Abstract
Matrine has shown therapeutic and/or adjuvant therapeutic effects on the treatment of some patients with breast cancer. However, its mechanisms of action are largely unknown. To disclose the mechanisms, we investigated in vitro and ex vivo effects of matrine on the cancer cells. Our results confirmed that matrine significantly suppressed the proliferation of highly-metastatic human breast cancer MDA-MB-231 cell line. Matrine displayed synergistic effects with existing anticancer agents celecoxib (the inhibitor of cyclooxygenase-2), trichostatin A (the histone deacetylase inhibitor) and rosiglitazone against the proliferation and VEGF excretions in MDA-MB-231 cells. Matrine induced the apoptosis and cell cycle arrest by reducing the ratios of Bcl-2/Bax protein and mRNA levels in the cancer cells. Matrine significantly reduced the invasion, MMP-9/MMP-2 activation, Akt phosphorylation, nuclear factor κB p-65 expression and DNA binding activity, and mRNA levels of MMP-9, MMP-2, EGF and VEGFR1 in MDA-MB-231 cells. Collectively, our results suggest that matrine inhibits the cancer cell proliferation and invasion via EGF/VEGF-VEGFR1-Akt-NF-κB signaling pathway.
Keywords: Matrine, Anticancer agents, Human breast cancer, Proliferation, Invasion, MMP-9/MMP-2, Akt signaling, Nuclear factor κB
Introduction
Breast cancer is the second leading cause of cancer related deaths among females worldwide (Jemal et al. 2008; Park et al. 2008), and its rate in China and other Asian countries is also increasing rapidly (Park et al. 2008; Ziegler et al. 2008). To find novel natural compounds with low toxicity and high selectivity of killing cancer cells is an important area in cancer research. To date, chemotherapy has been the most frequently used treatment for breast cancer and other cancers. However, some normal cells are destroyed as well by this method of treatment. Due to their wide range of biological activities and low toxicity in animal models, some natural products have been used as alternative treatments for cancers including breast cancer. Matrine is a naturally occurring small-molecule compound from Traditional Chinese Medicine Sophora flavescens Ait. In China, matrine as a clinical drug has been used to treat breast cancer as well as other diseases such as viral hepatitis, cardiac arrhythmia and skin inflammations. The chemical structure of matrine is shown in Fig. 1A. Matrine induced the apoptosis of murine hepatoma cells in vitro and in vivo as well as inhibited tumor growth (Ma et al. 2008). Matrine also inhibited the invasiveness and matastasis of human malignant melanoma cell line A375 (Liu et al. 2008). However, the mechanisms of action of matrine against cancer such as human breast cancer are largely unknown. In this study, we investigated the effects of matrine on proliferation and invasion of highly-metastatic human breast cancer cells and its mechanisms of action. We confirmed that matrine inhibited the proliferation and invasion of the human breast cancer cells as well as induced the apoptosis and cell cycle arrest in the cancer cells. We have demonstrated that the anticancer activities of matrine are associated with Akt signaling and its upstream and downstream targets by suppression of the related proteins and mRNA levels as well as reduction of activation of MMP-9 and MMP-2 in the cancer cells. Matrine also displayed synergistic effects with anticancer agents against the breast cancer cells.
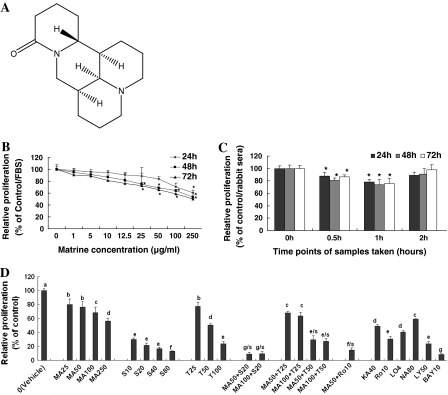
Fig. 1.
