Abstract
In the thymus, the transcription factor ThPOK is essential for the development of the CD4 helper T cell lineage, whereas active repression of ThPOK is critical for the development of the CD8 cytotoxic T cell lineage. ThPOK gene silencing is thought to be irreversible in peripheral CD8 T cells. We noticed that ThPOK repression is readily abrogated upon in vitro TCR stimulation of peripheral CD8 T cells. This observation prompted us to investigate a role for ThPOK in the CD8 T cell response to an acute viral infection. We observed that a functional deficiency of ThPOK does not affect CD8 T cell differentiation into effector T cells and the long-term persistence of Ag-specific memory T cells. However, in the absence of functional ThPOK, clonal expansion is significantly less in both primary and secondary CD8 T cell responses. Long-lived, Ag-specific CD8 T cells with a functional deficiency in ThPOK fail to produce high amounts of IL-2 and also fail to express high levels of granzyme B upon rechallenge. Our data reveal an unexpected role for ThPOK in CD8 T cells in promoting expansion and boosting the response to antigenic challenge.
Following an acute infection, memory CD8 T cells survive and persist for a long period of time after most effector CD8 T cells die upon pathogen clearance. Memory CD8 T cells acquire unique features that distinguish them from naive and effector CD8 T cells (1). The key features of memory CD8 T cells include not only a long life, but also more robust proliferation, more rapid reactivation of cytotoxic molecules, and more rapid production of cytokines than naive T cells after encountering Ag (2–5). Memory CD8 T cells express higher CD127 expression levels and produce higher amount of IL-2 than effector CD8 T cells; however, they lose those features during the secondary response (6, 7). Unknown transcription factor networks regulate this dynamic change of features in memory CD8 T cells.
The transcription factor ThPOK, also known as Zbtb7b, Zfp67, or cKrox, is essential for CD4 helper T cell development (8, 9). A spontaneous point mutation in the ThPOK gene is responsible for the helper T cell-deficient phenotype in the helper-deficient (HD)4 mutant (called “ThPOKhd/hd mice” in this article) (8). In ThPOKhd/hd or ThPOK-deficient mice, MHC class II-restricted thymocytes are redirected to develop into the CD8 cytotoxic T cell lineage and to up-regulate CD8 cytotoxic T cell-specific genes (10-12). Conversely, transgenic ThPOK expression forces MHC class I-restricted thymocytes to develop into the CD4 helper T cell lineage (8, 9). It has been reported that ThPOK inactivation in mature CD4 T cells results in the acquisition of some features of the CD8 T cell lineage, including induction of CD8 expression and high levels of granzyme B expression and IFN-γ production upon activation, indicating that ThPOK is essential for maintaining the characteristics of the CD4 T cell lineage (13). On the other hand, repression of ThPOK is essential for the development of the CD8 cytotoxic T cell lineage. Recent studies have identified critical cis-regulatory elements at the ThPOK locus (14, 15) and demonstrated that the repression of ThPOK expression through its silencer region is essential for CD8 T cell development (15). In the periphery, forced expression of ThPOK in mature CD8 T cells results in the loss of some CD8 T cell characteristics and the gain of CD4 T cell characteristics, including enhanced IL-2 production (16). Because of this, it is believed that active repression of ThPOK must be maintained permanently in the CD8 cytotoxic lineage for its cells to keep their identity. However, it is unknown whether permanent ThPOK gene silencing in mature CD8 T cells is important for their differentiating into effector and memory CD8 T cells.
We show here that peripheral CD8 T cells re-express ThPOK upon in vitro or in vivo TCR stimulation. In particular, memory CD8 T cells retain expression of ThPOK at higher levels than effector CD8 T cells, albeit at much lower levels than CD4 T cells. Therefore, we went on to evaluate a physiological role of ThPOK in peripheral CD8 T cells in the primary and secondary responses to an acute viral infection.
Materials and Methods
Mice
C57BL/6 and B6.PL (Thy1.1) mice were purchased from The Jackson Laboratory and housed under specific pathogen-free conditions in the animal facilities at the University of Washington (Seattle, WA). P14 TCR transgenic and ThPOKwt/gfp (where WT is wild type) knock-in mice (12) were bred at our facilities. ThPOKhd/hd mice, which were backcrossed to B6 mice >12 times, were provided by D. Kappes (Fox Chase Cancer Center, Philadelphia PA) (8). Mice were infected at 8–12 wk of age.
Cell preparations and adoptive transfer
Single-cell suspensions were prepared from spleens of P14 mice on a ThPOKhd/hd or ThPOKwt/wt background. For priming, naive CD44lowCD4− P14 T cells were isolated using an untouched CD8 isolation kit (Miltenyi Biotech) plus biotin-conjugated anti-CD44. Purified CD44lowCD4− P14 T cells (104) were transferred i.v. into recipient mice. P14 T cells were identified by Thy1.1 staining. For the secondary response, B cells were depleted from the spleen cells of immune mice using the MACS system (Miltenyi Biotech). Cells were stained with biotin-conjugated anti-CD19 and then labeled with anti-biotin microbeads and passed through LS columns. The flow-through cells containing 104 P14 cells in the negative fraction were transferred into new B6 hosts after determining the percentage of P14 T cells (Thy1.1+Va2+CD8+).
Microarray analysis
RNA was isolated with TRIzol (Invitrogen) from FACS-sorted memory P14 T cells after the enrichment of CD8 T cells using negative selection. RNA was prepared using an RNeasy mini kit (Qiagen) before amplification. The message was amplified using MessageAmp aRNA kit (Ambion). Duplicated samples per each group were labeled and hybridized to Affymetrix mouse 430 2.0 arrays at the Center for Array Technologies (University of Washington, Seattle, WA). Results were normalized and analyzed with GeneShifter (Geospiza). Microarray data in this study are available through the National Center for Biotechnology Information Gene Expression Omnibus database (www.ncbi.nlm.nih.gov/geo/) under accession numbers GSM426400, GSM426401, GSM426402, and GSM426403.
