Abstract
Purpose of review
Disease of the nose and sinuses is the most common co-morbidity associated with asthma. Rhinitis, sinusitis and asthma may represent part of one disease process with manifestations at different sites. The purpose of this review is to highlight significant new findings on the epidemiological and pathophysiological link between the upper and lower airway. Finally we will review recent data assessing the impact of treating sinonasal disease on both the development of asthma, and asthma control.
Recent findings
Studies illustrate that rhinitis is very common in asthma, and associated with worse asthma control. Rhinitis typically precedes the development of asthma. Even in patients with rhinitis without asthma there is evidence of subclinical change in the lower airways as measured by physiological changes and the presence of inflammatory mediators. There is much interest on the impact of treating allergic rhinitis on the development of asthma.
Summary
Rhinitis, sinusitis and asthma are likely part of one disease process. Treatment of established rhinitis may have some impact on measures of airway obstruction, but an effect on lower airway inflammation is yet to be established. Prospective studies are required to determine if treatment of rhinitis can prevent the development of asthma, and/or decrease airway inflammation to improve asthma outcomes in those with established asthma.
Keywords: rhinitis sinusitis asthma
Introduction
Sinonasal pathology is the most common comorbidity among patients with asthma. The co-existence of these conditions has been recognized for centuries. However, the nature of the link between the upper and lower airway has been poorly understood until recent years. The impact of effectively treating the upper airway on both the development of asthma and the presence of established asthma are important clinical questions. Important studies addressing these issues have recently been published. In this article we will discuss studies relating both rhinitis and sinusitis to asthma, as rhinitis and sinusitis represent two ends of a disease spectrum afflicting the upper airway which are closely related to asthma.
Epidemiology
The presence of allergic rhinitis is a risk factor for the presence of asthma. This has been shown in numerous cross-sectional studies.[1] Longitudinal studies show that allergic rhinitis is a risk factor for the future development of asthma. Burgess et al reported that childhood allergic rhinitis was significantly associated with the presence of asthma: 42% of participants with allergic rhinitis had asthma, compared with only 12.9 % of those without allergic rhinitis.[**2] The presence of allergic rhinitis before seven years of age predicted the subsequent development of asthma. The presence of allergic rhinitis at the age of seven was associated with an approximate three-fold risk of subsequently developing asthma compared to those without allergic rhinitis. This has led to use of the term “The Allergic March” to describe the progression of allergic disease from the nose and sinuses down to the airways of the lung.[*3]
The allergic march may begin before the development of rhinitis. The presence of atopy in infants with eczema is associated with an increased risk of subsequently developing allergic rhinitis and asthma compared with infants with non-atopic eczema. In fact eczema may predict the subsequent development of allergic sensitization, which in turn predicts the development of both allergic rhinitis and asthma. This further supports the concept of an “Allergic March”, that these diseases are all different, and perhaps progressive manifestations of allergy in children.[*4] The impact of early treatment of eczema on the development of allergic rhinitis and asthma is not known.
Environmental factors may affect the progression of disease to the lower airway in patients with allergic rhinitis. Polosa et al studied a cohort of clinic patients with allergic rhinitis, and found that smoking increased the risk of the development of asthma by approximately three-fold.[**5] Another common factor that is now recognized as a risk factor for asthma, obesity, does not appear to affect the presence or progression of “The Allergic March”. A number of recent publications suggest that obesity is a risk factor for asthma,[6] but this does not appear to be true for asthmatics with rhinitis. A population based study showed that obesity was associated with an increased prevalence of asthma, but not allergic rhinitis, suggesting that the pathogenesis of asthma in the obese may be through a different pathway than that linking allergic rhinitis and asthma.[7]
These studies confirm previous reports showing that rhinitis and sinusitis are common in asthmatics. An important emerging theme is that rhinitis is a risk factor for the future development of asthma. If rhinitis is a forerunner of asthma, intervening with rhinitis may afford an opportunity to prevent the development of asthma. One environmental factor that should be addressed in these high risk patients is certainly tobacco smoke.