In vitro (B) and (D) and ex vivo (C) effects of matrine on the proliferation of MDA-MB-231(B) and (D) cells as well as its synergistic effects with anticancer agents against MDA-MB-231 cell proliferation (D). (A) The chemical structure of matrine. The cells were treated with matrine (B) at the concentrations indicated in figure or 10% of the sera (C) taken from matrine-fed rabbits (n = 6 for each group at different time points), respectively. The cells in control group were treated with DMSO (0.1%, final concentration). The rate of cell relative proliferation was determined by the MTT assay. (D) Synergistic effects of matrine (MA) with anticancer agents against MDA-MB-231 proliferation. The cells were treated for 48 h with the indicated concentrations of matrine (MA, 25–250 μM), celecoxib (S, 10–80 μM), trichostatin A (T, 25–100 μg/L), carmofur (KA40, 40 mg/L), navelbine (NA80, 80 nM), rosiglitazone (Ro10, 10 μM), lovastatin (LO4, 4 μM), Ly294002 (LY, 50 μM), and Bay (10 μM) in the absence or presence of the synergistic anticancer agents S (20 μM), T (25 and 50 μg/L), and Ro (10 μM). The data are presented as the mean ± SD (Bar) for each group (n = 6). The figures (B, C and D) are the representative of 3 similar experiments performed. * P < 0.05. Values with different letters (a–f) differ significantly (P < 0.05). g/s, e/s and f/s represent the significant synergistic effects (MA50 + S20, P < 0.001; MA100 + S20, P < 0.001, two-way ANOVA; MA50 + T50, P < 0.001; MA100 + T50, two-way ANOVA; MA50 + Ro10, P < 0.001, two-way ANOVA) compared with the treatment with its individual compound alone
Materials and methods
Chemicals and antibodies
Matrigel and Boyden chambers were purchased from BD Bioscience (Bedford, MA) and Costar (Corning, NY), respectively. The primary antibodies to human Bcl-2, Bax, nuclear factor (NF-κB p-65), Akt, p-Akt, and β-actin were purchased from Cell Signaling Technology Inc. (Beverley, MA). Matrine, trichostatin A, Ly294002 (LY), Bay 11-7082 (Bay), DMEM, penicillin, streptomycin, propidium iodide, gelatin 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), fetal bovine serum (FBS), trypsin/EDTA, Hoechst 33258 and all other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Celecoxib, carmofur, navelbine, rosiglitazone, and lovastatin were obtained from Yuhuangding Hospital of Yantai, Yantai, China. LightShift™ chemiluminescent EMSA kit (Product # 20148) and Biotin 3′ End DNA Labeling Kit (Product # 89818) were purchased from Pierce, Rockford.
Animal experimentation and preparation of sera from matrine-fed rabbits
These were done according to our published methods with slight modifications (Zhang et al. 2000). In brief, New Zealand White female rabbits (3.5–4 kg; from Luye Pharmaceutical Company, Yantai, China) were treated in accordance with guidelines established by the Animal Care and Use Committee at Yantai University. Matrine was orally intubated into the rabbits once daily at a dose of 10 mg/mL/kg body weight for 3 days. On the third day, the blood was then collected at 0, 0.5, 1, and 2 h from the rabbits (fasted for 16 h) after oral intubation of matrine. The prepared sera were aliquoted, and stored at −80 °C until ex vivo assays.
Cell culture and in vitro and ex vivo proliferation assays
The highly metastatic human breast cancer cell line MDA-MB-231 was obtained from the American Type Culture Collection. The cell line was cultured in DMEM medium containing 10% FBS, glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 μg/ml) at 37 °C in a humidified incubator with 95% air/5% CO2 atmosphere. The in vitro and ex vivo assays were done according to our published methods (Zhang et al. 2000, 1999). The cells in control group were treated with DMSO (0.1%, final concentration). The cells were cultured in DMEM supplemented with 10% FBS (in the case of in vitro assay) containing different concentrations of matrine or in combination with or without an existing anticancer agent (celecoxib, trichostatin A, carmofur, navelbine, rosiglitazone, lovastatin, Ly294002 and Bay), or 10% the prepared rabbits sera (in the case of ex vivo assay) mentioned above. The rate of relative cell proliferation was measured 24, 48 and 72 h after the treatments using a MTT assay kit. Each experiment was repeated three times.
Apoptosis assays and cell cycle analysis
These were done according to our published methods (Zhang et al. 2000). The treated cells were stained with Hoechst 33258. The morphological changes in the nuclear chromatin were observed under a fluorescent microscope (Nikon, TE2000-U, Japan), using 40× lens. For flow cytometry for cell cycle analysis and apoptosis, the treated cells were labeled with propidium iodide solution containing RNase A. The DNA content was analyzed by flow cytometry (Becton Dickinson FACS Vantage SE, San Jose, CA).
In vitro invasion assay
Tumor cell invasion was measured by examining cell invasion through matrigel-coated polycarbonate filters, using modified transwell chambers. MDA-MB-231 cells (5 × 104) were seeded into the upper chamber in 200 μL of serum-free medium containing matrine at different concentrations; the lower compartment was filled with 0.66 ml of DMEM medium supplemented with 10% of FBS. The cells in control group were treated with DMSO (0.1%, final concentration). After incubation for 16 h at 37 °C, the cells that invaded to the lower surface of the filter were fixed and stained using propidium iodide. The cells on the upper side of the filter were removed using a rubber scraper. The invaded cells on the underside of the filter were counted and recorded for images under a fluorescent microscope (Nikon, TE2000-U, Japan). Experiments were performed in triplicate.
Gelatin zymography
This was performed according to the method of Cheung et al. (2006) The supernatants from the upper chamber of the transwell chamber in the aforementioned invasion assay, which was serum-free, were analyzed for MMP-9/MMP-2 activation. Each experiment was repeated three times.
