Abstract
In the retina of warm-blooded vertebrates, photoreceptors are specified many days before the onset of synaptogenesis and the expression of photopigments. The factors that regulate the maturation of photoreceptors in the developing retina remain unknown. We report here that photoreceptors transiently express LIM-domain transcription factors during the development of the chicken retina. We examined the differentiation of photoreceptors through the normal course of embryonic development and at the far periphery of the postnatal retina, where the differentiation of photoreceptors is slowed and persists across a spatial gradient. In the embryonic retina, we find visinin-positive photoreceptors that transiently express Islet2 and Lim3 starting at E8 and ending around E15, but persisting in far peripheral regions of the retina through the first 2 weeks of postnatal development. During early stages of photoreceptor maturation, there is coincident and transient expression of the LIM-domain factors with axonin1, a cell surface glycoprotein that is a member of the immunoglobulin super family. Coincident with the down-regulation of Islet2 and Lim3, we find the up-regulation of calbindin, red/green opsin, rhodopsin and a synaptic marker in the OPL (dystrophin). In the periphery of the postnatal retina, photoreceptors that express Islet2, Lim3 and axonin1 do not overlap with photoreceptors that express calbindin, red/geen opsin, rhodopsin, and dystrophin. We propose that Islet2 and Lim3 may promote the expression of genes that are involved in the early stages of differentiation, but may suppress the expression of genes that are required in the mature photoreceptors.
Keywords: Islet2, Lim3, axonin1, retina, photoreceptor
Introduction
The differentiation of photoreceptors involves many different processes that are ordered in space and time (Cornish et al., 2004; Cornish et al., 2005; Hendrickson and Hicks, 2002; Sears et al., 2000). Photoreceptor differentiation involves many complex cellular functions including fate specification, axon extension, formation of specialized synapses with bipolar and horizontal cells, formation of outer segments, assembly of disks, expression of photo-transduction machinery, and establishing interactions with retinal pigmented epithelium (RPE) cells. These developmental processes are regulated by external and internal cues (Cayouette et al., 2006). Much of the internal control over the development of photoreceptors is elicited by transcription factors that activate or suppress the expression of target genes. Despite differences in the numbers, types and distribution of photoreceptors, these cells develop via similar mechanisms in different vertebrate species. The genes that control the production and differentiation of photoreceptors are conserved across vertebrate species. The development of photoreceptors is driven by a set of transcriptional regulators that are known to include Crx, Nrl, Nr2e3, and NeuroD (reviewed by Adler and Raymond, 2007; Cepko, 1999; Morrow et al., 1998). In the retinas of warm-blooded vertebrates, Crx, Nrl, Nr2e3 and NeuroD are expressed either during fate specification or shortly after the prospective photoreceptors have exited the cell cycle.
In many warm-blooded vertebrates, the full differentiation of the photoreceptors is delayed for many days after terminal mitosis. For example, during the development of the chicken retina the expression of opsins is delayed by about 8-10 days after the terminal mitosis (Bruhn and Cepko, 1996; Fischer et al., 2007b). In central regions of the retina, most of the rod and cone photoreceptors are generated between embryonic day 4 (E4) and E6 (Prada et al., 1991). The differentiation of cone photoreceptors may begin as early as E6 with the onset of visinin expression, whereas red and green opsins are first expressed in central retina at about E14, rhodopsin at E15, and blue and violet opsins at E16 (Bradford et al., 2005; Bruhn and Cepko, 1996). At the time of hatching, between E21 and E22, the chicks are able to see, indicating that the retina contains mature, functional photoreceptors at this time.
Transcription factors that are likely to be involved in the maturation of retinal neurons may include members of the LIM-domain family of transcriptional regulators. For examples, the LIM-domain transcription factors, Islet1, Islet2, and Lim3, have been identified in the developing spinal cord. These factors are expressed by subclasses of motor neurons that segregate into columns in the spinal cord and produce axons that follow distinct pathways (Tsuchida et al., 1994). Islet1 is a LIM-domain homeotic transcription factor that is best known for its ability to regulate insulin production in the pancreas, but also has many important roles in the development of the central nervous system (Cai et al., 2003; Tsuchida et al., 1994). In the retina, Islet1 is known to be expressed by ganglion cells, cholinergic amacrine cells, many bipolar cells and a subset of horizontal cells early during development (Edqvist et al., 2006; Galli-Resta et al., 1997). The expression of Islet1 is maintained by these cells in the mature retina (Fischer et al., 2002a; Fischer and Reh, 2003). Nothing is known about the functions of Islet1 in retinal neurons; Islet1 null mutations in mice are lethal with the embryos dying at E10.5 (Cai et al., 2003). Islet2 is another LIM-domain homeotic transcription factor that is known to be expressed by ganglion cells in the retina. Loss of function mutations in Islet2 result in aberrant formation of visceral motor neurons in the spinal cord (Thaler et al., 2004) and path-finding deficits in retinal ganglion cells (Pak et al., 2004). It remains unknown whether Islet2 contributes to aspects of retinal development in addition to the guidance of retinal ganglion cell axons. Another LIM-domain transcription factor that is expressed in the retina is Lim3, also known as Lim Homeobox Gene 3 (Lhx3). Lim3 is normally expressed by post-mitotic bipolar cells in the developing retina (Edqvist and Hallbook, 2004). Lim3 is known to participate in the specification of motor neurons and interneurons in the developing spinal cord (Thaler et al., 2002), whereas nothing is currently known about the roles of Lim3 in the retina. Despite the demonstration of important roles of LIM-domain transcription factors in the developing spinal cord, very little is known about the roles of these factors in the retina. This paper describes the transient expression of LIM-domain transcription factors in differentiating photoreceptors and implicates these factors in maintaining photoreceptors in an immature state.
Methods and Materials
Animals
The use of animals in these experiments was in accordance with the guidelines established by the National Institutes of Health and the Ohio State University. Fertilized eggs and newly hatched White Leghorn chickens (Gallus gallus domesticus) were obtained from the Department of Animal Sciences at the Ohio State University. Chicks were housed in a stainless steel brooder at about 30°C, kept on a cycle of 12 hours light, 12 hours dark (lights on at 7:00 am), and received water and Purinatm chick starter ad libitum.
Reverse transcriptase PCR
At E14 and P7 retinas from 4 chickens were pooled for each time-point and placed in 3.0 ml of Trizol Reagent (Invitrogen). Total RNA was isolated according to the Trizol protocol and re-suspended in 50 μl RNAse-free water. Genomic DNA was removed by using the DNA FREE kit (Ambion). cDNA was synthesized from mRNA by using Superscripttm III First Strand Synthesis System (Invitrogen) and oligo dT primers according to the manufacturer's protocol. Control reactions were performed using all components with the exception of the reverse transcriptase to exclude the possibility that primers were amplifying genomic DNA.
PCR primers were designed by using the web-based program Primer 3 from the Whitehead Institute for Biomedical Research (http://www-genome.wi.mit.edu/cgi-bin/primer/primer3_www.cgi). Primer sequences are as follows: Islet1 forward = 5′ CGC CTG ATT TCC CTA TGT GT 3′, Islet1 reverse = 5′ CGT ATC TGG GAG CTG AGA GG 3′, Islet2 forward = 5′ CCT ACT GCA AGC GGG ACTGCT GCT TCA TGA GGA TGG AC 3′, Islet2 reverse = 5′ GAC TCG GAG AAG GAG ACC AG 3′, Lim3 forward = 5′ CTG GTG TGC AAG GCT GAC TA 3′, and Lim3 reverse 5′ AGG TCG TGG TAC TGG TCC TG 3′. Predicted product sizes (in base pairs) were 981 (Islet1), 531 (Islet2), and 515 (Lim3). PCR reactions were performed by using standard protocols and an Eppendorf thermal cycler. The cDNA for Islet2 is very GC-rich (∼76%). Accordingly, the PCR reactions with Islet2 primers were added with 2 mM betaine (Sigma) to overcome the high GC-content. PCR products were run on an agarose gel to verify the predicted product sizes.
TOPO cloning
PCR products were produced with Platinumtm Taq polymerase (Invitrogen), run on an agarose gel, extracted and purified by using Qiagen's Qiaex II kit according to the manufacturer's instructions. TOPO cloning was performed using Invitrogen's TOPO TAtm Cloning Kit and the pCR–II vector according to manufacturer's instructions.
Tissue dissection, fixation and sectioning
Tissues were fixed, cryosectioned and immunolabeled as described elsewhere (Fischer et al., 1998; Fischer and Stell, 1999). In short, enucleated eyes were hemisected equatorially and the gel vitreous removed from the posterior eye cup. Eye cups were fixed in 4% paraformaldehyde plus 3% sucrose in 0.1 M phosphate buffer, pH 7.4, for 30 min at 20°C. Fixed tissues were washed three times in PBS (phosphate-buffered saline; 0.05 M sodium phosphate, 195 mM NaCl, pH 7.4), cryoprotected in PBS plus 30% sucrose, immersed in embedding medium (OCT-compound; Tissue-Tek), and freeze-mounted onto sectioning blocks. Vertical sections, nominally 12 μm thick, were cut consistently from the posterior pole of the eye in the nasotemporal (mid-horizontal) plane, and thaw-mounted onto SuperFrost Plustm slides (Fisher Scientific). Sections were air-dried and stored at -20°C until use.
