Abstract
The purpose of this study is to investigate in vitro and ex vivo effects of matrine on the growth of human lung cancer and hepatoma cells and the cancer cell migration as well as the expressions of related proteins in the cancer cells. Matrine significantly inhibited the in vitro and ex vivo growth of human non-small cell lung cancer A549 and hepatoma SMMC-7721 cells. Matrine induced the apoptosis in A549 and SMMC-7721 cells. Western blot analysis indicated that matrine dose-dependently down-regulated the expression of anti-apoptotic protein Bcl-2 and up-regulated the level of pro-apoptotic protein bax, eventually leading the reduction of ratios of Bcl-2/Bax proteins in A549 and SMMC-7721 cells. Furthermore, matrine significantly suppressed the A549 cell migration without reducing the cell viability. In addition, matrine dramatically reduced the secretion of vascular endothelial growth factor A in A549 cells. More importantly, matrine markedly enhanced the anticancer activity of anticancer agent trichostatin A (the histone deacetylase inhibitor) by strongly reducing the viability and/or the ratio of Bcl-2/Bax protein in A549 cells. Our findings suggest that matrine may have the broad therapeutic and/or adjuvant therapeutic application in the treatment of human non-small cell lung cancer and hepatoma.
Keywords: Matrine; Trichostatin A; Non-small cell lung cancer; Hepatoma, growth; Migration; Bcl-2/Bax; Vascular endothelial growth factor
Introduction
Lung adenocarcinoma is the leading cause of cancer-related deaths among both men and women in the world (Parkin et al. 2005; Jemal et al. 2008). Despite recent advances in diagnosis and treatment, the mortality rates with an overall 5-year survival of only 15%. This high mortality is probably attributable to early metastasis, which is related to abnormal growth, migration, invasion and angiogenesis in the cancer cells. The non-small cell lung cancer (NSCLC), which constitutes ~80% of lung cancer cases, remains an aggressive lung cancer associated with a poor patient survival. The patients with advanced disease have a median survival of approximately 10 months when treated with standard platinum-based therapy. Hepatocellular carcinoma is also one of the leading causes of worldwide cancer mortality. Hence, there is an imminent need for better therapies and/or anticancer agents for human NSCLC and hepatoma. To date, chemotherapy has been the most frequently used treatment for lung cancer, hepatoma and other cancers. However, some normal cells are destroyed as well by this method of treatment. Due to their wide range of biological activities and low toxicity in animal models, some natural products have been used as alternative treatments for cancers including lung cancer and hepatoma. Matrine is a naturally occurring small-molecule compound from Traditional Chinese Medicine Sophora flavescens Ait. In China, matrine as a clinical drug has been used to treat cancer as well as other diseases such as viral hepatitis, cardiac arrhythmia and skin inflammations. Matrine induced the apoptosis of murine hepatoma cells in vitro and in vivo as well as inhibited tumor growth (Ma et al. 2008). Matrine also inhibited the invasiveness and matastasis of human malignant melanoma cell line A375 (Liu et al. 2008). Some studies reported that matrine induced gastric cancer MKN45 cell apoptosis (Luo et al. 2007) and reduced Hela cell adhesion and migration (Zhang et al. 2007). However, the effects of matrine on human NSCLC and hepatoma as well as the mechanisms of action are largely unknown. Whether or not matrine has the potential therapeutic and/or adjuvant therapeutic application in the treatment of human NSCLC and hepatoma is unclear. In order to address these issues, we investigated the in vitro and ex vivo effects of matrine on the growth of human NSCLC A549 and hepatoma SMMC-7721 cell lines and the migration of the A549 cells as well as the mechanisms of action. We also studied the effects of matrine in combination with anticancer agent trichostatin A on the growth of A549 cells. We showed that matrine significantly inhibited the in vitro and ex vivo growth of A549 and SMMC-7721 cells and regulated the expressions of bcl-2 and bax proteins in the cancer cells in a dose-dependent manner. Matrine also markedly suppressed A549 cell migration and reduced the secretion of vascular endothelial growth factor A (VEGF-A) in A549 cells. In addition, matrine enhanced the anticancer activity of anticancer agent trichostatin A by strongly reducing the viability and/or ratios of Bcl-2/Bax proteins in A549 cells.