Infections
The lymphocytic choriomeningitis virus (LCMV) Armstrong 53b was grown and titered as described previously (17). Listeria monocytogenes expressing the LCMV-derived GP33–41 epitope (LM-GP33) provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) was grown as previously described (17). Mice were infected i.p. with 2 × 105 PFU of LCMV Armstrong for immunization and rechallenge sequentially after P14 transfer. For analysis of ThPOK/GFP expression after rechallenge, immune mice were infected i.v. with 2 × 105 LM-GP33.
Flow cytometry
Mice were sacrificed at the time points indicated and single-cell suspensions were prepared from spleens and from livers after perfusion. RBC were eliminated with ACK lysis buffer. Cells were typically stained with anti-CD8 and anti-Thy1.1. The following Abs were used: anti-CD4, anti-CD27, anti-CD28, anti-CD127, anti-KLRG1, anti-CD62L, anti-CD44, anti-Vα2, anti-T-bet, isotype control for T-bet (BD Biosciences and eBioscience), or anti-granzyme B (Caltag Laboratories). For detection of Bcl-2, we used the Bcl-2 staining kit (BD Biosciences). For CCR7 detection, cells were stained with CCL19-IgG fusion supernatant (eBioscience). H-2Db tetramers bound to the LCMV-derived peptide KAVYNFATM were generated as described (18). To exclude dead cells, 7-aminoactinomycin D (BD Biosciences) was used for some experiments. Cells were analyzed using a FACSCanto flow cytometer (BD Biosciences) and the acquired data were analyzed using FlowJo (Tree Star) software.
T cell stimulation in vitro
T cells were stimulated in 48-well plates with immobilized anti-CD3 at various concentrations as indicated and anti-CD28 at a concentration of 1 μg/ml in complete medium containing DMEM (Invitrogen) supplemented with 10% FCS, HEPES, l-glutamine, penicillin, streptomycin, gentamicin, and 50 μM 2-ME. After 2 days of stimulation, cells were removed from anti-CD3-coated plates and cultured in complete medium supplemented with 10 U/ml IL-2. Cells were analyzed for ThPOK/GFP expression on the indicated days.
Intracellular cytokine staining
Intracellular cytokine staining was done using the Cytofix/Cytoperm kit (BD Pharmingen) following the manufacturer's instructions. Spleen cells (2 × 106 per well) were cultured in 96-well plates with or without 1 μg/ml GP33 peptide supplemented with complete medium in the presence of GolgiPlug (BD Pharmingen). After 5 h of incubation, cells were stained with anti-CD8 and anti-Thy1.1, fixed and permeabilized, and stained intracellularly with anti-IFN-γ, anti-IL-2, or anti-TNF-α (BD Biosciences and eBioscience).
BrdU incorporation
Mice were injected once with 1 mg of BrdU (Sigma-Aldrich) i.p. on the indicated days. After 6 h of labeling, single-cell suspensions were prepared from the spleens or peripheral blood. Cells were stained using a BrdU flow kit (BD Biosciences) according to the manufacturer's protocol.
Statistical analysis
All statistical analyses were performed by an unpaired Student's t test.
Results
TCR stimulation in vitro induces ThPOK expression in mature CD8 T cells
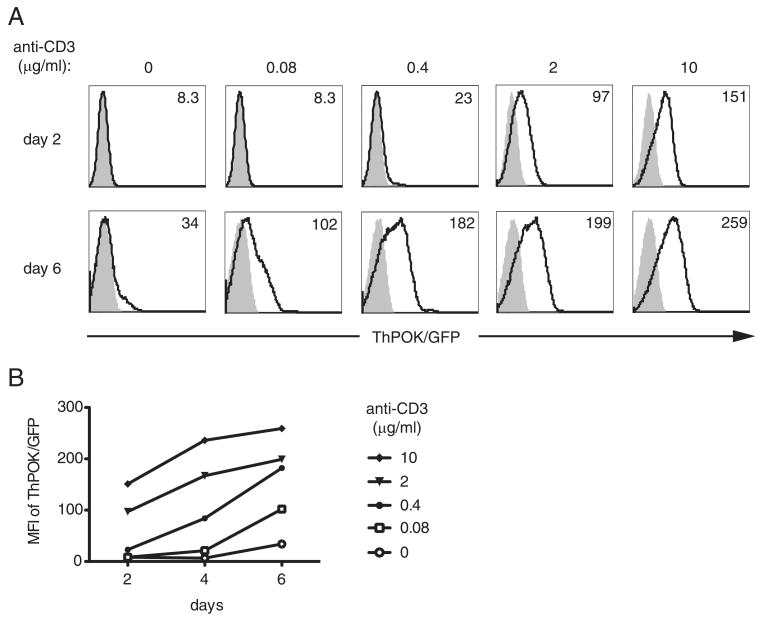
To examine whether ThPOK expression is permanently silenced in CD8 T cells, we used ThPOKwt/gfp knock-in mice that were generated by replacing the ThPOK coding sequence with a cDNA encoding GFP, rendering the ThPOK locus nonfunctional (12). Heterozygous mice serve as reporters for ThPOK expression. We purified CD8 T cells from ThPOKwt/gfp knock-in or ThPOKwt/wt control mice and stimulated them in vitro with titrated doses of immobilized anti-CD3 mAb and a fixed dose of soluble anti-CD28 mAb. We observed that stronger TCR stimulation, i.e., 2 or 10 μg/ml anti-CD3 mAb, induced ThPOK expression in CD8 T cells as early as 2 days after stimulation, and the expression levels continued to increase over time (Fig. 1). On day 6, ThPOK expression was even detectable with stimulation as low as 0.08 μg/ml CD3 mAb. These data show that CD8 T cells express ThPOK after TCR stimulation.
FIGURE 1.
Activation of peripheral CD8 T cells leads to ThPOK up-regulation. A, Splenic CD8 T cells derived from ThPOKwt/gfp (open histograms) or ThPOKwt/wt (shaded histograms) mice were exposed to the indicated concentrations of anti-CD3 and a fixed concentration of soluble anti-CD28 (1 μg/ml). After 2 days the cultures were removed from anti-CD3-coated plates and cultured in the presence of rIL-2 (10 U/ml). At 2, 4, and 6 days of culture the level of ThPOK/GFP expression was assessed by flow cytometry. The numbers inserted in the histograms refer to the GFP MFI. B, MFI of GFP expression vs time in culture is plotted. Results are representative of three independent experiments.