Upper Airway Inflammation
Epidemiological studies suggest that rhinitis (and perhaps even earlier eczema) predict the development of asthma. This may occur either because these disease entities are all manifestations of a progressive disease, or they may reflect separate disease processes that can afflict a susceptible population. A common disease process is strongly supported by data showing that: (1) the upper airway disease experienced by asthmatics is somewhat different than that experienced by the general population, (2) the inflammatory process in the upper and lower airways share common features, and (3) the severity of disease in the upper airway parallels that in the lower airway.
Sinonasal disease in asthmatics appears to differ somewhat from that of the general population. Rolla et al assessed patients presenting with nasal symptoms to determine if the clinical characteristics of sinonasal disease were related to the presence of disease in the lower airway. They found that persistent allergic rhinitis and chronic rhinosinusitis were more often associated with asthma than non-allergic rhinitis[*8] Asthmatics with sinusitis are more likely to have nasal polyps complicating their sinus disease than non-asthmatics, and asthmatics are more likely to have persistent disease over years that requires multiple surgeries.[*9] *[10] [11]
In patients with asthma, inflammation in the nose and sinuses share features of disease in the lung. For example, in nasal polyposis inflammation is characterized by eosinophilic inflammation and local IgE production (Figure 2).[10] Although common inflammatory mediators are implicated in the upper and lower airway, it has been hard to distinguish pathways that distinguish sinonasal inflammation in asthmatics from non-asthmatics. A recent study that compared gene expression in sinus mucosa of patients with nasal polyposis and aspirin sensitive asthma to those with chronic sinusitis found no difference in gene expression between these two groups (though one of the latter patients had a diagnosis of asthma).[*12] Future gene array studies that categorize patients based on careful physiological testing of the lower airway may be helpful to better understand how sinonasal disease in the asthmatic is different than the non-asthmatic.
Figure 2.
Gradation of airway disease
Many publications have suggested that the severity of nasal and sinus disease parallel that of the lower airway. A study by Ponte et al of 557 patients with severe asthma found that those with more severe rhinitis had significantly more severe asthma.[**13] This suggests that rhinitis, sinusitis and asthma are all manifestations of a single systemic disease. This is supported by a publication by Mehta et al[.*14] These authors reviewed data on sinus CT in asthmatics: they found that sinus CT score correlated with eosinophils in induced sputum and peripheral blood, and findings of osteitis on the CT scan also correlated with eosinophils from blood and sputum. Inflammation in the lung parallels that in the sinuses and also systemic inflammation as measured by circulating eosinophils, therefore the the severity of rhinitis and sinusitis parallels that of asthma.
These recent studies support earlier work which suggested that asthmatics have features of their rhinitis and sinusitis that distinguish them from the general population, lymphocyte and eosinophil inflammation are features of disease in the upper and lower airway, and that the severity of disease in the lower airway parallels that in the upper airway (Figure 1).
Figure 1.