Materials and methods
Materials
Fibronectin and Boyden chambers were purchased from BD Bioscience (Bedford, MA) and Corning (Corning, NY), respectively. Matrine (MA), trichostatin A (TSA), celecoxib (S), Bay 11-7082 (Bay), RPMI 1640 and DMEM medium, penicillin, streptomycin, 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), propidium iodide (PI), fetal bovine serum (FBS), trypsin/EDTA, and all other chemicals employed in this study were purchased from Sigma Chemical Co. (St. Louis, MO.)
Animal experimentation and preparation of sera from matrine-treated rabbits
These were done according to our published methods with slight modifications (Zhang et al. 2000, 2001). In brief, New Zealand White rabbits (3.5–4 kg; from Luye Pharmaceutical Company, Yantai, China) were treated in accordance with guidelines established by the Animal Care and Use Committee at Yantai University. Matrine was orally intubated to the rabbits once daily at a dose of 100 mg/mL/kg body weight for 3 days. On the third day, the blood was then collected at 0, 0.5, 1, and 2 h from the rabbits (fasted for 16 h) after oral intubation of matrine. The collected blood was left to clot for 2 h at room temperature and centrifuged twice at 3000×g at 4 °C for 20 min. The sera were sterilized by filtration and then heated at 56 °C for 30 min. The prepared sera were aliquoted, and stored at −80 °C until ex vivo cell growth assay.
Cell culture and in vitro and ex vivo cell growth assays
The A549 lung adenocarcinoma and hepatoma SMMC-7721 cell lines were obtained from the American Type Culture Collection. The human cancer cell lines A549 and SMMC-7721 were cultured in RPMI 1640 and DMEM medium, respectively, containing 10% heat-inactivated fetal bovine serum (FBS), glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37 °C in a humidified incubator with 95% air/5% CO2 atmosphere. The in vitro and ex vivo assays were done according to our published methods (Zhang et al. 1999, 2001). The cells in control group were treated with DMSO (0.1%, final concentration). The cells were respectively incubated in RPMI 1640 and DMEM medium supplemented with 10% FBS (in the case of in vitro assay) containing different concentrations of matrine, or in the absence or presence of the existing anticancer agents (TSA and Bay), or 10% rabbit sera (in the case of ex vivo assay) obtained at different time points after matrine was orally intubated to rabbits. Cell viability was measured 24, 48, and 72 h after the treatments using MTT assay kit. The MTT method is based on the method of Zhang et al. (1999). Each experiment was repeated three times.
Morphological evaluation of apoptotic cells
This was done according to our published methods (Zhang et al. 2000). In brief, A549 and SMMC-7721 cells at 70% confluence were respectively treated for 48 h with matrine at concentrations of 0 (0.1% DMSO, vehicle as control), 100 and 500 μg/mL (in the case of A549 cells) and 0.5 and 1 mg/mL (in the case of SMMC-7721 cells). The treated cells were fixed with 1% glutaraldehyde in PBS for 30 min at room temperature, washed in PBS, and stained with 1 mM Hoechst 33258 for 30 min at room temperature. The morphological changes in the nuclear chromatin were observed under a fluorescent microscope (Nikon, TE2000-U, Japan), using 40× lens.
Western blot analysis
This was performed according to the method of (Chen et al. 2001). In brief, A549 and SMMC-7721 cells were treated with matrine at different concentrations in the absence or presence of trichostatin A (TSA, 5 μg/L). The cells in control group were treated with DMSO (0.1%, final concentration). Bay (2.5 μM) and celecoxib (S, 10 μM) were used as positive control. The treated cells were collected at 48 h. Equal amounts of cell extracts were resolved by SDS–PAGE, transferred to nitrocellulose membranes, and probed with primary antibodies to human Bcl-2, Bax, and β-Actin and then horseradish peroxidase-conjugated secondary antibodies, respectively. Anti-β-Actin antibody was used as a loading control. Detection was done using an enhanced chemiluminescence system (GE Healthcare Life Sciences).