Ag-specific CD8 T cells increase ThPOK expression levels as they develop into effector and memory cells
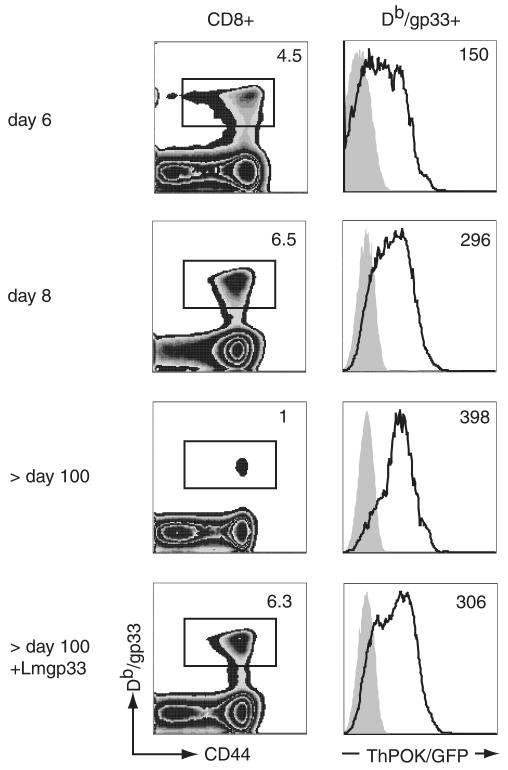
Next, we infected ThPOKwt/gfpand ThPOKwt/wt mice with LCMV and analyzed ThPOK expression levels in CD8 T cells specific for the immunodominant GP33 on days 6, 8, and ≥ 100 (memory state) after infection. Consistent with the in vitro findings, GP33-specific CD8 T cells up-regulated ThPOK expression upon priming with LCMV (Fig. 2). On day 6 we also examined ThPOK expression levels in short-lived effector KLRG1high and memory T cell precursor KLRG1low CD8 T cells (19, 20) and found that both subsets express ThPOK at similar levels (data not shown). The expression level of ThPOK increased gradually, with the highest expression in long-lived GP33-specific T cells (Fig. 2). We also checked ThPOK mRNA levels of CD44low CD8 naive T cells and GP33-specific CD8 T cells from B6 mice infected with LCMV using quantitative real-time PCR. ThPOK mRNA levels in GP33-specific T cells were up-regulated on days 8, 20, and 46 after infection compared with naive CD8 T cells (supplemental Fig. 1).5 It should be pointed out that the ThPOK expression level in long-lived GP33-specific CD8 T cells was much lower than that in naive CD4 T cells. In contrast to expanding Ag-specific CD8 T cells, we observed that activated CD44high CD4 T cells in an LCMV infection down-regulated ThPOK expression slightly compared with naive cells (supplemental Fig. 2).
FIGURE 2.
Foreign Ag-specific effector and memory T cells up-regulate ThPOK expression during an infection. Left panels, ThPOKwt/gfp and ThPOKwt/wt mice were infected with LCMV Armstrong. T cells specific for the LCMV-derived GP33 epitope in the spleen were identified using Db/GP33 tetramer and anti-CD8 staining at 6, 8, and >100 days postinfection and 5 days after rechallenge of immune mice (>100 days after the primary infection) with recombinant L. monocytogenes expressing GP33. The numbers indicate the percentage of tetramer-positive cells among CD8 T cells in ThPOKwt/gfp mice. Right panels, ThPOK/GFP expression in Db/GP33 tetramer-positive CD8 T cells was determined by flow cytometry. Shaded histograms represent ThPOKwt/wt tetramer+ cells. Numbers indicate the GFP MFI for ThPOKwt/gfp cells. Results are representative of three independent experiments.
When immune ThPOKwt/gfp and immune control mice (> 100 days postinfection) were rechallenged with LM-GP33, ThPOK expression levels in GP33-specific CD8 T cells were slightly decreased 5 days after the secondary challenge (Fig. 2). These data suggest that the transcription factor ThPOK might play a role in peripheral CD8 T cells after activation.
ThPOK expression increases the clonal burst size of Ag-specific T cells after LCMV infection
ThPOKgfp/gfp mice show a similar CD4 T cell deficiency as ThPOKhd/hd mice. It has been demonstrated that the peripheral CD8 T pool in both ThPOKhd/hd and ThPOKgfp/gfp mice contains MHC class II-restricted CD8 T cells (10, 12). In line with this, we also observed that CD8 T cells of ThPOKhd/hd mice produced IFN-γ in response to MHC class II-restricted peptides (data not shown). It has also been shown that GFP-expressing CD8 T cells in ThPOKgfp/gfp mice are MHC class II-restricted T cells (12). To exclude MHC class II-restricted CD8 T cells from our analysis, we used P14 TCR transgenic mice, which carry a transgenic TCR that recognizes the LCMV-derived, H-2Db-restricted epitope GP33. In naive mice, 70% of CD44high CD8 T cells of P14 TCR transgenic ThPOKgfp/gfp mice were GFP+ cells, and some GFP+ cells were negative for Vα2 TCR expression (supplemental Fig. 3). In contrast, CD44low P14 T cells in ThPOKgfp/gfp mice contained very few GFP+ cells (supplemental Fig. 3), indicating that class II-restricted CD8 T cells are excluded from CD44low ThPOKgfp/gfp P14 T cells. For our experiments we therefore used purified CD44low P14 T cells to determine a role for ThPOK in MHC class I-restricted CD8 T cells.
To do this, we crossed P14 TCR transgenic mice with ThPOKhd/hd mice that had been backcrossed onto a B6 background for >12 generations. We used ThPOKhd/hd mice instead of ThPOKgfp/gfp mice, because the latter are on a mixed genetic background. Expression levels and patterns of activation and naive markers (CD28, CD27, KLRG1, CD127, CD62L, and CCR7) were similar between CD44low ThPOKhd/hd and CD44low ThPOKwt/wt P14 T cells (supplemental Fig. 4A).
