Abstract
Müller glia in the mature retina have the capacity to become progenitor-like cells in a many different vertebrate classes. The cell-signaling pathways that control the ability of mature Müller glia to become progenitor-like cells remain uncertain. The purpose of this study was to investigate the roles of the Mitogen-Activated Protein Kinase (MAPK) pathway in regulating the activity of Müller glia in the chicken retina. In response to acute retinal damage, we found that Müller glia accumulated phosphorylated ERK1/2 and phospho-CyclicAMP Response Element Binding-protein (pCREB), and transiently expressed immediate early genes, cFos and Egr1, that are known to be downstream of MAPK-signaling. Egr1 and pCREB were normally expressed by retinal progenitors in the circumferential marginal zone (CMZ), whereas cFos and pERK1/2 were not. In addition, small molecule inhibitors of MEK (UO126) and the FGF-receptor (SU5402) suppressed the proliferation of Müller glia-derived progenitor-like cells. These inhibitors suppressed the accumulation of Egr1 and pCREB, whereas levels of cFos were unaffected in the glial cells. These findings suggest that Egr1 and pCREB are downstream of the-signaling cascade activated by FGF-receptors and ERK1/2. Further, our findings suggest that Egr1 and pCREB may promote glial proliferation. We propose that activation of both the FGF-receptor and ERK1/2-pathway is required for the proliferation and transdifferentiation of Müller glia into progenitor-like cells.
Keywords: retina, insulin, FGF2, Egr1, cFos, pCREB, progenitor
Introduction
There is growing evidence that glial cells of the central nervous system (CNS) might be a source of neural regeneration. For example, Müller glia in the retina can be stimulated to become progenitor-like cells in a variety of different vertebrates, including zebrafish (Bernardos et al. 2007; Fausett and Goldman 2006), rodents (Ooto et al. 2004), and chickens (Fischer 2005; Fischer et al. 2002b; Fischer and Reh 2001b). Under normal conditions, Müller glia are the predominant type of support cell in retina, providing structural, nutritive and metabolic support to neurons. However, “normal” Müller glia share many genes (~68%) with retinal progenitors (Blackshaw et al. 2004). In the post-hatch chicken retina, we have reported that Müller glia are a potential source of neural regeneration (Fischer and Reh 2001a). In response to acute excitotoxic damage, numerous Müller glia de-differentiate, re-enter the cell-cycle, and express genes normally found in embryonic retinal progenitors. These genes include Cash1 (ascl1a), Pax6, Chx10, PCNA (Fischer and Reh 2001a), Six3 (Fischer 2004; Fischer 2005), Notch1, Sox2 (Hayes et al. 2007) and the nestin-related intermediate filament transitin (Fischer and Omar 2005). In response to sufficient neuronal damage, numerous Müller glia re-enter the cell cycle and undergo only one round of division in vivo, whereas these cells continue to proliferate when dissociated from the intact retina and are grown in culture (Fischer and Reh 2001a). The proliferation of the Müller glia is an integral step in becoming progenitor-like cells and transdifferentiating into neuronal cells (Fischer 2005; Fischer and Reh 2003; Reh and Fischer 2001). The term “transdifferentiation”, as it applies to Müller glia, involves de-differentiation, re-entry into the cell cycle (proliferation), and expression of genes that are normally expressed by retinal progenitors. The majority of cells that are generated by proliferating glia remain as un-differentiated progenitor-like cells, whereas some differentiate into new Müller glia and a few differentiate into neurons (reviewed by (Fischer 2005; Fischer and Reh 2003). Although few neurons are regenerated, Müller glia produce thousands of undifferentiated progenitor-like cells that represent a large pool of cells that could be stimulated to differentiate and significantly regenerate the retina to restore vision. Thus, understanding the different signaling pathways that control the ability of Müller glia to transdifferentiate holds the potential to treat sight-threatening, neurodegenerative diseases of the retina.
The factors that stimulate the neurogenesis from Müller glia are slowing being revealed. In the rodent, Wnt-signaling has been shown to stimulate the proliferation and generation of new retinal neurons from Müller glia in response NMDA-induced damage (Osakada et al. 2007). In the chicken, Notch-signaling is increased in Müller glia-derived cells and may be required for re-entry into the cell cycle in NMDA-damaged retinas (Hayes et al. 2007). In the absence of damage, consecutive daily injections of the combination of insulin and FGF2, but not either factor alone, stimulate Müller glia to transdifferentiate and produce a few new neurons (Fischer et al. 2002b). Sustained exposure to the combination of insulin and FGF2 induces a response in Müller glia similar to that observed in NMDA-damaged retinas (Fischer and Reh 2003). Insulin and FGF2 are known to bind to receptor tyrosine kinases which can active MAPK-signaling pathways. Thus, it is possible that MAPK-signaling is involved in the proliferation and transdifferentiation of Müller glia. In this study we investigate whether MAPK-signaling is active in Mülller glia following acute retinal damage, and whether the inhibition of the MAPK-pathway influences the proliferation of Müller glia-derived progenitors.
Materials and Methods
Animals
The use of animals in these experiments was in accordance to the guidelines established by the National Institutes of Health and The Ohio State University. Newly hatched leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University and kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am). Chicks were housed in a brooder at about 28°C and received water and Purina™ chick starter ad libitum.
Intraocular injections
Chickens were anesthetized by inhalation of 2.5% isoflurane in oxygen, as described previously (Fischer et al. 1999a; Fischer et al. 1999b; Fischer et al. 1998b). Injections were made using a 25-μl Hamilton syringe and a 26-gauge needle with a beveled, curved tip. Penetration of the needle was consistently made into the dorsal quadrant of the vitreous chamber. In all experiments, 20 μl of vehicle containing the test compound was injected into the experimental (left) eye, and 20 μl of vehicle was injected into the control (right) eye. The vehicle was sterile saline containing bovine serum albumin (50 μg/ml) as carrier, and, in some experiments, 5-bromo-2′-deoxyuridine (BrdU; 2 μg/dose) to label proliferating cells. Test compounds included NMDA (50 nmol or 2000 nmol/dose; Sigma-Aldrich) or small molecule inhibitors SU5402 (0.8 μg/dose diluted in 30% DMSO; Calbiochem, Canada) and UO126 (1.7 μg/dose diluted in 30% DMSO; Calbiochem).
Fixation, sectioning and immunocytochemistry
Tissues were fixed, sectioned and immunolabeled similar to previous descriptions (Fischer et al. 1998b; Fischer and Stell 1999). In short, enucleated eyes were hemisected equatorially and the gel vitreous removed from the posterior eye cup. Eye cups were fixed (4% paraformaldehyde plus 3% sucrose in 0.1 M phosphate buffer, pH 7.4, 30 min at 20°C), washed three times in PBS (phosphate-buffered saline; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4), cryoprotected in PBS plus 30% sucrose, immersed in embedding medium (OCT-compound; Tissue-Tek), and freeze-mounted onto sectioning blocks. Vertical sections, nominally 12 μm thick, were cut consistently from the posterior pole of the eye in the nasotemporal plane, and thaw-mounted onto SuperFrost Plus™ slides (Fisher Scientific). Sections from control and treated eyes from the same individual were placed consecutively on each slide to ensure equal exposures to reagents. Sections were stored at −20°C until use.
