Abstract
The effect of eight vitamin E analogues (d-α-, dl-α-, d-β-, d-γ-, and d-δ-tocopherols, d-α- and dl-α-tocopheryl acetates) and 2,2,5,7,8-pentamethyl-6-hydroxychroman (PMC) on melanogenesis were compared in mouse B16 melanoma cells. D-β-tocopherol at 250 μg ml−1 inhibited not only 28% of melanin synthesis in B16 cells, but also 34% of the tyrosinase activity, a very important cascade enzyme involved in the synthesis of melanin in melanoma cells. D-γ-tocopherol also strongly inhibited up to 39% of melanin synthesis and 45% of the tyrosinase enzyme activity at the same concentration. The inhibitory activity of both d-β- and d-γ-tocopherols was observed without cytotoxicity up to a concentration of 250 μg ml−1. Weak activity was also observed with d-δ-tocopherol at 8 μg ml−1 and with PMC at 16 μg ml−1, with 19% and 25% inhibition of melanin synthesis, respectively. However, PMC did not directly inhibit tyrosinase, as was observed with d-β-, d-γ-, and d-δ-tocopherols. Analysis by reverse transcription-polymerase chain reaction showed that the mechanism of melanogenesis inhibition by d-β- and d-γ-tocopherols in cells might be attributed to reduced expression of tyrosinase and tyrosinase related protein-2 mRNA in addition to direct inhibition of the tyrosinase. These findings suggest that both d-β-tocopherol and d-γ-tocopherol might be useful as effective ingredients in whitening cosmetics with lower skin toxicity to prevent or improve skin pigmentation such as skin spots and freckles caused by UV exposure.
Keywords: Enzyme inhibition, Vitamin E, Analogue, Melanin, B16 cells, Melanoma
Introduction
Recently women desiring skin whitening treatment have increased in Japan and the market for whitening cosmetics expands every year. Whitening cosmetics and ultraviolet absorption and dispersion cosmetics are used to protect their skin from pigmentation, blotches, and freckles caused by ultraviolet radiation. Natural product compounds are especially of interest as whitening cosmetics. Many contain plant extracts and a wide variety of the biomass is believed to be an attractive bioresource to screen for inhibitors of melanin synthesis (An et al. 2005; Cho et al. 2006; Hermanns et al. 2002; Hwang and Lee 2007; Kim et al. 2002; Lee et al. 2003; Lin et al. 2007; Tanimoto et al. 2006; Thongchai et al. 2007; Wang et al. 2006).
In this background, we also searched for new biological compounds in natural products and screened for inhibitors of melanin synthesis in over 300 species of marine algae from the Japanese coastlines. We found that β-carotene from several species of marine algae is an active antimelanogenic compound (Kamei 2001). Since β-carotene has antioxidant activity, other antioxidant compounds like vitamin E analogues may also inhibit melanin synthesis. Indeed, one of the vitamin E analogues, α-tocopherol has been reported to have antimelanogenic activity (Funasaka et al. 1999; Funasaka et al. 2000; Hachinohe and Matsumoto 2005; Yamamura et al. 2002). Melanin biosynthesis is regulated by tyrosinase which is a key enzyme in melanogenesis in melanocytes (Körner and Pawelek 1982) and other enzymes in the tyrosinase-family such as tyrosinase related protein (TRP)-1 and TRP-2 (Jackson et al. 1992; Jimènez-Cervantes et al. 1994; Kobayashi et al. 1994; Tsukamoto et al. 1992). However, Funasaka et al. (1999) reported that α-tocopherol not only directly inactivates tyrosinase, but may also affect the post-translation levels of tyrosinase, TRP-1 and TRP-2. Thus since α-tocopherol has antimelanogenic activity, other vitamin E analogues may also suppress melanogenesis in pigment cells by directly inhibiting the enzymes or the translation of mRNAs for tyrosinase-family enzymes.
In this study, we used mouse B16 melanoma cells to evaluate the antimelanogenic activity of vitamin E because B16 cells have been known as a model melanoma cell line to evaluate such an biological activity (Kamaraju et al. 2004). Eight vitamin E analogues, d-α-, dl-α-, d-β-, d-γ-, and d-δ-tocopherols, d-α- and dl-α-tocopheryl acetates, and 2,2,5,7,8-pentamethyl-6-hydroxychroman were evaluated for antimelanogenic activity to mouse B16 melanoma cells which might possibly be developed as vitamin E analogues for skin whitening cosmetics. In addition, the mechanism of melanogenesis inhibition by active vitamin E compounds was analyzed.