In situ hybridization
Standard procedures were used for in situ hybridization, as described elsewhere (Fischer et al., 2002a; Fischer et al., 2004a). Digoxigenin-labeled riboprobes were synthesized by using a kit provided by Roche, and stored at -80°C until use. Embryonic (E12) eyes were dissected in RNase-free Hanks Balanced Salt Solution (HBSS), fixed overnight at 4°C in 4% paraformaldehyde (PFA) buffered in 0.1 M dibasic sodium phosphate (pH 7.4), and embedded in OCT-compound. Cryosections were processed for in situ hybridization as described previously (Fischer et al., 2002a; Fischer et al., 2004a). In short, slides were warmed to room temperature, sections ringed using a grease pen, and endogenous phosphatases inactivated by washing in 0.2 M HCl for 15 min. Sections were then treated with 0.5 ug/ml of Protease K for 10 min at 37°C, washed in PBS in DEPC-treated H20, and fixed in 4% PFA for 15 min. Sections were soaked in 0.25% acetic acid for 15 min, washed in DEPC-treated H20, and then incubated at 60°C with a hybridization solution (200-500ng of riboprobe, 50% formamide, 4× SSC, 10× Denhardt's solution, 500 μg of salmon sperm DNA and torula RNA). Post-hybridization treatment consisted of washing in decreasing concentrations of standard sodium citrate (SSC) at 60°C followed by a RNAse A digestion for 20 minutes at 37°C. Sections were washed at room temperature in a MABT solution (0.05M maleic acid buffer, 0.1% Tween-20) and incubated overnight at 4°C with Fab fragments raised to dioxygenin (DIG) that were conjugated to alkaline phosphatase (anti-DIG-AP; Roche) plus 10% normal goat serum, 10 mM levamisole, and 10 mM glycine in MABT. NBT/BCIP (BioChemika) in 0.1 M NaCl, 0.1 M tris-HCl pH 9.5, 0.05 M MgCl2 and 0.01% Tween-20 was used to precipitate chromophore from the anti-DIG-AP.
Immunocytochemistry
Retinal sections were immunolabeled as described elsewhere (Fischer et al., 1998; Fischer and Stell, 1999). In short, sections were ringed with rubber cement, washed three times in PBS, covered with primary antibody solution (200 μl of antiserum diluted in PBS plus 5% normal goat serum, 0.2% Triton X-100, and 0.01% NaN3), and incubated for about 24 hours at 20°C in a humidified chamber. The slides were then washed three times in PBS, covered with secondary antibody solution, and incubated for at least 1 hour at 20°C in a humidified chamber. Finally, the slides were washed three times in PBS, the rubber cement removed, and coverglass mounted on the slides with 4:1 (v:v) glycerol:water.
Working dilutions and sources of antibodies used in this study included the following. (i) Two different mouse monoclonal antibodies to Islet1 that were both raised to the C-terminus (amino acids 247-349) of rat Islet1 and used at 1:50 (40.2D6 and 39.3F7; Developmental Studies Hybridoma Bank – DSHB; University of Iowa). The 40.2D6 monclonal antibody is known to recognize both Islet1 and Islet2, and has a well-established pattern of labeling in the chicken retina (Fischer et al., 2002a; Fischer et al., 2007a). By comparison, the 39.3F7 monoclonal antibody recognizes Islet1 alone (see results). (ii) Mouse anti-Islet2 was raised to recombinant full-length chicken Islet2 fused to GST and used at 1:50 (51.4H9; DSHB). In the chicken retina, the monoclonal antibody to Islet2 is known to selectively label a subset of ganglion cells (Edqvist et al., 2006). (iii) Mouse anti-Lim3 was raised to recombinant full-length murine Lim3 fused to GST and used at 1:50 (67.4E12; DSHB). In the chicken retina, the monoclonal antibody to Lim3 is known to selectively label the nuclei of bipolar cells (Edqvist and Hallbook, 2004; Edqvist et al., 2006). (iv) Mouse anti-visinin was raised to purified bovine visinin and used at 1:100 (7G4; DSHB). In the chicken retina, this monoclonal antibody is known have specificity for visinin which is expressed by rod and cone photoreceptors (Fischer et al., 2007a; Fischer et al., 2004b; Toy et al., 2002). (v) Mouse anti-calbindin was raised to calbindin D28k purified from chicken gut and used at 1:400 (300; Swant Immunochemicals; Bellinzona, Switzerland). In the chicken retina, antibodies to calbindin are known to label a few amacrine, bipolar and ganglion cells, as well as cone photoreceptors including their axon terminals in the outermost stratum of the OPL (Ellis et al., 1991; Fischer and Reh, 2000; Pasteels et al., 1990; Rogers, 1989). (vi) Mouse anti-Inter Photoreceptor Retinoid Binding Protein (IRBP) was raised to purified bovine IRBP and used at 1:1000 (F7; Dr. J. Saari; University of Washington). This monoclonal antibody is known to have specificity for IRBP which occupies the intercellular spaces between the outer-segments of photoreceptors in the chicken retina (Fischer et al., 1999). (vii) Rabbit anti-axonin1 was raised to purified chicken axonin1 and used at 1:1000 (Dr. E. Stoeckli; University of Zurich). The polyclonal antibodies to axonin1 are known to selectively label differentiating neurons in the embryonic chick spinal cord (Stoeckli and Landmesser, 1995) and retina (Drenhaus et al., 2004). Mouse anti-axonin1 (TAG-1) was raised to E5 chick spinal cord membranes and used at 1:300 (23.4-5; DSHB). This monoclonal is known to recognize axonin1 and produced labeling in the chick retina that is identical to that observed with the polyclonal to axonin1 (see results) and match patterns of labeling produced from in situ hybridization (Morino et al., 1996). (viii) Rabbit anti-red/green opsin was raised to recombinant human red/green opsin and used at 1:400 (AB5405; Chemicon; Temecula, CA). In the chicken retina, the polyclonal antibody to red/green opsin is known to label the outer-segments and ellipsoids of cone photoreceptors (Fischer et al., 2007a). (ix) Mouse anti-rhodopsin was raised to purified bovine rhodopsin and used at 1:200 (rho4D2; Dr. R. Molday; University of British Columbia). In the chicken retina, the monoclonal antibody to rhodopsin is known to selectively label the outer-segments of rod photoreceptors (Fischer et al., 2007a; Fuhrmann et al., 1998; Montiani-Ferreira et al., 2005; Xie and Adler, 2000). (x) Mouse anti-dystrophin was raised to recombinant human dystrophin fragment encoded by exons 45-46 and used at 1:50 (MANDYS1 clone 3B7; DSHB). (xi) Mouse anti-CASK-1 was raised to amino acids 318-415 of rat CASK/Lin2 and used at 1:50 (K56A/50.1; NeuroMab; Davis, CA). The specificity of the monoclonal to CASK-1 has been determined by western blot analysis (Khanna et al., 2006). (xii) Mouse anti-PSD-95 was raised to amino acids 77-299 of human PSD-95/SAP-90 and used at 1:50 (K28/43; NeuroMab). The specificity of the monoclonal to PSD-95 has been determined by western blot analysis (Naisbitt et al., 2000). (xiii) Mouse anti-SV2 was raised synaptic vesicles that were purified from the Ommata electric organ and used at 1:50 (SV2; DSHB). The SV2 monoclonal antibody is known to recognize a ∼95 kDa (on western blots) transmembrane proteoglycan that is present at synapses in the central and peripheral nervous system (Bindra et al., 1993; Buckley and Kelly, 1985; Feany et al., 1992). In the embryonic chicken retina, antibodies to dystrophin, CASK-1, PSD-95 and SV2 label the axon terminals of photoreceptors in the OPL (Bergmann et al., 2000; Bergmann et al., 2002; Blank et al., 1997; Wahlin and Adler, 2007).
We evaluated the specificity of primary antibodies by comparison with published examples of labeling results and immunoassays. None of the observed labeling was due to non-specific binding of secondary antibody or auto-fluorescence in the fixed tissues, because sections labeled with secondary antibodies alone were devoid of fluorescence. Secondary antibodies used in these studies included goat-anti-rabbit-Alexa488 or -568 and goat-anti-mouse-Alexa488, -568 or -647 (Invitrogen), diluted to 1:1000 in PBS plus 0.2% Triton X-100.
Photography, measurements, cell counts, and statistical analyses
Photomicrographs were obtained by using a Leica DM5000B microscope equipped with epifluorescence and a 12 megapixel Leica DC500 digital camera. Confocal microscopy was done with a Zeiss LSM 510 meta. Images were optimized for color, brightness and contrast, and double-labeled images overlaid by using Adobe Photoshop™6.0. Measurements from digital images were made by using Image Pro Plus 6.2 (Mediacybernetics). Cell counts and measurements were made on at least 5 sections from 4 different animals, and means and standard errors calculated for those data sets. Cell counts were consistently made from the same region of the retina for each data set to avoid the possible confounding variable of region-specific differences within the retina.
Results
A recent study by Edqvist and colleagues has described the expression of Islet1, Islet2 and Lim3 in the outer nuclear layer (ONL) of the embryonic chick retina from E3 through E9 (Edqvist et al., 2006). The expression of these LIM-domain factors may be in differentiating photoreceptors, but unambiguous evidence is currently lacking. Thus, we sought to identify the time course of expression of the LIM-domain factors in the presumptive ONL in the embryonic chick retina and assess whether the expression resides in developing photoreceptors. Accordingly, sections of the retina were obtained from different developmental stages and were labeled with antibodies to Islet1, Islet2 and Lim3. At E8, when the ONL becomes morphologically distinct from the INL, we found numerous nuclei in the ONL that were immunoreactive for Islet1, Islet2 and Lim3 (Figs. 1a, 1g and 1m). This pattern of labeling persisted at E10 and E12 (Figs. 1b, 1h, 1i, 1n and 1o). By E14, levels of Islet1-immunoreactivity in the nuclei of cells in central regions of the ONL were reduced compared to levels seen in inner retinal neurons (Fig. 1c). By comparison, peripheral regions of the E14 retina contained Islet1-immunoreactive nuclei in the ONL with relatively high levels of immunofluorescence (Fig. 1d). These findings are consistent with those of Edqvist and colleagues (2006). However, since the 40.2D6 monoclonal is known to recognize both Islet1 and Islet2, we tested whether an antibody specific to Islet1 alone produced similar patterns of labeling in the embryonic retina. We found that the 39.3F7 monclonal antibody to Islet1 labeled inner retinal neurons in a pattern identical to that of the 40.2D6 antibody (Fig. 1e). However, the 30.3F7 antibody did not label nuclei in the embryonic ONL at any stage of development (Fig. 1e). Thus, Islet1 may not be expressed by differentiating photoreceptors.
Figure 1.