Sections were thawed, ringed with rubber cement, washed twice in PBS, covered with primary antibody (200 μl of antiserum diluted in PBS plus 5% serum, 0.2% Triton X-100, and 0.01% NaN3), and incubated for 24 hrs at 20°C in a humidified chamber. The slides were washed twice in PBS, covered with secondary antibody, and incubated for at least 1 hour in a humidified chamber. Finally, samples were washed twice in PBS, rubber cement removed from the slides, and coverglass mounted on 4:1 (v:v) glycerol to water.
Working dilutions and sources of antibodies used in this study included; (1) rabbit pERK1/2 used at 1:200 (137F5; Cell Signaling Technologies); (2) the Müller glial marker, mouse monoclonal 2M6 used at 1:100 (Dr. Paul Linser, University of Florida); (3) goat anti-Sox2 used at 1:1000 (Y-17; Santa Cruz Immunochemicals); (4) rabbit anti-cFos used at 1:400 (K-25; Santa Cruz Immunochemicals); (5) goat anti-Egr1 raised to amino acids 282-433 of recombinant human Egr1 used at 1:1000 (AF2818; R&D Systems); (6) rabbit anti-pCREB used at 1:1000 (87G3; Cell Signaling Technologies); (7) mouse anti-Pax6 used at 1:50 (PAX6; Developmental Studies Hybridoma Bank - DSHB); (8) mouse anti-n-cadherin used at 1:30 (6B3; DSHB); (9) mouse anti-transitin used at 1:600 (7A3B5; Dr. P. Henion, The Ohio State University); (10) mouse anti-vimentin was used at 1:100 (H5; DSHB); (11) mouse anti-PCNA used at 1:1000 (PC10; Dako Immunochemicals); (12) rat anti-BrdU used at 1:200 (OBT0030S; Serrotec); (13) mouse anti-BrdU used at 1:100 (G3B4; DSHB); (14) mouse anti-HuC/D used at 1:200 (16A11; Invitrogen); mouse anti-PKC used at 1:100 (554207; BD Biosciences Pharmingen); and (15) mouse anti-Islet1 used at 1:50 (40.2D6; DSHB). None of the observed labeling appeared to be due to non-specific binding of secondary antibodies because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies included donkey-anti-goat-Alexa488/568, goat-anti-rabbit-Alexa488/568/647, goat-anti-mouse-Alexa488/568/647, goat-anti-mouse-IgM-Alexa568 (Invitrogen) diluted to 1:1000 in PBS plus 0.2% Triton X-100.
TUNEL
To identify dieing cells that contained fragmented DNA we used the TUNEL method. We used the In Situ Cell Death Kit (TMR red; 1215679910) supplied by Roche Applied Science, as per the manufacturer's instructions.
Western blots
Western blots were performed by using standard techniques, similar to previous descriptions (Fischer et al. 1998a; Fischer et al. 2005). In short, control and treated retinas were pooled from 3 individuals for each condition, placed into extraction buffer (Bio-Rad), sonicated and heated to 95°C for 5 minutes. Protein samples were loaded onto 4-20% Tris-Ready Gels (Bio-Rad) and separated at 95V for 90 minutes. Protein was transferred to 0.2 μm pore PVDF membranes (Invitrogen) overnight at 25V. Membranes were immunolabeled for GAPDH at 1:1000, Egr1 at 1:1000, cFos at 1:1000, pCREB at 1:2000, and pERK at 1:2000. Secondary antibodies to goat, mouse and rabbit were used at 1:5000 (GE Healthcare). Blots were imaged using standard chemi-luminescent techniques and developing solutions from GE Healthcare and X-ray film (Denville Scientific). Densitometry was performed using ImagePro6.2 by summating the pixel intensities for each band and standardizing these to the loading control (GAPDH).
Photography, measurements, cell counts, and statistical analyses
Photomicrographs were obtained using a Leica DM5000B microscope equipped with epifluorescence and Leica DC500 digital camera. Images were optimized for color, brightness and contrast, and double-labeled images overlaid by using Adobe Photoshop™6.0. Cell counts were made from at least 5 different animals, and means and standard deviations calculated on data sets. To avoid the possibility of region-specific differences within the retina, cell counts were consistently made from the same region of retina for each data set. Images of the CMZ were taken at the far peripheral edge of the retina, images of peripheral retina were taken between 1 and 3 mm from the CMZ, and images of central retina were taken within 2 mm of the posterior pole of the eye in the nasotemporal plane.
Immunofluorescence was quantified by using ImagePro 6.2. Identical illumination, microscope and camera settings were used to obtain images for quantification. Areas (800 × 200 pixels or 232 × 58 μm) were sampled from 5.4 MP digital images. These areas were randomly sampled over the INL where the nuclei of the Müller glia were observed. Measurements were made for regions containing pixels with intensity values of 72 or greater (0 = black, 255 = saturated green); a threshold that included labeling in the nuclei Müller glia (see Fig. 7c for an example). The total area was calculated for regions with pixel intensities > 72 and with areas >190 pixels to exclude any debris within the field. The average pixel intensity was calculated for all pixels within thresholded regions. The density sum was calculated as the total of pixel values for all pixels within thresholded regions. These calculations were determined for INL regions sampled from 6 different retinas for each experimental condition.
Figure 7.
Small molecule inhibitors of MEK and the FGF-receptor suppress the expression of Egr1 in Müller glia that results from NMDA-treatment. Images of Egr1-labeled retinas where obtained using identical camera exposures and microscope illumination settings. Retinas were processed for immunolabeling 24 hours after treatment with 2000 nmol of NMDA alone, NMDA + 1.7 μg MEK inhibitor (UO126), or NMDA + 0.8 μg FGF-receptor inhibitor (SU5402). As described in the Methods, ImagePro 6.2 was used to obtain measurements of total area for pixel intensities > 72 (0 = black, 255 = saturated green), average pixel intensity, and the density sum. The small numbers and red outlines in panels c, d, h and i indicate the areas designed by ImagePro 6.2 for each object that met the threshold criteria. The calibration bar (50 μm) in panel b applies to a and b, and the bar in i applies to c, d, h and i. Means and standard deviations are displayed in histograms for total area (e and j), average intensity (f and k), and density sum (g and l) for areas with pixels above threshold. Significance of difference (*p=0.001, **p<0.0005 or ***p<0.0001) was determined by using a two-tailed, unpaired Student's t-test. Abbreviation: INL – inner nuclear layer.