In vitro migration assay
Cancer cell migration was measured by examining cell migration through fibronectin-coated polycarbonate filters, using modified transwell chambers. In brief, A549 cells (5 × 104) were seeded into the upper chamber in 200 μL of serum-free medium containing matrine at concentrations of 0–100 μg/mL, respectively; the cells in control group were treated with DMSO (0.1%, final concentration); the lower compartment was filled with 0.66 mL of RPMI 1640 medium supplemented with 10% of FBS (as a chemoattractant). After incubation for 6 h at 37°C, the cells that migrated to the lower surface of the filter were fixed and stained using propidium iodide. The cells on the upper side of the filter were removed using a rubber scraper. The migrated cells on the underside of the filter were counted and recorded for images under a fluorescent microscope (Nikon, TE2000-U, Japan). Experiments were performed in triplicate.
ELISA for detection of human VEGF-A secretion in A549 cells
For detection of the effects of matrine on the secretion of vascular endothelial growth factor A (VEGF-A) in A549 cells, the cells were treated for 24 h with matrine at the concentrations of 50–500 μg/mL. Then each supernatant of the cell culture was respectively collected and analyzed by ELISA using kit (VEGF-A) from R & D Systems (Minneapolis, MN). ELISA was done according to the instructions of the manufacturer. Each experiment was repeated three times.
Statistical analysis
The data were expressed as mean ± standard deviation (SD) and analyzed by the SPSS 13.0 software to evaluate the statistical difference. One-way or two-way ANOVA followed by the appropriate post hoc test (Bonferroni) was used to establish whether significant differences existed between groups. For confirming the synergistic effect between matrine and TSA, comparison was made by two-way ANOVA followed by Bonferroni post hoc test. Differences were considered significant at P < 0.05. Cell viability or migration (%) and mean concentrations are shown for each group; Asterisk P < 0.05, double asterisk P < 0.01. For all tests, P values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Results and discussion
In vitro and ex vivo effects of matrine on the growth in A549 and SMMC-7721 cells and its synergistic effect with trichostatin A against A549 cell growth
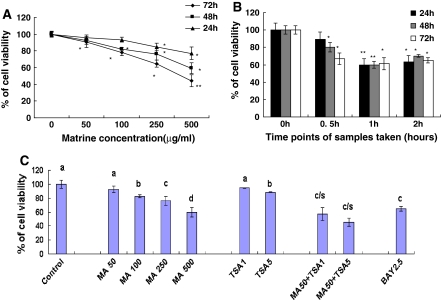
The in vitro cell growth assay showed that matrine reduced the viability of human lung cancer cell line A549 in dose- and time-dependent manners after the cells were treated with matrine at 50–500 μg/mL for 24, 48 and 72 h, respectively (Fig. 1A). The ex vivo assay indicated that the rabbit sera obtained 0.5, 1 and 2 h after oral administration of matrine significantly reduced the viability of A549 cells after the cells were treated with these sera for 24, 48 and/or 72 h, respectively (Fig. 1B). Furthermore, matrine at the concentration of 50 μg/mL enhanced the anticancer activity of the anticancer agent trichostatin A at 1 and 5 μg/L (the histone deacetylase inhibitor) by strongly reducing the viability of A549 cells after the cells were treated for 48 h with the agent (Fig. 1C). In addition, matrine at concentrations of 0.1–1.5 mg/mL dose-dependently and time-dependently decreased the viability of SMMC-7721 cells in vitro after the cells were treated with matrine for 24, 48 and 72 h, respectively (Fig. 2A). Moreover, the rabbit sera obtained 0.5 and 1 h after oral administration of matrine significantly