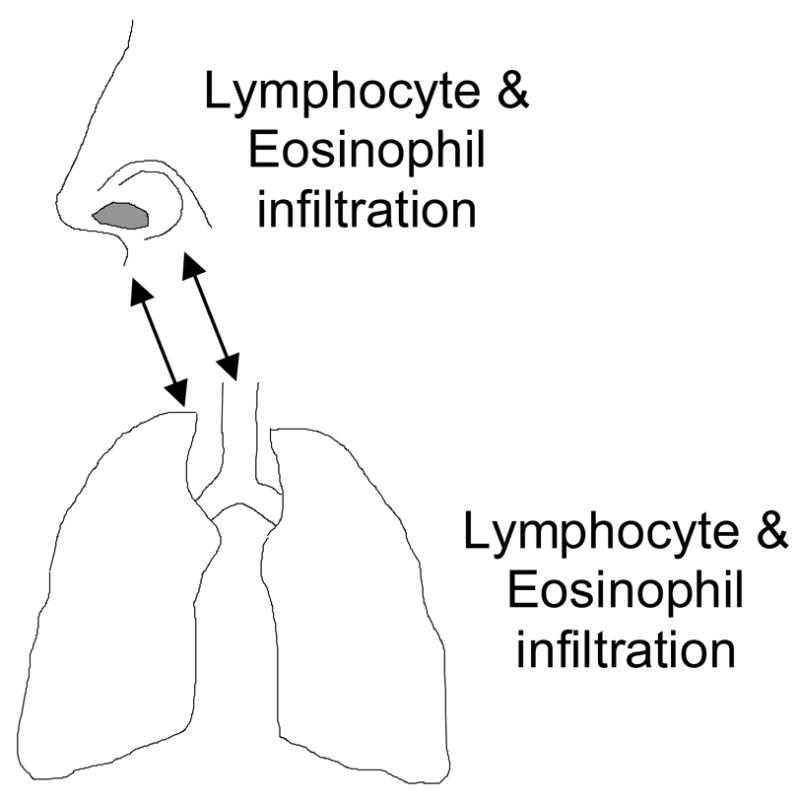
Functional Relationship between upper and lower airway: inflammation in the nose, sinuses and lungs is characterized by similar inflammatory profile, and disease severity in nose and sinuses parallels that in the lungs of asthmatics
Lung Function in patients with allergic rhinitis
The link between sinonasal disease and asthma is also supported by the finding that patients with allergic rhinitis (even without asthma) have subtle abnormalities in their lower airway. This is illustrated by the fact that young children with allergic rhinitis have an increased prevalence of bronchial hyperreactivity, and persistent (compared with intermittent) allergic rhinitis is a significant independent risk factor for bronchial hyperreactivity[15]. A study of patients with allergic rhinitis showed that a significant number actually had impaired lung function, and that impaired lung function was related to the duration of rhinitis and sensitization to house dust mites.[*16]
An elegant study by Crimi et al suggests that even those without obvious physiological abnormalities may have evidence of subtle dysfunction. [17] A lack of bronchodilator response to a deep inhalation is one of the characteristic abnormalities of patients with asthma,[**18] and suggests altered smooth muscle function in the intrathoracic airways of these patients. Crimi et al performed methacholine challenge tests on healthy controls and participants with allergic rhinitis (but without airways hyperresponsiveness by standard testing), they found that those with rhinitis had decreased improvement in lung function parameters with deep inhalations taken after the methacholine testing – this blunted response to a deep inhalation suggests that the airway smooth muscle function may be altered in people with allergic rhinitis, and is suggestive of physiological alterations in the lower airway of these patients.[17]
These studies suggest that many individuals with allergic rhinitis may have subclinical abnormalities of their intrathoracic airways and be at risk of developing the clinical disease of asthma (Figure 2).
Inflammation
In addition to abnormal physiology in the lower airway of patients with allergic rhinitis, recent publications also suggest there are subtle inflammatory changes in the lungs of patients with allergic rhinitis. A paper by Tufvesson et al showed that exhaled nitric oxide from the periphery of the lung is progressively elevated in patients with (1) rhinitis only, (2) patients with allergic rhinitis and bronchial hyperreactivity and (3) patients with allergic rhinitis and asthma.[*19] The same authors showed a trend towards increased sputum eosinophilic cationic protein going from (1) normals, to (2) rhinitis, to (3) rhinitis with bronchial hyperreactivity, to (4) rhinitis with asthma.[*20] Sohn et al assessed lower airway disease by induced sputum and found that levels of interleukin-5 (involved with eosinophilic inflammation) and vascular endothelial growth factor (involved in angiogenesis) were increased going from (1) allergic rhinitis only, to (2) patients with allergic rhinitis with airway hyperreactivity compared with (3) patients with allergic rhinitis and asthma.[*21] Bronchial biopsies show increased submucosal eosinophils and mast cells when comparing (1) normal controls, to (2) rhinitis and (3) asthmatics.[**22]
These studies show progressive inflammation detectable when comparing four groups of patients: (1) normal controls, (2) patients with allergic rhinitis without bronchial hyperreactivity/asthma, (3) patients with allergic rhinitis and bronchial hyperreactivity (without asthma), and (4) patients with allergic rhinitis and asthma. This suggests that there is a spectrum of airway disease from isolated allergic rhinitis to allergic rhinitis with asthma (Figure 2).