Results
Müller glia accumulate pERK1/2 in response to acute retinal damage
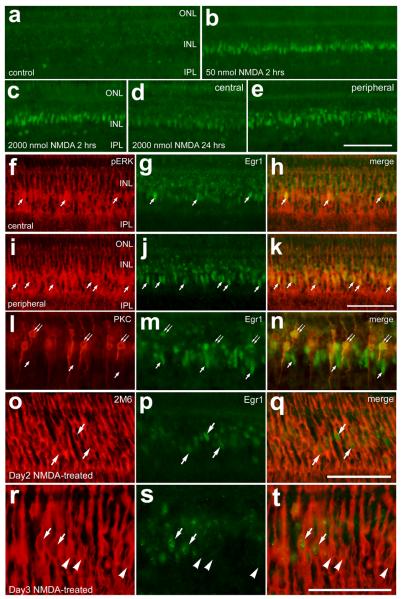
In response to damage, growth factors, including CNTF, IGFs and FGFs are produced at increased levels in the rodent retina (Cao et al. 2001; Kostyk et al. 1994; Valter et al. 1998; Walsh et al. 2001; Wen et al. 1995). Similarly, mRNA levels for CNTF, IGF-II, FGF1 and FGF2 are elevated, whereas levels of IGF-I are reduced in damaged chicken retinas (Fischer et al. 2004a). These findings suggest that CNTF, IGFs and FGFs could be involved in the responses of Müller glia to damage. Insulin, IGFs and FGFs are known to activate MAPK-signaling pathways (Grewal et al. 1999). If these secreted factors are involved in the glial responses to damage, then it is expected that MAPK-signaling may be active in Müller glia in damaged retinas. Activation of receptor tyrosine kinases triggers a signaling cascade that can culminate in the phosphorylation of ERK1/2 (i.e. ligand→receptor→Ras→Raf→MEK1/2→ERK1/2). Thus, assays for pERK1/2 are a read-out of MAPK-signaling through receptor tyrosine kinases. In undamaged retinas, we found that antibodies to pERK1/2 labeled a few amacrine cells in the proximal INL, along with neurites stratified in different laminae in the IPL (Fig. 1a). In addition, weak pERK-immunoreactivity was observed in vertical processes that span the retinal layers from the inner limiting membrane (ILM) to the outer limiting membrane (OLM; Fig. 1a). This weak pERK1/2-immunolabeling was likely in Müller glia. The distribution of pERK1/2-immunoreactivity in the retina changed dramatically with NMDA-induced damage. One day after NMDA-treatment, pERK1/2-immunoreactivity accumulated in Müller glia-like cells (Fig. 1b). The pERK1/2 was concentrated in somata located near the middle of the INL, and significant levels of pERK1/2 were observed in processes spanning the retina from ILM to OLM (Fig. 1b). The pERK1/2-positive cells were found throughout central and peripheral regions of retina, with the exception of retinal folds and detachments, where there was a stark absence of pERK1/2-immunoreactivity (Fig. 1c). Folds and detachments often occur following an excitotoxic insult and likely result from edemic swelling of the retina (Fischer et al. 1998b).
Figure 1.
In response to NMDA-treatment, pERK1/2 accumulates transiently in Müller glia. Vertical sections of the retina were labeled with antibodies to pERK1/2 (green) and the glial marker 2M6 (red; d, f and g) or the transcription factor Sox2 (red; h, j and k). Retinas were obtained from eyes that were injected with saline (a) or 2000 nmol of NMDA (b-o). Retinas were harvested at 1 (b-k), 3 (l and m) and 5 (n and o) days after NMDA-treatment. The translucent yellow lines in panel a indicate the OLM and ILM. The yellow boxes in panels l and m are enlarged 2.5-fold in l′ and m′. Arrows indicate Müller glia that are co-labeled for pERK1/2 and Sox2. The calibration bar (50 μm) in panel b applies to panels a and b, the bar in c applies to c alone, the bar in k applies to e-g and i-k, and the bar in o applies to d,l,m,n and o. Abbreviations: OLM – outer limiting membrane, ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, ILM - inner limiting membrane.
To confirm that pERK1/2-immunoreactivity accumulated in the Müller glia 24hrs after NMDA-treatment, sections were labeled with antibodies to pERK1/2 and the glial marker 2M6. The monoclonal antibody 2M6 is known to selectively label differentiating Müller glia in the embryonic chicken retina (Linser et al. 1997). We found significant overlap of immunoreactivities for 2M6 and pERK1/2 in NMDA-treated retinas (Fig. 1d-g). In addition, we assessed whether pERK1/2-immunoreactivity overlapped with labeling for Sox2, which is in the nuclei of proliferating Müller glia (Hayes et al. 2007). We found a complete overlap of oblong Sox2-positive nuclei with pERK1/2-immunoreactivity in the Müller glia (Fig. 1h-k). The small, round Sox2-positive/pERK1/2-negative nuclei in the proximal INL were those of type-I cholinergic amacrine cells (data not shown).
At 2 days after NMDA-treatment, immunoreactivity for pERK1/2 remained widespread in the Müller glia, similar to that seen at 1 day after NMDA-treatment (data not shown). By contrast, at 3 days after NMDA-treatment, we observed a decrease in pERK1/2-immunolabeling in the Müller glia. The Müller glia that remained immunoreactive for pERK1/2 appeared as clusters of 5-12 cells in central regions of the retina (Fig. 1l), whereas pERK1/2-positive Müller glia in peripheral regions of the retina were not clustered together (Fig. 1m). By 5 days after NMDA-treatment, pERK1/2-immunoreactivity was reduced to levels comparable to those seen in undamaged retinas, with the exception of intense labeling remaining in a few scattered Müller glia in central and peripheral regions of the retina (Fig. 1n and 1o).
Retinal progenitors normally accumulate Egr1 and pCREB
Immediate early genes are often expressed as a result of activated MAPK-signaling (Cahill et al. 1996). In addition, CREB, a bZIP transcription factor, can be activated by different branches of the MAPK-cascade by kinases including p90RSK and MAPKAP kinase-2 (Ribar et al. 2000; Tan et al. 1996; Xing et al. 1998). Thus, pCREB may accumulate and immediate early genes, such as Egr1 and cFos, may be expressed by Müller glia-derived progenitors. However, it remains unknown whether progenitors in the chicken retina normally accumulate cFos, Egr1 or pCREB.
To assess whether retinal progenitors accumulate pERK, pCREB, Egr1 or cFos, we probed for these markers in sections of the far peripheral retina and circumferential marginal zone (CMZ). The CMZ of the chicken retina is known to contain proliferating progenitors that add new neurons to the peripheral edge of the retina throughout post-hatch development (Fischer 2005; Fischer et al. 2002a; Fischer et al. 2005; Fischer and Reh 2000). In addition, at the peripheral edge of the retina there is a gradient of maturation from “early” progenitors through gradually maturing neurons, which allows for examination across all stages of development and differentiation (Fischer et al. 2008a; Ghai et al. 2008).
We found immunoreactivity for pERK1/2 at the peripheral edge of the retina, but this labeling was not in the CMZ progenitors (Fig. 2a). Instead, the pERK1/2 was in the axon terminals of bullwhip cells that are densely clustered at the far peripheral edge of the retina (data not shown). The terminals of the bullwhip cells are known to ramify among the CMZ progenitors and release glucagon to suppress proliferation and equatorial eye growth (Fischer et al. 2005; Fischer et al. 2008b; Fischer et al. 2006). In addition, CMZ progenitors were not immunoreactive for cFos (Fig. 2b). These findings indicate that pERK1/2 and cFos are not normally expressed by progenitors in the CMZ. By contrast, we consistently observed nuclei in the CMZ that were immunoreactive for Egr1 (Fig. 2c). We found Egr1 in the narrow, oblong nuclei of CMZ progenitors that were weakly immunoreactive for Pax6, whereas Egr1 was not observed in the Pax6-positive nuclei of differentiating amacrine cells in the far peripheral INL (Figs. 2e-g). Consistent with these findings, Egr1 was present in the narrow, oblong nuclei of CMZ progenitors that were immunoreactive for Sox2 (Figs. 2h-j). By contrast, Egr1-immunoreactivity was absent from the Sox2-positive nuclei of differentiating Müller glia in the far peripheral retina and absent from the non-pigmented epithelial (NPE) cells of the ciliary body anterior to the CMZ (Figs. 2h-j). Consistent with these findings, Egr1 was present in CMZ progenitors that express n-cadherin (Figs. 2k-m). Egr1-positive/n-cadherin-negative cells were observed toward the neural retina, away from the CMZ, and Egr1-negative/n-cadherin-positive cells were found in the NPE anterior to the CMZ (Figs. 2k-m). By comparison, we found a near-perfect coincidence of Egr1-positive nuclei among the transitin-positive CMZ progenitors (Figs. 2n-p). Transitin, an intermediate filament that is the avian homologue of mammalian nestin, is known to be expressed by CMZ progenitors and transdifferentiating Müller glia (Fischer and Omar 2005). The transitin-positive progenitors were immunoreactive for pCREB (Figs. 2q-s). However, high levels of pCREB-immunoreactivity extended from the NPE cells in the ciliary body, through the CMZ, and into the peripheral edge of the INL (Figs. 2q-s). In summary, CMZ progenitors normally do not contain significant levels of pERK1/2 or cFos, whereas both Egr1 and pCREB are present at high levels.
