Abstract
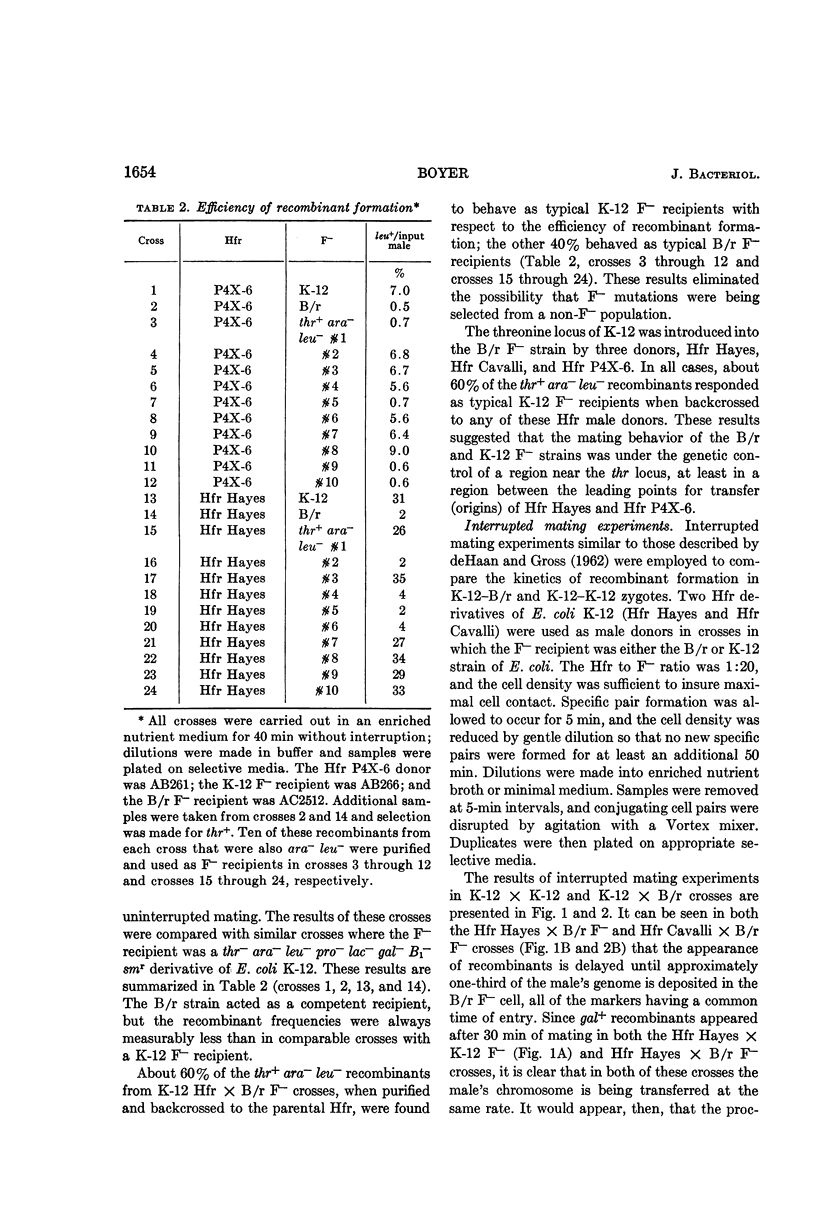
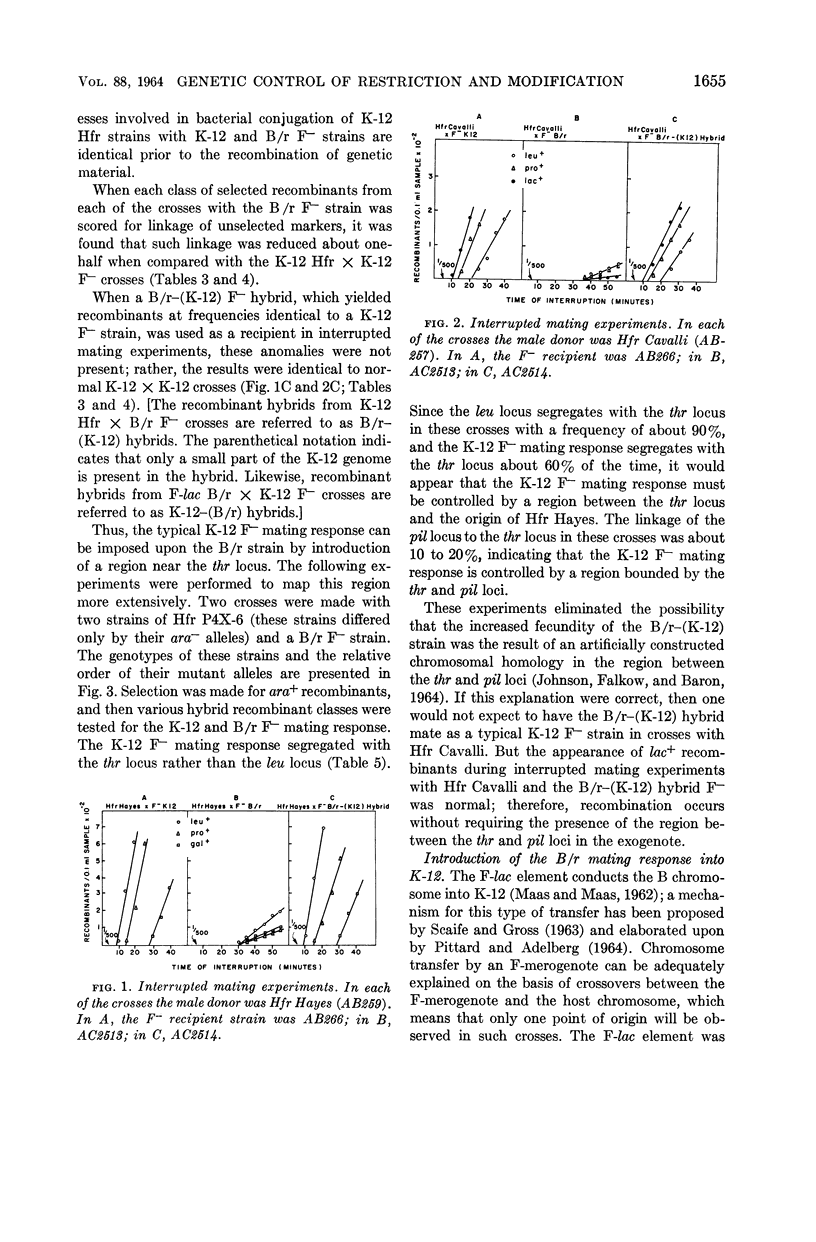
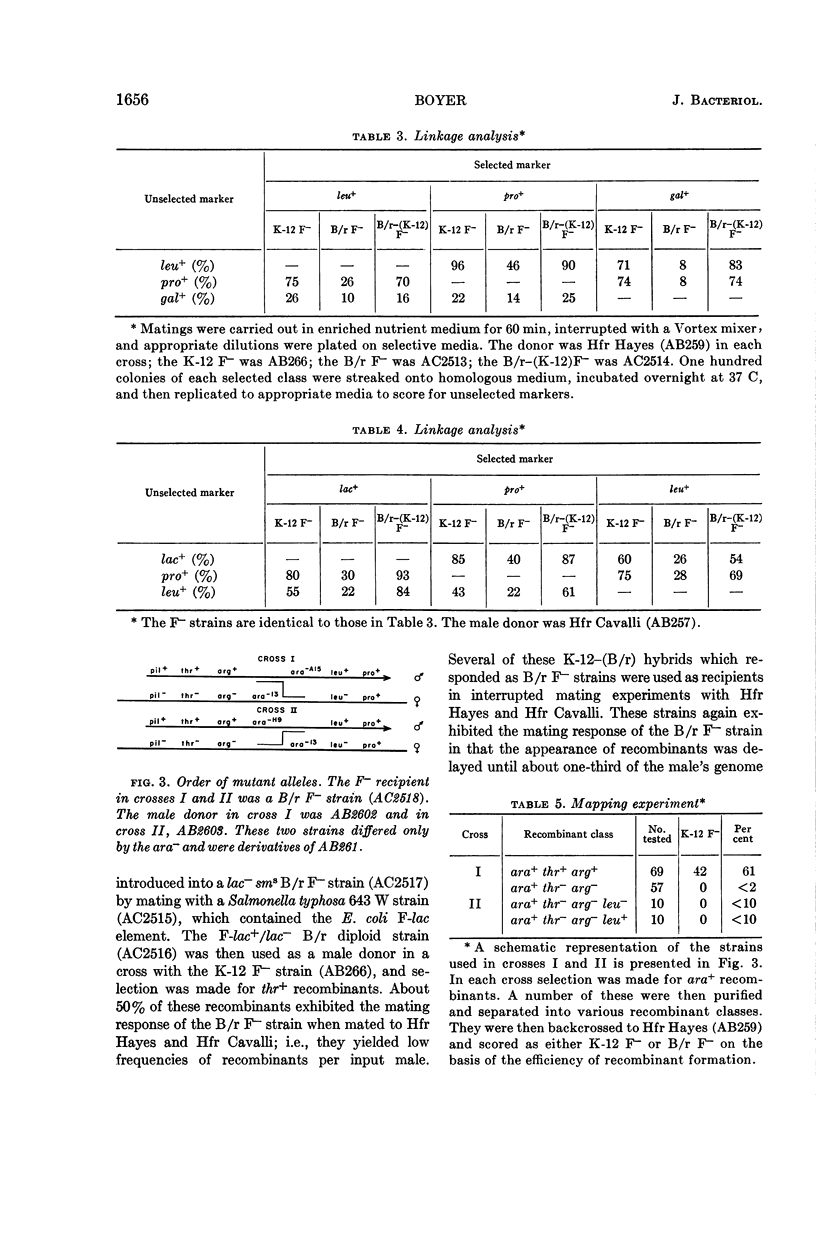
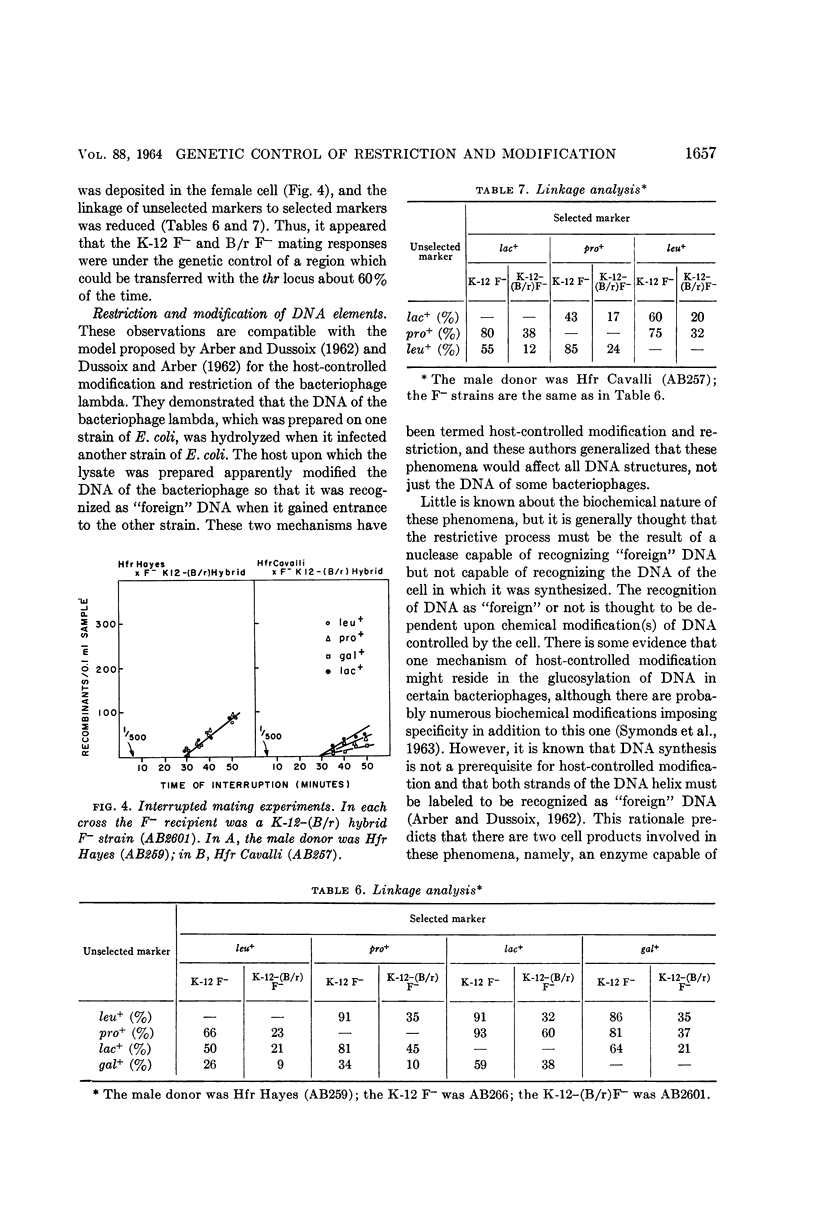
Boyer, Herbert (Yale University, New Haven, Conn.). Genetic control of restriction and modification in Escherichia coli. J. Bacteriol. 88:1652–1660. 1964.—Bacterial crosses with K-12 strains of Escherichia coli as Hfr donors (Hfr Hayes, Hfr Cavalli, and Hfr P4X-6) and B/r strains of E. coli as F− recipients were found to differ from crosses between K-12 Hfr donors and K-12 F− recipients in two ways: (i) recombinants (leu, pro, lac, and gal) did not appear at discrete time intervals but did appear simultaneously 30 min after matings were initiated, and (ii) the linkage of unselected markers to selected markers was reduced. Integration of a genetic region linked to the threonine locus of K-12 into the B/r genome resulted in a hybrid which no longer gave anomalous results in conjugation