Summary
T cells causing autoimmunity must escape tolerance. We observed that CD8+ T cells with high avidity for an antigen expressed in the pancreas, kidney, and thymic medulla were efficiently removed from a polyclonal repertoire by central and peripheral tolerance mechanisms. However, both mechanisms spared low-avidity T cells from elimination. Neither the introduction of activated, self-antigen-specific CD4+ helper T cells nor a global inflammatory stimulus were sufficient to activate the low-avidity CD8+ T cells and did not break tolerance. In contrast, challenge with a recombinant bacterium expressing the self antigen primed the low-avidity T cells, and the animals rapidly developed autoimmune diabetes. We suggest that whereas thymic and peripheral tolerance mechanisms remove cells that can be primed by endogenous amounts of self antigen, they do not guard against tissue destruction by low-avidity effector T cells, which have been primed by higher amounts of self antigen or by crossreactive antigens.
Introduction
Negative selection of maturing thymocytes, referred to as central tolerance, plays a key role in eliminating autoreactive cells from the developing T cell repertoire (Palmer, 2003; Starr et al., 2003; von Boehmer et al., 2003). Promiscuous or ectopic expression of tissue-restricted antigens (TRA) by medullary thymic epithelial cells (mTECs) is crucial for central tolerance, as it provides antigens that are otherwise expressed only in peripheral tissues (Derbinski et al., 2001; Kyewski and Klein, 2006). The importance of promiscuous antigen expression in mTECs for establishing tolerance is underlined by the fact that mice and humans with defective TRA expression by mTECs, caused by mutations in the AIRE gene, show impaired negative selection and suffer from multiple autoimmune disorders (Anderson et al., 2002; Liston et al., 2004).
Most studies of central tolerance use TCR transgenic mice, but in these models, all T cells posses receptors with the same high affinity for a self antigen, and it is not possible to analyze how cells bearing TCR with lower affinity for the self antigen are dealt with. Conversely, due to the low frequency of cells with specificity for a particular antigen in the endogenous repertoire, TCR nontransgenic models allow only limited insight into the selection process. Thus, despite the clearly established importance of central tolerance, it remains unclear how efficiently negative selection removes autoreactive T cells from the repertoire. This lack of information is particularly true for TRA-specific T cells, because few studies have focused on central tolerance against such antigens (Anderson et al., 2005; Gallegos and Bevan, 2004; Liston et al., 2003). TCR transgenic, immature double-positive (DP) thymocytes are known to be more sensitive than peripheral T cells in responding to peptide-MHC complexes (pMHC) that interact weakly with the TCR (Davey et al., 1998; Lucas et al., 1999). Furthermore, pMHC with affinities below the threshold for activating peripheral T cells can nevertheless induce thymic-negative selection (Pircher et al., 1991; Sant'Angelo and Janeway, 2002). It has therefore been concluded that the high sensitivity of immature thymocytes for pMHC enables central tolerance to remove cells carrying receptors with low affinity for self antigens, thereby providing a safety window for self tolerance (Knobloch et al., 1992; Pircher et al., 1991; Sant'Angelo and Janeway, 2002). However, it remains unclear whether this observation made with TCR transgenic thymocytes recognizing a widely distributed antigen also holds true for a polyclonal T cell repertoire and for the induction of central tolerance to a TRA expressed at low levels only in the thymic medulla. In addition, because only 1%–3% of mTECs express a particular TRA (Klein et al., 2001), the question arises whether some self-reactive T cells might stochastically escape negative selection in the thymus (Liston et al., 2005). After T cells leave the thymus, peripheral mechanisms of tolerance are also capable of eliminating TRA-specific autoreactive T cells (Kurts et al., 1997b; Morgan et al., 1999; Probst et al., 2003; Redmond and Sherman, 2005), raising the question to what degree central and peripheral mechanisms individually contribute to repertoire selection against TRA.
To analyze how efficiently central and peripheral tolerance mechanisms eliminate autoreactive T cells specific for a TRA, we utilized a double transgenic mouse model. The Rip-mOva transgene drives expression of membrane bound ovalbumin in the pancreatic β cells and in the proximal tubules of the kidney, as well as ectopically in mTECs (Gallegos and Bevan, 2004; Kurts et al., 1997b). The Kb-Ova epitope is constitutively crosspresented by dendritic cells (DC) in the lymph nodes draining the pancreas and kidney, and this presentation can induce peripheral tolerance when high-avidity OT-I TCR transgenic CD8+ T cells are injected into the mice (Kurts et al., 1997b). In the thymus of Rip-mOva mice, ectopic expression of the Ova transgene also results in deletion (central tolerance) of developing CD8+ and CD4+ TCR transgenic cells (Anderson et al., 2005; Gallegos and Bevan, 2004). The Rip-mOva mice were crossed to TCRβ-only transgenic mice (Vβ5) expressing the same TCRβ chain as OT-I that skews CD8+ T cells to recognition of the Kb-Ova ligand (Dillon et al., 1994). The TCRβ transgene, in combination with endogenously rearranged TCRα chains, results in a detectable polyclonal population of CD8+ T cells with heterogeneous avidity for Kb-Ova. Thus, we were able to visualize TRA-specific T cells of a range of avidities in the naive repertoire and after immunization.
By using these Vβ5×Rip-mOva mice, we report that central and peripheral tolerance were equally potent in eliminating high-avidity Kb-Ova-specific CD8+ T cells. However, both mechanisms spared T cells with low avidity for Kb-Ova. Interestingly, the CD8+ T cells that escaped tolerance in Vβ5×Rip-mOva mice were completely refractory to priming in lymph nodes by DC crosspresenting tissue-derived, endogenous Ova peptide. Neither a strong inflammatory stimulus caused by lymphocytic choriomeningitis virus (LCMV) or Listeria monocytogenes (Lm) infection nor licensing of crosspresenting DC via the introduction of Ova-specific CD4+ T cells resulted in diabetes. In contrast, both treatments led to diabetes when a small number of high-avidity OT-I T cells were transferred into the mice. Despite this failure to cause autoimmunity by relying on the presentation of endogenous levels of Ova, infecting Vβ5×Rip-mOva mice with Lm that express Ova (Lm-Ova) resulted in rapid development of autoimmune diabetes caused by low-avidity effector T cells. Thus, central and peripheral tolerance eliminated T cells that could be primed with the endogenous level of a TRA but did not ensure the complete elimination of all cells that, once activated, by higher levels of self antigen or by a mimic of the self antigen, could cause harmful autoimmune damage.