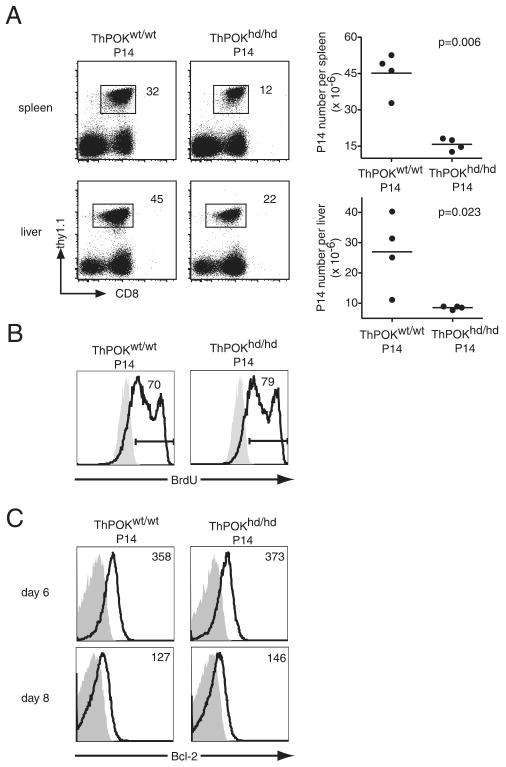
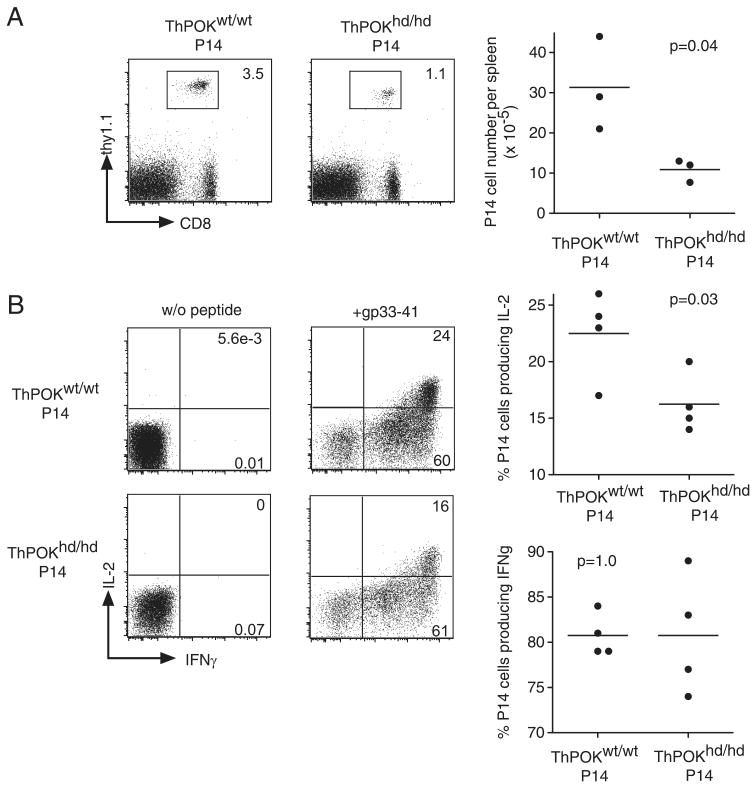
We transferred 104 CD44low ThPOKhd/hd or ThPOKwt/wt P14 T cells (Thy1.1+) into B6 mice and then infected the recipients with LCMV. On day 4 postinfection, P14 T cells could not be detected in blood, however similar numbers of P14 T cells were detected in the spleen of both groups (supplemental Fig. 5A). On day 5 postinfection, the percentage of ThPOKhd/hd P14 T cells in blood declined slightly but not significantly compared with ThPOKwt/wt P14 T cells (supplemental Fig. 5B). By day 6 postinfection, the percentage of ThPOKhd/hd P14 T cells in blood had decreased significantly compared with ThPOKwt/wt P14 T cells (supplemental Fig. 5B), indicating that ThPOK expression is important during the expansion phase, at least from day 6 after infection. At day 8 after infection, the number of ThPOKhd/hd P14 T cells in the spleen was less than that of ThPOKwt/wt T cells (Fig. 3A). Similarly as in the spleen, the reduction of ThPOKhd/hd P14 T cell numbers was also observed in the liver (Fig. 3A), indicating that the decline of ThPOKhd/hd P14 T cell numbers is not due to an altered tissue distribution. Next, we checked the turnover of ThPOKhd/hd P14 and ThPOKwt/wt P14 T cells. Six days after infection, BrdU was administered to the mice for a 6-h period. BrdU+ cells accounted for 70–80% of ThPOKhd/hd P14 T cells and ThPOKwt/wt P14 T cells, suggesting that impaired expansion of ThPOKhd/hd P14 T cells is not due to defects in proliferation (Fig. 3B). Bcl-2 expression levels were comparable between ThPOKhd/hd P14 T cells and ThPOKwt/wt P14 T cells on days 6 and 8 after infection (Fig. 3C), suggesting the possibility that other antiapoptotic molecules play an important role in the ThPOK-mediated amplification of CD8 T cell clonal expansion.
FIGURE 3.
Reduced accumulation of effector P14 T cells in the absence of functional ThPOK. A, CD44lowCD4−ThPOKhd/hd or ThPOKwt/wt P14 cells (Thy1.1) (104) were transferred into B6 hosts that were subsequently immunized with LCMV. At 8 days postinfection, the fraction of both types of P14 T cells and their absolute numbers in spleen and liver were determined. The results are representative of eight independent experiments for spleen and two independent experiments for liver. B, Host mice were injected with 1 mg of BrdU i.p. (open histograms) or left untreated (shaded histograms) at 6 days postinfection. Six hours later, cells harvested from the spleen were analyzed for BrdU incorporation. The numbers in the histograms are the percentages of BrdU+ cells among P14 cells. C, At the indicated time points the levels of Bcl-2 expression (open histograms) in ThPOKhd/hd P14 or ThPOKwt/wt P14 cells were determined by flow cytometry. Shaded histograms represent isotype controls and numbers refer to the MFI of Bcl-2.