reduced the viability of SMMC-7721 cells ex vivo after the cells were treated with these sera for 24, 48 and 72 h, respectively (Fig. 2B). These results confirm that matrine has a certain bioavailability by oral administration and the peak inhibition of the cancer cell growth is at 1 h after oral administration of matrine in rabbits. There have been reports showing that the peak plasma concentration of matrine is at 1–2 h after oral administration of matrine in rats (Zhang et al. 2008; Gao and Law 2009). Matrine also appeared in human blood after oral administration of matrine in human volunteers (Wu et al. 2006). Our present results of the in vitro and ex vivo experiments partially explain why matrine has its therapeutic and/or adjuvant therapeutic effects on treatment for some cancer patients. In the present study, the 50% inhibitory concentrations (IC50) for the growth of SMMC-7721 and A549 cells were more than 1.0 mg/mL and less than 0.5 mg/mL, respectively after the cells were treated with matrine for 72 h. It means that A549 cells are more sensitive than SMMC-7721 cells to matrine. The ex vivo assay also confirmed that the sera from the matrine-treated rabbits had much stronger inhibitory effect on growth of A549 cells than on growth of SMMC-7721 cells (Figs. 1, 2). The inhibitory effects of matrine at the different concentrations on the growth of both cancer cell lines may be the cancer-specific. We have not detected a significant inhibition of the growth in normal human liver cells after the cells were treated for 72 h with matrine at a concentration of 1.5 mg/mL (data not shown). Matrine had an activity of protecting liver function from damage (Lao 2005).
Fig. 1.
In vitro (A, C) and ex vivo (B) effects of matrine and sera from matrine-treated rabbits on the growth of human lung cancer A549 cells and the synergistic effects of matrine with anticancer agent trichostatin A against A549 cell growth. The cells were treated for 24, 48, and 72 h with matrine at the concentrations indicated (A) or the sera taken from rabbits (n = 6 for each group at different time points) at 0 (as the control group), 0.5, 1 and 2 h after oral administration of matrine in rabbits (B), respectively. C Synergistic effects of matrine with anticancer agent trichostatin A against A549 cell growth. The cells were treated for 48 h with matrine at 50–500 μg/mL (MA50, MA100, MA250, and MA500) in the absence or presence of the anticancer agent trichostatin A at 1 and 5 μg/L (TSA1 and TSA5, the histone deacetylase inhibitor) and Bay at 2.5 μM (BAY2.5, the inhibitor of NF-κB). The in vitro and ex vivo effects on A549 cell growth were examined by the MTT assay as described in the section of “Materials and methods”. The data are presented as the mean ± SD (Bar) for each group (n = 6). The figures (A, B and C) are the representative of 3 similar experiments. Comparison was made by two-way ANOVA followed by Bonferroni post hoc test to establish whether significant differences existed between the groups. Values with different letters (a–d) differ significantly (P < 0.05). c/s represents the significant synergistic effects (P < 0.05) compared with the treatment with its individual compound alone. Statistically significant synergistic effects on the growth were observed in A549 cells treated with MA50 + TSA1 or MA50 + TSA5 compared with the individual MA50, TSA1, and/or TSA5 treatment alone (MA50 + TSA1, P < 0.001, two-way ANOVA; MA50 + TSA5, P < 0.001, two-way ANOVA)
Fig. 2.