Materials and methods
Experimental reagents
Eight vitamin E analogues, d-α-, dl-α-, d-β-, d-γ-, d-δ-tocopherol, d-α-, dl-α-tocopheryl acetates and PMC were compared for their ability to inhibit melanin synthesis in mouse B16 melanoma cells. All these samples were dissolved in dimethyl sulfoxide (DMSO). Dulbecco’s modified Eagle’s medium (DMEM, low glucose) was purchased from Gibco RBL (Rockville, MD). Fetal bovine serum (FBS) was purchased from Sigma-Aldrich (St. Louis, MO). N-[2-hydroxy ethyl]piperazine-N′-[2-ethanesulfonic acid](HEPES)was from Katayama Chemical Industry. TRIZOL reagent for isolating total RNA for use in reverse transcription-polymerase chain reactions (RT-PCR) was purchased from Gibco BRL. All other chemicals were purchased from Sigma–Aldrich.
Cell culture
Mouse B16 melanoma cells (JCRB0202) were obtained from the Japanese Cancer Research Resources Bank (JCRB) and cultured in DMEM supplemented with 120 U ml−1 of penicillin G, 200 μg ml−1 of streptomycin, 25 μg ml−1 of ampicillin, 3.6 mg ml−1 of HEPES, and 10% FBS at 37 °C in a humidified 5%-CO2 incubator.
Cell pellet assay
B16 melanoma cells were plated onto 50 mm culture dishes at an initial density of 1.6 × 105 cells dish−1 and cultured under the same conditions described above. The cells were harvested by centrifugation, then the color of the cell pellets were evaluated visually after being cultured in the presence of vitamin E analogues for 3 days.
Antimelanogenesis assay
B16 melanoma cells were plated at 2.4 × 104 cells 300 μl−1 well−1 in a 48-well plate and cultured in DMEM for 24 h. After incubation, the medium was refreshed with medium containing one of eight vitamin E analogues serially diluted 1/2 to concentrations ranging from 3.9 to 500 μg ml−1 and the cells incubated for another 3 days. After incubation, the cells were washed with phosphate-buffered saline (PBS) and completely lysed by sonication and dissolved in 100 μl of 2 M NaOH. The melanin content in the cell lysates was estimated by use of a standard curve made with synthetic melanin. The absorbance at 405 nm was measured in a microplate reader (Model 450, Bio-Rad). The inhibition of melanin synthesis was evaluated by comparing samples with a negative control that did not contain a vitamin E analogue.
Cytotoxicity test
The cytotoxicity of vitamin E analogues in B16 cells was tested in vitro by MTT assay (Mosmann 1983). The B16 cells were seeded into a 48-well plate and cultured in the presence of vitamin E samples as described above. After a 3-day incubation, 30 μl of 5 mg ml−1 3-[4,5-dimethylthiazol-2-ly]-2,5-diphenyltetrazolium bromide (MTT) was added to 300 μl of cell culture in the plates. After dissolving the cells in 300 μl of 10% SDS in 0.01 M HCl, the cells were incubated at 37 °C for 4 h and the absorbance at 570–655 nm was measured in a microplate reader. The growth rate as an index of cytotoxicity was calculated by dividing the test cell number by the numbers of cells in the corresponding control sample.
Tyrosinase inhibition assay
Tyrosinase activity was determined by colorimetric assay of 3,4-dihydroxyphenylalanine (DOPA) oxidase activity. B16 cells (2.0 × 103 cells 100−1 μl well−1) were plated into 96-well microplates and cultured in DMEM containing 0.5 mM theophylline for 2 days. The culture medium was refreshed with medium containing one of vitamin E analogues as describe above and cultured for another 3 days. The medium was removed and the cells were washed with PBS. The cells were lysed with 100 μl of 1% triton X-100 in PBS. The reaction was initiated by addition of 10 μl of 10 mM L-DOPA. The absorbance was measured at 490 nm immediately after adding the L-DOPA. The plate was incubated at 37 °C for 1 h and the absorbance at 490 nm was measured. The inhibition of tyrosinase inhibiting activities by the vitamin E analogues was evaluated by comparing them with the activity of a negative control containing no analogues.