Link between upper and lower airway
Nasal challenge may increase lower airway inflammation, this has been shown in adults.[23] Marucci et al found that exhaled nitric oxide was increased following dust mite nasal challenge during the winter in allergic asthmatic children and children with allergic rhinitis. [*24] Previous studies have shown that allergen challenge in asthmatics can lead to the release of eosinophil precursors from the bone marrow,[25] nasal challenge in a mouse model of allergic rhinitis leads to the release of eosinophil precursors from the bone marrow[26] suggesting that rhinitis and asthma could be separate anatomic compartments affected by one systemic disease.[27]
Asthma Control
Patients with nasal symptoms appear to experience worse asthma control. This was supported by a recent cross-sectional studies looking at the relationship between nasal symptom scores and asthma symptoms.[28] [29] Many of the studies that have looked at the relationship between asthma control and rhinitis have been cross-sectional or retrospective. However, a prospective cohort study of patients with severe asthma showed that those with rhinitis had increased emergency room visits, and that the severity of rhinitis correlated with the severity of asthma as assessed by standardized questionnaires.[**13]
Outcomes from Treatment
There has been a great deal of interest in determining if early treatment of patients with allergic rhinitis may prevent the development of asthma. A separate, and equally important questions is whether treating sinonasal disease may improve asthma control. There are recent publications addressing both of these questions.
If treatment of allergic rhinitis could prevent the development of asthma, it would have a major impact on public health. There are some interesting studies to suggest this may warrant further investigation. A long term follow up of 147 children treated with subcutaneous specific immunotherapy for allergic rhinoconjunctivitis showed a significant reduction in the number of patients developing asthma in the treated group compared with the control group.[**30] In addition, an interesting (uncontrolled) study was published this last year in which patients with allergic rhinitis and evidence of airflow obstruction measured by FEF25-75 were treated with an antihistamine and nasal corticosteroids. Airflow obstruction improved after 3 months of treatment.[*31]
There has been much interest over the years in how treating established rhinitis and sinusitis may affect asthma. Retrospective studies suggested that treating allergic rhinitis improves asthma outcomes,[32, 33] though prospective studies have been disappointing.[34] It has been hypothesized that effective treatment of nasal disease could affect lower airway inflammation by decreasing systemic eosinophilic inflammation. However, a recent study found that treatment of allergic rhinitis did not affect lower airway inflammation (as measured by exhaled NO). In this randomized double-blinded placebo controlled trial, 40 children were randomized to treatment with nasal steroid versus placebo. There was an effect on nasal inflammation (measured by levels of eosinophilic cationic protein) and systemic inflammation (measured by eosinophil count), but no effect on lower airway inflammation (as measured by exhaled nitric oxide).[**35] This contrasts with an earlier study of adults, which did identify a decrease in exhaled NO in patients treated with nasal steroids, though in the latter study, participants were deliberately selected who had a high NO at baseline.[36] These studies suggest that only certain patient subgroups may respond to treatment of nasal disease. Many studies suggest that surgical treatment of sinus disease improves asthma outcomes: a retrospective chart review of asthmatic patients undergoing endoscopic sinus surgery showed decreased asthma severity and decreased use of inhaled corticosteroids, there was a greater improvement in asthma in patients who had a history of aspirin intolerant asthma.[37] Controlled trials of interventions for severe sinusitis are difficult, as the sinus disease warrants treatment outside of considerations for asthma.
Studies published this last year still leave open the question as to how well treating sinonasal disease can improve asthma control. Certain patient populations may have a benefit in their asthma from treating sinonasal disease, though the mechanisms and populations that benefit requires further investigation. Further studies are also required to determine if early aggressive treatment of rhinitis and/or sinusitis could prevent the development of asthma (Figure 3).