Figure 2.
Retinal progenitors in the circumferential marginal zone (CMZ) are not immunoreactive for pERK1/2 or cFos, but are positive for pCREB and Egr1. Vertical sections of the far peripheral retina and CMZ were labeled with antibodies to pERK (a), cFos (b), Egr1 (green; d,f,g,i,j,l,m,o and p), pCREB (green; d, r and s), Pax6 (red; e and g), Sox2 (red; h and j), n-cadherin (red; k and m) and/or transitin (red; n,p,q and s). The double-ended arrows indicate the domain of the CMZ at the peripheral edge of the retina. For all micrographs the neural retina is to the left and the non-pigmented epithelium of the ciliary body is to the right. The calibration bar (50 μm) in panel d applies to panels a-d, and the bar in s applies to e-s. Abbreviations: INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Müller glia transiently up-regulate cFos, Egr1 and pCREB in response to acute damage
We next assessed whether cFos was expressed by Müller glia in NMDA-damaged retinas. Although central regions of saline-treated retinas contained little immunoreactivity for cFos (Fig. 3a), we found that cFos was induced in the nuclei of Müller glia within 2 hours of an intraocular injection of 2000 nmol of NMDA (Figs. 3b-d). In addition, cFos was observed in the nuclei of a few cells in the amacrine cell layer of the INL shortly after NMDA-treatment (Figs. 3b-d). Twenty-four hours after NMDA-treatment, cFos was prevalent in the nuclei of 2M6-positive Müller glia and presumptive amacrine cells in the proximal INL (Figs. 3e-h). We confirmed that cFos was expressed in amacrine cells by combining labeling for cFos and the amacrine marker HuC/D (Figs. 3i-k). At 2 days after NMDA-treatment, Müller glia are known to re-enter S-phase of the cell cycle (Fischer 2005; Fischer and Reh 2001a). At this time, levels of cFos in the nuclei of Müller glia were reduced compared to those seen at 24 hours after treatment, and most of the cFos-positive glial nuclei were near the center of the INL in central regions of the retina (Fig. 3l). In peripheral regions of the retina at 2 days after NMDA-treatment, cFos remained apparent in the nuclei of Müller glia, however more of the glial nuclei were de-laminated and had migrated to distal layers of the INL (nuclei indicated by arrows in Fig. 3m). At one and two days after NMDA-treatment, 100% of the Müller glia contained cFos (n=324 cells at day 1; n=381 cells at day 2; Figs. 3n-p). At 3 days after NMDA-treatment, levels of cFos-immunoreactivity were decreased (compare Figs. 3q and 3r) and many of the Müller glia no longer contained detectable levels of labeling (Figs. 3s-v).
Figure 3.
The expression of cFos appears in the nuclei of Müller glia shortly after treatment with NMDA and is gradually decreased in the days following the treatment. Vertical sections of the retina were labeled with antibodies to cFos (green; a, c-e, g-i, k-m, o-r, u and v) and vimentin (red; b and d) HuC/D (red; j and k), 2M6 (red in f, h, n, p; magenta in s and v) or Sox2 (red; t and v). Retinas were obtained at 2 hrs (b-d), 1 day (e-k and q), 2 days (l-p), and 3 days (r-v) after NMDA-treatment. Arrows indicate the nuclei of Müller glia in the inner nuclear layer, small double-arrows indicate cFos-negative Müller glia and arrow-heads indicate nuclei of cFos-positive amacrine cells. Images in panels q (cFos at 1 day after NMDA-treatment) and r (3 days after treatment) were obtained using identical microscope and camera settings to illustrate the time-dependant decline in cFos expression following NMDA-treatment. The calibration bar (50 μm) in panel p applies to panels a-d and n-p, the bar in r applies to e, l, m, q and r, and the bar in v applies to f-k and s-v. Abbreviations: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer.
Since Egr1 is normally expressed by neural progenitors in the post-hatch chicken retina (see Fig. 2), we predicted that Egr1 would be expressed by proliferating Müller glia. Indeed, we found that Egr1 was rapidly expressed by glia after NMDA-treatment. Saline-treated retinas contained low levels of Egr1-immunolabeling. Egr1 was present in a few scattered nuclei in the amacrine cell layer of the INL (Fig. 4a), consistent with previous descriptions (Fischer et al. 1999a). Within 2 hours of treatment with 50 nmol NMDA, numerous Müller glial nuclei in the middle of the INL were immunoreactive for Egr1 (Fig. 4b). Glial expression of Egr1 in response to 50 nmol NMDA subsided to control levels by one day after treatment (data not shown). By comparison, a 2000 nmol dose of NMDA stimulated Egr1 expression in Müller glia within 2 hours after injection (Fig. 4c) and this pattern of expression was maintained at 24 hours after treatment in peripheral regions of the retina (Fig. 4d and e). At one day after NMDA-treatment, glial expression of Egr1 coincided with the accumulation of pERK1/2, and this was more prevalent in peripheral regions of the retina compared to central regions (Figs. 4d-k). Increased numbers of Müller glia are known to transdifferentiate in peripheral regions of the retina (Fischer and Reh 2003). In central regions of the retina approximately 1 in 5 glial cells (17.4 ± 6.3%; n=345) expressed detectable levels of Egr1 (Figs. 4f-h). By comparison, in peripheral regions of the retina the majority (87.6 ± 8.1%; n=249 cells) of the Müller glia expressed Egr1 (Figs. 4i-k). In addition many PKC-positive bipolar cells expressed Egr1 at 1 day after NMDA-treatment (Figs. 4l-n). At 2 days after NMDA-treatment, when Müller glia are known to re-enter S-phase of the cell cycle, we found relatively few Müller glia that were Egr1-positive and these cells were found in peripheral regions of the retina (Figs. 4o-q). At day 3, the Müller glia that continued to express Egr1 were found predominantly in peripheral regions of the retina (Fig. 4r-t), coincident with where Müller glia are known to generate new cells in response to NMDA-treatment (Fischer and Reh 2003).
Figure 4.
The immediate early gene Egr1 is transiently expressed by Müller glia in response to acute retinal damage. Vertical sections of the retina were labeled with antibodies to Egr1 (green) and pERK1/2 (red; f,h,i and k), the bipolar cell marker PKC (l and n), or the glial marker 2M6 (red; o,q,r and s). Retinas were obtained from saline-treated eyes (a) or from eyes that were injected with 50 nmol (b) or 2000 nmol (c-t) of NMDA at P7 and harvested 2 hrs (b and c), 1 day (d-n), 2 days (o-q), or 3 days later (r-t). Photomicrographs were obtained from central (a-c and f-h) and peripheral (d,e and i-t) regions of the retina. The calibration bar (50 μm) in panel e applies to panels a-e, the bar in k applies to f-k, the bar in q applies to o-q, and the bar in t applies to l-n and r-t. Arrows indicate Müller glia with Egr1-positive nuclei, and arrow-heads indicate Müller glia that are negative for Egr1. Abbreviations: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer.