experiments. A similar region of the B strain was introduced into the K-12 strain, which then behaved as a typical B F− recipient. These observations are interpreted as the manifestation of host-controlled modification and restriction on the E. coli chromosome. This was verified by experiments on the restriction and modification of the bacteriophage lambda, F-lac, F-gal, and sex-factor, F1. It was found that the genetic region that controlled the mating responses of the K-12 and B/r strains also controlled the modification and restriction properties of these two strains. The genes responsible for the restricting and modifying properties of the K-12 and B strains of E. coli were found to be allelic, linked to each other, and linked to the threonine locus.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., ADELBERG E. A. Bacterial conjugation. Annu Rev Microbiol. 1962;16:289–319. doi: 10.1146/annurev.mi.16.100162.001445. [DOI] [PubMed] [Google Scholar]
- DUSSOIX D., ARBER W. Host specificity of DNA produced by Escherichia coli. II. Control over acceptance of DNA from infecting phage lambda. J Mol Biol. 1962 Jul;5:37–49. doi: 10.1016/s0022-2836(62)80059-x. [DOI] [PubMed] [Google Scholar]
- Echols H. PROPERTIES OF F' STRAINS OF ESCHERICHIA COLI SUPERINFECTED WITH F-LACTOSE AND F-GALATOSE EPISOMES. J Bacteriol. 1963 Feb;85(2):262–268. doi: 10.1128/jb.85.2.262-268.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON E. M., FALKOW S., BARON L. S. RECIPIENT ABILITY OF SALMONELLA TYPHOSA IN GENETIC CROSSES WITH ESCHERICHIA COLI. J Bacteriol. 1964 Jan;87:54–60. doi: 10.1128/jb.87.1.54-60.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS R., MAAS W. K. Introduction of a gene from Escherichia coli B into HFR and F-strains of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1887–1893. doi: 10.1073/pnas.48.11.