Figure 3.
Potential Opportunities for intervention in the development of asthma?
Conclusions
Studies published during this last year confirm that rhinitis and sinusitis are very important co-morbidities in people with asthma. The temporal sequence of disease and parallel inflammatory pathways involved suggest that they may be progressive manifestations of a common disease process. As such, studies investigating treatment of early disease on the development of asthma are of great interest. Retrospective studies suggest that treatment of chronic upper airway diseased in established asthma can lead to improved asthma outcomes, though this has not been established in controlled clinical trials.
Acknowledgments
Funding: NIH: RR019965
References
- 1.Dixon AE, Kaminsky DA, Holbrook JT, et al. Allergic rhinitis and sinusitis in asthma: differential effects on symptoms and pulmonary function. Chest. 2006 Aug;130(2):429–35. doi: 10.1378/chest.130.2.429. [DOI] [PubMed] [Google Scholar]
- 2**.Burgess JA, Walters EH, Byrnes GB, et al. Childhood allergic rhinitis predicts asthma incidence and persistence to middle age: a longitudinal study. J Allergy Clin Immunol. 2007 Oct;120(4):863–9. doi: 10.1016/j.jaci.2007.07.020. A longitudinal study of participants in the Tasmanian Asthma Study, originally enrolled in 1968, now followed over a 36 year period which found that childhood allergic rhinitis increased likelihood of subsequently developing asthma and having persistent asthma into middle age. [DOI] [PubMed] [Google Scholar]
- 3*.Almqvist C, Li Q, Britton WJ, et al. Early predictors for developing allergic disease and asthma: examining separate steps in the ‘allergic march’. Clin Exp Allergy. 2007 Sep;37(9):1296–302. doi: 10.1111/j.1365-2222.2007.02796.x. A report from the Childhood Asthma Prevention Study that examined predictors for developing asthma which found that eczema, but not wheeze, asthma or rhinitis predicted the subsequent development of sensitization. [DOI] [PubMed] [Google Scholar]
- 4*.Lowe AJ, Hosking CS, Bennett CM, et al. Skin prick test can identify eczematous infants at risk of asthma and allergic rhinitis. Clin Exp Allergy. 2007 Nov;37(11):1624–31. doi: 10.1111/j.1365-2222.2007.02822.x. A study of an Australian birth cohort which found that atopy in children with eczema predicted the development of childhood asthma. [DOI] [PubMed] [Google Scholar]
- 5**.Polosa R, Knoke JD, Russo C, et al. Cigarette smoking is associated with a greater risk of incident asthma in allergic rhinitis. J Allergy Clin Immunol. 2008 Jun;121(6):1428–34. doi: 10.1016/j.jaci.2008.02.041. A study of an Italian adult population followed at an outpatient allergy clinic which reports that smoking predicts the later development of asthma in patients with allergic rhinitis. [DOI] [PubMed] [Google Scholar]
- 6.Camargo CA, Jr, Weiss ST, Zhang S, et al. Prospective study of body mass index, weight change, and risk of adult-onset asthma in women. Archives of internal medicine. 1999 Nov 22;159(21):2582–8. doi: 10.1001/archinte.159.21.2582. [DOI] [PubMed] [Google Scholar]
- 7.Loerbroks A, Apfelbacher CJ, Amelang M, Sturmer T. Obesity and adult asthma: potential effect modification by gender, but not by hay fever. Annals of epidemiology. 2008 Apr;18(4):283–9. doi: 10.1016/j.annepidem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 8*.Rolla G, Guida G, Heffler E, et al. Diagnostic classification of persistent rhinitis and its relationship to exhaled nitric oxide and asthma: a clinical study of a consecutive series of patients. Chest. 2007 May;131(5):1345–52. doi: 10.1378/chest.06-2618. A study of patients referred to an allergy clinic for nasal symptoms. Found that asthma was significantly more common in alllergic rhinitis and chronic rhinosinusitis than in patient with non-allergic rhinitis. [DOI] [PubMed] [Google Scholar]