We next sought to examine whether pCREB accumulated in the nuclei of Müller glia in response to acute damage. In undamaged retinas, pCREB-immunoreactivity was detected in the nuclei of photoreceptors in the ONL, cells in the bipolar cell layer of the INL, and a few scattered cells in the amacrine layer of the INL and GCL (Fig. 5a). By comparison, 2 hours after NMDA-treatment, levels of pCREB-immunoreactivity were increased in numerous cells in the INL, including glial nuclei near the center of the INL (Fig. 5b). We confirmed that pCREB was present in bipolar cell nuclei by combining immunolabeling for pCREB with Islet1 (Figs. 5e-g), a transcription factor that is known to be expressed by many bipolar cells in the chick retina (Fischer et al. 2002a; Fischer et al. 2008a). At 1 day after NMDA-treatment, levels of pCREB remained high in the nuclei of presumptive Müller glia and amacrine cells, whereas levels in presumptive bipolar cell nuclei were reduced (Fig. 5c). We confirmed that Müller glia were positive for pCREB by combining labeling for pCREB and Sox2 (Fig. 5d). Levels of pCREB-immunoreactivity remained high in Müller glia at 2 days after NMDA-treatment (Figs. 5h-j). By comparison, after glial cell division at 3 days after NMDA-treatment, pCREB-immunoreactivity was detectable in nearly two-thirds (63.9 ± 10.6%; n=324 cells) of the Sox2-positive Müller glia (Figs. 5k-m). By 4 days after treatment, the levels and distribution of pCREB-immunoreactivity were similar to those seen in control retinas, with some labeling in bipolar cell and photoreceptor nuclei (Figs. 5n-p). At this time, the majority of Müller glia-derived cells that were Sox2-positive contained little or no pCREB-immunoreactivity (Figs. 5n-p).
Figure 5.
pCREB accumulates in Müller glia in response to acute damage. Vertical sections of the retina were labeled with antibodies to pCREB (green) and the transcription factor Islet1 (red; e and g), the glial marker 2M6 (red; h and j) or the transcription factor Sox2 (red; d,k,m,n and p). Retinas were obtained from eyes that were treated with saline (a) or 2000 nmol of NMDA at P7 and harvested 2 hrs (b, e-g), 1 day (c and d), 2 days (h-j), 3 days (k-m) or 4 days (n-p) after NMDA-treatment. The areas indicated by the yellow boxes in panels a, b and c are enlarged approximately 3-fold in panels a′, b′ and c′. Arrows indicate pCREB-positive Müller glial that are immunoreactive for 2M6 or Sox2. In panels e-g the arrow-heads indicate bipolar cells that are labeled for pCREB and Islet1, whereas arrow-heads in k-p indicate Sox2-positive Müller glia nuclei that contain low levels of pCREB. The calibration bar (50 μm) in panel c applies to panels a-c, the bar in j applies d and h-j, and the bar in p applies to e-g and k-p. Abbreviations: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer.
Inhibition of MEK and the FGF-receptor suppresses glial proliferation in response to damage
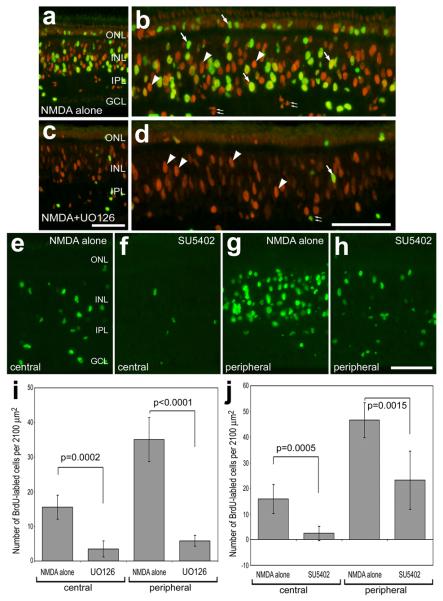
To assess whether MAPK-signaling is required for the proliferation of Müller glia in damaged retinas, we applied small molecule inhibitors after NMDA-treatment and probed for glial proliferation by labeling for BrdU and PCNA. In retinas treated with NMDA alone, we found numerous proliferating Müller glia with nuclei in the INL and ONL that were positive for BrdU and PCNA (Figs. 6a and 6b), consistent with previous reports (Fischer and Reh 2001a). All of the large fusiform nuclei in the INL and ONL that are positive for BrdU and/or PCNA are known to arise from Müller glia in NMDA-damaged retinas (Fischer and Reh 2001a). In eyes treated with the MEK-inhibitor UO126, we found significantly fewer proliferating Müller glia (Figs. 6c, d and i). With NMDA-treatment at P7 or later, the majority of Müller glia that transdifferentiate are found in peripheral regions of the retina, whereas few glia in central retinal regions undergo transdifferentiation (reviewed by Fischer 2005; Fischer and Reh 2003). The proliferation-suppressing affects of UO126 were consistent across central and peripheral regions of the retina (Fig. 6i). Similar to the effects of UO126, we found significantly fewer proliferating Müller glia in retinas treated with the FGF-receptor inhibitor SU5402. These effects were consistent across central and peripheral regions of the retina (Figs. 6e-h and j). These findings indicate that inhibition of MEK or FGF-receptors suppresses the proliferation of Müller glia-derived progenitors in response to acute retinal damage. We failed to find evidence that the UO126 or SU5402 were toxic and induced cell death by using the TUNEL method to detect dying cells that contained fragmented DNA (data not shown).
Figure 6.
Small molecule inhibitors of MEK (UO126) and the FGF-receptor (SU5402) suppress the proliferation of Müller glia in acutely damaged retina. Retinas were obtained from NMDA-treated eyes that were injected with vehicle or inhibitor (1.7 μg/dose UO126 or 0.8 μg/dose SU5402) starting at P7. Inhibitors were injected 6 hours after NMDA at P7 and again at 24 hrs after NMDA on P8. Then 2 μg of BrdU was injected into both eyes at 2 days after NMDA on P10 and retinas were harvested at 3 days after NMDA on P11. Vertical sections of the retina were labeled with antibodies to PCNA (red; a-d) and BrdU (green; a-h). Arrows indicate the nuclei of Müller glia that are immunoreactive for BrdU and PCNA, arrow-heads indicate the nuclei of Müller glia that are immunoreactive for PCNA alone, and small double-arrows indicate the nuclei of proliferating microglia in the IPL. Numbers of BrdU-labeled cells were counted separately in central (a-f) and peripheral (g and h) regions of the retina. The calibration bar (50 μm) in panel c applies to panels a and c, the bar in d applies to b and d, and the bar in h applies to e-h. Significance of difference was assessed by using a Student's t-test. Abbreviations: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer, CMZ – circumferential marginal zone.