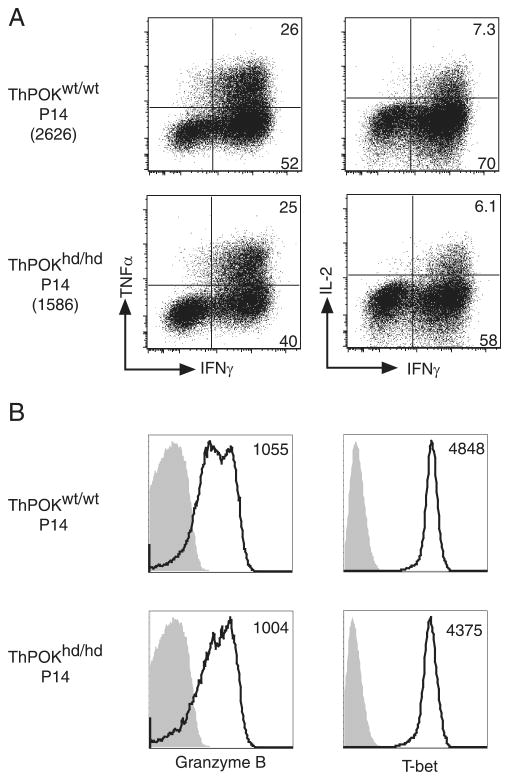
We also compared the phenotype and effector function of ThPOKhd/hd and ThPOKwt/wt P14 T cells at day 8 after infection. We did not detect significant differences in the expression levels of CD28, CD27, KLRG1, CD127, CD62L, and CCR7 between the two groups (supplemental Fig. 4B). ThPOKhd/hd P14 T cells produced IFN-γ, TNF-α, and IL-2 at similar percentages as those of ThPOKwt/wt P14 T cells in response to GP33 peptide stimulation, although the mean fluorescence intensity (MFI) of IFN-γ in ThPOKhd/hd P14 T cells decreased slightly (Fig. 4A). Granzyme B and T-bet expression levels in ThPOKhd/hd P14 T cells were also equivalent to those in control P14 T cells (Fig. 4B). These data indicate that CD8 T cells can develop into effector T cells in the absence of functional ThPOK.
FIGURE 4.
Normal differentiation of effector T cells in the absence of functional ThPOK. A, Splenocytes harvested at day 8 from mice that received either ThPOKhd/hd or ThPOKwt/wt P14 cells plus LCMV were stimulated in vitro with the GP33 peptide for 5 h. The percentages of P14 T cells producing IFN-γ, TNF-α, and IL-2 after stimulation are shown. The numbers in parenthesis indicate the MFI of IFN-γ of P14 T cells. B, ThPOKhd/hd or ThPOKwt/wt P14 cells were analyzed for granzyme B and T-bet expression 8 days postinfection. Shaded histograms are isotype controls and numbers refer to the MFI of granzyme B or T-bet.
HD mice harbor a point mutation within the second of four zinc finger domains of the ThPOK gene that is predicted to diminish its DNA binding ability. Polyclonal ThPOKwt/hd GP33-specific CD8 T cells in mixed bone marrow chimeras and ThPOKwt/hd P14 T cells exhibited milder reductions of their number 8 days after LCMV infection (supplemental Fig. 6, A and B), suggesting that the decline of ThPOKwt/hd GP33-specific CD8 T cell number depends on the quantity of ThPOK expression. However, there are other possible explanations for this heterozygous effect. First, mutated ThPOK might bind to other sites on DNA to which normal ThPOK does not. Second, mutated ThPOK may function as a dominant negative. To exclude these possibilities, we bred ThPOKwt/gfp heterozygous knockout mice to P14 TCR transgenic mice. We transferred 104 CD44low P14 T cells purified from ThPOKwt/gfp or ThPOKwt/wt littermates (both groups were from mice backcrossed to B6 for six generations) into congenic B6 hosts (Ly5.1) and infected the recipients with LCMV. Similarly as for ThPOKwt/hd P14 T cells, the number of ThPOKwt/gfp P14 T cells was decreased 8 days after infection (supplemental Fig. 6C), demonstrating that the effect of ThPOK on the clonal expansion of CD8 T cells depends on the quantity of ThPOK.
Memory CD8 T cells persist in the absence of functional ThPOK but show reduced ability to produce IL-2
ThPOKhd/hd P14 T cells developed into functional effector T cells, although their number was smaller than those of control P14 T cells. We asked whether they can develop into memory T cells. The percentage of ThPOKhd/hd P14 T cells in peripheral blood was approximately one-third that of ThPOKwt/wt P14 T cells at day 20 as well as at day 8 after infection (data not shown), indicating that ThPOKhd/hd P14 T cells exhibited a similar degree of contraction as control P14 T cells. During the memory phase, >100 days after infection, the percentages and numbers of ThPOKhd/hd P14 T cells were one-third those of ThPOKwt/wt P14 T cells in the spleen (Fig. 5A), preserving the ratio observed during the effector phase (Fig. 3A; 8 days after infection). During the memory phase, there was no significant difference in the expression levels of CD28, CD27, KLRG1, CD127, CD62L, and CCR7 between ThPOKhd/hd P14 and ThPOKwt/wt P14 long-lived CD8 T cells (supplemental Fig. 4C). We also checked their cytokine production ability in response to GP33 peptide stimulation. ThPOKhd/hd P14 T cells produced IFN-γ at a similar level as that of ThPOKwt/wt P14 T cells (Fig. 5B). However, IL-2 production was significantly reduced in ThPOKhd/hd P14 memory T cells compared with ThPOKwt/wt P14 T cells (Fig. 5B).
FIGURE 5.
P14 T cells lacking functional ThPOK can form long-lived T cells that exhibit impaired ability to produce IL-2. A, The percentages and the absolute numbers of ThPOKhd/hd and ThPOKwt/wt P14 T cells in the spleen at >100 days postinfection with LCMV were determined by CD8, Vα2, and Thy1.1 expression. B, Fractions of both types of long-lived P14 T cells that produce IFN-γ and IL-2 in response to in vitro stimulation with the GP33 peptide for 5 h.
Thus, these data clearly demonstrate that ThPOK is not essential for the formation and long-term maintenance of memory CD8 T cells, however, a ThPOK-regulated transcriptional pathway amplifies IL-2 production in long-lived P14 CD8 T cells, not in effector CD8 T cells.