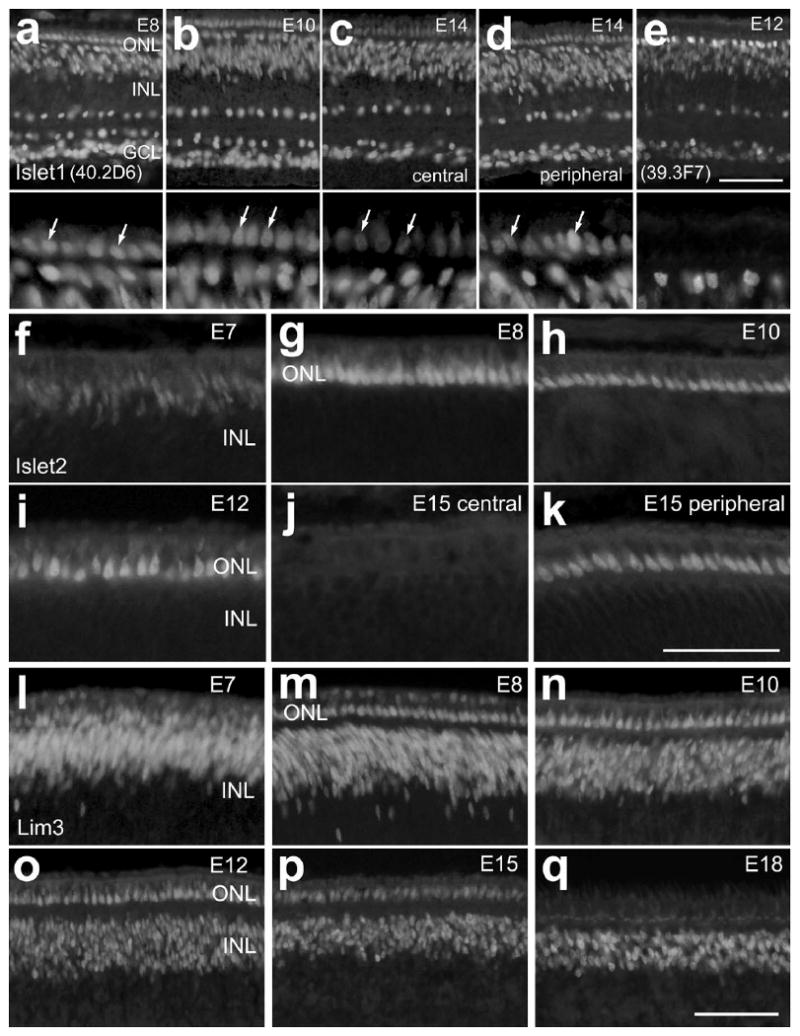
Immunoreactivities for Islet1, Islet2 and Lim3 are transiently observed in the nuclei of cells in the outer nuclear layer of the chick retina. Retinal sections were obtained from chick embryos on embryonic day 7 (E7), E8, E10, E12, E14 and postnatal day 4 (P4). Sections were labeled with antibodies to Islet1 (monoclonal 40.2D6; a-d), Islet1 (monoclonal 39.3F7; e), Islet2 (f-k) or Lim3 (l-q). For panels a through e, the lower portions of each panel are high-magnification views of the ONL at the different stages of development. The calibration bar (50 μm) in panel e applies to panels a-e, the bar in k applies to f-k, and the bar in q applies to l-q. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, GCL – ganglion cell layer.
To assess whether Islet2 and Lim3 are expressed by differentiating photoreceptors we immunolabeled retinal sections from different stages of development. At E7, when the ONL is not yet morphological distinct, we observed a mixture of nuclei that were weakly and strongly immunoreactive for Islet2 (Fig. 1f) and Lim3 (Fig. 1l) in the distal layers of the retina. Although, the identity of the cells that were weakly immunoreactive for Islet2 remains uncertain, the majority of Lim3-immunoreactive cells likely were differentiating bipolar cells, since Lim3 is known to be expressed by differentiating bipolar cells (Edqvist et al., 2006). At E8, when the ONL becomes morphologically distinct from the INL, we found numerous nuclei in the ONL that were immunoreactive for Islet2 and Lim3 (Figs. 1a, 1g and 1m). This pattern of labeling persisted at E10 and E12 (Figs. 1b, 1h, 1i, 1n and 1o). At E15, immunoreactivity for Islet2 was no longer detectable in central regions of the ONL (Fig. 1j). By comparison, peripheral regions of the E15 retina contained Islet2-positive nuclei in the ONL (Fig. 1k). By contrast, Lim3-immunoreactive nuclei were detected in central regions of the ONL at E15, but were absent 3 days later at E18 (Fig. 1p and 1q) with the exception of cells in far peripheral regions of the ONL (data not shown). In addition to the expression of the LIM-domain factors in presumptive photoreceptors, we observed; (1) persistent Islet1-specific immunoreactivity in horizontal, bipolar, cholinergic amacrine, and ganglion cells; (2) persistent Islet2-immunoreactivity in a sub-set of ganglion cells; and (3) persistent Lim3-immunoreactivity in bipolar cells. Taken together, our findings, for the most part, are in agreement with those of Edqvist and colleagues (2006).
To verify the findings of the immunolabeling studies we used RT-PCR and in situ hybridization (ISH). We found distinct PCR products for Islet1, Islet2 and Lim3 in E14 and P7 retinas (Fig. 2a). These products were cloned and sequenced to verify the identity of the cloned cDNA fragments. Consistent with the findings using the Islet1 antibody, we found ISH-labeling in the GCL (not shown) and distal INL (Fig. 2b). However, we failed to detect significant levels of Islet1 mRNA in the E12 ONL (Fig. 2b), suggesting that Islet1 may not be expressed by developing photoreceptors. We failed to successfully detect Islet2 mRNA by using ISH. This likely occurred because the Islet2 fragment that we sub-cloned is 76% GC-rich and, thus, we obtained low-quality riboprobe. Consistent with the immunolabeling studies, we detected mRNA for Lim3 in presumptive bipolar cells in the distal INL and in the ONL (Fig. 2c). The ISH-product in the photoreceptors was concentrated in the distal portion of the developing cells (Fig. 2c). Sense probes to Islet1 and Lim3 failed to produce labeling (not shown).
Figure 2.
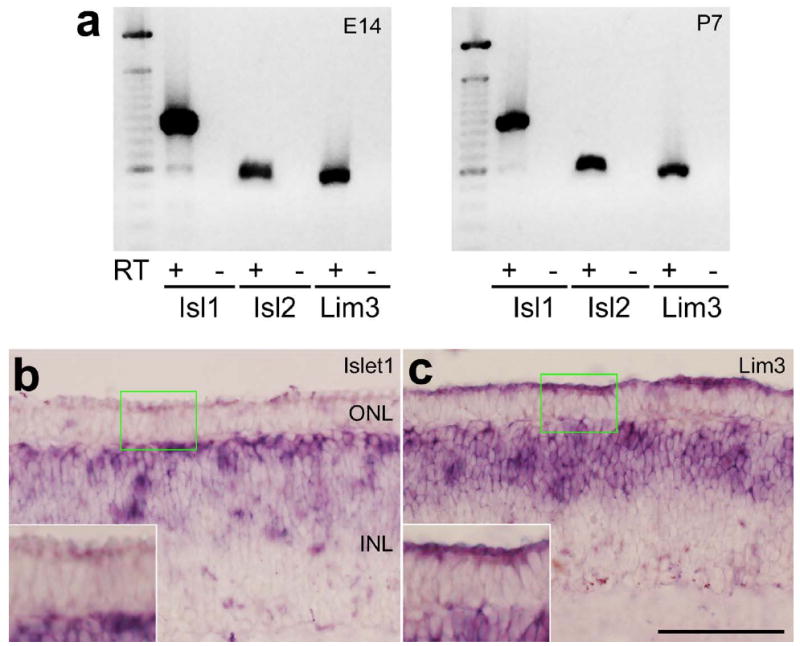
RT-PCR and in situ hybridization for Islet1, Islet2 and Lim3 in the embryonic chicken retina. Panel a; mRNA was harvested from retinas obtained from E14 and P7 chickens, reverse-transcribed to generate cDNA, amplified by using PCR, run on an agarose gel, and stained with ethidium bromide. The left-most lanes contain base-pair standards Vertical sections of the E12 retina were hybridized with riboprobes to Islet1 (b) or Lim3 (c). The calibration bar (50 μm) in panel c applies to panels b and c. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, RT – reverse transcriptase, Isl1 – Islet1, Isl2 – Islet2.
To determine whether the Islet2- and Lim3-immunoreactive nuclei that were observed in the embryonic ONL were those of photoreceptors, we double-labeled sections of the retina with antibodies to the LIM-domain factors and visinin. Visinin is the avian homolog of mammalian recoverin and is known to be expressed by all types of photoreceptors in the chicken retina (Bruhn and Cepko, 1996; Fischer et al., 2004b; Yamagata et al., 1990). Since both of these antibodies were raised in mouse, we applied the antibodies in sequence with the antibodies to the LIM-domain factors applied first and the anti-visinin applied second. At E12, we found that all (n=129) Islet2-positive nuclei in the ONL were localized to visinin-positive photoreceptors (Figs. 3a-d). High levels of Islet2-immunoreactivity were observed in visinin-positive photoreceptors with nuclei located in the proximal half of the ONL, whereas low levels of Islet2-immunoreactivity was observed in nuclei in the distal half of the ONL (Figs. 3b-d). The nuclei in the distal half of the ONL appeared to be those of the accessory member of double cones. We found few instances (about 3%) of visinin-positive photoreceptors that were negative for Islet2. At this stage of development the photoreceptors have not yet extended axons nor established connections with inner retinal neurons, but begin to form outer segments (Fig. 3a). In addition, visinin was transiently expressed at low levels by a few bipolar cells and many amacrine cells with somata located near the middle of the INL (Fig. 3e). To assess whether the Lim3-immunoreactive nuclei in the ONL were those of photoreceptors, we labeled E12 sections sequentially with antibodies to Lim3 and visinin. We found that all (n=176) Lim3-positive nuclei were localized to visinin-positive photoreceptors (Fig. 3e-h). However, not all visinin-positive photoreceptors at E12 were immunoreactive for Lim3 (69.7 ± 8.7%), indicating that Lim3 may be expressed by a subset of differentiating photoreceptors (Figs. 3f-h). The Lim3-positive nuclei were found primarily in the proximal half of the ONL and confined to photoreceptors with robust visinin-immunoreactivity (Figs. 3f-h).
Figure 3.