Western blot analysis
The cells were treated with either matrine at the different concentrations, or 10% of rabbit sera prepared as mentioned above, respectively and collected at 45 min (for detection of p-Akt) or 48 h. The cells in control group were treated with DMSO (0.1%, final concentration). The treated cells were analyzed by Western Blotting according to the method of Chen et al. (2001).
Cell fractionation and electrophoretic mobility shift assay
Cells were grown and treated as indicated. They were then pelleted by centrifugation at 1,000 rpm for 5 min at 4 °C and resuspended in ice-cold buffer A (10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethysulfonylfluoride (PMSF), 1 μg/mL leupeptin, 5 μg/mL aprotinin). Following the addition of 25 μL 10% NP40, the suspension was vortexed and centrifuged at 14,500 rpm for 1 min at 4 °C; the supernatant was designated as the cytoplasmic fraction. Nuclei were resuspended in 50 μL of ice-cold buffer B (20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 25% glycerol, 1 μg/mL leupeptin, 5 μg/mL aprotinin) and centrifuged at 14,500 rpm for 5 min. The supernatant was used as the nuclear fraction and protein concentration determined by the Bradford method. Electrophoretic mobility shift assay (EMSA) was done with 10-μg nuclear protein using the Biotin 3′ End DNA Labeling Kit (Pierce, Rockford, Product # 89818) and Biotinlabeled NF-κB consensus oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′). Nuclear protein-DNA complexes were separated by 4% PAGE. Transferred DNAs were UVcross-linked to the membrane and detected using horseradish peroxidase-conjugated streptavidin (LightShift™ chemiluminescent EMSA kit, Pierce, Rockford, Product # 20148) according to the manufacturer’s instructions.
ELISA for detection of human VEGF protein levels secreted by human breast cancer cells
For detection of effects of matrine and its synergistic anticancer agents on the secretion of vascular endothelial growth factor (VEGF) in MDA-MB-231 cells, the cells were treated for 48 h with the indicated concentrations of matrine and the anticancer agents mentioned above. Then each supernatant of the cell culture was respectively collected and analyzed by ELISA using a kit (VEGF) from R & D Systems (Minneapolis, MN). ELISA was done according to the instructions of the manufacturer. Each experiment was repeated three times.
Semi-quantitative reverse transcription-PCR
Total cellular RNA was extracted using TRIzol reagent (Invitrogen, USA) from the cancer cells treated for 48 h with matrine at different concentrations or the prepared rabbit sera mentioned above, respectively according to the manufacturer’s instructions, and quantified by spectrophotometry. The cells in control group were treated with DMSO (0.1%, final concentration). RT reaction was done using total RNA as a template and a RT-for-PCR kit (Promega, Madison, WI). PCR amplification was carried out with the following primers:
Bcl-2, 5′-GGAGGATTGTGGCCTTCTTT-3 and 5′-TCACTTGTGGCTCAGATAGGC-3′;
Bax, 5′-TCTGACGGCAACTTCAACTG-3′ and 5′-CACTGTGACCTGCTCCAGAA-3′;
β-Actin, 5′-ATCATGTTTGAGACCTTCAACACC-3′ and 5′-TAGCTCTTCTCCAGGGAGG-3′;
MMP-2, 5′-GGATGATGCCTTTGCTCG-3′ and 5′- CAGTGGACATGGCGGTCT-3′;
MMP-9, 5′-TCCCTGGAGACCTGAGAACC-3′ and 5′-GGCAAGTCTTCCGAGTAGTTT-3′;
EGF, 5′-TGCCAACTGGGGGTGCACAG-3′ and 5′-CTGCCCGTGGCCAGCGTGGC-3′;
VEGFR1, 5′-GAGAATTCACTATGGAAGATCTGATTTCTTACAGT-3′ and 5′-GAGCATGCGGATAAATACACATGTGCTTCTAG-3′.
PCR conditions for the expression of human genes included an initial denaturation of 3 min at 94 °C followed by 30 cycles of denaturation for 45 s at 94 °C, annealing for 1 min at 60 °C, and extension for 1 min at 72 °C. Aliquots (10 μL) of the amplification products were separated by electrophoresis through a 1.5% agarose gel and visualized by ethidium bromide staining. The intensity of each band was quantified using Scion Image software (Scion, Frederick, MD). Results for each detected band intensity were normalized to β-Actin band intensity values. RNA only samples that gave completely negative results in PCR without reverse transcriptase were used to rule out the presence of genomic DNA contamination.