A ThPOK-regulated transcriptional pathway is required for a robust recall response
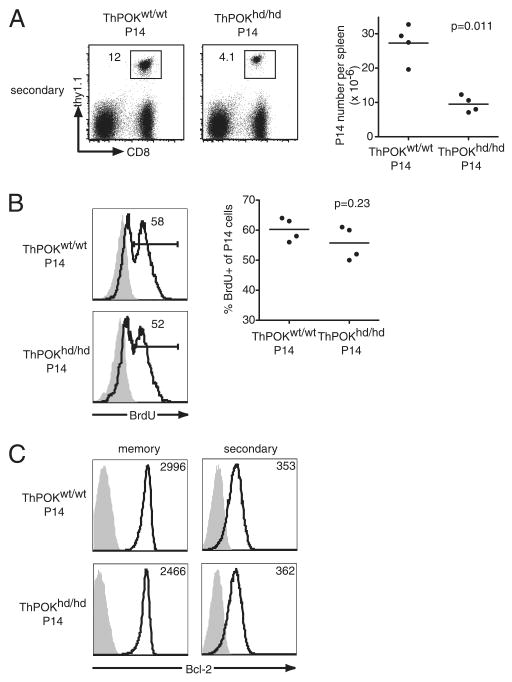
The most important features of memory T cells are vigorous proliferation and rapid reactivation of effector functions after rechallenge. We therefore examined whether long-lived ThPOKhd/hd P14 T cells are functional memory CD8 T cells or not. We transferred B cell-depleted spleen cells, including 104 long-lived ThPOKhd/hd P14 T cells and ThPOKwt/wt P14 memory T cells, into secondary B6 hosts and then rechallenged with LCMV. The two groups of memory cells showed similar expression levels of CD62L and CCR7 before the secondary transfer (supplemental Fig. 4C). Seven days following rechallenge, the percentages and numbers of ThPOKhd/hd P14 T cells in the spleen were smaller than those of ThPOKwt/wt P14 T cells (Fig. 6A). A similar decrease of ThPOKhd/hd P14 T cell number was observed in the liver (data not shown), suggesting that the decline of ThPOKhd/hd P14 T cells is not caused by an altered tissue distribution. Similar to the effector phase, BrdU incorporation in ThPOKhd/hd P14 T cells was equivalent to that in ThPOKwt/wt P14 T cells 6 days after rechallenge (Fig. 6B). Down-regulation of Bcl-2 expression levels in ThPOKhd/hd P14 T cells and ThPOKwt/wt P14 T cells also followed similar kinetics (Fig. 6C). Therefore, these data suggest that ThPOK regulates the expression of survival factors other than Bcl-2 directly or indirectly for CD8 T cell recall response.
FIGURE 6.
P14 memory T cells lacking ThPOK show reduced accumulation upon rechallenge. A, Ninety to 180 days postinfection, CD19+ cell-depleted spleen cells containing 104 ThPOKhd/hd or ThPOKwt/wt P14 cells were transferred into new B6 hosts, and rechallenged with LCMV. The percentages and absolute numbers of each type of P14 T cell 7 days after rechallenge were analyzed for CD8 and Thy1.1 expression. Results are representative of four independent experiments. B, B6 host mice were injected with (open histograms) or without (shaded histograms) 1 mg of BrdU i.p. 6 days after rechallenge. The percentages of BrdU incorporation in ThPOKhd/hd or ThPOKwt/wt P14 cells in PBL were analyzed 6 h after BrdU injection. Results are representative of two independent experiments. C, Bcl-2 expression levels (open histograms) in ThPOKhd/hd and ThPOKwt/wt P14 cells from the spleen before (90–180 days postinfection) or 7 days after the secondary challenge. Shaded histograms are isotype controls. The numbers in the histograms indicate MFI of Bcl-2.
To identify genes either directly or indirectly regulated by ThPOK, we performed a microarray analysis of ThPOKhd/hd and ThPOKwt/wt memory P14 T cells. We analyzed gene expression profiles of memory cells, because they showed the highest and most uniform ThPOK expression compared with not only naive but also effector CD8 T cells as shown in Fig. 2. Ninety-one genes were found to be differentially expressed (with >1.5-fold difference) between these two populations of CD8 T cells. Four genes whose products are involved in DNA repair and three genes encoding adhesion molecules showed decreased expression in ThPOKhd/hd P14 T cells compared with WT P14 T cells (supplemental Table I). In contrast, CD160, which was reported to serve as a coinhibitory signal (21), was up-regulated in ThPOKhd/hd P14 T cells compared with ThPOKwt/wt P14 T cells (supplemental Table I). Altered expression of these genes might contribute to the impaired clonal expansion of functional ThPOK-deficient CD8 T cells.
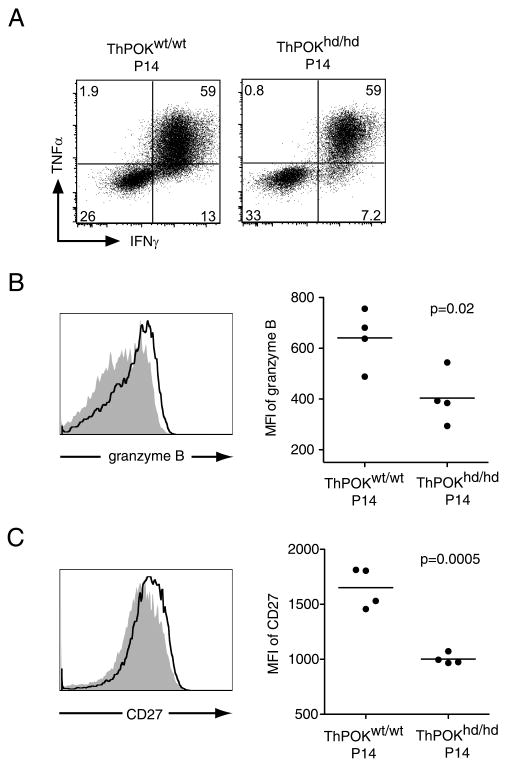
We also analyzed the reactivation of effector functions, namely cytokine production and granzyme B expression, in mutant and control P14 T cells after rechallenge. ThPOKhd/hd P14 T cells produced IFN-γ and TNF-α at similar levels as those of ThPOKwt/wt P14 T cells in response to GP33peptide stimulation (Fig. 7A). However, the level of granzyme B re-expression in ThPOKhd/hd P14 T cells upon rechallenge was lower than that in ThPOKwt/wt P14 T cells 5 days after rechallenge (Fig. 7B). Seven days after rechallenge, the cell surface expression level of CD27, which was reported to contribute to clonal expansion in the secondary response to LCMV (22), was significantly down-regulated on ThPOKhd/hd P14 T cells compared with control P14 T cells in the spleen (Fig. 7C) and in the liver (data not shown).