In vitro (A) and ex vivo (B) effects of matrine and sera from matrine-treated rabbits on the growth of human hepatoma SMMC-7721 cells. The cells were treated for 24, 48, and 72 h with matrine at the concentrations indicated (A) or the sera taken from rabbits (n = 6 for each group at different time points) at 0 (as the control group), 0.5, 1 and 2 h after oral administration of matrine in rabbits (B), respectively. The in vitro and ex vivo effects on the SMMC-7721 cell growth were examined by the MTT assay as described in the section of “Materials and methods”. The data are presented as the mean ± SD (Bar) for each group (n = 6). The figures (A, B) are the representative of 3 similar experiments performed. Statistical analysis was carried out using the one-way ANOVA followed by Bonferroni post hoc test. * P < 0.05
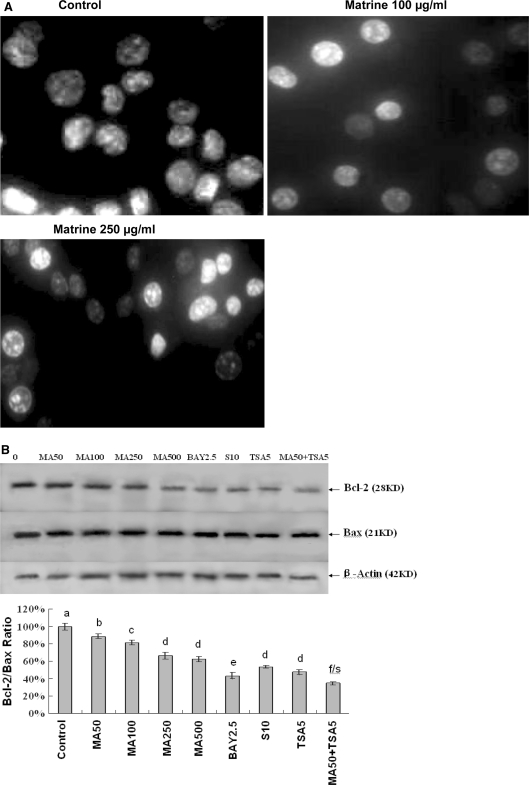
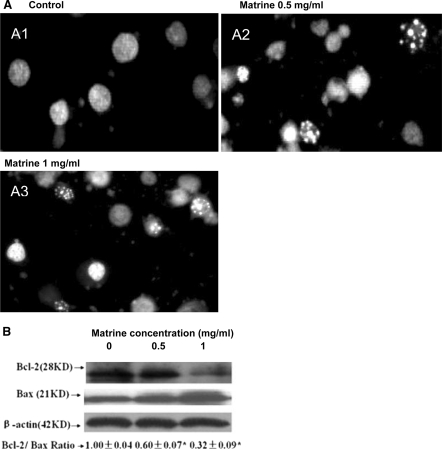
Matrine induced apoptosis by reducing the Bcl-2/Bax protein ratios in A549 and SMMC-7721 cells
To understand the mechanisms of action of matrine on the growth in human lung cancer and hepatoma cells, we investigated the effects of matrine on apoptosis and the expression of Bcl-2 and Bax proteins in A549 cells. Hoechst 33258 staining showed that the typical morphological changes, such as formation of apoptotic bodies, appeared in A549 cells after the cells were treated for 48 h with matrine at 100 and 250 μg/mL, whereas the control cells without matrine treatment did not show the evident apoptotic changes in morphology (Fig. 3A). Furthermore, Western blot analysis confirmed that matrine dose-dependently down-regulated the expression of anti-apoptotic protein Bcl-2 and affected the level of pro-apoptotic protein bax, eventually leading the reduction of Bcl-2/Bax protein ratio in A549 cells (Fig. 3B). Matrine at 50 μg/mL also enhanced the anticancer activity of anticancer agent TSA at 5 μg/L by reducing the Bcl-2/Bax protein ratio in A549 cells (Fig. 3B). In addition, Matrine at concentrations of 0.5–1 mg/mL induced apoptosis of SMMC-7721 cells (Fig. 4A) and reduced the Bcl-2/Bax protein ratio by down-regulating Bcl-2 expression and up-regulating Bax expression in the hepatoma cells (Fig. 4B). There have been studies showing that Bcl-2 and its dominant inhibitor Bax are key regulators of cell proliferation and apoptosis. Overexpression of Bcl-2 enhances cell survival by suppressing apoptosis, but overexpression of Bax accelerates cell death (Oltvai et al. 1993). Induction of apoptosis and decrease in the Bcl-2/Bax protein ratios by matrine may be one of the important mechanisms of action of matrine against the growth of human lung cancer and hepatoma cells.
Fig. 3.