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTARD J., ADELBERG E. A. GENE TRANSFER BY F' STRAINS OF ESCHERICHIA COLI K-12. III. AN ANALYSIS OF THE RECOMBINATION EVENTS OCCURRING IN THE F' MALE AND IN THE ZYGOTES. Genetics. 1964 Jun;49:995–1007. doi: 10.1093/genetics/49.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J. Effect of phage-controlled restriction on genetic linkage in bacterial crosses. J Bacteriol. 1964 May;87(5):1256–1257. doi: 10.1128/jb.87.5.1256-1257.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYMONDS N., STACEY K. A., GLOVER S. W., SCHELL J., SILVER S. The chemical basis for a case of host-induced modification in phage T2. Biochem Biophys Res Commun. 1963 Jul 26;12:220–222. doi: 10.1016/0006-291x(63)90193-1. [DOI] [PubMed] [Google Scholar]
- UETAKE H., TOYAMA S., HAGIWARA S. ON THE MECHANISM OF HOST-INDUCED MODIFICATION. MULTIPLICITY ACTIVATION AND THERMOLABILE FACTOR RESPONSIBLE FOR PHAGE GROWTH RESTRICTION. Virology. 1964 Feb;22:202–213. doi: 10.1016/0042-6822(64)90005-4. [DOI] [PubMed] [Google Scholar]