FIGURE 7.
Reduced expression levels of granzyme B and CD27 during the secondary response in the absence of ThPOK. A, Five days after the secondary LCMV challenge, spleen cells from rechallenged mice were stimulated in vitro with GP33 peptide for 5 h. The production of IFN-γ and TNF-α in ThPOKhd/hd or ThPOKwt/wt P14 cells are shown. B, Granzyme B expression levels of ThPOKhd/hd (shaded histogram) or ThPOKwt/wt (open histogram) P14 cells in the spleen 5 days after rechallenge were analyzed. C, CD27 expression on ThPOKhd/hd P14 (shaded histogram) or ThPOKwt/wt P14 cells (open histogram) 7 days after rechallenge was analyzed by flow cytometry. Results are representative of two independent experiments.
Collectively, these data demonstrate that a ThPOK-regulated transcriptional pathway is important for the function and expansion of memory CD8 T cells.
Discussion
During thymocyte development, the transcription factor ThPOK has been shown to regulate lineage choice into CD4 or CD8 T cells. In naive peripheral T cells, ThPOK expression is restricted to CD4 T cells, and its expression is essential for maintenance of the lineage. It has been also demonstrated that active repression of ThPOK through its silencer element is critical for CD8 T cell development. Previously, repression of ThPOK in the CD8 lineage was believed to be maintained permanently, because forced ThPOK overexpression resulted in the loss of some features of the CD8 T cell lineage and the acquisition of certain features of the CD4 lineage. However, our study reveals that TCR stimulation readily breaks the repression of ThPOK in mature CD8 T cells and that physiological expression levels of ThPOK in mature CD8 T cells promote robust T cell proliferation in both primary and secondary responses. Thus, our study reveals a function of ThPOK beyond the CD4 vs CD8 T cell lineage fate decision.
We initially showed that TCR signals combined with CD28 ligation are sufficient to induce ThPOK expression and that ThPOK expression levels correlated with the levels of anti-CD3 stimulation in vitro. This indicates that TCR engagement is an upstream signaling pathway that leads to ThPOK expression in peripheral CD8 T cells. It has been shown that strong TCR signals impact the magnitude of clonal expansion in vivo but do not influence the formation of memory CD8 T cells (23, 24). Massive clonal expansion induced by strong TCR ligation might be regulated through a ThPOK-regulated transcriptional pathway. Which other signaling pathways may also lead to the expression of ThPOK in CD8 T cells remains unknown. Costimulation and inflammation are other important factors that strongly influence CD8 T cell responses. Costimulatory signals, such as those from CD27, CD28, and 4-1BB, have been reported to facilitate the clonal burst size of CD8 T cell responses (25–27). Inflammatory signals, including those of IL-12 and type I IFNs, have been reported to promote the survival of activated CD8 T cells after L. monocytogenes or LCMV infection, respectively (28, 29). Therefore, these factors, IL-12, type I IFN, and costimulatory molecules, might be candidates that support the induction of ThPOK expression in CD8 T cells that receive weak TCR signaling.
Contrary to physiological ThPOK expression in activated CD8 T cells, forced expression of high levels of ThPOK in mature CD8 T cells has been reported to lead to significant down-regulation of CD8 lineage features including the expression of the CD8 coreceptor, eomesodermin (Eomes), perforin, granzyme B, and IFN-γ, while simultaneously up-regulating CD4 T cell features such as IL-2, IL-4, and GATA-3 expression (16). Interestingly, activated CD8 T cells express ThPOK at rather low levels equal to about one-tenth the level of that in naive CD4 T cells. It is important to note that physiological expression levels of ThPOK in activated CD8 T cells do not induce trans-differentiation of CD8 T cells. These observations suggest that re-expressing ThPOK at such a low level after activation is critical for maintaining the features of the CD8 T cell lineage. It remains to be determined which mechanisms, such as cis-elements of the ThPOK locus, repression of ThPOK enhancer/promoter activity, or enhancement of silencer activity, contribute to maintaining ThPOK expression at low levels in Ag-activated CD8 T cells.
Memory CD8 T cells produce more IL-2 than effector CD8 T cells. We observed mild yet significantly higher production of IL-2 in ThPOKwt/wt CD8 T cells than in ThPOKhd/hd cells during the memory phase, but not during the effector phase. The differential requirement of ThPOK for IL-2 production between effector and memory CD8 T cells is likely to be ascribed to different ThPOK expression levels or collaboration with another factor that is specifically expressed in memory CD8 T cells. It has been reported that the methylation status of IL-2 promoter in memory CD8 T cells is decreased compared with that in effector and naive cells, suggesting that the IL-2 locus is more accessible to transcription factors during the memory phase (30). It is well known that naive CD4 T cells can produce more IL-2 upon activation compared with other cell types. Thus, there might be common features of IL-2 gene regulation mediated by ThPOK in IL-2-producing CD4 T cells and in memory CD8 T cells.
Granzyme B is a key cytotoxic molecule. To date, it has been reported that Runx3 and Eomes are required for the up-regulation of granzyme B expression in activated CD8 T cells (31, 32). Recent evidence shows that ThPOK disruption in peripheral CD4 T cells leads to up-regulation of granzyme B expression (13), suggesting the possibility that ThPOK might repress granzyme B expression directly or indirectly in peripheral CD4 T cells. Our data show the differential contribution of ThPOK to granzyme B expression between the primary and secondary responses of CD8 T cells. ThPOKhd/hd P14 T cells expressed granzyme B at similar levels as control P14 T cells during the primary response, but much lower levels during the secondary response. It is possible that Runx3 or Eomes rather than ThPOK has a dominant role in the regulation of granzyme B expression during the primary response. The role of ThPOK in the regulation of granzyme B expression in CD8 T cells during the secondary response and in mature CD4 T cells is apparently contradictory, i.e., negative regulation in the CD4 T cell lineage and positive regulation in the secondary CD8 T cell response. However, two possibilities might explain this. First, if ThPOK regulates granzyme B expression directly, a lineage-specific transcriptional network might play a dominant role in the regulation of granzyme B expression. Second, if ThPOK does not bind to the cis-elements of the granzyme B locus, ThPOK may be important for the cellular fitness of the CD4 T cell lineage and memory CD8 T cells through different target molecules that it regulates directly. Further analysis of ThPOK target genes in the different T cell lineages will evaluate these possibilities.