Results
The Kb-Ova Repertoire in Naive Vβ5 and Vβ5×Rip-mOva Mice
Functional studies showed previously that unimmunized Vβ5 transgenic mice on a B6 background have a high frequency of CD8+ T cells that can respond to the Kb-Ova epitope (Dillon et al., 1994). We originally employed tetramer staining to visualize these cells. In comparison to B6 mice, peripheral cells from Vβ5 mice showed a readily detectable population of CD8+ T cells that stained brightly with Kb-Ova tetramers (Figures 1A and 1B). In addition to this brightly staining population, Vβ5 mice contained CD8+ T cells that stained less brightly with tetramer at levels in excess of those seen in control B6 mice. CD8+ T cells in Vβ5 mice that also carry the Rip-mOva transgene lacked the tetramer-bright population, but retained the increased number of cells that stained at lower levels (Figure 1C). These results suggested that CD8+ T cells with high-avidity TCR for Kb-Ova are largely eliminated in the double transgenic Vβ5×Rip-mOva mice, but low-avidity T cells may still be present in these mice.
Figure 1.
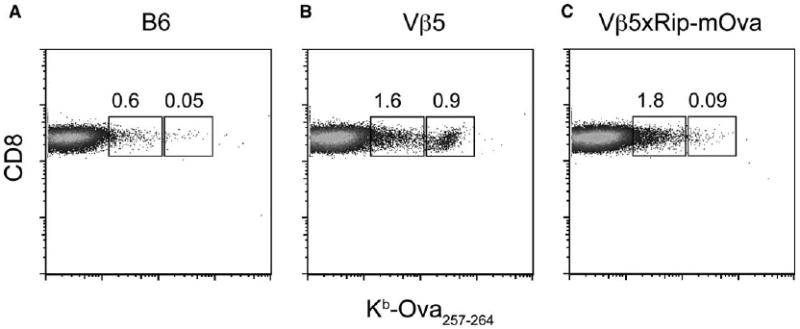
Tetramer Staining of Naive B6, Vβ5, and Vβ5×Rip-mOva (C) Mice
Peripheral blood cells from B6 (A), Vβ5 (B), and Vβ5×Rip-mOva (C) mice were stained with anti-CD8 and Kb-Ova tetramer. The representative plots shown are gated on CD8+ T cells. The regions highlight cells staining at high and low levels with tetramer, and the numbers represent their frequency within the CD8+ T cell population. The data are representative of three independent experiments.
Assessing the Functional Avidity of Ova-Specific T Cells
We devised a method to allow us to study more precisely the tetramer-dull, presumably low-avidity, T cells. Peripheral T cells from naive mice were activated in vitro by stimulation with anti-CD3 plus anti-CD28-coated beads for 6 days. This led to a nonbiased, 150- to 200-fold expansion of CD8+ T cells and the acquisition of effector function, including the ability to make IFNγ after brief peptide stimulation. Without restimulation, less than 1% of the bead-stimulated blasts made IFNγ in quantities that could be detected by intracellular cytokine staining (ICS). After short-term restimulation with PMA plus ionomycin, however, the vast majority of cells made cytokine (Figure 2A). The stimulation of cytokine production by a range of concentrations of the Ova peptide provided a way to measure the functional avidity of T cells for the Kb-Ova epitope. The experiment in Figure 2A shows a representative comparison of T cells isolated from control B6 mice, T cells from OT-I mice that uniformally express a high-affinity TCR, and T cells from Vβ5 and Vβ5×Rip-mOva mice. Even at the highest concentration of Ova peptide, B6 T cells showed no IFNγ production above the unstimulated control, indicating that the precursor frequency of Kb-Ova-specific CD8+ T cells in these mice is below the limit of detection of this assay. In contrast, almost all of the cells from OT-I mice made IFNγ with peptide concentration as low as 10−10 M, and about half of the OT-I cells made IFNγ at 10−11 M Ova peptide. About 5% of T cells from Vβ5 mice, and about 4% of cells from Vβ5×Rip-mOva mice, produced IFNγ above background at 10−5 M and 10−6 M Ova peptide. The response of the Vβ5 cells remained above background levels when the peptide concentration was titered down to 10−11 M, indicating the existence of cells with TCR of affinity similar to that of OT-I. In contrast, IFNγ production by the T cells from Vβ5×Rip-mOva mice reached background levels at 10−9 M peptide, suggesting the absence of high-avidity cells (Figure 2A).
Figure 2.

Detection of Kb-Ova-Reactive T Cells in Nonimmunized Mice
(A) Peripheral blood CD8+ T cells from OT-I, Vβ5, Vβ5×Rip-mOva, and control B6 mice were expanded with anti-CD3 plus anti-CD28 beads. 6 days later, cells were stimulated with congenically marked splenocytes in the presence of PMA and Ionomycin, no peptide, or the indicated concentrations of Ova257-264 peptide and analyzed for intracellular accumulation of IFNγ. Representative flow data gated on expanded CD8+ T cells are shown.
(B and C) Peptide dose-response curves graphing the mean percentage of peripheral blood CD8+ T cells in Vβ5×Rip-mOva, Vβ5, and B6 (B) or of CD8+ T cells isolated from pancreatic lymph nodes of Vβ5×Rip-mOva and Vβ5 mice (C) that specifically produce IFNγ in response to Ova257-264 stimulation. Bars represent standard deviation of data from 3–5 mice. Results are representative of at least three experiments.
The raw data, obtained from several naive mice, were corrected for background and plotted as a dose-response curve (Figure 2B). About 1% of CD8+ T cells from naive Vβ5 mice recognized antigen with high avidity and responded to 10−11 M and 10−10 M peptide, while such cells were absent in Vβ5×Rip-mOva mice. This frequency of high-avidity Kb-Ova-specific T cells is similar to the number of tetramer-bright CD8+ T cells observed specifically in the Vβ5 mice (Figure 1B). Moreover, sorting Kb-Ova tetramer-bright cells from Vβ5 mice and analyzing their functional avidity by ICS after expansion with anti-CD3 plus anti-CD28 beads confirmed that the tetramer-bright cells are the high-avidity T cells detected in the ICS assay (data not shown). Starting from a concentration of 10−9 M Ova peptide, the curves for Vβ5 and Vβ5×Rip-mOva increased in parallel, indicating that both types of mice contained a similar fraction of cells recognizing Kb-Ova with low avidity, representing about 4% of total CD8+ T cells. Because only about 1% tetramer-dull cells were observed (Figure 1), this suggests that the ICS assay is more sensitive than tetramer staining in detecting low-avidity T cells.