Inhibition of MEK and the FGF-receptor results in reduced levels of Egr1 and pCREB
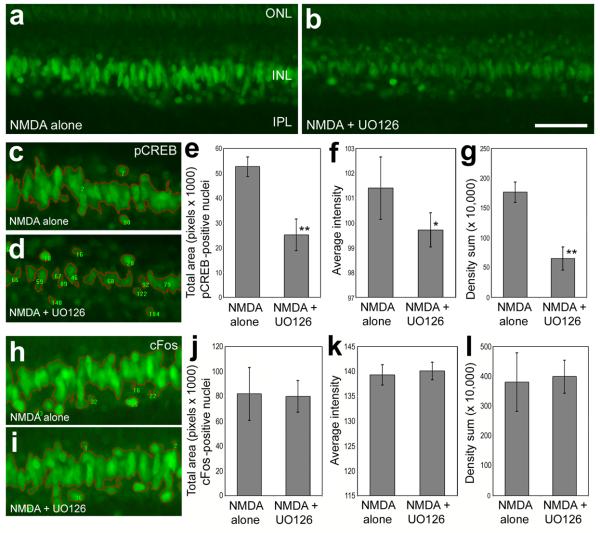
To assess whether the UO126 and SU5402 influenced the downstream targets of MAPK-signaling, we measured levels of immunofluorescence for Egr1, pCREB and cFos in damaged retinas that were treated with inhibitors. We found that UO126 and SU5402 reduced glial expression of Egr1 and inhibited phosphorylation of CREB in NMDA-damaged retinas (Fig. 7). The UO126 or SU5402 were applied with the NMDA at P7 and 20 hours later on P8, and retinas were harvested 4 hours later. There was an apparent reduction in Egr1-immunofluorescence in Müller glia in damaged retinas treated with UO126 (compare Figs. 7a and 7b). We quantified this difference by using ImagePro 6.2, as described in the methods. There was a significant reduction in Egr1-immunofluorescence, predominantly in the nuclei of Müller glia, in damaged retinas treated with UO126 (Figs. 7c-g) or SU5402 (Figs. 7h-l) compared to that measured in retinas treated with NMDA alone. Similarly, UO126 resulted in an apparent decrease in immunoreactivity for pCREB in the INL; diminished pCREB was predominant in the oblong nuclei of Müller glia (compare Figs. 8a and 8b). Measurements indicated significant decreases in pCREB-immunofluorescence in the INL of retinas treated with UO126 (Figs. 8c-g). By comparison, we failed to find a significant decrease in pCREB-immunoreactivity in retinas treated with SU5402 (data not shown). In addition, we failed to find a significant decrease in cFos-immunoreactivity in the INL of retinas treated with UO126 (Figs. 8h-l) or SU5402 (data not shown). Taken together, these findings indicate that UO126 and SU5402 effectively inhibit signaling in the Müller glia and, consequently, reduce glial expression of the immediate early gene Egr1 and reduce phosphorylation of CREB, at least for the UO126.
Figure 8.
A small molecule inhibitor (UO126) of MEK suppresses the accumulation of pCREB in Müller that results from NMDA-treatment, whereas cFos is not affected. Images were obtained using identical camera exposures and microscope illumination settings. Retinas were processed for immunolabeling 24 hours after treatment with 2000 nmol of NMDA alone or NMDA + 1.7 μg MEK inhibitor (UO126). As described in the Methods, ImagePro 6.2 was used to obtain measurements of total area for pixel intensities >72 for pCREB and >100 for cFos (0 = black, 255 = saturated green), average pixel intensity, and the density sum. The small numbers and red outlines in panels c, d, h and i indicate the areas designed by ImagePro 6.2 to each object that met the threshold criteria. Means and standard deviations are displayed in histograms for total area (e and j), average intensity (f and k), and density sum (g and l) for areas with pixels above threshold. Significance of difference (*p=0.001, **p<0.0005 or ***p<0.0001) was determined by using a two-tailed, unpaired Student's t-test. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer.
Western blots confirm the findings of immunofluorescence
To verify the findings of the quantitative immunofluorescence, we used Western blots and densitometry. In parallel to the observations of immunofluorescence, densitometry of Western blots indicated that levels of pERK, pCREB, cFos and Egr1 were increased at 1 day after NMDA-treated and were decreased by 3 days after treatment, with the exception of pCREB which remained relatively high (Figs. 9a and b). Since the Western blots represent protein levels across all retinal cell types, the levels of pCREB are elevated at day 3 likely because of persistent accumulations in the ONL (see Fig. 5). The GAPDH loading controls were decreased by 3 days after treatment likely because of the loss of many inner retinal neurons.
Figure 9.
Western blot analysis indicates that levels of pERK, Egr1, pCREB and cFos are elevated in the retina after NMDA-treatment, and these increases can be inhibited by treatment with UO126 or SU5402. Protein extracts obtained from control retinas and from NMDA-treated retinas at 1 and 3 days after treatment (a and b), and from NMDA-damaged retinas that were treated with UO126 or SU5402 (c and d). Densitometry was performed using ImagePro6.2 by summating the pixel intensities for each band and standardizing the summated intensities to the loading control (GAPDH) for each experimental condition (b and d).
In agreement with the findings of the quantitative immunofluorescence, Western blot analysis indicated that levels of pERK, Egr1 and pCREB, but not cFos, were reduced in NMDA-damaged retinas that were treated with the MEK inhibitor UO126 (Figs. 9c and d). In addition, Western blot analysis indicated that levels of pERK, Egr1 and pCREB were reduced in retinas treated with the FGF-receptor inhibitor SU5402 (Figs. 9c and d). By comparison, we failed to observe a decrease in pCREB in SU5402-treated Müller glia by using quantitative immunofluorescence. This likely occurred because the Western blot analysis measures protein levels from all retinal cell types, unlike the quantitative immunofluorescence which allows for measurements of relative protein levels in discrete populations of cells.
Discussion
We report that MAPK-signaling is transiently active in Müller glia shortly after an excitotoxic retinal insult. In response to acute retinal damage, the manifestations of MAPK activation in Müller glia include phosphorylation of ERK1/2 and CREB, and expression of the immediate early genes cFos and Egr1. Subsequent to the activation of the MAPK pathway, the Müller glia de-differentiate, proliferate and become progenitor-like cells in damaged retinas. Consistent with the hypothesis that MAPK-signaling is required for the transdifferentiation of Müller glia, we find that inhibitors of MEK and FGF-receptors reduce numbers of glia that proliferate in response to retinal damage. Further, we find that the MEK inhibitor suppresses the accumulation of Egr1 and pCREB in Müller glia, whereas the FGF-receptor inhibitor suppresses the expression of Egr1 alone. Since Egr1 is normally expressed by retinal progenitors in the CMZ, our findings imply that MAPK-induced expression of Egr1 in Müller glia is an important step in transdifferentiation. The absence of pERK1/2 in the CMZ is consistent with reports suggesting that the post-hatch retinal progenitors are normally quiescent because of low microenvironmental levels of growth factors, including those that activate MAPK-signaling (Fischer 2005; Fischer et al. 2002a; Fischer and Reh 2000).
Sustained MAPK-signaling and the consequential expression of Egr1 may be required to stimulate the proliferation of Müller glia in damaged retinas. Consistent with this hypothesis, we found that a low dose of NMDA, which fails to stimulate glial proliferation, results in short-lived expression of Egr1 (a read-out of MAPK-signaling) in the Müller glia. Further, reduced glial expression of Egr1, resulting from inhibition of MEK or FGF-receptors shortly after NMDA-treatment, was coincident with suppressed proliferation. In addition, intraocular injections of insulin and FGF2, that presumably activate the MAPK pathway, induce the transdifferentiation of Müller glia without damage to the retina (Fischer et al. 2002b). Notably, no less than 3 consecutive daily injections of insulin and FGF2 are required to stimulate glial transdifferentiation (Fischer et al. 2002b). Taken together, these findings suggest that the activation of the MAPK pathway in Müller glia must be sustained for several days to stimulate proliferation, and perhaps the early steps in transdifferentiation.