- 9*.Seybt MW, McMains KC, Kountakis SE. The prevalence and effect of asthma on adults with chronic rhinosinusitis. Ear, nose, & throat journal. 2007 Jul;86(7):409–11. A cross-sectional study of 145 patients referred to an otolaryngology clinic for chronic rhinosinusitis which found that nasal polyps and symptoms such as nasal congestion were significantly more common in asthmatics than non-asthmatics. [PubMed] [Google Scholar]
- 10*.Bugten V, Nordgard S, Romundstad P, Steinsvag S. Chronic rhinosinusitis and nasal polyposis; indicia of heterogeneity. Rhinology. 2008 Mar;46(1):40–4 . A study of 102 patients undergoing function endoscopy sinus surgery: the authors report that asthma was significantly more common in patients with nasal polyps than patients with chronic rhinosinusitis. [PubMed] [Google Scholar]
- 11.Matsuwaki Y, Ookushi T, Asaka D, et al. Chronic rhinosinusitis: risk factors for the recurrence of chronic rhinosinusitis based on 5-year follow-up after endoscopic sinus surgery. International archives of allergy and immunology. 2008;146( Suppl 1):77–81. doi: 10.1159/000126066. [DOI] [PubMed] [Google Scholar]
- 12*.Stankovic KM, Goldsztein H, Reh DD, et al. Gene expression profiling of nasal polyps associated with chronic sinusitis and aspirin-sensitive asthma. Laryngoscope. 2008 May;118(5):881–9. doi: 10.1097/MLG.0b013e31816b4b6f. A genome-wide microarray of sinonasal mucosa from normal controls compared with patients with chronic rhinosinuisitis alone and chronic rhinosinusitis with asthma: the study found a similar pattern of gene expression in both populations with chronic rhinosinuisitis, which was distinct from healthy controls. [DOI] [PubMed] [Google Scholar]
- 13**.Ponte EV, Franco R, Nascimento HF, et al. Lack of control of severe asthma is associated with co-existence of moderate-to-severe rhinitis. Allergy. 2008 May;63(5):564–9. doi: 10.1111/j.1398-9995.2007.01624.x. This prospective cohort study of patients with severe asthma found that severity of rhinitis was associated with poor asthma control and asthma exacerbations. [DOI] [PubMed] [Google Scholar]
- 14*.Mehta V, Campeau NG, Kita H, Hagan JB. Blood and sputum eosinophil levels in asthma and their relationship to sinus computed tomographic findings. Mayo Clinic proceedings. 2008 Jun;83(6):671–8. doi: 10.4065/83.6.671. This retrospective review of asthmatic patients with sinus CT and blood and sputum eosinophil data available found a positive relationship between eosinophils and severity of sinus disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi SH, Kim do K, Yoo Y, et al. Comparison of deltaFVC between patients with allergic rhinitis with airway hypersensitivity and patients with mild asthma. Ann Allergy Asthma Immunol. 2007 Feb;98(2):128–33. doi: 10.1016/s1081-1206(10)60684-9. [DOI] [PubMed] [Google Scholar]
- 16*.Ciprandi G, Cirillo I, Pistorio A. Impact of allergic rhinitis on asthma: effects on spirometric parameters. Allergy. 2008 Mar;63(3):255–60. doi: 10.1111/j.1398-9995.2007.01544.x. This prospective study of naval personnel found that duration of allergic rhinitis and sensitization to house mites were significantly associated with impaired lung function. [DOI] [PubMed] [Google Scholar]
- 17.Skloot G, Togias A. Bronchodilation and bronchoprotection by deep inspiration and their relationship to bronchial hyperresponsiveness. Clin Rev Allergy Immunol. 2003 Feb;24(1):55–72. doi: 10.1385/CRIAI:24:1:55. [DOI] [PubMed] [Google Scholar]