Immunoreactivity for Islet2 and Lim3 are present in the nuclei of visinin-positive photoreceptors in E12 retinas. Vertical sections of E12 retinas were labeled with antibodies to visinin (red), and Islet2 (green; a-d) or Lim3 (green; e-h). Both of the antibodies to visinin and the LIM-domain factors were mouse monoclonal. Thus, the antibodies were applied sequentially with the anti-Islet2 or anti-Lim3 applied first and the anti-visinin applied second. It was expected that the second secondary antibody (anti-mouse Alexa-568) recognized the first primary antibody. Accordingly, the visinin-positive photoreceptors that express Islet2 or Lim3 have nuclei with some red fluorescence. Arrows indicate developing, visinin-positive photoreceptors that express Islet2 or Lim3 and arrow-heads indicate visinin-positive photoreceptors that are not immunoreactive for Lim3. The calibration bar (50 μm) in panel e applies to panels a and e, and the bar in h applies to b-d and f-h. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
Axonin1 is transiently expressed by differentiating photoreceptors
We found that axonin1 is expressed in a spatial and temporal pattern similar to that seen for the LIM-domain factors in differentiating photoreceptors. Axonin1 is a member of the immunoglobulin superfamily and is known to function as a cell adhesion molecule to guide the formation neurites in the developing CNS (Perrin et al., 2001; Sonderegger et al., 1998; Stepanek et al., 2005; Stoeckli, 1998; Stoeckli and Landmesser, 1995). In the retina, axonin1 has been shown to be expressed in differentiating amacrine and ganglion cells (Drenhaus et al., 2004). In addition, there is a brief account that axonin1 mRNA is expressed by photoreceptors in the embryonic chicken retina (Morino et al., 1996). We found that immunoreactivity for axonin1 occurs transiently in differentiating photoreceptors that express visinin (Figs. 4a-f). In parallel to the time-course of expression of Islet2 and Lim3 in photoreceptors, the expression of axonin1 in the ONL begins at E8 (Figs 4a and 4b), peaks at E12 (Fig. 4c), and decreases dramatically at about E15 (Fig. 4d). By E18, immunoreactivity for axonin1 in the photoreceptors is greatly diminished (Fig. 4e), similar to levels of immunoreactivity in the distal retina of the postnatal eye (Fig. 4f).
Figure 4.
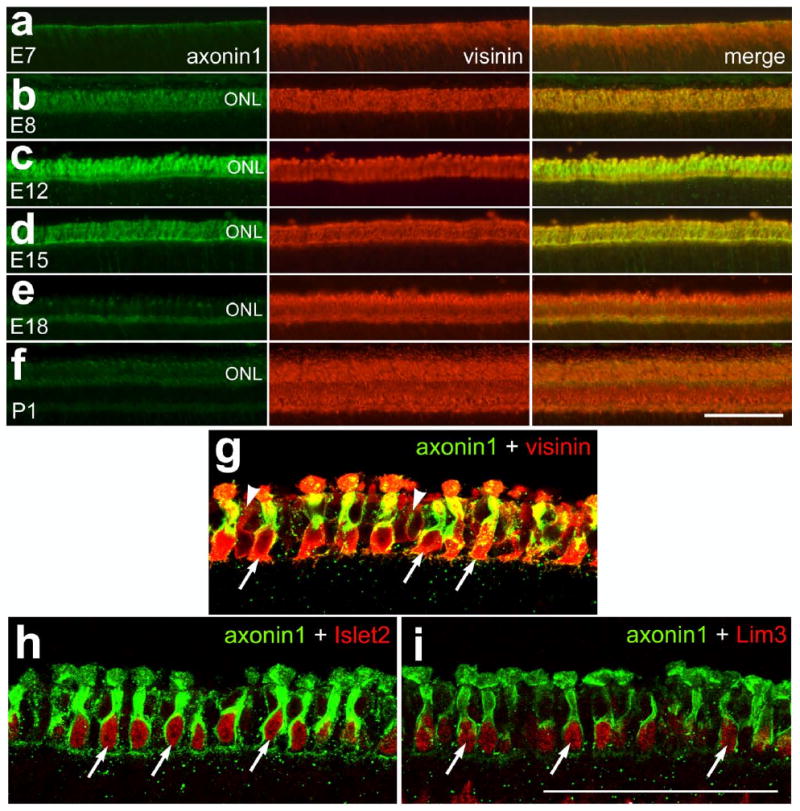
Axonin1 is transiently expressed by differentiating photoreceptors. Retinal sections were obtained at E7, E8, E12, E15, E18 and P1. Expression of axonin1 in photoreceptors begins at E8, peaks at E12 and decreases by E15. (a-f) Sections were labeled with antibodies to axonin1 (green) and visinin (red). (g-j) In the embryonic retina axonin1 is expressed by photoreceptors that are positive for Islet1, Islet2 and Lim3. Sections of E10 retina were labeled with antibodies to axonin1 (green) and Islet1, Islet2 or Lim3 (red). The images in panels g-j were obtained by using confocal microscopy and projection of two 1μm-thick optical sections. Arrows indicate examples of double-labeled cells. The calibration bar (50 μm) in panel f applies to panels a-f, and the bar in i applies to g-i. Abbreviation: ONL – outer nuclear layer.
To assess whether axonin1 was present in photoreceptors that expressed the LIM-domain transcription factors we used double-immunolabeling and confocal microscopy. In the E12 retina, we found that most of the visinin-positive photoreceptors were immunoreactive for axonin1 (Fig. 4g). The majority of visinin-immunoreactive photoreceptors were also labeled for axonin1. By contrast, a small number of photoreceptors that were weakly immunoreactive for visinin failed to label for axonin1 (Fig. 4g). At E12, axonin1-immunoreactivity in the ONL is co-localized to cells that express Islet2 and Lim3 (Figs. 4h and 4i). All of the axonin1-positive photoreceptors are immunoreactive for Islet2 or Lim3. Similarly, all of the nuclei in the ONL at E12 that are immunoreactive for Islet2 and Lim3 are positive for axonin1. In the embryonic retina, the patterns of immunolabeling were identical for both rabbit polyclonal and mouse monoclonal antisera to axonin1 (data not shown). These findings suggest that the expression of axonin1 overlaps temporal with the expression of Islet2 and Lim3 in differentiating photoreceptors in the embryonic chicken retina.
The slowed maturation of photoreceptors in far peripheral regions of the postnatal retina coincides with the transient expression of LIM-domain transcription factors
The retinas of postnatal chickens are known to contain a zone of progenitors that proliferate and continue to add neurons to the peripheral edge of the retina (Fischer, 2005; Fischer et al., 2002b; Fischer and Reh, 2000). This zone of progenitors in the chicken retina is similar to the well-described circumferential marginal zone (CMZ) of frogs and fish (Hitchcock et al., 2004; Raymond and Hitchcock, 1997; Raymond and Hitchcock, 2000; Reh and Fischer, 2001; Reh and Levine, 1998). However, unlike the CMZ of frogs and fish, the CMZ of chickens does not produce photoreceptors (Fischer and Reh, 2000). The photoreceptors near the CMZ are known to be generated during embryonic development (Fischer and Reh, 2000; Ghai et al., 2007). However, the maturation of neurons in far peripheral regions of the retina is greatly slowed compared to the maturation of neurons that are generated in central regions of the retina (Ghai et al., 2007). Thus, we used sections of far peripheral regions of the retina to make observations of photoreceptor maturation across the gradient of maturity that extends centrally away from the CMZ.
In the P1 eye, immunoreactivity for Islet2 and Lim3 was detected in nuclei in the ONL that were confined to far peripheral regions of the retina (Fig. 5a and 5b). Similar to results observed in the embryonic retina, the 39.3F7 antibody to Islet1 failed to label nuclei in the ONL of the postnatal retina, whereas the 40.2D6 antibody labeled nuclei in the ONL in a pattern similar to that seen for Islet2 and Lim3 (data not shown). We found that the levels of expression of Islet2 and Lim3 in the ONL gradually decrease with increasing distance from the CMZ. The zone of Islet2/Lim3-expressing cells in the ONL decreased in breadth as postnatal development proceeded from P1 to P7 (Figs. 5a-d), and was nearly absent by P14 (Figs. 5e and 5f). In peripheral temporal regions of P1 retina, the domain of photoreceptors that was immunoreactive for Islet2, Lim3 or axonin1 was nearly 1000 μm in radial diameter; 906.8 ± 49.7 μm, 963.4 ± 48.6 μm and 918.3 ± 48.6 μm, respectively (n=6). By comparison, at P14 the domain of photoreceptors that was immunoreactive for Islet2, Lim3 or axonin1 was less than 50 μm in radial diameter; 21.8 ± 6.9 μm, 35.8 ± 4.9 and 22.5 ± 6.3 μm, respectively, (n=6). These observations are consistent with the hypotheses that Islet2 and Lim3 are expressed by photoreceptors during post-mitotic maturation and this differentiation is greatly slowed in far peripheral regions of the retina.
Figure 5.
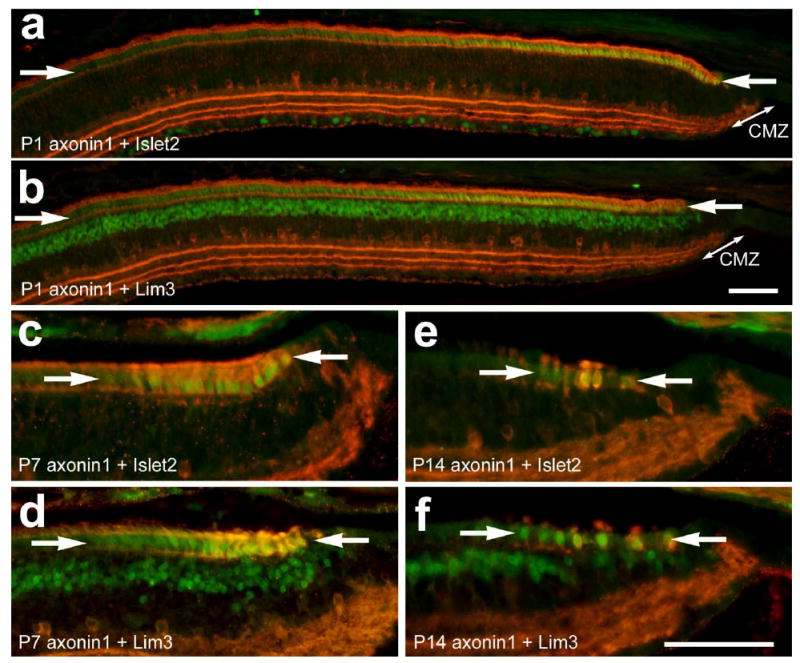
Immunoreactivities for Islet2, Lim3 and axonin1 are present in the nuclei of differentiating photoreceptors in the far peripheral regions of retina of postnatal chickens. Retinal sections were labeled with antibodies to axonin1 (red) and Islet2 or Lim3 (green). Tissues were obtained at P1 (a and b), P7 (c and d) and P14 (e and f). The arrows indicate the domain of photoreceptors in the ONL that are immunoreactive for the LIM-domain factors and axonin1. The calibration bar (50 μm) in panel b applies to panels a and b, and the bar in f applies to c-f. Abbreviations: CMZ – circumferential marginal zone.