In vitro effects of matrine on induction of apoptosis (A) and reduction of Bcl-2/Bax protein ratio (B) in A549 cells by matrine with its synergistic anticancer agent trichostatin A. A Induction of apoptosis in A549 cells by treatment for 48 h with matrine at concentrations of 0 (0.1% DMSO vehicle as the control), 100 and 250 μg/mL. The treated cells were stained with Hoechst 33258 and the apoptotic morphological changes in the nuclear chromatin were observed under a fluorescent microscope as described in the “Materials and methods” section. B Reduction of Bcl-2/Bax protein ratio in A549 cells by treatment for 48 h with matrine at the concentrations of 50–500 μg/mL (MA50, MA100, MA250, and MA500) in the absence or presence of Bay at 2.5 μM (BAY2.5, the inhibitor of NF-κB), celecoxib at 10 μM (S10, the inhibitor of cyclooxygenase-2), and trichostatin A at 5 μg/L (TSA5). The protein expressions of Bcl-2 and Bax were analyzed by Western blotting as described in the “Materials and methods” section. Anti-β-Actin antibody was used as a sample loading control. The ratio of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of 0 (0.1% DMSO vehicle) as the control (designated as 100%). For one experiment, 3 assays were carried out and only one set of gels is shown. Comparison was made by two-way ANOVA followed by Bonferroni post hoc test to establish whether significant differences existed between the groups. Values with different letters (a–f) differ significantly (P < 0.05); f/s represents the significant synergistic effect (P < 0.05) compared with the treatment with its individual compound alone. Statistically significant synergistic effect on the Bcl-2/Bax ratio was observed in A549 cells treated with MA50 + TSA5 compared with the individual MA50 or TSA5 treatment alone (P < 0.001, two-way ANOVA)
Fig. 4.
In vitro effects of matrine on induction of apoptosis (A) and reduction of Bcl-2/Bax protein ratios (B) in SMMC-7721 cells by matrine. A Induction of apoptosis in SMMC-7721 cells by treatment for 48 h with matrine at concentrations of 0 (0.1% DMSO vehicle as the control), 0.5 and 1 mg/mL. The treated cells were stained with Hoechst 33258 and the apoptotic morphological changes in the nuclear chromatin were observed under a fluorescent microscope as described in the “Materials and methods” section. B Reduction of Bcl-2/Bax protein ratios in SMMC-7721 cells by treatment for 48 h with matrine at concentrations of 0 (0.1% DMSO vehicle as the control), 0.5 and 1 mg/mL. The protein expressions of Bcl-2 and Bax were analyzed by Western blotting as described in the “Materials and methods” section. Anti-β-Actin antibody was used as a sample loading control. The ratio of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of 0 (0.1% DMSO vehicle) as the control (designated as 1.00). For one experiment, 3 assays were carried out and only one set of gels is shown. Statistical analysis was carried out using the one-way ANOVA followed by Bonferroni post hoc test. * P < 0.05
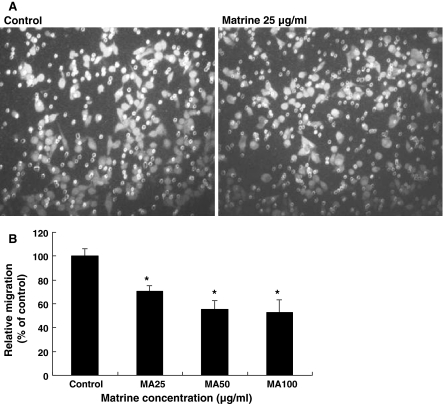
In vitro effects of matrine on the A549 cell migration
Abnormal growth and metastasis of cancer cells are regarded as the important biological characteristics of cancers. The presence of metastasis is the main causes of morbidity and mortality in millions of patients with cancer. During the complicated process of metastasis, the invasion of cancer cells is the most important and characteristic step. The migration of cancer cells is one of the important steps during the invasion. Therefore, we examined the effects of matrine on the migration in A549 cells. The migration assay indicated that matrine at the concentrations of 25–100 μg/mL significantly reduced the rate of A549 cell migration more than 30–48% after the cells were treated for 6 h with matrine (Fig. 5). Matrine at the concentrations did not reduce the viability of A549 cells after the cells were treated for 24 h with matrine. This suggests that the inhibition of A549 cell migration by matrine is not the result from the reduction of A549 viability. However, the mechanisms of action of matrine against the A549 cell migration require further investigation.
