Abstract
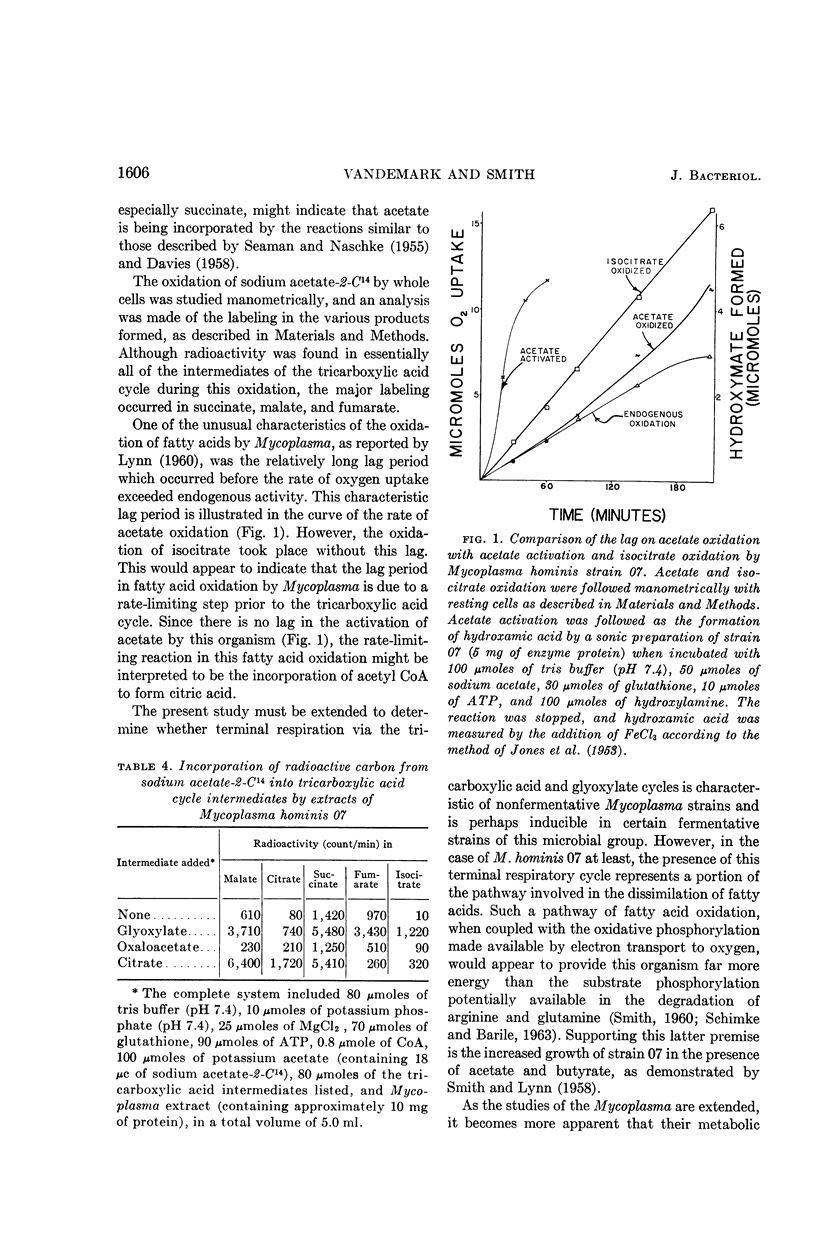
VanDemark, P. J. (Cornell University, Ithaca, N.Y.), and P. F. Smith. Evidence for a tricarboxylic acid cycle in Mycoplasma hominis. J. Bacteriol. 88:1602–1607. 1964.—Resting cells of acetate-grown Mycoplasma hominis strain 07 oxidized the various intermediates of the tricarboxylic and glyoxylate cycles, with the exception of sodium citrate and glyoxylate. Extracts of these cells possessed isocitric dehydrogenase, isocitratase, α-ketoglutaric dehydrogenase, succinic dehydrogenase, fumarase, malic dehydrogenase, citratase, and acetyl coenzyme A kinase activities. With the assay conditions employed, condensing enzyme, malate synthetase, and phosphotransacetylase activities were negligible. Incubation of sodium acetate-2-C14 with the various intermediates of the tricarboxylic acid cycle in the presence of cell-free extracts resulted in exchange of the isotope with these compounds as well as the formation of other labeled intermediates of the tricarboxylic acid cycle. Oxidation of sodium acetate-2-C14 alone resulted in the formation of labeled succinate, fumarate, and malate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASTREJON-DIEZ J., FISHER T. N., FISHER E., Jr Acetokinase reaction in several pleuropneumonia-like organisms. Biochem Biophys Res Commun. 1962 Nov 27;9:416–420. doi: 10.1016/0006-291x(62)90026-8. [DOI] [PubMed] [Google Scholar]
- CASTREJON-DIEZ J., FISHER T. N., FISHER E., Jr GLUCOSE METABOLISM OF TWO STRAINS OF MYCOPLASMA LAIDLAWII. J Bacteriol. 1963 Oct;86:627–636. doi: 10.1128/jb.86.4.627-636.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ABRAMO F., ROMANO M., RUFFO A. Inhibition of citrate oxidation by glyoxylate in rat-liver homogenates. Biochem J. 1958 Feb;68(2):270–275. doi: 10.1042/bj0680270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES D. D. Synthesis of succinate from acetate by an enzyme system of pig heart muscle. Nature. 1958 Feb 1;181(4605):339–340. doi: 10.1038/181339a0. [DOI] [PubMed] [Google Scholar]
- GILL J. W. Culture and metabolism of Mycoplasma gallisepticum. J Bacteriol. 1962 Feb;83:213–218. doi: 10.1128/jb.83.2.213-218.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES M. E., BLACK S., FLYNN R. M., LIPMANN F. Acetyl coenzyme a synthesis through pyrophosphoryl split of adenosine triphosphate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):141–149. doi: 10.1016/0006-3002(53)90133-4. [DOI] [PubMed] [Google Scholar]
- LECCE J. G., MORTON H. E. Metabolic studies on three strains of Pleuropneumonia-like organisms isolated from man. J Bacteriol. 1954 Jan;67(1):62–68. doi: 10.1128/jb.67.1.62-68.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNN R. J. Oxidative metabolism of pleuropneumonialike organisms. Ann N Y Acad Sci. 1960 Jan 15;79:538–542. doi: 10.1111/j.1749-6632.1960.tb42720.x. [DOI] [PubMed] [Google Scholar]