Consistent with the hypothesis that the axonin1 is coincident with the expression of LIM-domain transcription factors in differentiating photoreceptors, we found a near-complete overlap of axonin1 and Islet2 or Lim3 in photoreceptors at the peripheral edge of the postnatal retina (Fig. 5). Similar to the patterns of labeling seen for the LIM-domain factors in the ONL, we found that the number of photoreceptors in the ONL that express axonin1 is decreased in far peripheral regions of the postnatal retina from P1 through P14 (Fig. 5). In the postnatal retina, the patterns of immunolabeling for axonin1 were identical for both the rabbit polyclonal and mouse monoclonal antisera (data not shown).
The onset of expression of calbindin and red/green opsin is delayed in photoreceptors that are found in far peripheral regions of the postnatal retina (Ghai et al., 2007). Thus, we sought to assess whether the expression of LIM-domain factors in photoreceptors overlapped with that of calbindin and red/green opsin. In the P7 retina, we found that as levels of immunoreactivity for Islet2 (Figs. 6a-d) and Lim3 (Figs. 6e-h) gradually decreased, there was a coincident increase in labeling for calbindin and red/green opsin (Fig. 6). In some instances, we observed Lim3-positive nuclei that were found within the domain of calbindin-positive photoreceptors; these cells failed to express calbindin and red/green opsin (Fig. 6i).
Figure 6.
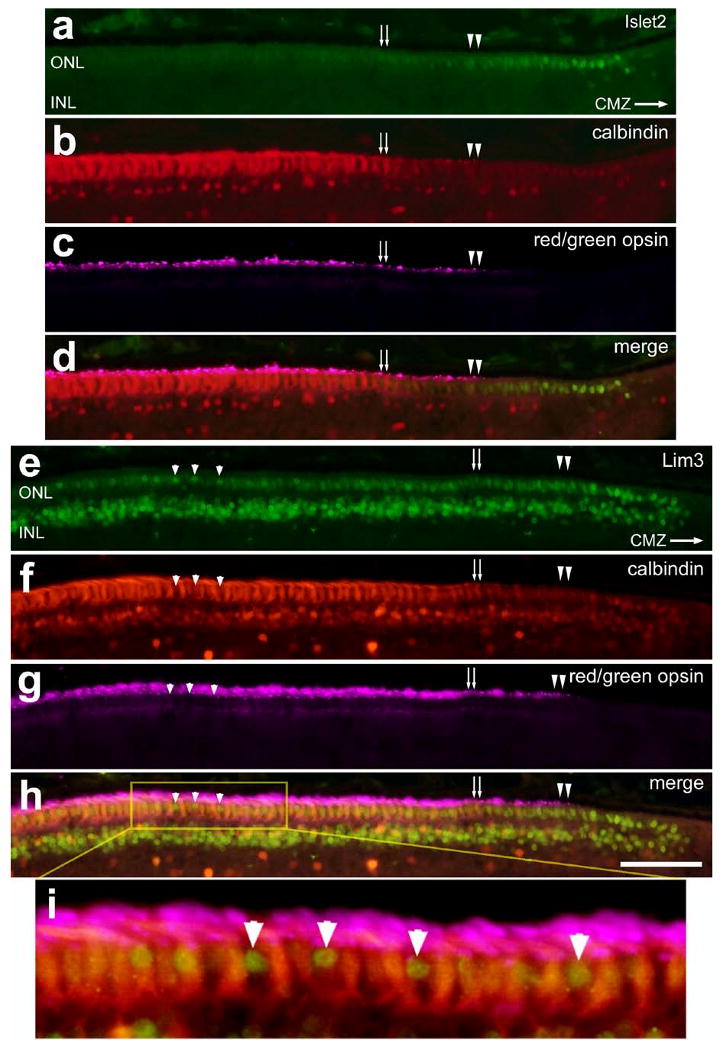
The up-regulation of calbindin and red-green opsin coincides with the down-regulation of Islet2 and Lim3 expression in photoreceptors in the far peripheral regions of the retina. Vertical sections of the far peripheral retina (adjacent to the CMZ) were labeled with antibodies to calbindin (red; b,d,f,h and i), red-green opsin (magenta; c,d and g-i), and Islet2 (green; a and d) or Lim3 (green; e and h). Tissues were obtained at P7. Small double-arrows indicate the region of the ONL that contains photoreceptors that begin to express calbindin, double arrow-heads indicate the first photoreceptors that are immunoreactive for red-green opsin, and the small arrow-heads indicate Lim3-positive/calbindin-negative photoreceptors mixed among. The calibration bar (50 μm) in panel h applies to panels a-h. Panel i is a four-fold enlargement of the boxed-out area in panel h. Abbreviations; ONL – outer nuclear layer, INL – inner nuclear layer, CMZ – circumferential marginal zone.
Similar to the gradual increase in immunoreactivity for red/green opsin in photoreceptors in the far peripheral retina, we observed a gradual increase in immunoreactivity for rhodopsin in outer segments with increasing distance from the CMZ (Figs. 7a and 7c). At P1 in far peripheral regions of the temporal retina, the region of photoreceptors that were rhodopsin-negative was about 700 μm in radial diameter (Figs 7a and 7c). By comparison, the region of photoreceptors that was rhodopsin-negative was reduced to less than 50 μm at P14 (Fig. 7f). Since all of the photoreceptors in the periphery of the retina are generated in the embryo prior to E14, our findings indicate that there is a gradual maturation of rod photoreceptors during the last week of embryonic development and over the first 2 weeks of postnatal development. The onset of rhodopsin expression in the photoreceptors was complimentary to the gradual decrease in immunoreactivity for Islet2 (Fig. 7b) and Lim3 (Fig. 7d). In other words, the region of photoreceptors that begin to express rhodopsin coincides with the photoreceptors that down-regulate the expression of the LIM-domain factors.
Figure 7.
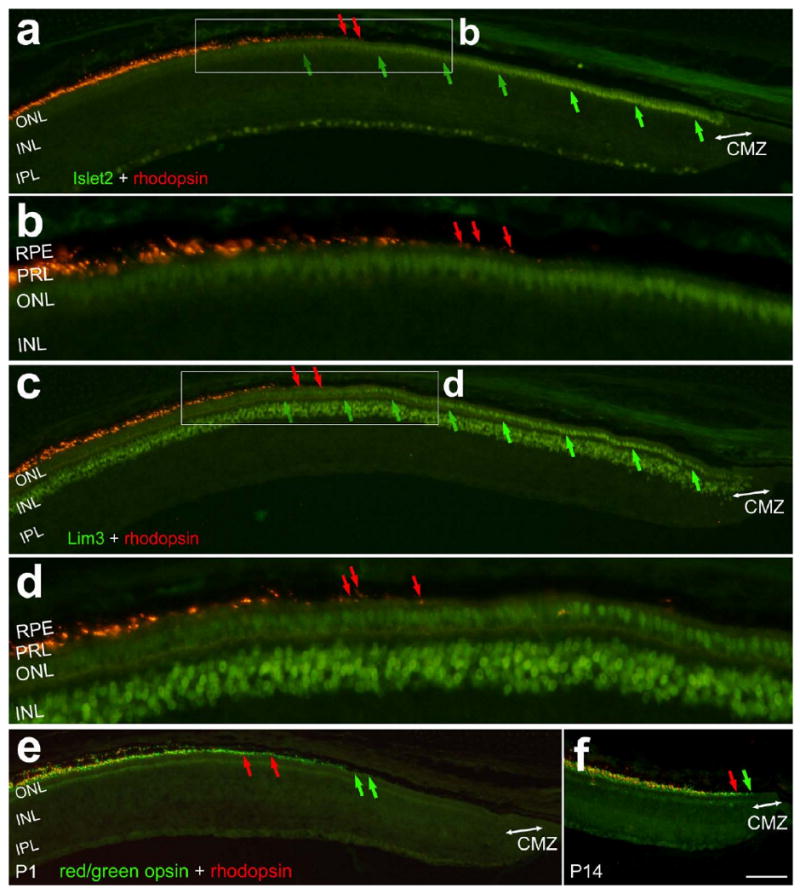
The onset of expression of rhodopsin in the far peripheral retina coincides with the down-regulation of expression or Islet1, Islet2 and Lim3. Sections of far temporal regions of the P1 (a-e) or P14 (f) retina were labeled with antibodies to rhodopsin (red) and Islet2 (green; a and b), Lim3 (c and d), or red-green opsin (green; e and f). The red arrows indicated the onset of rhodopsin immunoreactivity in rod outer segments, the green arrows in panels a-d indicate the gradual down-regulation of Islet2 or Lim3 in photoreceptor nuclei, and the green arrows in panels e and f indicate the onset of red/geen opsin expression in cone outer segments. The double-ended arrows indicate the CMZ at the peripheral edge of the retina. The calibration bar (50 μm) in panel f applies to panels a,c,e and f. Panels b and d are three-fold enlargements of the areas that are boxed-out in panels a and c, respectively. Abbreviations: RPE – retinal pigmented epithelium, PRL – photoreceptor layer, ONL – outer nuclear layer, INL – inner nuclear layer, CMZ – circumferential marginal zone.