RT-PCR assay
Total RNA was extracted from 1 × 106 cells ml−1 B16 cells cultured in the presence of 62.5,125, and 250 μg ml−1 of each vitamin E analogue in a 100 mm-dish for 3 days. The total RNA was reverse transcribed at 42 °C for 60 min in the presence of oligo(dT) 20 primers (Toyobo) and reverse transcriptase (ReverTra Ace, Toyobo). cDNA was amplified by the polymerase chain reaction (PCR) at 94 °C for 5 min by 1 cycle, 98 °C for 20 s, 68 °C for 7 min followed by a 10 min elongation cycle at 72 °C using a GeneAmp PCR System 2400 (ABI). The oligonucleotides primers for mouse tyrosinase (forward, 5′-TGGCCAAATGAACAATGGGTCAAC-3′; reverse, 5′-GGTCCCTCAGGTGTTCCA-TCGCAT-3′), mouse TRP-2 (forward, 5′-GATGTT-AGAGGAGCTTCGGATG-3′; reverse, 5′-GAAGGATATAAGGGCCACTCC-3′), and mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control (forward, 5′-GGTGAAGGTCGGTGTGAACG-3′; reverse, 5′-CTT-GGAGGCCATGTAGGCCATG-3′) were used. The PCR products from the PCR amplification of tyrosinase and TRP-2 mRNA was electrophoresed at 100 V for 40 min on a 2% agarose gel in TAE buffer (40 mM Tris-acetate, 2 mM EDTA, pH 8.0) containing 0.5 μg ml−1 ethidium bromide. After electrophoresis, the signal intensity of each band was quantified with NIH-Image Software Ver. 1.62 and decrease in expression of tyrosinase and TRP-2 mRNA by vitamin E analogues was evaluated relative to the internal control PCR product.
Results
Depigmentation of B16 cell pellets
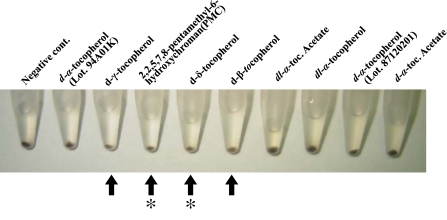
The eight vitamin E analogues (d-α-, dl-α-, d-β-, and d-δ-tocopherols, d-α- and dl-α-tocopheryl acetates, and PMC) used in this study are shown in Fig. 1. These vitamin E analogues as well as d-α-tocopherol which was from a different lot number from that of another d-α-tocopherol were tested for their ability to depigment B16 cells by direct observation of the cell pellets after treatment. A reduction in melanogenesis in B16 cells compared with a negative control was observed in cells treated with four of the tested analogues, d-β-, d-γ-, and d-δ-tocopherols, and PMC. However, d-δ-tocopherol and PMC also reduced the amount of cells, probably due to cytotoxicity (Fig. 2).
Fig. 1.
Vitamin E analogues tested for antimelanogenic activity assay
Fig. 2.
Pellets of mouse B16 melanoma cells after cells were cultured in the presence of vitamin E analogues. Arrows indicates cell pellets where melanogenesis was inhibited in B16 melanoma cells cultured in the presence of vitamin E analogues; asterisks indicates growth inhibition of B16 cells by vitamin E analogues
Melanogenesis inhibition in B16 cells
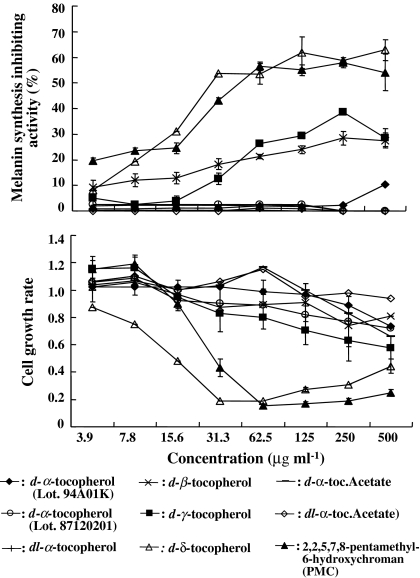
Four vitamin E analogues, d-β-, d-γ-, and d-δ-tocopherols, and PMC also exhibited intracellular antimelanogenic activity in B16 cells when the melanin content was measured directly based on a standard curve made using synthetic melanin (Fig. 3). However, antimelanogenic activity was not found with d-α-tocopherol in this experiment although α-tocopherol has been reported to have antimelanogenic activity. D-δ-tocopherol and PMC, both had cytotoxic effects on B16 cells at higher concentrations of over 16 μg ml−1. The antimelanogenic activity of both d-β- and d-γ-tocopherols were dose dependant.