Fig. 5.
In vitro effects of matrine on the migration of human lung cancer A549 cells. The A549 cell migration was examined by the migration assay as described in the section of “Materials and methods”. A The photos show the propidium iodide-stained A549 cells that migrated through the fibronectin-coated transwell chamber. The cells were treated for 6 h with either the vehicle (0.1% DMSO as the control) or matrine at 25 μg/mL. B Suppression of A549 cell migration by matrine. The cells were treated for 6 h with matrine at the concentrations indicated in figure. Relative migration (%) ± SD (n = 6) are shown for the indicated matrine concentrations and the control received DMSO vehicle (0.1%, final concentration). The figure is the representative of 3 similar experiments. Statistical analysis was carried out using the one-way ANOVA followed by Bonferroni post hoc test. * P < 0.05
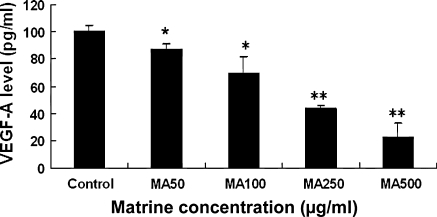
Reduction of VEGF-A secretion in A549 cells by matrine
Vascular endothelial growth factor is a positive regulator of angiogenesis, and its expression is up-regulated in many carcinomas. There has been the report showing that suppression of VEGF expression in human lung cancer cell lines including A549 cells leads the inhibition of growth in the lung cancer in vitro and in vivo (Liu et al. 2009). The clinical study suggested that angiogenic factors including VEGF are poor prognostic indicators for tumor aggressiveness and survival in human NSCLC; assessments of circulating levels of VEGF may be valuable future tools for treatment planning and monitoring of treatment effect and relapse in the lung cancer patients (Bremnes et al. 2006). The recent study has shown that VEGF-A modulates migration and invasion of lung cancer via the PI3K/AKT pathway (Chen et al. 2009). Hence, we investigated the effects of matrine on the VEGF-A secretion in A549 cells. The results from ELISA detection indicated that matrine dose-dependently reduced the VEGF-A secretion in A549 cells after the cells were treated for 24 h with matrine at the concentrations of 50–500 μg/mL (Fig. 6). This reduction of VEGF-A secretion in A549 cells by matrine may be associated with suppression of the growth and migration of A549 cells.
Fig. 6.
In vitro effects of matrine (MA) on the excretion of VEGF-A in A549 cells. The cells were treated for 24 h with matrine at concentrations of 50–500 μg/mL. Then each supernatant of the cell culture was respectively collected and analyzed by using an ELISA kit (VEGF-A) as described in the section of “Materials and methods”. Values are shown as mean ± SD (bar) for the indicated concentration (n = 3). The figure is the representative of 3 similar experiments. Statistical analysis was carried out using the one-way ANOVA followed by Bonferroni post hoc test. * P < 0.05.; ** P < 0.01
In the present study, we have confirmed that matrine significantly suppresses the growth of human lung cancer A549 and hepatoma SMMC-7721 cells in vitro and ex vivo. Furthermore, we have also demonstrated that the induction of apoptosis by reducing ratios of the Bcl-2/Bax protein levels in A549 and SMMC-7721 cells is one of the important mechanisms of action of matrine against cancer cell growth. In addition, our result indicates that matrine reduces the rate of A549 cell migration more than 30–48% at concentrations without affecting cell viability. Moreover, our present result has also suggested that the reduction of VEGF-A secretion in A549 cells by matrine may be one of important mechanisms of action of matrine against the migration and growth in A549 cells. More importantly, we show that matrine enhances the anticancer activity of the anticancer agent TSA by reducing the viability and/or the Bcl-2/Bax protein ratio in A549 cells. All these findings suggest that matrine may have the wide therapeutic and/or adjuvant therapeutic application in the treatment of human NSCLC and hepatoma.
