM
Confocal laser scanning microscopy
- CTF
C-terminal fragment
- GFP
Green fluorescent protein
- HAI-1
Hepatocyte growth factor activator inhibitor type I
- MDCK cells
Madin–Darby canine kidney cells
- NTF
N-terminal fragment
- pIgR
Polymeric immunoglobulin receptor
References
- Aroeti B, Kosen PA, Kuntz ID et al (1993) Mutational and secondary structure analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol 123:1149–1160 [DOI] [PMC free article] [PubMed]
- Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447 [DOI] [PubMed]
- Désilets A, Béliveau F, Vandal G et al (2008) Mutation of G827R in matriptase causing autosomal recessive ichthyosis with hypotrichosis yields an inactive protease. J Biol Chem 283:10535–10542 [DOI] [PMC free article] [PubMed]
- Futter CE, Gibson A, Allchin EH et al (1998) In polarized MDCK cells basolateral vesicles arise from clathrin-γ-adaptin-coated domains on endosomal tubules. J Cell Biol 141:611–623 [DOI] [PMC free article] [PubMed]
- Godiksen S, Selzer-Plon J, Pedersen ED et al (2008) Hepatocyte growth factor activator inhibitor-1 has a complex subcellular itinerary. Biochem J 413:251–259 [DOI] [PMC free article] [PubMed]
- Kataoka H, Suganuma T, Shimomura T et al (1999) Distribution of hepatocyte growth factor activator inhibitor type-1 (HAI-1) in human tissues: cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J Histochem Cytochem 47:673–682 [DOI] [PubMed]
- Kojima K, Tsuzuki S, Fushiki T et al (2008) Roles of functional and structural domains of hepatocyte growth factor activator inhibitor type 1 in the inhibition of matriptase. J Biol Chem 283:2478–2487 [DOI] [PubMed]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed]
- Lee SL, Dickson RB, Lin CY (2000) Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem 275:36720–36725 [DOI] [PubMed]
- List K, Hobson JP, Molinolo A et al (2007) Co-localization of the channel activating protease prostasin/(CAP1/PRSS8) with its candidate activator, matriptase. J Cell Physiol 213:235–245 [DOI] [PubMed]
- Oberst MD, Singh B, Ozdemirli M et al (2003) Characterization of matriptase expression in normal human tissues. J Histochem Cytochem 51:1017–1025 [DOI] [PubMed]
- Oberst MD, Chen LY, Kiyomiya K et al (2005) HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol 289:C462–C470 [DOI] [PubMed]
- Odorizzi G, Trowbridge IS (1997) Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol 137:1255–1264 [DOI] [PMC free article] [PubMed]
- Reich V, Mostov K, Aroeti B (1996) The basolateral sorting signal of the polymeric immunoglobulin receptor contains two functional domains. J Cell Sci 109:2133–2139 [DOI] [PubMed]
- Satomi S, Yamasaki Y, Tsuzuki S et al (2001) A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem Biophys Res Commun 287:995–1002 [DOI] [PubMed]
- Takeuchi T, Harris JL, Huang W et al (2000) Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J Biol Chem 275:26333–26342 [DOI] [PubMed]
- Tsuzuki S, Kokado Y, Satomi S et al (2003) Purification and identification of a binding protein for pancreatic secretory trypsin inhibitor: a novel role of the inhibitor as an anti-granzyme A. Biochem J 372:227–233 [DOI] [PMC free article] [PubMed]
- Tsuzuki S, Murai N, Miyake Y et al (2005) Evidence for the occurrence of membrane-type serine protease1/matriptase on the basolateral sides of enterocytes. Biochem J 388:679–687 [DOI] [PMC free article] [PubMed]