Fig. 3.
Antimelanogenic activity and cytotoxicity of vitamin E analogues in mouse B16 melanoma cells
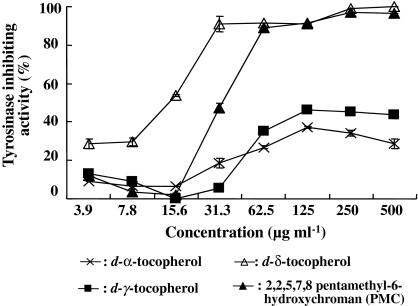
Tyrosinase inhibition in B16 cells
Since d-β-, d-γ-, and d-δ-tocopherols, and PMC had intracellular antimelanogenic activity (Fig. 3), these 4 vitamin E analogues were tested to see if they directly inactivated tyrosinase in B16 cells. These tocopherols exhibited inhibitory activity against intracellular tyrosinase that was similar to the intracellular antimelanogenesis observed. The most potent inactivation of tyrosinase was found with d-δ-tocopherol at lower concentrations of below 31 μg ml−1, with over 90% inhibition of tyrosinase activity (Fig. 4). PMC was also a potent inhibitor at over 125 μg ml−1. However, although both d-β-tocopherol and d-γ-tocopherol had lower activity compared with d-δ-tocopherol and PMC, both still had relatively high tyrosinase-inhibiting activity of over 30% at 63 μg ml−1. At these concentrations, no cytotoxicity by d-β-tocopherol and d-γ-tocopherol was observed as shown in Fig. 3.
Fig. 4.
Tyrosinase enzyme inhibition by vitamin E analogues
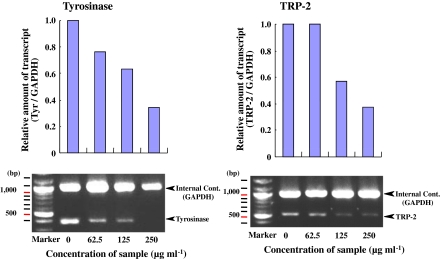
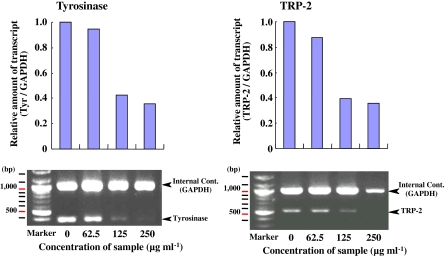
Inhibition of tyrosinase and TRP-2 mRNA levels by d-β-tocopherol and d-γ-tocopherol
Before attempting to elucidate the mechanisms of antimelanogenesis of B16 cells by active vitamin E analogues in B16 cells by RT-PCR analysis of tyrosinase and TRP-2 mRNA levels, we first determined the quantity of total RNA for RT-PCR. At concentrations ranging from 0.08 to 10 ng μl−1 of total RNA, the quantity of both tyrosinase and TRP-2 mRNA were observed at concentrations ranging from 0.63 ng μl−1 to 2.5 ng μl−1 (data not shown). From these results, therefore, 1.3 ng μl−1 total RNA was chosen for use in RT-PCR to determine tyrosinase and TRP-2 mRNA levels. Under these conditions, d-β-tocopherol reduced tyrosinase and TRP-2 mRNA expression levels in a dose dependant manner at concentrations of 62.5 μg ml−1 to 250 μg ml−1 (Fig. 5). D-γ-tocopherol also reduced the mRNA levels of both enzymes in a dose dependant manner at the same concentrations (Fig. 6). In particular, these compounds reduced the expression of mRNAs for these enzymes beyond a concentration of 125 μg ml−1 of d-γ-tocopherol. These results indicate that d-γ-tocopherol also suppresses the mRNA levels of tyrosinase and TRP-2 in B16 cells.
Fig. 5.
Decreased expression of tyrosinase and TRP-2 mRNAs by d-β-tocopherol evaluated by RT-PCR
Fig. 6.