Bruhn and Cepko (1996) reported that mRNA for rhodopsin first appears at E15, about 1 day after the mRNA for red/green opsin is first detected. Thus, we postulated that the onset of rhodopsin-immunoreactivity should occur farther away from the CMZ in the postnatal retina than the onset of immunoreactivity for red/green opsin. Indeed, at P1 in the far peripheral retina, the onset of rhodopsin-immunoreactivity was about 100 μm further away from the CMZ than the onset of expression of red-green opsin (Fig. 7e). At P14, this difference was greatly reduced to about 10 μm (Fig. 7f). Taken together, these findings suggest that the spatial gradient of maturation at the peripheral edge of the retina faithfully recapitulates the temporal gradient of maturation in the embryonic retina.
Unlike calbindin, red/green opsin and rhodopsin, we found that the onset of IRBP expression precedes the off-set of expression of the LIM-domain factors. We found that immunoreactivity for IRBP in the retina first appears at E12 and is increased by E15 (Fig. 8a and 8b). In peripheral regions of the P7 retina, immunoreactivity for IRBP was present among the photoreceptor outer-segments that were found near the CMZ (Figs. 8c-e). Levels of IRBP-immunoreactivity gradually increased with increasing distance from the CMZ. The expression of Islet2 and Lim3 was present in many photoreceptors that were immunoreactive for IRBP (Figs. 8c-e). These findings indicate that the down-regulation of LIM-domain transcription factors does not coincide with the onset of IRBP expression.
Figure 8.
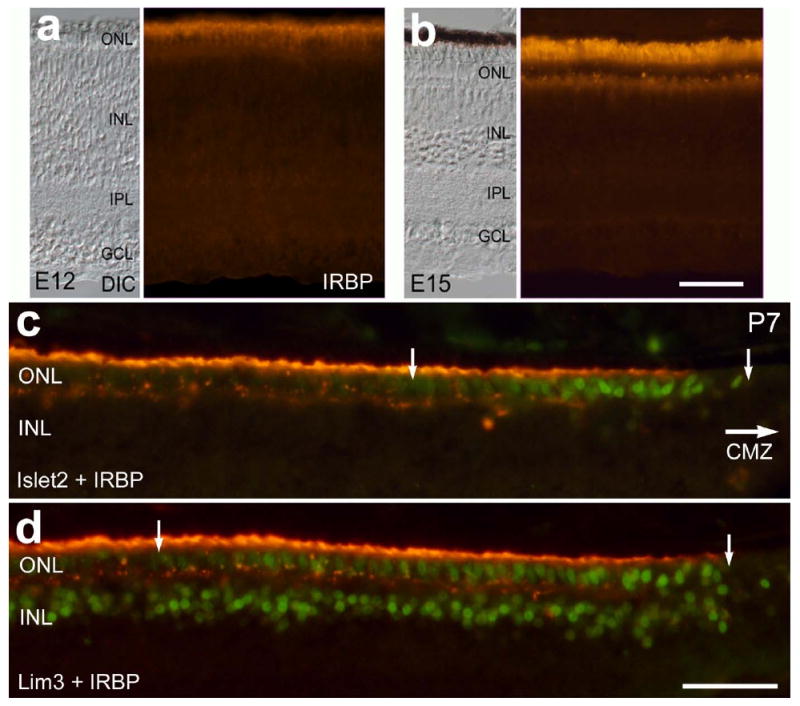
The onset of expression for IRBP does not coincide with the down-regulation of LIM-domain factors in photoreceptors. Vertical sections of the central (a and b) and far peripheral (c-d) retina were obtained at E12 (a), E15 (b) and P7(c-d). Sections were labeled with antibodies to IRBP (red) and Islet1, Islet2 and Lim3. Panels a and b include images obtained by using bright-field differential interference contrast and epifluorescence. Arrows in panels c-e indicate the region of ONL where photoreceptors are immunoreactive for the LIM-domain transcription factors. The calibration bar (50 μm) in panel b applies to all panels a and b, and the bar e applies to c-e. Abbreviations: ONL – outer nuclear layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer, DIC – differential interference contrast, CMZ – circumferential marginal zone.
The expression of synaptic proteins by maturing photoreceptors
The maturation of photoreceptors involves the establishment of synaptic contacts with inner retinal neurons, and the synaptogenesis requires the expression of proteins such as dystrophin, CASK-1, PSD-95 and SV2. During the development of the chicken retina, immunoreactivity for CASK-1, PSD-95 and SV2 first appear in the OPL at E12-E13, whereas dystrophin first appears at about E15 (Bergmann et al., 2000; Blank et al., 1997; Wahlin and Adler, 2007). In the central regions of the P1 retina, we found immunoreactivity for dystrophin, CASK-1, and PSD-95 at low levels in the IPL, whereas immunoreactivity for SV2 was high in the IPL (9a-d). By comparison, immunoreactivities for dystrophin, CASK-1, PSD-95 and SV2 were very intense in the OPL of central regions of retina (Fig. 9a-d). In the OPL, crescent-shaped structures were labeled for dystrophin, CASK-1, PSD-95 and SV2; these structures were present in the inner and outer strata of the OPL. These crescent-shaped structures likely are the axon terminals of rod and cone photoreceptors. Consistent with this hypothesis, labeling for dystrophin, CASK-1, PSD-95 and SV2 in the distal OPL was localized to the axon terminals of calbindin-positive cones, whereas labeling for the synaptic markers in the proximal OPL was in the terminals of calbindin-negative photoreceptors (Fig. 9e-v). Since the antibodies to calbindin and the synaptic markers were all raised in mouse, we used sequential, reciprocal double-labeling to assess whether these proteins are co-localized in the axon terminals of photoreceptors. Our findings are consistent with previous reports that have detected synaptic proteins in the axon terminals of photoreceptors in the chicken retina (Bergmann et al., 2000; Blank et al., 1997; Wahlin and Adler, 2007).
Figure 9.
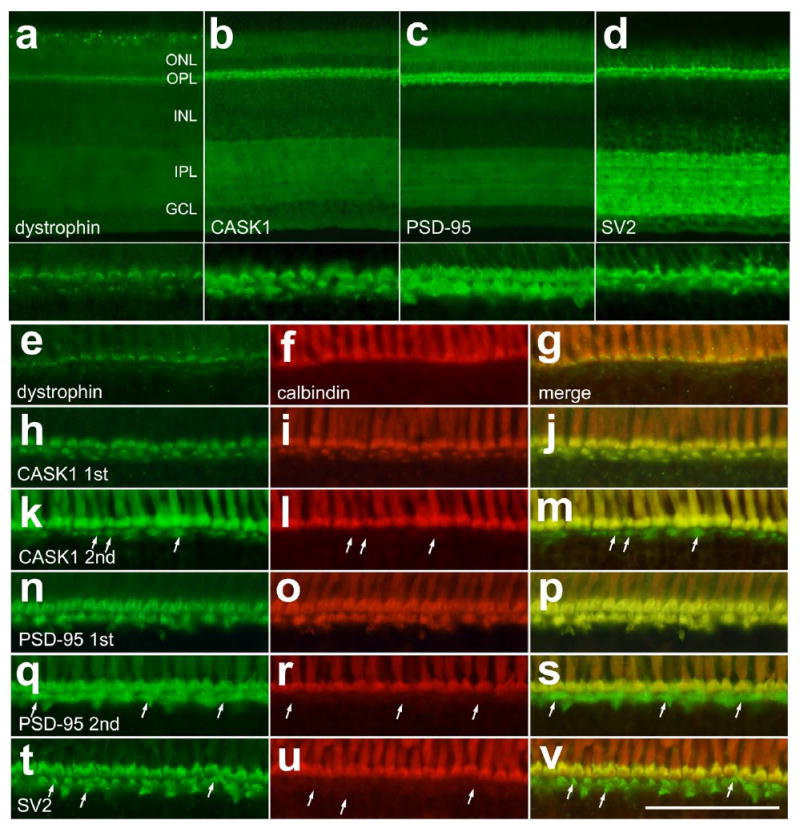
The synaptic proteins dystrophin, CASK-1, PSD-95 and SV2 are concentrated in the OPL and in the axon terminals of calbindin-positive photoreceptors in central regions of the postnatal retina. Vertical sections of the retina were labeled with antibodies to dystrophin (a, e and g), CASK-1 (b, h, j, k and m), PSD-95 (c, n, p, q, and s), SV2 (d, t and v), and calbindin (red). Since the antibodies to calbindin and the synaptic markers were all raised in mouse, sequential reciprocal double-immunolabeling was used to distinguish photoreceptor axon terminals that were positive for calbindin and the synaptic markers from those that were positive for the synaptic markers alone. Co-localization of the synaptic markers and calbindin within the axon terminals appears yellow. Arrows indicate axon terminals in the proximal OPL that are labeled for CASK-1, PSD-95 or SV2 that are negative for calbindin when the antibodies to the synaptic markers were applied second, after the anti-calbindin (k-m and q-v). By contrast, the synaptic markers appear in both channels when the antibodies to these markers were applied first, after the anti-calbindin (h-j and n-p). The calibration bar (50 μm) in panel d applies to panels a-d, and the bar in p applies to panels e-p. Abbreviations: ONL – outer nuclear layer, OPL – outer plexiform layer, INL – inner nuclear layer, IPL – inner plexiform layer, GCL – ganglion cell layer.
We tested the hypothesis that the onset of expression of synaptic proteins in the axon terminals of photoreceptors is gradually established over the gradient of maturation that exists at the peripheral edge of the postnatal retina. In far peripheral regions of the retina, we found spatial gradients for levels of expression and gradual accumulation within the OPL of dystrophin, CASK-1, PSD-95 and SV2. Immunolabeling for dystrophin, CASK-1, PSD-95 and SV2 in the OPL increased with increasing distance from the CMZ (Fig. 10). At P1, the onset of immunoreactivity for dystrophin appeared about 400 μm into the neural retina away from the CMZ (Fig. 10a). At P14, by comparison, the onset of immunoreactivity for dystrophin appeared about 80 μm away from the CMZ (Fig. 10b). Unlike the patterns of labeling observed for dystrophin, immunoreactivity for CASK-1, PSD-95 and SV2 was observed in photoreceptors near (<50 μm) for the CMZ at P1 (Figs. 10c, 10e and 10f). Similar to prior reports (Bergmann et al., 2000; Wahlin and Adler, 2007), immunoreactivities for CASK-1, PSD-95 and SV2 first appear in the distal aspects of differentiating photoreceptors near the outer limiting membrane, and become concentrated in axon terminals as photoreceptors mature; this is evident in the far peripheral OPL at P14 (Figs. 10d, 10f and 10h). These findings suggest that these proteins may have important functions during the early stages of photoreceptor differentiation in addition to functions during the establishment and maintenance of synapses in the OPL.