- 18**.Crimi E, Saporiti R, Bartolini S, et al. Airway responsiveness to methacholine and deep inhalations in subjects with rhinitis without asthma. J Allergy Clin Immunol. 2008 Feb;121(2):403–7. doi: 10.1016/j.jaci.2007.09.009. This was a study of 10 healthy subjects and 10 subjects with rhinitis without asthma, looking at the effects of a deep breath on responsiveness to methacholine: the effect of a deep breath was attenuated in those with rhinitis compared to healthy people. [DOI] [PubMed] [Google Scholar]
- 19*.Tufvesson E, Aronsson D, Ankerst J, et al. Peripheral nitric oxide is increased in rhinitic patients with asthma compared to bronchial hyperresponsiveness. Respir Med. 2007 Nov;101(11):2321–6. doi: 10.1016/j.rmed.2007.06.015. A study that compared patients with rhinitis only, rhinitis and bronchial hyperreactivity and rhinitis and asthma, and found trend towards progressive increase in peripheral nitric oxide which correlated with peripheral obstruction in response to methacholine. [DOI] [PubMed] [Google Scholar]
- 20*.Tufvesson E, Aronsson D, Bjermer L. Cysteinyl-leukotriene levels in sputum differentiate asthma from rhinitis patients with or without bronchial hyperresponsiveness. Clin Exp Allergy. 2007 Jul;37(7):1067–73. doi: 10.1111/j.1365-2222.2007.02746.x. This study compared asthmatics, rhinitis with bronchial hyperreactivity, rhinitics without bronchial hyperreactivity and normal controls, and found increased cysteinyl leukotrienes and hyaluronan in the sputum of asthmatics compared to other groups. [DOI] [PubMed] [Google Scholar]
- 21*.Sohn SW, Lee HS, Park HW, et al. Evaluation of cytokine mRNA in induced sputum from patients with allergic rhinitis: relationship to airway hyperresponsiveness. Allergy. 2008 Mar;63(3):268–73. doi: 10.1111/j.1398-9995.2007.01550.x. This cross-sectional study of patients with allergic rhinitis only, allergic rhinitis with bronchial hyperreactivity and asthma found progressive increase in interleukin-5 and vascular endothelial growth factor across the three groups. [DOI] [PubMed] [Google Scholar]
- 22**.Brown JL, Behndig AF, Sekerel BE, et al. Lower airways inflammation in allergic rhinitics: a comparison with asthmatics and normal controls. Clin Exp Allergy. 2007 May;37(5):688–95. doi: 10.1111/j.1365-2222.2007.02695.x. A cross-sectional study of endobroncial biopsies from normal controls, allergic rhinitis and patients with asthma: there was progressive airway inflammation across the three groups. [DOI] [PubMed] [Google Scholar]
- 23.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001 Mar;107(3):469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 24*.Marcucci F, Passalacqua G, Canonica GW, Frati F, Salvatori S, Di cara G, et al. Lower airway inflammation before and after house dust mite nasal challenge: an age and allergen exposure-related phenomenon. Respir Med. 2007 Jul;101(7):1600–8. doi: 10.1016/j.rmed.2006.06.006. Comparison of upper and lower airway response to dust mite nasal challenge in healthy control, allergic asthmatic and rhinitic children studied both during winter and summer months. [DOI] [PubMed] [Google Scholar]
- 25.Dorman SC, Sehmi R, Gauvreau GM, Watson RM, Foley R, Jones GL, et al. Kinetics of bone marrow eosinophilopoiesis and associated cytokines after allergen inhalation. Am J Respir Crit Care Med. 2004 Mar 1;169(5):565–72. doi: 10.1164/rccm.200307-1024OC. [DOI] [PubMed] [Google Scholar]