Glial expression of cFos may be related to reactivity rather than the proliferation of Müller glia in damage retinas. Levels of cFos-immunoreactivity in Müller glia were not decreased by inhibitors of FGF-receptors or MEK, whereas glial proliferation was suppressed by the inhibitors. In other words, glial expression of cFos occurs independent of the activation of FGF-receptors or MEK and is not symptomatic of proliferation. NMDA may act directly at glutamate receptors that are expressed by the Müller glia (Lamas et al. 2005) to promote the expression of cFos and phosphorylation of CREB (Lamas et al. 2005; Lamas et al. 2007). These findings are consistent with our observations that inhibition of MEK and FGF-receptors fail to suppress the expression of cFos, and that the phosphorylation of CREB is partially prevented by MEK inhibitor and is not prevented by FGF-receptor inhibitor in NMDA-treated retinas. Thus, it seems likely that glial expression of cFos resulted from the activation of NMDA receptors rather than the activation of receptor tyrosine kinases and the MAPK pathway.
Our findings that cFos and pERK accumulate in Müller glia in response to NMDA are consistent with observations in the rodent retina. In the mouse retina, Nakazawa and colleagues (Nakazawa et al. 2008) demonstrated that pERK and cFos accumulate in Müller glia within 1 hour of treatment with NMDA and the activity of ERK1 promotes glial-mediated protection of retinal neurons from damage. In addition, these findings are consistent with a report demonstrating MAPK-signaling in Müller glia in the rat retina following an ischemic insult (Roth et al. 2003). However, the link between active MAPK-signaling, downstream immediate early genes and Müller glial proliferation has not been established previously.
Our findings are consistent with the hypothesis that FGF2 stimulates the proliferation of Müller glia. For example, FGF2 has been shown to stimulate the proliferation of modest numbers of Müller glia in the rabbit retina (Lewis et al. 1992), human Müller glial cell lines (Hollborn et al. 2004), and in the intact chicken retina (Fischer et al. 2002b). MAPK-signaling in damaged retinas and the proliferation of Müller glia may be activated by factors in addition to FGF2. In the rodent retina, for example, light-induced retinal damage stimulates the Müller glia to up-regulate expression of the EGF-receptor and, subsequently, proliferate in response to exogenous EGF (Close et al. 2006). EGF is capable of acting through the ERK1/2 pathway, but it remains unknown whether signaling through EGF-receptors influences glial proliferation in the chick retina. Several recent reports have indicated that non-MAPK pathways stimulate the proliferation of Müller glia in damaged retinas. For example, Wnt-signaling has been shown to stimulate the proliferation and neuronal regeneration from Müller glia in NMDA-damaged rodent retina (Osakada et al. 2007). However, it remains unknown whether Wnt-signaling influences Müller glia in the chicken retina, and whether Wnts can stimulate glial transdifferentiation in the absence of damage. In addition, there is a brief report that Sonic Hedgehog stimulates the proliferation of Müller glia-derived cells in damaged rodent retina (Wan et al. 2007). Finally, a recent report has demonstrated that the activation of the Notch-pathway promotes the proliferation of Müller glia in NMDA-damaged chick retinas (Hayes et al. 2007). Further studies are required to determine whether these different signaling pathways work in parallel or in serial to stimulate the transdifferentiation of Müller glia.
To further complicate the mechanisms that control retinal regeneration from Müller glia, several different signaling pathways may suppress the proliferation and transdifferentiation of Müller glia. For example, CNTF has been shown to suppress the proliferation of Müller glia in response to NMDA-induced retinal damage (Fischer et al. 2004b). Interestingly, CNTF and JAK/STAT-signaling have been shown to promote the differentiation of Müller glia in the postnatal rodent retina (Goureau et al. 2004). Similarly, TGF-β2 has been shown to suppress the proliferation of late-stage progenitors and differentiating Müller glia during early stages of postnatal retinal development in the rodent (Close et al. 2005). Collectively, these findings suggest that the proliferation and transdifferentiation of Müller glia are regulated by several different signaling pathways. These different pathways can act to promote or suppress glial transdiffferentiation, and the decision of Müller glia to proliferate is likely determined by the summation of “push-pull” input for the different signal transduction cascades.
The threshold stimuli and transcriptional factors that trigger the proliferation of Müller glia remain uncertain. It is possible that Egr1 acts as transcription trigger that activates the proliferation of Müller glia. We find that Egr1 is expressed by Müller glia before entry into S-phase, and inhibitors that suppress glial proliferation also suppress the expression of Egr1. Alternatively, it is possible that the pro-neural bHLH transcription factor ash1/ascl1a acts as a trigger for glial transdifferentiation. In the chicken retina Cash1 is expressed by Müller glia entering S-phase 2 days after NMDA-treatment (Fischer and Reh 2001a). Knock-down of ascl1a in the zebrafish retina prevents the transdifferentiation and neuronal regeneration from Müller glia (Fausett et al. 2008). The influence of FGF, CNTF, Wnt, TGFβ, Notch1 and Shh-signaling pathways on glial expression of ash1 and Egr1 will be the focus of future studies.
Conclusions
We conclude that activation of FGF-receptors and the ERK1/2 pathway are required for Müller glia to re-enter the cell cycle and become progenitor-like cells in response to acute retinal damage. In addition, we conclude that retinal progenitors normally contain Egr1 and pCREB, and these factors may be required to drive the Müller glia back into the cell cycle. By contrast, glial expression of cFos occurs independent of the activation of FGF-receptors or ERK1/2, and this immediate early gene likely is not involved in glial proliferation or transdifferentiation.
Supplementary Material
Acknowledgements
We thank H.M. El-Hodiri, J. Stanke, and E.R. Ritchey for comments that contributed to the final form of this paper. We thank Drs. P. Henion and P. Linser for providing antibodies to transitin and 2M6, respectively. The vimentin, n-cadherin, BrdU, Islet1 and Pax6 antibodies developed by Drs J.R. Sanes, K.A. Knudsen, S.J. Kaufman, T. Jessell and A. Kawakami, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. This work was supported by grants from the National Institutes of Health, National Eye Institute to AJF (RO1 EY016043) and supporting WT (T35 EY007151).