To ask whether high-avidity, Ova-specific T cells accumulated in lymph nodes draining the pancreas of Vβ5×Rip-mOva mice, T cells were also isolated from the pancreatic lymph nodes of naive Vβ5 and Vβ5×Rip-mOva mice and stimulated with anti-CD3 plus anti-CD28 beads, and the resulting blasts were restimulated with titered concentrations of Ova peptide. Similar to the result with T cells isolated from spleen or blood, the pancreatic lymph nodes of Vβ5×Rip-mOva mice did not harbor high-avidity CD8+ T cells specific for the Ova peptide (Figure 2C). We conclude that about 1% of CD8+ T cells in naive Vβ5 mice have high avidity for Kb-Ova, comparable to that of OT-I T cells, and about 4% of cells have a much lower avidity. In Vβ5×Rip-mOva mice, the former are eliminated or silenced, but the later, low-avidity CD8+ T cells are spared.
Induction of Autoimmune Diabetes in Double Transgenic Mice
While unimmunized Vβ5×Rip-mOva mice do not spontaneously develop autoimmune diabetes, 83% of analyzed mice (20 out of 24 mice) developed diabetes between day 5 and 9 postinfection with Lm-Ova (Figures 3A and 3B). In contrast, infection with wild-type Lm (Lm-wt) did not induce diabetes in Vβ5×Rip-mOva mice (0 out of 8 mice). Histologically, the pancreas of Lm-Ova-infected Vβ5×Rip-mOva mice showed extensive islet infiltration and islet destruction. In contrast, the islets of Lm-Ova-infected Vβ5 mice or Lm-wt-infected Vβ5×Rip-mOva mice appeared normal (Figure 3B).
Figure 3.
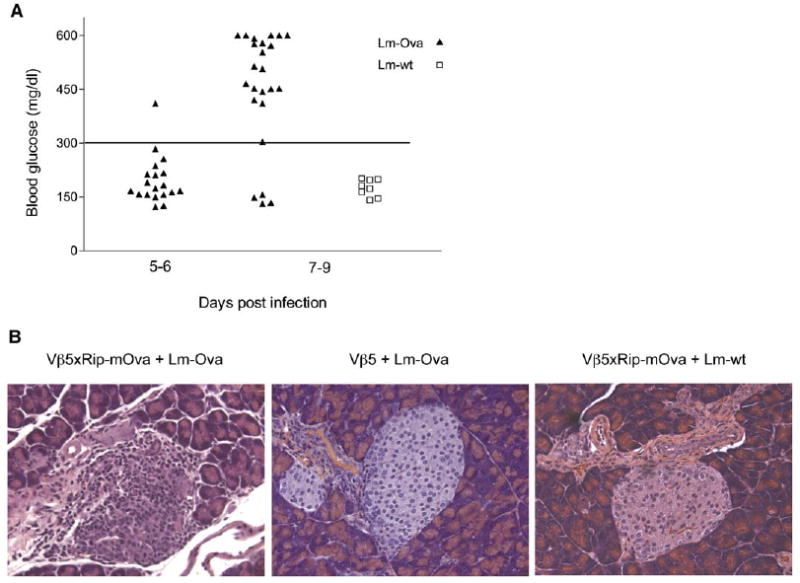
Incidence of Autoimmune Diabetes in Vβ5×Rip-mOva Mice after Lm-Ova Infection
Vβ5×Rip-mOva mice were immunized with Listeria monocytogenes-expressing ovalbumin (Lm-Ova) or wild-type Listeria (Lm-wt). Blood glucose was monitored starting from day 5–7 until day 11 postinfection, and mice with levels above 300 mg/dl were considered diabetic.
(A) Maximum blood glucose levels, detected between days 5–6 and days 7–9 post Lm-Ova (triangles) infection and days 7–9 after Lm-wt (squares) infection are shown.
(B) Representative 20× magnified, HE-stained pancreas sections of Lm-Ova-immunized Vβ5×Rip-mOva (left) and Vβ5 (middle) mice and Lm-wt-immunized Vβ5×Rip-mOva mice (right) are presented.
To asses whether diabetes was caused by the low-avidity anti-Kb-Ova T cells or whether a small number of high-avidity cells escaped tolerance and caused disease, splenocytes from Vβ5×Rip-mOva and Vβ5 mice were harvested on day 7 after Lm-Ova infection, immediately stimulated with serial dilutions of Ova peptide, and analyzed for their ability to make IFNγ. At the maximum concentration of 10−5 M peptide, about 5% of total CD8+ T cells in immunized Vβ5×Rip-mOva mice responded while immunized Vβ5 mice contained about four times as many responders (Figure 4A). Cells from uninfected Vβ5×Rip-mOva and Vβ5 mice did not produce IFNγ at any concentration of peptide, indicating that in these mice, the Kb-Ova-specific cells were naive (Figure 4A). In order to better compare the slope and thus the functional avidity of the cells making IFNγ in the immunized mice, the data were normalized to the response seen at 10−5 M peptide (Figure 4B). This revealed that about 100 times more peptide was required to induce a half-maximal response in the effector T cells from Lm-Ova-immunized Vβ5×Rip-mOva mice than the concentration that was required for cells from Vβ5 mice. Notably, as with polyclonal activation of naive CD8+ T cells (Figure 2), T cells able to make cytokine upon stimulation with 10−11 to 10−10 M peptide were absent in the double transgenic mice. It is also noteworthy that the shape of the peptide titration curve for Lm-Ova-immunized Vβ5 mice shifted compared to the curve for naive mice stimulated with anti-CD3 plus anti-CD28 beads, indicating a preferential enrichment of high-avidity cells after immunization (compare Figure 2B with Figure 4). To ask whether CD8+ T cells with higher avidities could be found in other organs of Vβ5×Rip-mOva mice, pancreas-infiltrating T cells and T cells from pancreatic lymph nodes from diabetic mice at day 7 postinfection with Lm-Ova were also analyzed. Because the cell yields from these organs were too low to allow direct analysis in a peptide titration assay, the cells were polyclonally expanded with anti-CD3 plus anti-CD28 beads, and after 6 days, assessed for their functional avidity. The avidity of the cells isolated from these organs resembled the avidity of cells found in the spleen of the same mice, once again revealing a deficiency of high-avidity cells (Figure 4B). This indicates that high-avidity Kb-Ova-reactive cells are efficiently removed from the repertoire of Vβ5×Rip-mOva mice, but low-avidity T cells capable of becoming effectors that cause autoimmunity after Lm-Ova infection remain.