Figure 10.
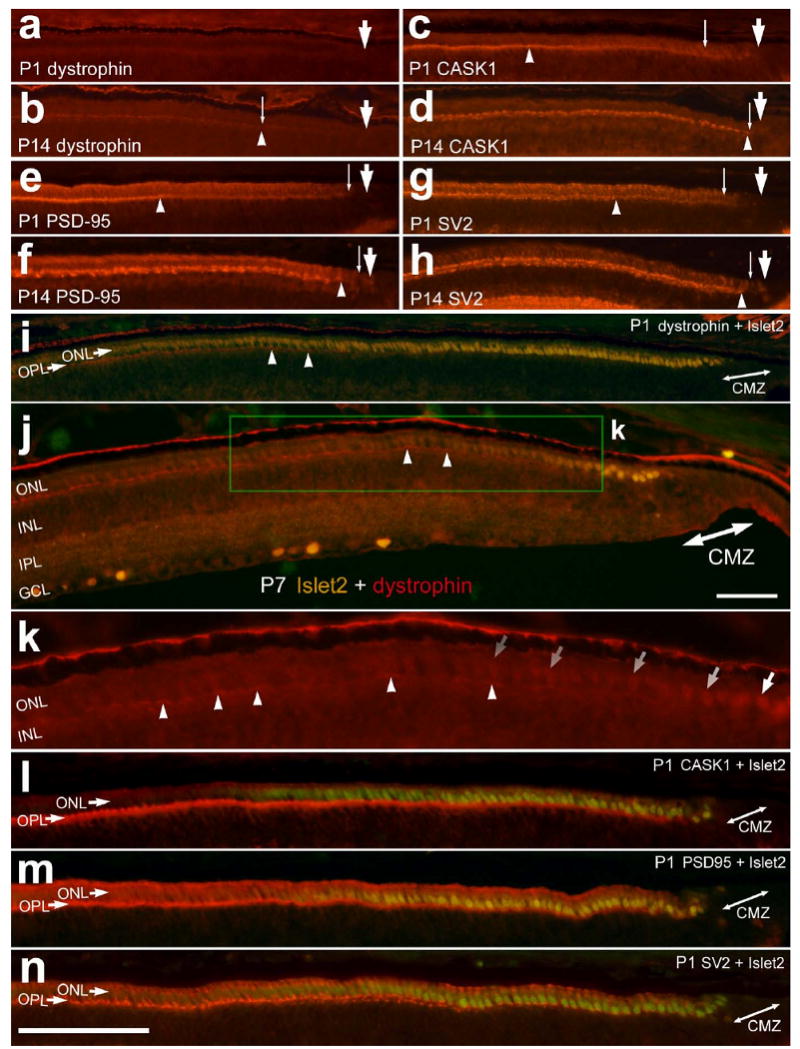
The onset of expression in photoreceptors and accumulation in the OPL of synaptic markers in the far peripheral retina overlap with the down-regulation of LIM-domain transcription factors. Vertical sections of the far peripheral retina were labeled with antibodies to dystrophin (a,b and i-k), CASK-1 (c, d and l), PSD-95 (e, f and m), SV2 (g, h and n) and Islet2 (green nuclei; i, j and l-m). Tissues were obtained at P1, P7 and P14. The small, thin arrows indicate the onset of expression of dystrophin, CASK-1 and PSD-95 in photoreceptors, the arrow-heads indicate the accumulation of the synaptic proteins in the OPL, the large arrows in panels a-h indicate the edge of the CMZ, and the double-ended arrows in panels i-n indicate the CMZ. The calibration bar (50 μm) in panel n applies to all panels and a-i and l-n, and the bar in j applies to j alone. Abbreviations; OPL – outer plexiform layer, ONL – outer nuclear layer, CMZ – circumferential marginal zone.
In peripheral regions of P1 and P7 retinas, we found that there was a coincident up-regulation of dystrophin-immunoreactivity in the OPL as Islet2-immunreactivity was down-regulated in the ONL (Figs. 10i-k). By contrast, immunoreactivities for CASK-1, PSD-95 and SV2 in the OPL overlapped with the region of ONL that contained photoreceptor nuclei that were immunoreactive Islet2 (Figs. 10l-m) and Lim3 (not shown).
Discussion
Here we present evidence that the LIM-domain transcription factors Islet2 and Lim3 are transiently expressed by differentiating photoreceptors in the chicken retina. In addition, we find that the expression of axonin1 coincides temporally and spatially with the LIM-domain factors in the differentiating photoreceptors. By contrast, as the LIM-domain factors are down-regulated in the photoreceptors, we find an up-regulation of red/green opsin, rhodopsin, calbindin, and the synaptic protein dystrophin. By comparison, the onset of expression of IRBP, PSD-95, CASK-1 and SV2 in differentiating photoreceptors precedes the down-regulation of the LIM-domain factors. A summary of these findings in the embryonic retina and in the far periphery of the postnatal retina is illustrated in Figure 11.
Figure 11.
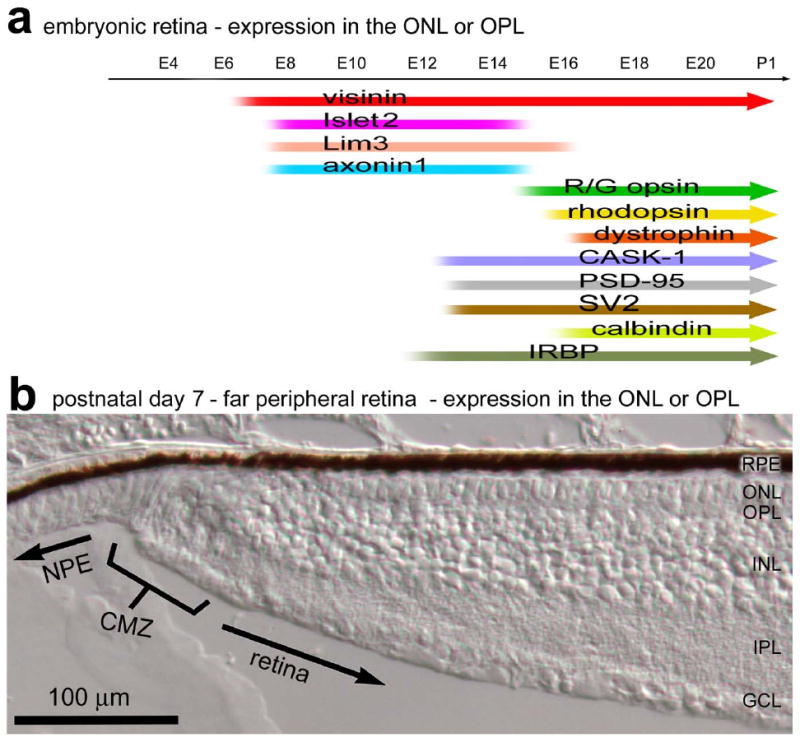
Schematic diagram summarizing the distribution of Islet1, Islet2, Lim3, axonin1, opsin, rhodopsin, calbindin, dystrophin, CASK-1, PSD-95, SV2 and IRBP in the outer layers of the developing retina and far periphery of the postnatal retinal. The distribution of these genes in the embryonic retina represents a temporal distribution in the ONL, whereas the distribution of these genes in the far periphery of the postnatal retina represents the spatial distribution within the ONL relative to the CMZ. Abbreviations: NPE – non-pigmented epithelium, CMZ – circumferential marginal zone, RPE – retinal pigmented epithelium, ONL – outer nuclear layer, OPL – outer plexiform layer.
Islet1 is likely not expressed by differentiating photoreceptors in the chicken retina. It is likely that the 40.2D6 monoclonal antibody that we used to Islet1 cross-reacted with Islet2 in developing photoreceptors. By comparison, the 39.3F7 antibody to Islet1 failed to label nuclei of differentiating photoreceptors. Consistent with the findings of the immunolabeling studies, we failed to detect significant levels of Islet1 mRNA in developing photoreceptors by using ISH. However, we detected Lim3 mRNA in presumptive bipolar cells and in developing photoreceptors cells at E12. The ISH-labeling for Lim3 in the photoreceptors was concentrated in the distal portion of the cells near the outer limiting membrane; similar to the patterns of labeled reported for a variety of photoreceptor-specific genes in the chicken retina (Bradford et al., 2005; Hackam et al., 2003). Taken together, these findings suggest that Islet2 and Lim3 are expressed in the differentiating photoreceptors, whereas Islet1 is not.
The photoreceptors in far peripheral regions of the retina appear to be immature and differentiate much more slowly than those in central regions of the retina. In central regions of the retina, most cone photoreceptors are generated between E5 and E7 (Prada et al., 1991), begin to express visinin shortly after terminal mitosis at about E7 (Bruhn and Cepko, 1996), and begin to express opsins by about E15 (Bruhn and Cepko, 1996). These findings indicate that cone photoreceptors in central regions of the retina may be photosensitive about 9 days after terminal mitosis. The photoreceptors in far peripheral regions of the retina do not express opsin or calbindin in the postnatal retina up to P14, more than 21 days after terminal mitosis, whereas visinin is expressed by these cells as early as E12 (Ghai et al., 2007). In addition to the delayed onset of expression of red/green opsin and calbindin, we report here that the immature photoreceptors in the peripheral retina do not express rhodopsin or dystrophin during the first 2 weeks of postnatal development. Taken together, our findings indicate that the maturation of photoreceptors in far peripheral regions of the retina is greatly slowed compared to the rates of maturation of photoreceptors in central regions of the retina. Furthermore, the immature phenotype of photoreceptors in the peripheral retina is coincident with the expression of Islet2, Lim3 and axonin1. Consistent with the hypothesis that Islet2 and Lim3 regulate the delayed onset of expression of certain genes in photoreceptors, several reports have demonstrated that LIM-domain factors can act as transcriptional repressors, often through interactions with LIM-domain binding proteins (Bach et al., 1999; Mochizuki et al., 2000; Ostendorff et al., 2002).