References
- Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27(26):7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH. Genomic analysis of mouse retinal development. PLoS Biol. 2004;2(9):E247. doi: 10.1371/journal.pbio.0020247. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill MA, Janknecht R, Nordheim A. Signalling pathways: jack of all cascades. Curr Biol. 1996;6(1):16–9. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- Cao W, Li F, Steinberg RH, Lavail MM. Development of normal and injury-induced gene expression of aFGF, bFGF, CNTF, BDNF, GFAP and IGF-I in the rat retina. Exp Eye Res. 2001;72(5):591–604. doi: 10.1006/exer.2001.0990. [DOI] [PubMed] [Google Scholar]
- Close JL, Gumuscu B, Reh TA. Retinal neurons regulate proliferation of postnatal progenitors and Muller glia in the rat retina via TGF beta signaling. Development. 2005;132(13):3015–26. doi: 10.1242/dev.01882. [DOI] [PubMed] [Google Scholar]
- Close JL, Liu J, Gumuscu B, Reh TA. Epidermal growth factor receptor expression regulates proliferation in the postnatal rat retina. Glia. 2006;54(2):94–104. doi: 10.1002/glia.20361. [DOI] [PubMed] [Google Scholar]
- Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26(23):6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausett BV, Gumerson JD, Goldman D. The proneural basic helix-loop-helix gene ascl1a is required for retina regeneration. J Neurosci. 2008;28(5):1109–17. doi: 10.1523/JNEUROSCI.4853-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chicken retina. Progress in Retinal and Eye Research. 2004 doi: 10.1016/j.preteyeres.2004.07.003. in press. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24(2):161–82. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002a;129(9):2283–91. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Foster S, Scott MA, Sherwood P. Transient expression of LIM-domain transcription factors is coincident with delayed maturation of photoreceptors in the chicken retina. J Comp Neurol. 2008a;506(4):584–603. doi: 10.1002/cne.21578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002b;22(21):9387–98. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, McGuire JJ, Schaeffel F, Stell WK. Light- and focus-dependent expression of the transcription factor ZENK in the chick retina. Nat Neurosci. 1999a;2(8):706–12. doi: 10.1038/11167. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998a;392(3):273–84. [PubMed] [Google Scholar]
- Fischer AJ, Morgan IG, Stell WK. Colchicine causes excessive ocular growth and myopia in chicks. Vision Res. 1999b;39(4):685–97. doi: 10.1016/s0042-6989(98)00178-3. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G. Transitin, a nestin-related intermediate filament, is expressed by neural progenitors and can be induced in Muller glia in the chicken retina. J Comp Neurol. 2005;484(1):1–14. doi: 10.1002/cne.20406. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin and FGF2 in the absence of damage. Molecular Vision. 2004a;10:973–986. [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Walton NA, Verrill TA, Unson CG. Glucagon-expressing neurons within the retina regulate the proliferation of neural progenitors in the circumferential marginal zone of the avian eye. J Neurosci. 2005;25(44):10157–66. doi: 10.1523/JNEUROSCI.3247-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Muller glia are a potential source of neural regeneration in the postnatal chicken retina. Nat Neurosci. 2001a;4(3):247–52. doi: 10.1038/85090. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Transdifferentiation of pigmented epithelial cells: a source of retinal stem cells? Dev Neurosci. 2001b;23(4-5):268–76. doi: 10.1159/000048710. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Potential of Muller glia to become neurogenic retinal progenitor cells. Glia. 2003;43(1):70–6. doi: 10.1002/glia.10218. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Ritchey ER, Scott MA, Wynne A. Bullwhip neurons in the retina regulate the size and shape of the eye. Dev Biol. 2008b doi: 10.1016/j.ydbio.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Schmidt M, Omar G, Reh TA. BMP4 and CNTF are neuroprotective and suppress damage-induced proliferation of Muller glia in the retina. Mol Cell Neurosci. 2004b;27(4):531–42. doi: 10.1016/j.mcn.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998b;393(1):1–15. [PubMed] [Google Scholar]
- Fischer AJ, Skorupa D, Schonberg DL, Walton NA. Characterization of glucagon-expressing neurons in the chicken retina. J Comp Neurol. 2006;496(4):479–94. doi: 10.1002/cne.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405(1):1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2008;(1192):76–89. doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goureau O, Rhee KD, Yang XJ. Ciliary Neurotrophic Factor Promotes Muller Glia Differentiation from the Postnatal Retinal Progenitor Pool. Dev Neurosci. 2004;26(5-6):359–370. doi: 10.1159/000082278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9(5):544–53. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Hayes S, Nelson BR, Buckingham B, Reh TA. Notch signaling regulates regeneration in the avian retina. Dev Biol. 2007;312(1):300–11. doi: 10.1016/j.ydbio.2007.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollborn M, Jahn K, Limb GA, Kohen L, Wiedemann P, Bringmann A. Characterization of the basic fibroblast growth factor-evoked proliferation of the human Muller cell line, MIO-M1. Graefes Arch Clin Exp Ophthalmol. 2004;242(5):414–22. doi: 10.1007/s00417-004-0879-x. [DOI] [PubMed] [Google Scholar]
- Kostyk SK, D'Amore PA, Herman IM, Wagner JA. Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci. 1994;14(3 Pt 2):1441–9. doi: 10.1523/JNEUROSCI.14-03-01441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas M, Lee-Rivera I, Lopez-Colome AM. Cell-specific expression of N-methyl-D-aspartate receptor subunits in Muller glia and neurons from the chick retina. Invest Ophthalmol Vis Sci. 2005;46(10):3570–7. doi: 10.1167/iovs.04-1398. [DOI] [PubMed] [Google Scholar]
- Lamas M, Lee-Rivera I, Ramirez M, Lopez-Colome AM. D-serine regulates CREB phosphorylation induced by NMDA receptor activation in Muller glia from the retina. Neurosci Lett. 2007;427(1):55–60. doi: 10.1016/j.neulet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Erickson PA, Guerin CJ, Anderson DH, Fisher SK. Basic fibroblast growth factor: a potential regulator of proliferation and intermediate filament expression in the retina. J Neurosci. 1992;12(10):3968–78. doi: 10.1523/JNEUROSCI.12-10-03968.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser PJ, Schlosshauer B, Galileo DS, Buzzi WR, Lewis RC. Late proliferation of retinal Muller cell progenitors facilitates preferential targeting with retroviral vectors in vitro. Dev Genet. 1997;20(3):186–96. doi: 10.1002/(SICI)1520-6408(1997)20:3<186::AID-DVG2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Shimura M, Ryu M, Nishida K, Pages G, Pouyssegur J, Endo S. ERK1 plays a critical protective role against N-methyl-D-aspartate-induced retinal injury. J Neurosci Res. 2008;86(1):136–44. doi: 10.1002/jnr.21472. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101(37):13654–9. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ooto S, Akagi T, Mandai M, Akaike A, Takahashi M. Wnt signaling promotes regeneration in the retina of adult mammals. J Neurosci. 2007;27(15):4210–9. doi: 10.1523/JNEUROSCI.4193-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58(5):296–305. doi: 10.1159/000057571. [DOI] [PubMed] [Google Scholar]
- Ribar TJ, Rodriguiz RM, Khiroug L, Wetsel WC, Augustine GJ, Means AR. Cerebellar defects in Ca2+/calmodulin kinase IV-deficient mice. J Neurosci. 2000;20(22):RC107. doi: 10.1523/JNEUROSCI.20-22-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Shaikh AR, Hennelly MM, Li Q, Bindokas V, Graham CE. Mitogen-activated protein kinases and retinal ischemia. Invest Ophthalmol Vis Sci. 2003;44(12):5383–95. doi: 10.1167/iovs.03-0451. [DOI] [PubMed] [Google Scholar]
- Tan Y, Rouse J, Zhang A, Cariati S, Cohen P, Comb MJ. FGF and stress regulate CREB and ATF-1 via a pathway involving p38 MAP kinase and MAPKAP kinase-2. Embo J. 1996;15(17):4629–42. [PMC free article] [PubMed] [Google Scholar]
- Valter K, Maslim J, Bowers F, Stone J. Photoreceptor dystrophy in the RCS rat: roles of oxygen, debris, and bFGF. Invest Ophthalmol Vis Sci. 1998;39(12):2427–42. [PubMed] [Google Scholar]
- Walsh N, Valter K, Stone J. Cellular and subcellular patterns of expression of bFGF and CNTF in the normal and light stressed adult rat retina. Exp Eye Res. 2001;72(5):495–501. doi: 10.1006/exer.2000.0984. [DOI] [PubMed] [Google Scholar]
- Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363(2):347–54. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- Wen R, Song Y, Cheng T, Matthes MT, Yasumura D, LaVail MM, Steinberg RH. Injury-induced upregulation of bFGF and CNTF mRNAS in the rat retina. J Neurosci. 1995;15(11):7377–85. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18(4):1946–55. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.