Figure 4.

Comparison of Functional Avidity of Kb-Ova-Reactive T Cells Found after Lm-Ova Infection in Vβ5×Rip-mOva and Vβ5 Mice
(A) Splenocytes harvested from Vβ5×Rip-mOva (n = 4) and Vβ5 (n = 6) mice on day 7 post Lm-Ova infection and from unimmunized Vβ5×Rip-mOva (n = 1) and Vβ5 (n = 1) mice were stimulated with the indicated concentrations of Ova peptide and analyzed for the percentage of CD8+ T cells producing IFNγ. The mean percentage of CD8+ T cells producing IFNγ is graphed against the concentration of Ova peptide used to stimulate the cells (error bars represent standard deviation).
(B) Comparison of the functional avidity of Ova peptide-reactive T cells found on day 7 post Lm-Ova infection in the pancreas (n = 1), pancreatic lymph nodes (n = 1), and spleen (n = 4) of Vβ5×Rip-mOva mice and in the spleen of immunized Vβ5 mice (n = 6). The extent of IFNγ production at a given peptide concentration is shown as the fraction of the responses induced by 10−5 M peptide. Data for splenocytes is derived from (A). Pancreas infiltrating and pancreatic lymph node CD8+ T cells were expanded with anti-CD3 plus anti-CD28 for 6 days, then stimulated with congenically marked splenocytes plus Ova peptide and analyzed for IFNγ production. Shown are mean values for the indicated number of mice used, and where applicable bars represent standard deviation.
DC in the lymph nodes draining the pancreas and kidneys of Rip-mOva mice constitutively present the Kb-Ova epitope to CD8+ T cells (Kurts et al., 1997b). However, this level of presentation is not sufficient to induce diabetes when double transgenic mice were injected with a nonspecific inflammatory stimulus such as wild-type Lm (Figure 3) or LCMV (Table 1). In contrast, transfer of as few as 5 × 104 high-affinity OT-I T cells into Rip-mOva mice and infection with LCMV led to rapid development of diabetes (Table 1). It should be noted that 5 × 104 OT-I cells is far below the number of cells that need to be transferred into Rip-mOva mice to cause diabetes without any further manipulation (Table 1; Kurts et al., 1997a). These data suggest strongly that the endogenous level of Kb-Ova presentation is sufficient to activate high- but not low-avidity CD8+ T cells, when a global inflammatory stimulus is provided.
Table 1.
LCMV Infection of Vβ5×Rip-mOva and Rip-mOva Mice
| Mice | Cell Transfer | Treatment | Diabetes Incidence |
|---|---|---|---|
| Rip-mOva | 5 × 105 OT-I | none | 0/3 |
| Rip-mOva | 5 × 105 OT-I | LCMV | 9/9 |
| Rip-mOva | 5 × 104 OT-I | LCMV | 4/4 |
| Rip-mOva | no transfer | LCMV | 0/6 |
| Vβ5×Rip-mOva | no transfer | LCMV | 0/9 |
Mice were injected with the indicated number of OT-I T cells and then infected with LCMV. Blood glucose levels were monitored every other day, starting from day 5 until day 11 postinfection, and mice with levels above 300 mg/dl were considered diabetic.
CD4+ helper T cells specific for Ova are capable of activating or licensing DC crosspresenting the Kb-Ova epitope in the pancreatic and renal lymph nodes of Rip-mOva mice, such that cotransfer of naive OT-II and small numbers of OT-I T cells causes diabetes in these mice (Kurts et al., 1997a). However, transfer of naive OT-II CD4+ T cells into Vβ5×Rip-mOva mice did not lead to diabetes (Table 2). Furthermore, even when these mice were subsequently injected with LPS-activated, OT-II peptide-pulsed DC, diabetes did not result. However, when these mice were subsequently infected with Lm-Ova, they developed diabetes within 6–7 days. Similarly, Vβ5×Rip-mOva mice remained free of disease after injecting a large number of OT-II T cells that had been activated in vitro with anti-CD3 plus anti-CD28 beads (Table 2). We conclude that the double transgenic mice do not contain CD8+ T cells that can be activated to cause autoimmunity when relying on DC activation and endogenous Ova presentation. Immunization with Listeria that produce Ova, however, resulted in diabetes caused by low-avidity CD8+ T cells.
Table 2.
Licensing of Ova Crosspresenting DC
| Experiment | Time Point | Treatment | Number Diabetic |
|---|---|---|---|
| 1 | day 0 | 1.5 × 106 naive OT-II | 0/4 (until day 13) |
| day 13 | 2.5 × 106 DC (activated, OT-II peptide pulsed) | 0/4 (until day 28) | |
| day 28 | Lm-Ova infection | 3/4 (until day 39) | |
| 2 | day 0 | 2 × 106 activated OT-II | 0/4 (until day 11) |
| day 11 | Lm-Ova infection | 4/4 (until day 18) | |
Naive OT-II T cells (experiment 1) or OT-II T cells activated for 24 hr with anti-CD3 plus anti-CD28 (experiment 2) were adoptively transferred into double transgenic Vβ5×Rip-mOva mice. Starting from day 5, blood glucose measurements were performed every other day. In experiment 1, mice also received 2.5 × 106 CD11c+ splenic dendritic cells on day 13, treated with LPS and OT-II peptide, and 3000 CFU Lm-Ova on day 28. Mice from experiment 2 were infected with Lm-Ova on day 11.