- NEIMARK H. C., PICKETT M. J. Products of glucose metabolism by pleuropneumonialike organisms. Ann N Y Acad Sci. 1960 Jan 15;79:531–537. doi: 10.1111/j.1749-6632.1960.tb42719.x. [DOI] [PubMed] [Google Scholar]
- OCHOA S., STERN J. R., SCHNEIDER M. C. Enzymatic synthesis of citric acid. II. Crystalline condensing enzyme. J Biol Chem. 1951 Dec;193(2):691–702. [PubMed] [Google Scholar]
- RACKER E. Spectrophotometric measurements of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta. 1950 Jan;4(1-3):211–214. doi: 10.1016/0006-3002(50)90026-6. [DOI] [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The breakdown of pyruvate by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):31–36. [PubMed] [Google Scholar]
- RODWELL A. W., RODWELL E. S. The pathway for glucose oxidation by Asterococcus mycoides, the organism of bovine pleuropneumonia. Aust J Biol Sci. 1954 Feb;7(1):37–46. [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. ARGININE METABOLISM IN PLEUROPNEUMONIA-LIKE ORGANISMS ISOLATED FROM MAMMALIAN CELL CULTURE. J Bacteriol. 1963 Aug;86:195–206. doi: 10.1128/jb.86.2.195-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN G. R., NASCHKE M. D. Reversible cleavage of succinate by extracts of Tetrahymena. J Biol Chem. 1955 Nov;217(1):1–12. [PubMed] [Google Scholar]
- SLATER E. C., BORNER W. D., Jr The effect of fluoride on the succinic oxidase system. Biochem J. 1952 Oct;52(2):185–196. doi: 10.1042/bj0520185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. Amino acid metabolism of PPLO. Ann N Y Acad Sci. 1960 Jan 15;79:543–550. doi: 10.1111/j.1749-6632.1960.tb42721.x. [DOI] [PubMed] [Google Scholar]
- SMITH P. F., LYNN R. J. Lipid requirements for the growth of pleuropneumonia-like organisms. J Bacteriol. 1958 Sep;76(3):264–269. doi: 10.1128/jb.76.3.264-269.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. THE CAROTENOID PIGMENTS OF MYCOPLASMA. J Gen Microbiol. 1963 Sep;32:307–319. doi: 10.1099/00221287-32-3-307. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- STERN J. R., SHAPIRO B., STADTMAN E. R., OCHOA S. Enzymatic synthesis of citric acid. III. Reversibility and mechanism. J Biol Chem. 1951 Dec;193(2):703–720. [PubMed] [Google Scholar]
- SWIM H. E., KRAMPITZ L. O. Acetic acid oxidation by Escherichia coli; evidence for the occurrence of a tricarboxylic acid cycle. J Bacteriol. 1954 Apr;67(4):419–425. doi: 10.1128/jb.67.4.419-425.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JACOBS R. E. Physiological and serologic comparisons of PPLO from various sources. Ann N Y Acad Sci. 1960 Jan 15;79:521–530. doi: 10.1111/j.1749-6632.1960.tb42718.x. [DOI] [PubMed] [Google Scholar]