Decreased expression of tyrosinase and TRP-2 mRNAs by d-γ-tocopherol evaluated by RT-PCR
Discussion
In a large screening programs of biologically active compounds from marine algae, we showed that the well-known antioxidant β-carotene has potent antimelanogenic activity in mouse B16 melanoma cells, after purifying and determining it to be the active compound in the extracts from an antimelanogenesis-positive marine algae using activity-guided purification techniques (Kamei 2001). Based on our previous result, we hypothesized that another well-known antioxidant, vitamin E also might potentially have antimelanogenic activity.
In this study, we determined the antimelanogenic activity of vitamin E analogues in B16 cells that might potentially be developed as ingredients in skin whitening cosmetics. Four of 8 vitamin E analogues tested had antimelanogenic activity in B16 cells. In particular, d-β-tocopherol and d-γ-tocopherol showed promising antimelanogenic activity with less cytotoxicity at relatively high concentrations of up to 250 μg ml−1. However, no strong antimelanogenic activity was observed with d-α-tocopherol or dl-α-tocopherol in this study although antimelanogenic activity of d-α-tocopherol alone (Yamamura et al. 2002) or in the presence of ferulic acid (Funasaka et al. 1999; Funasaka et al. 2000) has been reported. This might be attributed to the number of methyl residues bound to benzene ring of these tocopherols. α-Tocopherol has three methyl residues bound to its benzene ring, however, d-β-tocopherol and d-γ-tocopherol have two methyl residues bound to the benzene ring in their structure. This property may cause the different permeability of the compound through the membrane of cells. It is well known that the permeability of tocopherol through the cell membrane is different among the tocopherols. The order of the incorporation of tocopherol homologues into cells has been reported as β-tocopherol > δ-tocopherol > γ-tocopherol > α-tocopherol, and α-tocopherol is worst among them (Wu et al. 2000). This biological property might be attributed to the number of methyl residues bound to the benzene ring of α-tocopherol which has three methyl residues on the benzene ring of this structure. Such structural difference may cause antimelanogenic activity in B16 cells. Furthermore the difference in antimelanogenic activity with d-α-tocopherol in the previous report and in our present results might be due to the different property between synthesized and natural α-tocopherol products. We used the natural product of α-tocopherol extracted from soybean oil in this study. Antimelanogenic activity is also not likely based on the different optical forms (d- and dl-forms) of α-tocopherol isomers.
When we evaluated the mechanisms of antimelanogenic activity of d-β-tocopherol and d-γ-tocopherol in B16 cells, both tocopherols strongly inhibited intracellular tyrosinase activity. Furthermore, both tocopherols also suppressed the mRNA expression of tyrosinase and TRP-2. D-β-tocopherol and d-γ-tocopherol might specifically inhibit the production of tyrosinase and TRP-2 in B16 cells. From these results, the mechanism of antimelanogenic activity of d-β-tocopherol and d-γ-tocopherol appeared to be two-fold; direct inactivation of the tyrosinase enzyme and suppression of the mRNA expression of both tyrosinase and TRP-2. Since tyrosinase is a key enzyme in the melanin synthetic pathway in melanocytes, the strong inhibitory effects of d-β-tocopherol and d-γ-tocopherol directly against the tyroninase enzyme are likely to be very important in the suppression of melanogenesis in these cells. Such a direct inactivation of tyrosinase by vitamin E has been reported previously (Funasaka et al. 1999). In our study, we found potent inhibitory effects by d-δ-tocopherol and PMC on tyrosinase, however, these compounds were also cytotoxic at the same concentrations required to inhibit melanin synthesis which would prohibit their inclusion into skin care products like skin whitening cosmetics.
This is the first report showing tyrosinase enzyme inhibition by vitamin E analogues like d-β-tocopherol and d-γ-tocopherol. The results suggest that d-β-tocopherol and d-γ-tocopherol might be effective and suitable antimelanogenic components for use in whitening cosmetics to prevent or improve the undesirable skin pigmentation such as skin spots and freckles caused by UV exposure.
Acknowledgments
The authors wish to thank Dr. Etsushi Kitamura for technical advices on RT-PCR.