Central and Peripheral Tolerance Spare Lower-Avidity T Cells
Having characterized the extent to which the Kb-Ova repertoire is shaped in Vβ5×Rip-mOva mice, we were interested in analyzing how central and peripheral tolerance individually contribute to repertoire selection. To analyze the stringency of central tolerance, we transferred thymocytes from 9-week-old Vβ5×Rip-mOva or Vβ5 mice into Rag1−/− hosts, infected them with Lm-Ova, and analyzed the peptide dose response of Kb-Ova-reactive T cells from the spleen 7 days later. While 50% of CD8+ T cells derived from Vβ5 thymocytes produced IFNγ upon restimulation with 10−5 M Ova peptide, only 1.5%–13% of Vβ5×Rip-mOva CD8+ T cells responded to this peptide concentration (data not shown). Interestingly, the avidity spectrum of Kb-Ova-specific T cells found after adoptive transfer of double-transgenic thymocytes closely resembled that of T cells found in the periphery of Vβ5×Rip-mOva mice (Figure 5A). Similarly, as shown in Figure 4 for Lm-Ova-infected Vβ5 mice, the Kb-Ova-specific response in Lm-Ova-immunized Rag−/− mice, which received Vβ5 thymocytes, is dominated by high-avidity cells. Given that high-avidity Kb-Ova-specific T cells are preferentially expanded after Lm-Ova infection, the virtual absence of high-avidity T cells in Rag1−/− mice that received thymocytes from Vβ5×Rip-mOva mice demonstrated how efficiently central tolerance alone removed high-avidity T cells. These findings argue against leakiness in thymic selection and illustrate how rigorously central tolerance purges the repertoire of high-avidity T cells specific for TRA.
Figure 5.
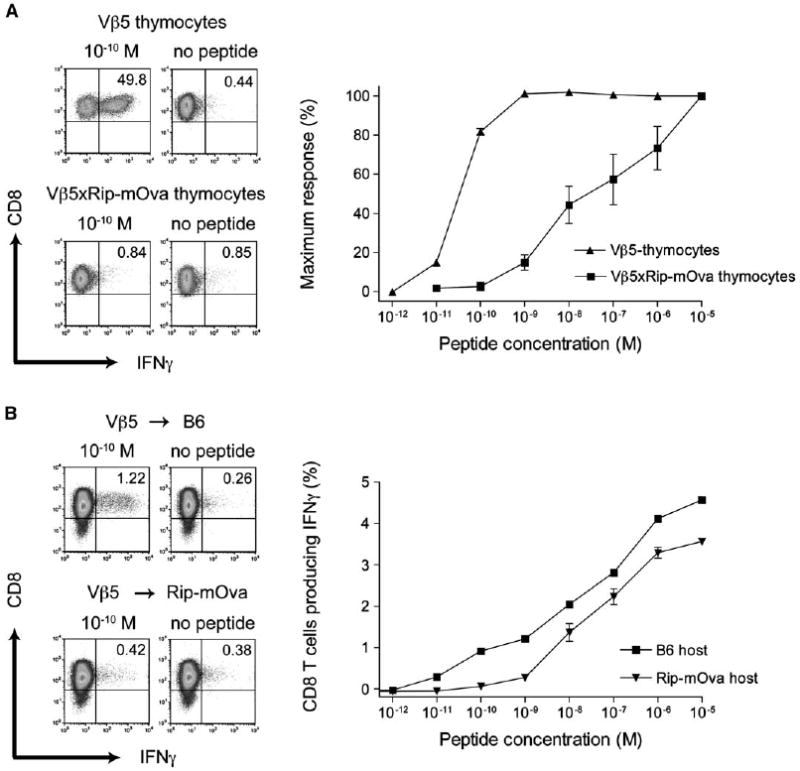
Influence of Central and Peripheral Tolerance on CD8+ T Cells Reactive to a Tissue-Restricted Antigen
(A) Thymocytes from 9-week-old Vβ5×Rip-mOva (n = 3) and Vβ5 (n = 3) mice were adoptively transferred into Rag1−/− mice at a ratio of one thymus per recipient. 24 hr later, the mice were infected with Lm-Ova, and 7 days after infection, splenocytes were harvested and analyzed for CD8+ T cells producing IFNγ in response to Ova peptide. The flow cytometry plots are representative of cells stimulated with 10−10 M Ova or no peptide control. The graph shows the complete peptide titration. Bars represent standard deviation of three mice.
(B) 107 splenic Ly5.1 CD8+ T cells from Vβ5 mice were adoptively transferred either into Rip-mOva (Ly5.2) transgenic mice (n = 2) or into control B6 (Ly5.2) mice (n = 2). 6 weeks later, grafted T cells were reisolated from the host spleen and expanded for 6 days with anti-CD3 plus anti-CD28 beads. After stimulation with Ova peptide-pulsed splenocytes (Ly5.2), the expanded cells were analyzed for intracellular IFNγ. The flow plots gated on Ly5.1 cells show representative stains for stimulation with 10−10 M Ova or no peptide control, and the complete titration curve for the donor cells is shown in the graph. Shown are mean values for the indicated number of mice and the bars represent the position of individual data points. The results are representative of at least two experiments.
Although these data indicate that peripheral tolerance does not play an obligatory role in selecting the repertoire of Vβ5×Rip-mOva mice, peripheral tolerance is important for tolerizing T cells against antigens that are not expressed in the thymus. Thus, we were interested in determining how stringently peripheral tolerance might shape the T cell repertoire. CD8+ T cells from the periphery of congenically marked Vβ5 mice were transferred into either Rip-mOva mice or B6 mice. After 6 weeks the cells were reisolated, stimulated with anti-CD3 plus CD28 beads, and analyzed for IFNγ synthesis in response to different concentrations of Ova peptide (Figure 5B). T cells hosted in Rip-mOva mice made IFNγ only when stimulated with peptide concentrations higher than 10−9 M, whereas cells that had resided for 6 weeks in B6 mice responded to concentrations of peptide as low as 10−11 M. Thus, although both hosts received the same T cells, high-avidity T cells were selectively tolerized in Rip-mOva hosts. Whether the high-avidity T cells were deleted, rendered anergic, or silenced in some way is not known. This experiment indicates that, in this system, peripheral tolerance is as potent in selecting the T cell repertoire as central tolerance and can serve as an efficient backup for inducing tolerance to antigens that are not expressed intrathymically.