Statistical analysis
The data were expressed as mean ± SD and analyzed by the SPSS 13.0 software to evaluate the statistical difference. One-way or two-way ANOVA followed by the appropriate post hoc test (Bonferroni) was used to establish whether significant differences existed among groups. For confirming the synergistic effect between matrine and celecoxib, trichostatin A, or rosiglitazone, comparison was made by two-way ANOVA followed by Bonferroni post hoc test. Values among different treatment groups at different times were compared. Mean concentrations and inhibition (%) are shown for each group; Asterisk P < 0.05. For all tests, P values less than 0.05 were considered statistically significant. All statistical tests were two-sided.
Results and discussion
Matrine shows in vitro and ex vivo inhibition of proliferation in MDA-MB-231 cells and synergistic activity with anticancer agents against MDA-MB-231 cell proliferation
We first confirmed that matrine reduced the rates of relative proliferation of highly-metastatic human breast cancer cell line MDA-MB-231 in a dose- and time-dependent manner after the cells were treated with matrine at 1–250 μg/mL for 24, 48 and 72 h, respectively (Fig. 1B). The ex vivo assay showed that the rabbit sera obtained 0.5 and 1 h after oral intubation of matrine significantly reduced the rate of relative proliferation of MDA-MB-231 cells after the cells were treated with these sera for 48 and 72 h, while the 2 h rabbit sera did not show the significant inhibitory effect (Fig. 1C). This result suggests that matrine has a certain bioavailability by oral administration and the peak inhibition of the cancer cell proliferation is at 1 h after oral intubation of matrine. More importantly, matrine displayed synergistic effects with existing anticancer agents such as celecoxib (the inhibitor of COX-2), trichostatin A (the histone deacetylase inhibitor) and rosiglitazone against the proliferation of MDA-MB-231 cells, which enhanced inhibitory activity on cancer cell proliferation more than twice. In addition, the anticancer agents celecoxib, trichostatin A, carmofur, rosiglitazone, lovastatin, navelbine, Ly294002 and Bay showed significant reduction of the relative proliferation rate of MDA-MB-231 cells (Fig. 1D). These results partially explain why matrine has its therapeutic and/or adjuvant therapeutic effects on treatment for some patients with breast cancer.
Matrine induced apoptosis and cell cycle arrest by reducing the ratios of Bcl-2/Bax protein and mRNA levels in MDA-MB-231 cells
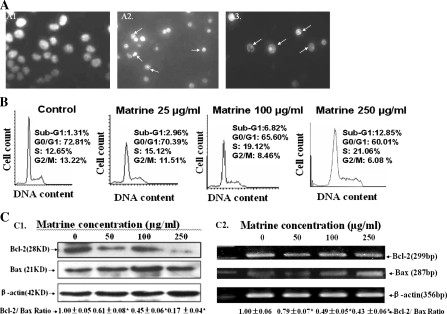
To understand the mechanisms of action of matrine against the proliferation in human breast cancer cells, we investigated the effects of matrine on apoptosis and cell cycle arrest in the cancer cells as well as the expressions of related protein and mRNA. Hoechst 33258 staining showed that the typical morphological changes, such as formation of apoptotic bodies appeared in both MDA-MB-231 cells after the cells were treated for 48 h with matrine at 100 and 250 μg/mL, whereas the control cells without matrine treatment did not show the evident apoptotic morphological changes (Fig. 2A). Flow cytometric analysis confirmed that matrine at concentrations of 25–250 μg/mL dose-dependently induced apoptosis and cell cycle arrest at the S phase in MDA-MB-231 cells (Fig. 2B). Furthermore, matrine down-regulated Bcl-2 protein and mRNA levels and up-regulated Bax protein and mRNA levels, eventually leading to the reduction of ratios of Bcl-2/Bax protein (Fig. 2C1) and mRNA (Fig. 2C2) levels in the cancer cells. There have been studies showing that Bcl-2 and its dominant inhibitor Bax are key regulators of cell proliferation and apoptosis. Overexpression of Bcl-2 enhances cell survival by suppressing apoptosis, but overexpression of Bax accelerates cell death (Oltvai et al. 1993). Induction of apoptosis and cell cycle arrest and decrease in the ratios of Bcl-2/Bax protein and mRNA levels by matrine may be one of the important mechanisms of action of matrine against the cancer cell proliferation.
Fig. 2.