Acknowledgments
This work is supported in part by grants from the Ministry of Education of the People’s Republic of China to G. Z, from the Ministry of Human Resources and Social Security of the People’s Republic of China to G. Z, Priority Project Fund of Yantai University to G. Z, and Projects from the Department of Science and Technology of Shandong Province to G. Z. (Y2008C71; 2009GG10002087).
Abbreviations
- MA
Matrine
- TSA
Trichostatin A
- S
Celecoxib
- NF-κB
Nuclear factor κB
- VEGF-A
Vascular endothelial growth factor A
- NSCLC
Non-small cell lung cancer
Footnotes
Ying Zhang (No. of student: 0725100168), Hui Zhang (No. of student: 200823130108), and Pengfei Yu contributed equally to this work.
References
- Bremnes RM, Camps C, Sirera R (2006) Angiogenesis in non-small cell lung cancer: the prognostic impact of neoangiogenesis and the cytokines VEGF and bFGF in tumours and blood. Lung Cancer 51:143–158 [DOI] [PubMed]
- Chen Q, Gong B, Mahmoud-Ahmed AS, Zhou A, Hsi ED, Hussein M, Almasan A (2001) Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood 98:2183–2192 [DOI] [PMC free article] [PubMed]
- Chen CH, Lai JM, Chou TY, Chen CY, Su LJ, Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J, Wu YC, Huang CY (2009) VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3 K/AKT pathway. PLoS ONE 4:e5052 [DOI] [PMC free article] [PubMed]
- Gao G, Law FC (2009) Physiologically based pharmacokinetics of matrine in the rat after oral administration of pure chemical and ACAPHA. Drug Metab Dispos 37:884–891 [DOI] [PMC free article] [PubMed]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed]
- Lao Y (2005) Clinical study of matrine injection on preventing liver function damage of anti-tumor drugs during chemotherapy of breast cancer. Zhong Yao Cai 28:735–737 [PubMed]
- Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM, Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL, Chen HC (2008) Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int J Dermatol 47:448–456 [DOI] [PubMed]
- Liu B, Peng XC, Zheng XL, Wang J, Qin YW (2009) MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. doi:10.1016/j.lungcan.2009.01.010 [DOI] [PubMed]
- Luo C, Zhu Y, Jiang T, Lu X, Zhang W, Jing Q, Li J, Pang L, Chen K, Qiu F, Yu X, Yang J, Huang J (2007) Matrine induced gastric cancer MKN45 cells apoptosis via increasing pro-apoptotic molecules of Bcl-2 family. Toxicology 229:245–252 [DOI] [PubMed]
- Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J (2008) Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med 74:245–251 [DOI] [PubMed]
- Oltvai ZN, Milliman C, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619 [DOI] [PubMed]
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108 [DOI] [PubMed]
- Wu XL, Hang TJ, Shen JP, Zhang YD (2006) Determination and pharmacokinetic study of oxymatrine and its metabolite matrine in human plasma by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal 41:918–924 [DOI] [PubMed]
- Zhang G, Miura Y, Yagasaki K (1999) Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology 31:37–44 [DOI] [PMC free article] [PubMed]
- Zhang G, Miura Y, Yagasaki K (2000) Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer 38:265–273 [DOI] [PubMed]
- Zhang G, Miura Y, Yagasaki K (2001) Inhibitory effects of theanine and sera from theanine-fed rats on receptor-mediated cancer cell beneath mesothelial-cell monolayers. Cytotechnology 36:195–200 [DOI] [PMC free article] [PubMed]
- Zhang L, Wang T, Wen X, Wei Y, Peng X, Li H, Wei L (2007) Effect of matrine on HeLa cell adhesion and migration. Eur J Pharmacol 563:69–76 [DOI] [PubMed]
- Zhang L, Liu W, Zhang R, Wang Z, Shen Z, Chen X, Bi K (2008) Pharmacokinetic study of matrine, oxymatrine and oxysophocarpine in rat plasma after oral administration of Sophora flavescens Ait. extract by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal 47:892–898 [DOI] [PubMed]