References
- An BJ, Kwak JH, Son JH, Park JM, Lee JY, Park TS, Kim SY, Kim YS, Jo C, Byun MW (2005) Physiological activity of irradiated green tea polyphenol on the human skin. Am J Chin Med 33:535–546 [DOI] [PubMed]
- Cho YH, Kim JH, Park SM, Lee BC, Pyo HB, Park HD (2006) New cosmetic agents for skin whitening from Angelica dahurica. J Cosmet Sci 57:11–21 [PubMed]
- Funasaka Y, Chakraborty AK, Komoto M, Ohashi A, Ichihashi M (1999) The depigmenting effect of alpha-tocopheryl ferulate on human melanoma cells. Br J Dermatol 141:20–29 [DOI] [PubMed]
- Funasaka Y, Komoto M, Ichihashi M (2000) Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res 8:170–174 [DOI] [PubMed]
- Hachinohe M, Matsumoto H (2005) Involvement of reactive oxygen species generated from melanin synthesis pathway in phytotoxicity of L-DOPA. J Chem Ecol 31:237–246 [DOI] [PubMed]
- Hermanns JF, Petit L, Piérard-Franchimont C, Paquet P, Piérard GE (2002) Assessment of topical hypopigmenting agents on solar lentigines of Asian women. Dermatology 204:281–286 [DOI] [PubMed]
- Hwang JH, Lee BM (2007) Inhibitory effects of plant extracts on tyrosinase, l-DOPA oxidation, and melanin synthesis. J Toxicol Environ Health A 70:393–407 [DOI] [PubMed]
- Jackson IJ, Chambers DM, Tsukamoto K, Copeland NG, Gilbert DJ, Jenkins NA, Hearing V (1992) A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J 11:527–535 [DOI] [PMC free article] [PubMed]
- Jimènez-Cervantes C, Solano F, Kobayashi T, Urabe K, Hearing VJ, Jose LA, Garcia-Borron JC (1994) A new enzymatic function in the melanogenic pathway. J Biol Chem 269:17993–18001 [PubMed]
- Kamaraju AK, Adjalley S, Zhang P, Chebath J, Revel M (2004) C/EBP-δ induction by gp130 signaling role in transition to myelin gene expressing phenotype in a melanoma cell line model. J Biol Chem 279:3852–3861 [DOI] [PubMed]
- Kamei Y (2001) Melanin synthesis inhibiting function of β-carotene. Fragrance J 29:28–32
- Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y (2002) Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 277:16340–16344 [DOI] [PubMed]
- Kobayashi T, Urabe K, Winder A, Jimenz-Cervantes C, Imokawa G, Brewington T, Solano F, Garcia-Borron JC, Hearing VJ, Hearing VJ (1994) Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J 13:5818–5825 [DOI] [PMC free article] [PubMed]
- Körner A, Pawelek J (1982) Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 217:1163–1165 [DOI] [PubMed]
- Lee KT, Lee KS, Jeong JH, Jo BK, Heo MY, Kim HP (2003) Inhibitory effects of Ramulus mori extracts on melanogenesis. J Cosmet Sci 54:133–142 [PubMed]
- Lin YP, Hsu FL, Chen CS, Chern JW, Le MH (2007) Constituents from the Formosan apple reduce tyrosinase activity in human epidermal melanocytes. Phytochemistry 68:1189–1199 [DOI] [PubMed]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival, application to proliferation and cytotoxicity assay. J Immunol Methods 65:55–63 [DOI] [PubMed]
- Tanimoto S, Tominaga H, Okada Y, Nomura M (2006) Synthesis and cosmetic whitening effect of glycosides derived from several phenylpropanoids. Yakugaku Zasshi 126:173–177 [DOI] [PubMed]
- Thongchai W, Liawruangrath B, Liawruangrath S (2007) High-performance liquid chromatographic determination of arbutin in skin-whitening creams and medicinal plant extracts. J Cosmet Sci 58:35–44 [PubMed]
- Tsukamoto K, Jackson IJ, Urabe K, Montague PM, Hearing VJ (1992) A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J 11:519–526 [DOI] [PMC free article] [PubMed]
- Wang KH, Lin RD, Hsu FL, Huang YH, Chang HC, Huang CY, Lee MH (2006) Cosmetic applications of selected traditional Chinese herbal medicines. J Ethnopharmacol 106:353–359 [DOI] [PubMed]
- Wu D, Meydani M, Beharka AA, Serafini M, Martin KR, Meydani SN (2000) In vitro supplementation with different tocopherol homologues can affect the function of immune cells in old mice. Free Rad Biol Med 28:643–651 [DOI] [PubMed]
- Yamamura T, Onishi J, Nishiyama T (2002) Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch Dermatol Res 294:349–354 [DOI] [PubMed]