In vitro effects of matrine on induction of apoptosis and cell cycle arrest as well as protein and mRNA levels of Bcl-2 and Bax in MDA-MB-231 cells. (A) Induction of apoptosis in MDA-MB-231 cells by treatment for 48 h with matrine at concentrations of 0 (A1: vehicle, 0.1% DMSO as the control), 100 μg/mL (A2) and 250 μg/mL (A3). The cells stained with Hoechst 33258 were observed under a fluorescent microscope. (B) Induction of apoptosis (cells in Sub-G1 phase) and cell cycle arrest (at S phase) in MDA-MB-231 cells by matrine was analyzed by flow cytometry. (C) Reduction of protein and mRNA levels in MDA-MB-231 cells by treatment for 48 h with matrine at the concentrations indicated in figure. The protein expressions (C1) and mRNA levels (C2) of Bcl-2 and Bax were analyzed by Western blotting and RT-PCR, respectively. ß-Actin was used as a sample loading control. The ratio of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of 0 (0.1% DMSO vehicle) as the control (designated as 1.0). For one experiment, 3 assays were carried out and only one set of gels is shown. * P < 0.05
Matrine suppressed the invasion and MMP-9/MMP-2 activation and reduced the mRNA levels of MMP-9, MMP-2, EGF, and VEGFR1 in MDA-MB-231 cells
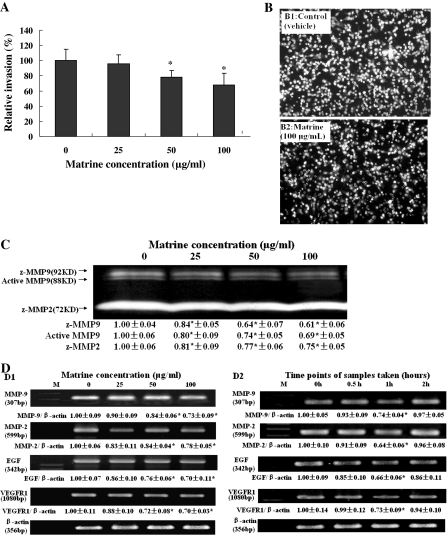
The presence of metastasis is the main cause of morbidity and mortality in millions of patients with cancer. During the complicated process of metastasis, the invasion of cancer cells is the most important and characteristic step. Clearly, an agent which could efficiently inhibit the proliferation and invasion of cancer cells would be a hopeful candidate to suppress cancer progression and metastasis and thus could reduce mortality. Therefore, we examined the effects of matrine on the invasion and related factors in cancer cells. The invasion assay indicated that matrine at 50–100 μg/mL significantly suppressed the invasion of MDA-MB-231 cells (Fig. 3A, B). In addition, Ly294002 and Bay significantly reduced the MDA-MB-231 invasion (data not shown). The gelatin zymography analysis further demonstrated that the activation of active MMP-9, and MMP-9/MMP-2 zymogens in the supernatants of invading MDA-MB-231 cells was significantly suppressed by matrine at 25–100 μg/mL (Fig. 3C). Moreover, RT-PCR detection indicated that matrine at 50–100 μg/mL significantly down-regulated the mRNA levels of MMP-9, MMP-2, VEGFR1 and EGF in MDA-MB-231 cells in vitro (Fig. 3D1). The rabbit sera obtained 1 h after oral intubation of matrine for 3 days significantly reduced the mRNA levels of MMP-9, MMP-2, VEGFR1 and EGF in MDA-MB-231 cells ex vivo (Fig. 3D2). These results suggest that the inhibition of invasion and MMP-9/MMP-2 activity as well as the reduction of mRNA levels of MMP-9, MMP-2, VEGFR1 and EGF by matrine may play an important role in the clinical effects of matrine against cancer progression.
Fig. 3.
Suppression of invasion and activation of MMP-9 and MMP-2 as well as reduction of mRNA levels of MMP-9, MMP-2, EGF and VEGFR1 in MDA-MB-231 cells by matrine. (A) Relative invasion (%) ±SD (n = 6) are shown for the indicated matrine concentrations and 0 (0.1% DMSO vehicle) is the control. (B) The photos show the propidium iodide-stained MDA-MB-231 cells invading through Matrigel-coated transwell chamber. The cells were treated for 16 h with 0 (B1: 0.1% DMSO vehicle as the control) and matrine at 100 μg/mL (B2). (C) The activation of active MMP-9 and MMP-9/MMP-2 zymogens (z-MMP9/z-MMP2) in the supernatants (serum-free) of invading MDA-MB-231 cells mentioned in (A) was determined by gelatin zymography analysis. Values (the relative activation of active MMP9, z-MMP9/z-MMP2) are shown as mean ± SD of 3 runs for each sample, only one set of gels is shown (n = 3). (D) In vitro (D1) and ex vivo (D2) effects of matrine on mRNA levels of MMP-9, MMP-2, EGF and VEGFR1 in MDA-MB-231 cells. The cells were treated for 48 h with matrine at the indicated concentrations (D1) or the matrine-fed rabbit sera (D2) obtained at the indicated time points. The mRNA levels were determined by semi-quantitative RT-PCR. For one experiment, 3 assays were carried out and only one set of gels is shown. The density of the band (normalized to β-actin) shown as mean ± SD is relative to that of the control (designated as 1.00). The figures (A, B, C and D) are the representative of 3 similar experiments performed. * P < 0.05