Discussion
In Rip-mOva transgenic mice, where Ova is expressed as a model TRA (Kurts et al., 1997b), central and peripheral tolerance mechanisms operate efficiently to delete all CD8+ T cells with the ability to respond to low levels of the Kb-Ova ligand. Central tolerance in this system is mediated by mTECs directly presenting ectopically expressed Ova and crosspresentation by DCs, when T cells cross from the thymic cortex to the medulla (Gallegos and Bevan, 2004). Peripheral tolerance in this system occurs when CD8+ T cells, with high avidity for Kb-Ova, traffic to the lymph nodes draining the pancreas and kidneys. These tolerance mechanisms are extremely efficient, as indicated by the fact that we were unable to detect any high-avidity Kb-Ova-specific T cells in double transgenic Vβ5×Rip-mOva mice. This was the case both when we examined the naive T cell repertoire in these mice by activating T cells nonspecifically with anti-CD3 plus anti-CD28, as well as after immunization with Lm-Ova. However, CD8+ T cells responding to Kb-Ova with lower avidity can be found to a similar extent (about 4% of total T cells) in the naive T cell repertoire of Vβ5×Rip-mOva and Vβ5 mice, indicating that they are largely spared from elimination in the thymus and the periphery. These low-avidity T cells acquired effector function after Lm-Ova immunization, as demonstrated by their ability to make IFNγ upon brief in vitro restimulation with high concentrations of peptide, but no high-avidity T cells were detectable in the spleen, pancreatic lymph nodes, or within the pancreas itself, even as the mice developed diabetes. We conclude that low-avidity CD8+ T cells with specificity for Kb-Ova routinely escape elimination and are the mediators of autoimmunity in this system.
Despite the constitutive presentation of Ova in the lymph nodes draining the pancreas and kidneys and the presence of these low-avidity T cells in the Vβ5×Rip-mOva mice, neither a global inflammatory stimulus caused by LCMV infection (Table 1) nor licensing of the crosspresenting DC by activated OT-II helper cells (Table 2) resulted in autoimmunity. It is noteworthy that both treatments are capable of activating high-avidity T cells and causing diabetes in Rip-mOva mice that received OT-I T cells (Table 1; Kurts et al., 1997a). Thus, it appears that Vβ5×Rip-mOva mice are refractory to autoimmune disease induced by endogenous levels of self-antigen presentation. However, the Vβ5×Rip-mOva mice rapidly developed disease after immunization with Lm-Ova. One likely explanation for this is that infection with Lm-Ova results in a higher density of Kb-Ova presentation than can be achieved by endogenous antigen presentation. The higher ligand density would allow the activation of naive, low-avidity T cells that, upon differentiation into effector cells, could recognize and damage pancreatic β cells expressing Ova. It would be informative to quantitate the ligand density on antigen-presenting cells in uninfected and Lm-Ova-infected Rip-mOva mice, but the staining reagent specific for this complex is not sensitive enough for this purpose. A second, not mutually exclusive possibility, is that Lm-Ova infection results in antigen presentation by DC with sufficient stimulatory capacity to activate even the low-avidity T cells. With stimulation of naive CD8+ T cells as a readout, constitutive presentation of pancreas-derived antigen and presentation of Lm-Ova-derived antigen have both been shown to be a property of the same CD8α DC subset (Belz et al., 2002, 2005). This second explanation would therefore require that the CD8α DC crosspresenting Lm-Ova-derived antigens are somehow more stimulatory than CD8α DC in pancreatic and renal lymph nodes crosspresenting tissue-derived Ova, even when the latter are activated by Ova-specific helper T cells or an inflammatory stimulus. A recent report on the induction of T cell responses to melanocyte antigens showed that melanoma tumor immunity and autoimmunity, read out by vitiligo, could be induced in normal mice by immunization with vectors encoding nonmutated self-antigens (Engelhorn et al., 2006). Although the mechanisms for activating these self-reactive T cells are unclear, these results provide evidence linking the induction of autoimmunity to more efficient presentation of the self-antigens.
At first glance, our contention that large numbers of low-avidity T cells able to inflict tissue damage escape central tolerance induction may seem to be at odds with studies showing that thymic-negative selection is able to delete virtually all cells with autoimmune potential. Experiments with “altered peptide ligands,” variants of antigenic peptides, have shown that immature, DP thymocytes are exquisitely sensitive to low-affinity ligands. They are able to respond by fluxing Ca2+ and up-regulating CD69 in the presence of low-affinity ligands that are unable to activate mature, single-positive T cells bearing the same receptor (Davey et al., 1998; Lucas et al., 1999). Also, in vivo experiments have established that thymic-negative selection can be induced by pMHC ligands that are of too low affinity to signal to mature T cells, thus providing a window of safety for self tolerance (Knobloch et al., 1992; Pircher et al., 1991; Sant'Angelo and Janeway, 2002). In our system, we did not observe such a broad safety window. However, these previous studies are not in conflict with our present findings, because they all compared the sensitivity of mature T cells with CD4+CD8+ DP thymocytes. They used systems in which the tolerogenic ligand was widely expressed and had access to the most immature thymocytes. In contrast, our study used the well-characterized Rip-mOva model for a self-antigen expressed ectopically at low levels only in the thymic medulla and not in the thymic cortex. As shown previously, the deletion of OT-I thymocytes in Rip-mOva transgenic mice occurs after positive selection of DP thymocytes, at the subsequent CD4+CD8lo or CD4loCD8lo stages of CD8+ T cell maturation (Gallegos and Bevan, 2004). A comparison of the TCR-triggering sensitivity of these late intermediates with that of mature T cells has not been performed. Thus, different outcomes in central tolerance induction against a ubiquitously expressed antigen versus a TRA might be explained by a loss of sensitivity for pMHC ligands occurring after positive selection.
As far as we could judge from the peptide titration assay, the efficiency of central and peripheral tolerance induction of Ova-specific CD8+ T cells in Rip-mOva transgenic mice is about equal (Figure 5). Both mechanisms eliminate CD8+ T cells able to make IFNγ in response to concentrations of Ova peptide below 10−9 M. However, this equality may not apply to all examples of self tolerance to TRA, as some self antigens may be expressed at different levels in the periphery or in the thymus. The observation that peripheral tolerance can efficiently delete the T cell repertoire of self-reactive T cells is particularly relevant for tumor immunology, because mutated or viral tumor-associated antigens may induce similar potent tolerization of the peripheral T cell repertoire. Recent observations that a spontaneously arising tumor can tolerize tumor-specific T cells provide support for this view (Willimsky and Blankenstein, 2005).