Microarray analysis identified genes that could be regulated either directly or indirectly by ThPOK in Ag-experienced CD8 T cells. We found that some molecules related to DNA repair, including antiapoptotic functions and adhesion molecules, were down-regulated, whereas CD160 was up-regulated in functional ThPOK-deficient CD8 T cells compared with WT CD8 T cells. ThPOK might up-regulate these antiapoptotic molecules directly and promote the survival of CD8 T cells during clonal expansion. ThPOK might up-regulate those adhesion molecules directly on the surface of CD8 T cells and induce the strong interaction with APCs in the secondary response. On the other hand, CD160 might be repressed directly by ThPOK and maximize the burst size of CD8 T cells. Questions that remain unsolved include the identity of the essential molecule directly regulated by ThPOK that maximizes the burst size of CD8 T cell response and whether this mechanism is common in both the primary and secondary responses. Significantly higher CD27 expression levels and higher granzyme B re-expression levels in WT T cells vs mutant T cells were detected in the secondary response, but not in the primary response. Therefore, there is the possibility that ThPOK amplifies the burst size via different mechanisms in the primary and in secondary responses. Future studies are needed to determine the molecules involved in the ThPOK-regulated transcription pathway that regulates the amplified CD8 T cell response in the primary and secondary immune responses.
Supplementary Material
Acknowledgments
We thank members of the laboratory, especially B. Dere, B. Paul, X. Pan, and K. Rider, for technical assistance in the breeding, maintaining and typing mouse colonies, D. Kamimura for helpful comments on this study, and D. Zehn and S. Hori for critical reading of the manuscript. We also thank Dietmar Kappes for his gifts of ThPOKhd/hd mice.
Footnotes
This work was supported by the Howard Hughes Medical Institute (to M.J.B.) and by National Institutes of Health Grant AI19335.
The microarray data presented in this article have been submitted to the Gene Expression Omnibus under accession numbers GSM426400, GSM426401, GSM426402, and GSM426403.
Abbreviations used in this paper: HD, helper deficient; Eomes, eomesodermin; LCMV, lymphocytic choriomeningitis virus; GP33, LCMV-derived GP33–41 epitope; LM, Listeria monocytogenes; MFI, mean fluorescence intensity; WT, wild type.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 2.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 5.Rocha B, Tanchot C. CD8 T cell memory. Semin Immunol. 2004;16:305–314. doi: 10.1016/j.smim.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- 7.Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X, He X, Dave VP, Zhang Y, Hua X, Nicolas E, Xu W, Roe BA, Kappes DJ. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 9.Sun G, Liu X, Mercado P, Jenkinson SR, Kypriotou M, Feigenbaum L, Galera P, Bosselut R. The zinc finger protein cKrox directs CD4 lineage differentiation during intrathymic T cell positive selection. Nat Immunol. 2005;6:373–381. doi: 10.1038/ni1183. [DOI] [PubMed] [Google Scholar]
- 10.Keefe R, Dave V, Allman D, Wiest D, Kappes DJ. Regulation of lineage commitment distinct from positive selection. Science. 1999;286:1149–1153. doi: 10.1126/science.286.5442.1149. [DOI] [PubMed] [Google Scholar]
- 11.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muroi S, Naoe Y, Miyamoto C, Akiyama K, Ikawa T, Masuda K, Kawamoto H, Taniuchi I. Cascading suppression of transcriptional silencers by ThPOK seals helper T cell fate. Nat Immunol. 2008;9:1113–1121. doi: 10.1038/ni.1650. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Wildt KF, Castro E, Xiong Y, Feigenbaum L, Tessarollo L, Bosselut R. The zinc finger transcription factor Zbtb7b represses CD8-lineage gene expression in peripheral CD4+ T cells. Immunity. 2008;29:876–887. doi: 10.1016/j.immuni.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Park K, Wang H, He X, Zhang Y, Hua X, Li Y, Kappes DJ. CD4-CD8 lineage commitment is regulated by a silencer element at the ThPOK transcription-factor locus. Immunity. 2008;28:346–358. doi: 10.1016/j.immuni.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Setoguchi R, Tachibana M, Naoe Y, Muroi S, Akiyama K, Tezuka C, Okuda T, Taniuchi I. Repression of the transcription factor Th-POK by Runx complexes in cytotoxic T cell development. Science. 2008;319:822–825. doi: 10.1126/science.1151844. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson SR, Intlekofer AM, Sun G, Feigenbaum L, Reiner SL, Bosselut R. Expression of the transcription factor cKrox in peripheral CD8 T cells reveals substantial postthymic plasticity in CD4-CD8 lineage differentiation. J Exp Med. 2007;204:267–272. doi: 10.1084/jem.20061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun JC, Williams MA, Bevan MJ. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat Immunol. 2004;5:927–933. doi: 10.1038/ni1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 19.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 22.Matter M, Mumprecht S, Pinschewer DD, Pavelic V, Yagita H, Krautwald S, Borst J, Ochsenbein AF. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur J Immunol. 2005;35:3229–3239. doi: 10.1002/eji.200535179. [DOI] [PubMed] [Google Scholar]
- 23.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;185:251–262. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 27.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 28.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 29.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Northrop JK, Thomas RM, Wells AD, Shen H. Epigenetic remodeling of the IL-2 and IFN-γ loci in memory CD8 T cells is influenced by CD4 T cells. J Immunol. 2006;177:1062–1069. doi: 10.4049/jimmunol.177.2.1062. [DOI] [PubMed] [Google Scholar]
- 31.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.