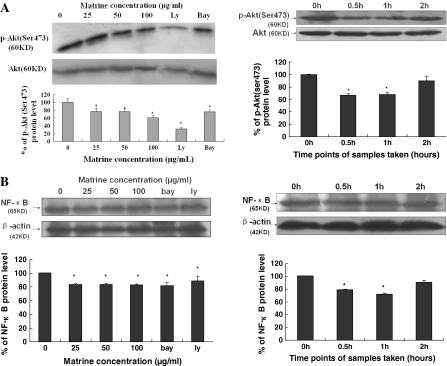
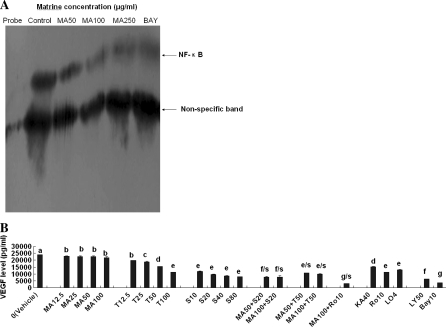
Matrine reduced the phosphorylation of Akt and expressions and activity of NF-κB p65 in vitro and/or ex vivo as well as VEGF secretions in MDA-MB-231 cells
Many reports indicated that in Akt signaling, pAkt, NF-κB, EGF, VEGF, VEGFR1, MMP-9 and MMP-2 play important roles in promoting proliferation, migration, invasion, angiogenesis, and metastasis of cancer cells (Price et al. 1999; Helbig et al. 2003; Rolli et al. 2003; Koivunen et al.1999; Kletsas et al. 2000; John and Tuszynski 2001; Shibata et al. 2002; Gibson et al. 2002; Lee et al. 2007). Thus, we next investigated if the suppression of the cancer cell proliferation and invasion by matrine is associated with Akt signaling. Western blot analysis confirmed that matrine at 25–100 μg/mL and the positive control Ly294002 (the inhibitor of PI3 K/Akt) and Bay (the inhibitor of NF-κB) significantly suppressed the phosphorylation of Akt (Fig. 4A) and NF-κB p-65 expression (Fig. 4B) in MDA-MB-231 cells in vitro. The rabbit sera obtained 0.5 and 1 h after oral intubation of matrine also significantly suppressed the phosphorylation of Akt (Fig. 4A) and NF-κB p-65 expression (Fig. 4B) in MDA-MB-231 cells ex vivo. Furthermore, the result of EMSA indicated that matrine at 50–250 μg/mL dose-dependently and significantly suppressed the activity of NF-κB p65 DNA binding (Fig. 5A) in MDA-MB-231 cells in vitro. In addition, matrine also displayed synergistic effects with anticancer agents celecoxib, trichostatin A, and rosiglitazone on reducing VEGF secretion in MDA-MB-231 cells (Fig. 5B). The anticancer agents celecoxib, trichostatin A, carmofur, rosiglitazone, lovastatin, Ly294002 and Bay also reduced VEGF secretion in MDA-MB-231 cells (Fig. 5B). These results suggest that the suppression of the cancer cell proliferation and invasion by matrine is associated with the inhibition of Akt signaling and its upstream targets such as EGF, VEGF and VEGFR1, and downstream targets such as NF-κB p-65, Bcl-2/Bax, MMP-9 and MMP-2 in the breast cancer cells. There have been studies reporting that EGF, VEGF and VEGFR1 protect from apoptosis in MDA-MB-231 cells (Gibson et al. 2002; Lee et al. 2007). EGF and NF-κB promote migration of breast cancer cells (Price et al. 1999; Helbig et al. 2003). MMP-9 is a NF-κB-regulated gene. MMP-9 and MMP-2 are not only associated with invasion and metastasis but also MMP-9 and VEGF have been implicated in angiogenesis, and hence they are considered to be the therapeutic targets of high priority (Rolli et al. 2003; Koivunen et al. 1999; Mitropoulou et al. 2003). Inhibition of NF-κB activity decreases the VEGF mRNA expression in MDA-MB-231 (Shibata et al. 2002). NF-κB is a nuclear transcription regulator with a specific motif for bcl-2 transcription (Marsden et al. 2002; Wang et al. 1996). Activation of p-Akt and the NF-κB/bcl-2 pathway leads to inhibition of chemotherapy-induced apoptosis, which results in treatment resistance (Wang et al. 1996). Therefore, the p-Akt, NF-κB, Bcl-2/Bax, EGF, MMP-9, MMP-2, VEGF and VEGFR1 in the Akt signaling have become the important targets of action by anticancer agents against the proliferation, invasion, angiogenesis and metastasis in cancer cells (Price et al. 1999; Helbig et al. 2003; Rolli et al. 2003; Koivunen et al. 1999; John and Tuszynski 2001; Gibson et al. 2002; Mitropoulou et al. 2003; Wang et al. 1996; Emi et al. 2005). Our present results have demonstrated that matrine significantly inhibits these important targets in the Akt signaling that is associated with the proliferation and invasion of MDA-MB-231 cells. These results suggest that the inhibition of both VEGF excretion and activation of pro-MMP-9/MMP-2 (z-MMP-9/z-MMP-2) and active MMP-9 as well as the reduction of MMP-9/MMP-2 mRNA levels by matrine may be one of the mechanisms of action of matrine against the proliferation and invasion of MDA-MB-231 cells. In addition, the suppression of the phosphorylation of p-Akt and the expression and activity of NF-κB as well as the EGF/VEGFR1 mRNA levels by matrine may also be the important mechanisms of action of matrine against the proliferation and invasion of the human breast cancer cells.