- 26.Saito H, Howie K, Wattie J, Denburg A, Ellis R, Inman MD, et al. Allergen-induced murine upper airway inflammation: local and systemic changes in murine experimental allergic rhinitis. Immunology. 2001 Oct;104(2):226–34. doi: 10.1046/j.0019-2805.2001.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Saito H, Crawford L, Inman MD, Cyr MM, Denburg JA. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005 Mar;114(3):386–96. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koga T, Matsuse H, Kohrogi H, Kohno S, Aizawa H. Impact of nasal condition on self-assessed disease control and treatment satisfaction in patients with asthma complicated by allergic rhinitis. Allergol Int. 2007 Dec;56(4):427–31. doi: 10.2332/allergolint.O-06-477. [DOI] [PubMed] [Google Scholar]
- 29.Magnan A, Meunier JP, Saugnac C, Gasteau J, Neukirch F. Frequency and impact of allergic rhinitis in asthma patients in everyday general medical practice: a French observational cross-sectional study. Allergy. 2008 Mar;63(3):292–8. doi: 10.1111/j.1398-9995.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- 30**.Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long-term preventive effect of seasonal and perennial asthma: 10-year follow-up on the PAT study. Allergy. 2007 Aug;62(8):943–8. doi: 10.1111/j.1398-9995.2007.01451.x. This paper reported on the long term effects of specific immunotherapy. At baseline children were randomized to treatment subcutaneous specific immunotherapy or no therapy. Seven years after completion of immunotherapy there was a significantly reduced risk of developing asthma in the treated group. [DOI] [PubMed] [Google Scholar]
- 31*.Kessel A, Halloun H, Bamberger E, et al. Abnormal spirometry in children with persistent allergic rhinitis due to mite sensitization: the benefit of nasal corticosteroids. Pediatr Allergy Immunol. 2008 Feb;19(1):61–6. doi: 10.1111/j.1399-3038.2007.00588.x. This was an uncontrolled study of the effects of nasal steroids for treatment of allergic rhinitis on lung function in children which found improvement in measures of airflow obstruction with treatment. [DOI] [PubMed] [Google Scholar]
- 32.Corren J, Manning BE, Thompson SF, Hennessy S, Strom BL. Rhinitis therapy and the prevention of hospital care for asthma: A case-control study. J Allergy ClinImmunol. 2004 Mar;113(3):415–9. doi: 10.1016/j.jaci.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002 Jan 19;109(1):57–62. doi: 10.1067/mai.2002.120554. [DOI] [PubMed] [Google Scholar]
- 34.Nathan RA, Yancey SW, Waitkus-Edwards K, Prillaman BA, Stauffer JL, Philpot E, et al. Fluticasone propionate nasal spray is superior to montelukast for allergic rhinitis while neither affects overall asthma control. Chest. 2005 Oct;128(4):1910–20. doi: 10.1378/chest.128.4.1910. [DOI] [PubMed] [Google Scholar]
- 35**.Pedroletti C, Lundahl J, Alving K, Hedlin G. Effect of nasal steroid treatment on airway inflammation determined by exhaled nitric oxide in allergic schoolchildren with perennial rhinitis and asthma. Pediatr Allergy Immunol. 2008 May;19(3):219–26. doi: 10.1111/j.1399-3038.2007.00613.x. A randomized placebo controlled trial of nasal steroids in 40 children with allergic rhinitis and asthma. The investigators found improvement in nasal measures of inflammation, but no changes in lower airway inflammation (measured by exhaled nitric oxide) [DOI] [PubMed] [Google Scholar]
- 36.Sandrini A, Ferreira IM, Jardim JR, Zamel N, Chapman KR. Effect of nasal triamcinolone acetonide on lower airway inflammatory markers in patients with allergic rhinitis. J Allergy Clin Immunol. 2003 Feb;111(2):313–20. doi: 10.1067/mai.2003.64. [DOI] [PubMed] [Google Scholar]
- 37.Awad OG, Fasano MB, Lee JH, Graham SM. Asthma outcomes after endoscopic sinus surgery in aspirin-tolerant versus aspirin-induced asthmatic patients. Am J Rhinol. 2008 Mar–Apr;22(2):197–203. doi: 10.2500/ajr.2008.22.3148. [DOI] [PubMed] [Google Scholar]