The presence of autoreactive T cells in the periphery can, in some cases, be attributed to the lack of antigen or epitope presentation in the thymus (Klein et al., 2000; Morgan et al., 1999; Ohashi et al., 1991; Oldstone et al., 1991). In line with our findings, an earlier study showed that even when a TRA is ectopically expressed in the thymus, self-reactive T cells may still be present in the periphery (von Herrath et al., 1994). It was unknown whether the escape from tolerance in these systems was due to leakiness in central and peripheral tolerance processes. Our data strongly argue that this is not the case. In our TCRβ chain transgenic mice with a skewed polyclonal repertoire, we could readily quantitate numbers and avidity of self-reactive cells. We showed that both tolerance mechanisms efficiently removed all high-avidity T cells, while they spared cells below a certain avidity threshold. Thus, mice expressing the TRA contained just as many low-avidity self-reactive T cells as mice that did not express the antigen, amounting in both cases to about 4% of total CD8+ T cells. Our data indicate that these low-avidity T cells, induced to effector activity by Lm-Ova immunization, are the causative agents of autoimmunity. Thus, we conclude that low-avidity T cells, although harboring self-destructive potential, might routinely escape central and peripheral mechanisms of tolerance.
In applying our findings to autoimmunity in general, we have to ask how low-avidity autoreactive T cells are induced to cause disease. We do not envisage that a viral or bacterial pathogen breaks tolerance by introducing an exact copy of the self peptide. As the affinity of the foreign pMHC-TCR interaction has been shown to be crucial for inducing autoimmunity (Gronski et al., 2004), we rather consider that these low-avidity anti-self T cells have TCR with high affinity for unknown foreign ligands and can be readily induced when the foreign ligand is introduced by infection. Molecular mimicry is one mechanism for how pathogens might trigger autoimmune responses (Oldstone, 1998). We propose that while self-reactive T cells that can be primed with endogenous levels of a self-antigen are completely eliminated by both peripheral and central tolerance, remaining low-avidity cells constitute a population of cells that can be activated by a crossreactive foreign antigen to cause autoimmunity. In conclusion, our findings imply that central and peripheral repertoire selection against TRA imposes a degree of safety that guards against priming with endogenous self-antigen but does not fully protect against tissue destruction when low-avidity anti-self T cells are induced to became effector cells by foreign antigen stimulation.
Experimental Procedures
Mice
Rip-mOva mice, expressing a membrane bound form of ovalbumin under the control of the rat insulin promoter (Kurts et al., 1996), Vβ5 TCRβ chain-only transgenic mice (Fink et al., 1992), Vβ5×Rip-mOva, Rag1−/−-deficient, OT-I, and OT-II TCR transgenic mice recognizing Kb-Ova257-264 and Ab/Ova323-337, respectively, as well as B6 and CD45.1 congenic B6 mice were bred and maintained in SPF facilities at the University of Washington and used at 5–8 weeks of age. All experiments with mice were performed in compliance with the University of Washington Institutional Animal Care and Use Committee regulations.
Listeria monocytogenes and LCMV Infections
Recombinant Listeria monocytogenes secreting ovalbumin (Lm-Ova) was provided by H. Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) (Pope et al., 2001). Frozen stocks of Lm-Ova and wild-type Listeria monocytogenes (Lm-wt) were grown in brain-heart infusion broth to mid log phase. Cell numbers were determined by measuring the OD at 600 nm, and 3000 CFU were injected in PBS intravenously. LCMV Armstrong (clone 53b) was grown on BHK cells and titered on vero cells. Frozen stocks were diluted in PBS and injected at 3 × 105 PFU per mouse intraperitoneally.
Blood Glucose Measurement and Histology
Blood was analyzed with OneTouchUltra blood glucose testing system (Lifescan, Miltipas, CA). Values above 300 mg/dl were considered diabetic. Mice were monitored for blood glucose levels every other day starting from day 5 or 6 (in some experiments starting from day 7) until, if not stated otherwise, day 11 postinfection or cell transfer. Pancreatic tissue isolated on day 7 post Lm-Ova infection was formalin fixed and sent to Histology Consultation Services (Everson, WA) for processing and HE staining.
Cell Isolation
Single-cell suspensions from spleen, thymus, and pancreatic lymph nodes were obtained by mashing organs through a 100 μM nylon cell strainer (BD Falcon, Bedford, MA). Red blood cells were eliminated from splenocytes with ACK lysis buffer. Mouse CD4+ or CD8+ T cell Isolation kit and LS separation columns (Miltenyi Biotec, Bergisch Gladbach, Germany) were used, according to the manufacturer's protocols, for untouched CD4+ or CD8+ T cell isolation from total splenocytes. For isolation of peripheral blood-derived CD8+ T cells, PBMC were purified from blood with lympholyte (Cedarlane Laboratories, Burlington, NC) density gradient centrifugation, and untouched CD8+ isolation was performed with MS separation columns (Miltenyi). Pancreas-infiltrating T cells were purified as described (Trembleau et al., 1999). Pancreatic tissue was harvested on day 7 post Lm-Ova, minced with a scalpel, and stirred for 10 min at 37°C in HBSS (Invitrogen, Grand Island, NY) supplemented with collagenase 4 at 1 mg/ml (Sigma, St. Louis, MO). Nondigested tissue was allowed to settle for 2 min, and then 2/3 of the supernatant was replaced with fresh HBSS and collagenase. The procedure was repeated two more times. The supernatants were diluted in 10% DMEM (Invitrogen), and T cells were isolated with anti-CD8 beads and MS separation columns (Miltenyi). Dendritic cells were isolated from the spleen as described (den Haan and Bevan, 2002) by means of combined digestion with 1 WU/ml liberase R1 and 50 μg/ml DNAase1 (Roche Diagnostics, Indianapolis, IN) and anti-CD11c-based magnetic cell separation (Miltenyi).
T Cell Stimulation and Expansion
2–5 × 105 CD8+ T cells were stimulated in 48-well plates with 10 μl anti-CD3 plus anti-CD28-coated beads (Dynal Biotech, Oslo, Norway) in RPMI (Invitrogen) supplemented with 10% FCS, antibiotics, 50 μM 2-ME, and T cell growth factor (α-methylmannopyranoside-blocked supernatant from concavalin A-stimulated rat splenocytes). Cells were diluted 1:3 to 1:5 on day 2, 3, and 5 and analyzed for Kb-Ova-specific T cells on day 6.