Fig. 4.
Effects of matrine on in vitro (left panels) and ex vivo (right panels) phosphorylation of Akt (A) and NF-κB p65 expressions (B) in MDA-MB-231 cells. The cells were treated for 48 h with matrine at the indicated concentrations or sera taken at the indicated time points from matrine-fed rabbits. p-Akt and NF-κB p65 were analyzed by Western Blotting. The density of the band (normalized to Akt) in (A) or (normalized to β-actin) in (B) shown as mean ± SD is relative to that of 0 μg/mL (0.1% DMSO vehicle) or 0 h as the control (designated as 100%). Ly (Ly294002) and Bay are the inhibitors of PI3 K/Akt and NF-κB, respectively. For one experiment, 3 assays were carried out and only one set of gels is shown. * P < 0.05
Fig. 5.
Effects of matrine on NF-κB activity (A) and its synergistic effects with anticancer agents on VEGF secretion (B) in MDA-MB-231 cells. (A) Suppression of the activity of NF-κB p65 DNA binding in MDA-MB-231 cells by matrine. The cells were treated for 48 h with matrine at the concentrations of 50–250 μg/mL and Bay (10 μM), respectively. NF-κB p65 DNA binding was analyzed by EMSA as described in the section of “Materials and methods”. (B) Reduction of VEGF secretion in MDA-MB-231 cells by matrine and its synergistic anticancer agents celecoxib (S), trichostatin A (T) and rosiglitazone (Ro). The cells were treated for 48 h with the indicated concentrations of matrine (MA, 12.5–100 μM), trichostatin A (T, 12.5–100 μg/L), celecoxib (S, 10–80 μM), carmofur (KA, 40 mg/L), rosiglitazone (Ro10, 10 μM), lovastatin (LO4, 4 μM), Ly294002 (LY, 50 μM), and Bay (10 μM) in the absence or presence of the synergistic anticancer agents S (20 μM), T (50 μg/L), and Ro (10 μM). The VEGF secreted in the supernatants of MDA-MB-231 cells was analyzed by ELISA as described in the section of “Materials and methods”. Values are shown as mean ± SD (bar) for the indicated concentration (n = 3). Values with different letters (a–g) differ significantly (P < 0.05). f/s, e/s and g/s represent the significant synergistic effects (MA50 + S20, P < 0.001; MA100 + S20, P < 0.001, two-way ANOVA; MA50 + T50, P < 0.001; MA100 + T50, two-way ANOVA; MA100 + Ro10, P < 0.001, two-way ANOVA) compared with the treatment with its individual compound alone
Abnormal proliferation and metastasis of cancer cells are regarded as the important biological characteristics of cancers. Our present results have confirmed that matrine significantly suppressed the proliferation and invasion of highly-metastatic human breast cancer MDA-MB-231 cells by affecting the expressions of Bcl-2/Bax/VEGF/p-Akt/NF-κB proteins and MMP-9/MMP-2/Bcl-2/Bax/EGF/VEGFR1 mRNA as well as activation of MMP-9/MMP-2 in the Akt signaling. The sera from matrine-fed rabbits also showed ex vivo inhibitory effects on the proliferation of MDA-MB-231 cells by affecting these protein and mRNA levels in the Akt signaling. Moreover, matrine displayed synergistic effects with the anticancer agents against the proliferation and VEGF excretion in MDA-MB-231 cells. All these findings suggest that matrine may have a wide therapeutic and/or adjuvant therapeutic application in the treatment of human breast cancer; the mechanisms of action of matrine against cancer cell proliferation and invasion are associated with the EGF/VEGF-VEGFR1-Akt-NF-κB signaling.
Acknowledgments
The authors would like to extend our thanks to Ronald E. Vincent (BIOCON Scientific) for his thoughtful reading and the help of Drs. Jianyuan Li and Shaohua Jin for FACS analysis. This work is supported in part by grants from the Ministry of Education of the People’s Republic of China to G.Z, from the Ministry of Human Resources and Social Security of the People’s Republic of China to G.Z, Projects of Yantai University to G.Z, and Projects from the Department of Science and Technology of Shandong Province to G.Z. (Y2008C71; 2009GG10002089).
Abbreviations
- MA
Matrine
- T
Trichostatin A
- LY
Ly294002
- Bay
Bay 11-7082
- S
Celecoxib
- KA
Carmofur
- NA
Navelbine
- Ro
Rosiglitazone
- LO
Lovastatin
- VEGF
Vascular endothelial growth factor
- VEGFR1
VEGF receptor-1
- EGF
Epidermal growth factor
- pro-MMP-9
Pro-matrix metalloproteinase-9
- pro-MMP-2
Pro-matrix metalloproteinase-2
- NF-κB
Nuclear factor κB
- EMSA
Electrophoretic mobility shift assay
Footnotes
Pengfei Yu and Qian Liu contributed equally to this work.
References
- Chen Q, Gong B, Mahmoud-Ahmed AS, Zhou A, Hsi ED, Hussein M, Almasan A (2001) Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood 98:2183–2192 [DOI] [PMC free article] [PubMed]
- Cheung LW, Leung PC, Wong AS (2006) Gonadotropin-releasing hormone promotes ovarian cancer cell invasiveness through c-Jun NH2-terminal kinase-mediated activation of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res 66:10902–10910 [DOI] [PubMed]
- Emi M, Kim R, Tanabe K, Uchida Y, Toge T (2005) Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res 7:R940–R952 [DOI] [PMC free article] [PubMed]
- Gibson EM, Henson ES, Haney N, Villanueva J, Gibson SB (2002) Epidermal growth factor protects epithelial-derived cells from tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by inhibiting cytochrome c release. Cancer Res 62:488–496 [PubMed]
- Helbig G, Christopherson KW, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE, Nakshatri H (2003) NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem 278:21631–21638 [DOI] [PubMed]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96 [DOI] [PubMed]
- John A, Tuszynski G (2001) The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res 7:14–23 [DOI] [PubMed]
- Kletsas D, Pratsinis H, Zervolea I, Handris P, Sevaslidou E, Ottaviani E, Stathakos D (2000) Fibroblast responses to exogenous and autocrine growth factors relevant to tissue repair. The effect of aging. Ann N Y Acad Sci 908:155–166 [DOI] [PubMed]
- Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, Sorsa T, Ruoslahti E, Pasqualini R (1999) Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol 1776:8–17774 [DOI] [PubMed]
- Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S (2007) Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med 4:e186 [DOI] [PMC free article] [PubMed]
- Liu XY, Fang H, Yang ZG, Wang XY, Ruan LM, Fang DR, Ding YG, Wang YN, Zhang Y, Jiang XL, Chen HC (2008) Matrine inhibits invasiveness and metastasis of human malignant melanoma cell line A375 in vitro. Int J Dermatol 47:448–456 [DOI] [PubMed]
- Ma L, Wen S, Zhan Y, He Y, Liu X, Jiang J (2008) Anticancer effects of the Chinese medicine matrine on murine hepatocellular carcinoma cells. Planta Med 74:245–251 [DOI] [PubMed]
- Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A (2002) Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 419(6907):634–637 [DOI] [PubMed]
- Mitropoulou TN, Tzanakakis GN, Kletsas D, Kalofonos HP, Karamanos NK (2003) Letrozole as a potent inhibitor of cell proliferation and expression of metalloproteinases (MMP-2 and MMP-9) by human epithelial breast cancer cells. Int J Cancer 104:155–160 [DOI] [PubMed]
- Oltvai ZN, Milliman CL, Korsmeyer SJ (1993) Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74:609–619 [DOI] [PubMed]
- Park S, Bae J, Nam BH, Yoo KY (2008) Aetiology of cancer in Asia. Asian Pac J Cancer Prev 9:371–380 [PubMed]
- Price JT, Tiganis T, Agarwal A, Djakiew D, Thompson EW (1999) Epidermal growth factor promotes MDA-MB-231 breast cancer cell migration through a phosphatidylinositol 3’-kinase and phospholipase C-dependent mechanism. Cancer Res 59:5475–5478 [PubMed]
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B (2003) Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100:9482–9487 [DOI] [PMC free article] [PubMed]
- Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H (2002) Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat 73:237–243 [DOI] [PubMed]
- Wang CY, Mayo MW, Baldwin AS Jr (1996) TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science 274:784–787 [DOI] [PubMed]
- Zhang G, Miura Y, Yagasaki K (1999) Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology 31:37–44 [DOI] [PMC free article] [PubMed]
- Zhang G, Miura Y, Yagasaki K (2000) Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer 38:265–273 [DOI] [PubMed]
- Ziegler RG, Anderson WF, Gail MH (2008) Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst 100:1339–1341 [DOI] [PubMed]