Adoptive Transfer of T Cells, Thymocytes, and Dendritic Cells
107 CD45.1 congenically marked CD8+ T cells from Vβ5 mice were injected into B6 and Rip-mOva CD45.2 host mice. 6 weeks later, cells were reisolated from the host spleen via magnetic depletion of host-derived CD45.2 cells, expanded with anti-CD3 plus anti-CD28 beads for 6 days, and analyzed for Kb-Ova-specific T cells. Total thymocytes from 9-week-old Vβ5 or Vβ5×Rip-mOva mice, at a ratio of one thymus per mouse, were injected into Rag1−/− mice. 24 hr after the transfer, mice were infected with Lm-Ova, and splenocytes from these mice were analyzed 7 days post Lm-Ova infection. Isolated DC were treated for 1 hr with 20 μg/ml of OVA323-337 peptide and 1 μg/ml LPS and then injected at 2.5 × 106 per mouse subcutaneously.
Detection of Kb-Ova-Specific T Cells
Total peripheral blood cells were stained for 2 hr with Kb-Ova257-264 phycoerythrin-labeled tetramers in the presence of anti-CD8 PerCP clone 53-6.7 (BD, San Diego, CA) at 4°C. For measuring Kb-Ova-specific T cells by intracellular cytokine staining (ICS), either 3 × 106 splenocytes from naive or infected mice (day 7 postinfection) or 1–1.5 × 106 expanded CD8+ T cells mixed with 1.5–2 × 106 stimulator cells (total splenocytes from naive CD45 congenic mice) were transferred into 96-well plates in DMEM medium (Invitrogen) containing 10% FCS, antibiotics, and 50 μM 2-ME. Ova257-264 SIINFEKL peptide dilutions were added and the samples were incubated at 37°C. Samples stimulated with 0.66 μg/ml PMA (Sigma) and 0.66 μg/ml Ionomycin (Calbiochem, San Diego, CA) served as positive controls. 45 min after adding peptide, the samples were supplemented with 7 μg/ml Brefeldin A (Sigma) and incubated for an additional 5.5 hr. Then samples were washed, stained with anti-CD8 and anti-CD45.1 or anti-CD45.2 (all BD), fixed and permeabilized with Cytofix and Cytoperm Kit (BD), and stained intracellularly with anti-IFNγ (BD).
Data Analysis
Flow cytometry data were acquired on a FACS-Canto machine (BD) and analyzed with FlowJo software (Tree Star Inc, Ashland, OR). All intracellular IFNγ measurements were performed in duplicate. The percentage of cells specifically producing IFNγ was determined by subtracting the response seen with the no peptide control from the percentage of IFNγ+CD8+ T cell at a given peptide concentration. Graphs were prepared with GraphPad Prizm 3.0, and they show average data and corresponding standard deviation. When indicated, data were normalized to the response seen at 10−5 M peptide.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and National Institutes of Health Grant AI19335 (to M.J.B.) and by a scholarship from the German Academic Exchange Service (DAAD) (to D.Z.). We thank Pamela J. Fink, Martin Prlic, Jilian A. Sacks, and Matthew A. Williams for helpful discussions and critical review of the manuscript and Xiaocun Pan and Beverley Dere for excellent technical support.
References
- Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med. 2002;196:1099–1104. doi: 10.1084/jem.20020861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- Dillon SR, Jameson SC, Fink PJ. V beta 5+ T cell receptors skew toward OVA+H-2Kb recognition. J Immunol. 1994;152:1790–1801. [PubMed] [Google Scholar]
- Engelhorn ME, Guevara-Patino JA, Noffz G, Hooper AT, Lou O, Gold JS, Kappel BJ, Houghton AN. Autoimmunity and tumor immunity induced by immune responses to mutations in self. Nat Med. 2006;12:198–206. doi: 10.1038/nm1363. [DOI] [PubMed] [Google Scholar]
- Fink PJ, Swan K, Turk G, Moore MW, Carbone FR. Both intrathymic and peripheral selection modulate the differential expression of V beta 5 among CD4+ and CD8+ T cells. J Exp Med. 1992;176:1733–1738. doi: 10.1084/jem.176.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- Klein L, Roettinger B, Kyewski B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur J Immunol. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Schonrich G, Schenkel J, Malissen M, Malissen B, Schmitt-Verhulst AM, Hammerling GJ, Arnold B. T cell activation and thymic tolerance induction require different adhesion intensities of the CD8 co-receptor. Int Immunol. 1992;4:1169–1174. doi: 10.1093/intimm/4.10.1169. [DOI] [PubMed] [Google Scholar]
- Kurts C, Heath WR, Carbone FR, Allison J, Miller JF, Kosaka H. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Carbone FR, Barnden M, Blanas E, Allison J, Heath WR, Miller JF. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997a;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C, Kosaka H, Carbone FR, Miller JF, Heath WR. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8(+) T cells. J Exp Med. 1997b;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lesage S, Gray DH, Boyd RL, Goodnow CC. Genetic lesions in T-cell tolerance and thresholds for autoimmunity. Immunol Rev. 2005;204:87–101. doi: 10.1111/j.0105-2896.2005.00253.x. [DOI] [PubMed] [Google Scholar]
- Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Kurts C, Kreuwel HT, Holst KL, Heath WR, Sherman LA. Ontogeny of T cell tolerance to peripherally expressed antigens. Proc Natl Acad Sci USA. 1999;96:3854–3858. doi: 10.1073/pnas.96.7.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Oldstone MB. Molecular mimicry and immune-mediated diseases. FASEB J. 1998;12:1255–1265. doi: 10.1096/fasebj.12.13.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Palmer E. Negative selection—clearing out the bad apples from the T-cell repertoire. Nat Rev Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. [DOI] [PubMed] [Google Scholar]
- Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Sant'Angelo DB, Janeway CA., Jr Negative selection of thymocytes expressing the D10 TCR. Proc Natl Acad Sci USA. 2002;99:6931–6936. doi: 10.1073/pnas.102182499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Trembleau S, Penna G, Gregori S, Chapman HD, Serreze DV, Magram J, Adorini L. Pancreas-infiltrating Th1 cells and diabetes develop in IL-12-deficient nonobese diabetic mice. J Immunol. 1999;163:2960–2968. [PubMed] [Google Scholar]
- von Boehmer H, Aifantis I, Gounari F, Azogui O, Haughn L, Apostolou I, Jaeckel E, Grassi F, Klein L. Thymic selection revisited: how essential is it? Immunol Rev. 2003;191:62–78. doi: 10.1034/j.1600-065x.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–242. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Willimsky G, Blankenstein T. Sporadic immunogenic tumours avoid destruction by inducing T-cell tolerance. Nature. 2005;437:141–146. doi: 10.1038/nature03954. [DOI] [PubMed] [Google Scholar]