In differentiating photoreceptors, the LIM-domain factors may regulate the delayed expression of many genes in addition to the genes described here. For example, the report by Bradford and colleagues (2005) describes the onset of expression of peripherin, guanylate cyclase activating protein (GCAP) and blue opsin in chick photoreceptors at about E15. The timing of expression of these photoreceptor-specific genes corresponds well with when the LIM-domain factors are down-regulated in the ONL. By contrast, the expression of some genes that are required by mature photoreceptors does not correlate with the down-regulation of LIM-domain factors. For example, we found that the onset of IRBP expression does not correlate with the down-regulation of LIM-domain factors in differentiating photoreceptors. We observed low levels of IRBP-immunoreactivity in the distal retina at E12, when there high levels of immunoreactivity for the LIM-domain factors in the ONL. By comparison, Bradford et al (2005) reported that mRNA for IRBP is detected in the embryonic chick retina from E5 through E18. Taken together, these findings indicate that mRNA for IRBP is present in differentiating photoreceptors several days before the protein, suggesting that the expression of IRBP is, in part, regulated post-translationally. Consistent with the findings in the embryonic retina, we found that IRBP was expressed by photoreceptors in the far peripheral regions of the postnatal retina that also express the LIM-domain factors. Thus, our findings suggest that the expression of IRBP may not be regulated by LIM-domain transcription factors. Similar to the expression pattern of IRBP, the onset of expression of PSD-95, CASK-1 and SV2 precedes the down-regulation of the LIM-domain factors across the gradient of maturity in the far periphery of the postnatal retina. Consistent with these findings, immunoreactivity for PSD-95 and CASK-1 appears in the embryonic retina in the OPL at about E12 (Wahlin and Adler, 2007), several days before the down-regulation of the LIM-domain factors. Although CASK-1, PSD-95 and SV2 are expressed before the down-regulation of the LIM-domain factors in differentiating photoreceptors, these synaptic proteins are trafficked and gradually concentrated in the axon terminals as Islet2 and Lim3 are down-regulated. It is possible that the expression of Islet2 and Lim3 in photoreceptors somehow influences the trafficking and concentration of CASK-1, PSD-95 and SV2 into axon terminals. Taken together, these findings suggest that the expression of CASK-1, PSD-95 and SV2 is not regulated by the LIM-domain factors. Alternatively, the expression may be a function of gene dosage, and the expression of CASK-1, PSD-95 and SV2 (among other genes) might be allowed with sufficient down-regulation of the LIM-domain factors.
The function of delayed photoreceptor maturation remains uncertain. It is possible that the delayed onset of expression of photopigments, and other genes involved in phototransduction, is required for normal development. It is possible that active phototransduction in immature photoreceptors is damaging, may attenuating survival, or somehow interferes with the establishment of proper connections with inner retinal neurons. In addition, it is possible that the onset of opsin expression may first require the establishment of the proper trafficking mechanisms and formation of outer segments to prevent phototransduction from occurring within the inner segments. For example, mistrafficking of the phototransduction machinery to the inner segments of photoreceptors is often observed in diseased retinas (Huttl et al., 2005; Michalakis et al., 2005; Sung and Tai, 2000). It has been postulated that phototransduction in the inner segments of photoreceptors is damaging and may lead to cell death (Kong et al., 2006; Michalakis et al., 2005; Nir and Agarwal, 1991; Rohrer et al., 2005). In developing photoreceptors, if the phototransduction machinery is expressed before the outer segments are formed, phototransduction will occur within the inner segments. Thus, in species where retinal development occurs with exposure to light, it may be important to delay the onset of expression of the phototransduction machinery until the cells mature sufficiently (i.e. form outer segments) to confine phototransduction to the outer segments. Further studies are required to determine why the maturation of photoreceptors (i.e. the delayed onset of expression of photopigments and components of the phototransduction machinery) is protracted in developing retinas.
The slowed maturation of photoreceptors in peripheral regions of the retina may be regulated by the local microenvironment and LIM-domain transcription factors. There is speculation that the progenitors in the CMZ are maintained into postnatal development by a variety of secreted growth factors (Fischer et al., 2002a; Fischer and Reh, 2000; Moshiri et al., 2005). It is possible that the microenvironment in the far peripheral retina serves to not only maintain proliferating progenitors in the CMZ, but also to greatly slow the maturation of photoreceptors. The precise mechanisms that control the rate of photoreceptor maturation remain uncertain. There are several possibilities that may account for the immature photoreceptors in the far peripheral retina; (1) the milieu of secreted factors that maintains the CMZ at the far peripheral edge of the retina inhibits the maturation of photoreceptors, (2) the factors that stimulate the maturation of photoreceptors are lacking in peripheral regions of the retina, (3) the photoreceptors in the peripheral retina are intrinsically programmed to mature slowly, or (4) a combination of these aforementioned possibilities is responsible for the slowed maturation of photoreceptors in the far peripheral regions of the retina. Based on the findings presented here, we propose that the slowed maturation of photoreceptors in far peripheral regions of the postnatal retina is, in part, regulated by the sustained expression of Islet2 and Lim3 in response the environmental cues.
Conclusions
We conclude that Islet2 and Lim3 are transiently expressed by differentiating photoreceptors in the chicken retina. In photoreceptors, the expression of Islet2 and Lim3 overlap temporally and spatially with high levels of expression for the cell surface glycoprotein axonin1. By comparison, as Islet2 and Lim3 are down-regulated in photoreceptors several genes are up-regulated; these genes include calbindin, rhodopsin, red-green opsin and dystrophin. By contrast, there was little correlation between the down-regulation of Islet2 and Lim3 and the onset of expression of IRBP, visinin, CASK-1, PSD-95 and SV2 in photoreceptors. We propose that Islet2 and Lim3 regulate aspects of photoreceptor maturation. Specifically, we propose that Islet2 and Lim3 may promote the expression of axonin1, but suppress the expression of red/green opsin, rhodopsin, calbindin and dystrophin.
Acknowledgments
We thank Ms. Kanika Ghai, Ms. Jennifer Stanke and Dr. Heithem El-Hodiri for providing comments that contributed to final form of the manuscript. We also wish to thank Drs. E. Stoeckli and J. Saari for kindly providing antibodies to axonin1 and IRBP, respectively. The antibodies developed by Drs T. Jessell (Islet1, Islet2, Lim3), J. Dodd (axonin1), G.E. Morris (dystrophin), K.M. Buckley (SV2) and C. Cepko (visinin), respectively, were obtained from the Developmental Studies Hybridoma Bank, which was developed under the auspices of the NICHD and is maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. Services and expert technical assistance were provided by Dr. R. Burry at the Campus Microscopy and Imaging Facility at The Ohio State University. This work was supported by grants from the National Science Foundation (#0413795) and the National Institutes of Health (EY016043-02) to AJF.
References
- Adler R, Raymond PA. Have we achieved a unified model of photoreceptor cell fate specification in vertebrates? Brain Res. 2007 doi: 10.1016/j.brainres.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach I, Rodriguez-Esteban C, Carriere C, Bhushan A, Krones A, Rose DW, Glass CK, Andersen B, Izpisua Belmonte JC, Rosenfeld MG. RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nat Genet. 1999;22(4):394–399. doi: 10.1038/11970. [DOI] [PubMed] [Google Scholar]
- Bergmann M, Grabs D, Rager G. Expression of presynaptic proteins is closely correlated with the chronotopic pattern of axons in the retinotectal system of the chick. J Comp Neurol. 2000;418(3):361–372. [PubMed] [Google Scholar]
- Bergmann M, Grabs D, Roder J, Rager G, Jeromin A. Differential expression of neuronal calcium sensor-1 in the developing chick retina. J Comp Neurol. 2002;449(3):231–240. doi: 10.1002/cne.10302. [DOI] [PubMed] [Google Scholar]
- Bindra PS, Knowles R, Buckley KM. Conservation of the amino acid sequence of SV2, a transmembrane transporter in synaptic vesicles and endocrine cells. Gene. 1993;137(2):299–302. doi: 10.1016/0378-1119(93)90024-w. [DOI] [PubMed] [Google Scholar]
- Blank M, Koulen P, Kroger S. Subcellular concentration of beta-dystroglycan in photoreceptors and glial cells of the chick retina. J Comp Neurol. 1997;389(4):668–678. [PubMed] [Google Scholar]
- Bradford RL, Wang C, Zack DJ, Adler R. Roles of cell-intrinsic and microenvironmental factors in photoreceptor cell differentiation. Dev Biol. 2005;286(1):31–45. doi: 10.1016/j.ydbio.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn SL, Cepko CL. Development of the pattern of photoreceptors in the chick retina. J Neurosci. 1996;16(4):1430–1439. doi: 10.1523/JNEUROSCI.16-04-01430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5(6):877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006 doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9(1):37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Cornish EE, Hendrickson AE, Provis JM. Distribution of short-wavelength-sensitive cones in human fetal and postnatal retina: early development of spatial order and density profiles. Vision Res. 2004;44(17):2019–2026. doi: 10.1016/j.visres.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Cornish EE, Madigan MC, Natoli R, Hales A, Hendrickson AE, Provis JM. Gradients of cone differentiation and FGF expression during development of the foveal depression in macaque retina. Vis Neurosci. 2005;22(4):447–459. doi: 10.1017/S0952523805224069. [DOI] [PubMed] [Google Scholar]
- Drenhaus U, Morino P, Rager G. Expression of axonin-1 in developing amacrine cells in the chick retina. J Comp Neurol. 2004;468(4):496–508. doi: 10.1002/cne.10986. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Hallbook F. Newborn horizontal cells migrate bi-directionally across the neuroepithelium during retinal development. Development. 2004;131(6):1343–1351. doi: 10.1242/dev.01018. [DOI] [PubMed] [Google Scholar]
- Edqvist PH, Myers SM, Hallbook F. Early identification of retinal subtypes in the developing, pre-laminated chick retina using the transcription factors Prox1, Lim1, Ap2alpha, Pax6, Isl1, Isl2, Lim3 and Chx10. Eur J Histochem. 2006;50(2):147–154. [PubMed] [Google Scholar]
- Ellis JH, Richards DE, Rogers JH. Calretinin and calbindin in the retina of the developing chick. Cell Tissue Res. 1991;264(2):197–208. doi: 10.1007/BF00313956. [DOI] [PubMed] [Google Scholar]
- Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70(5):861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- Fischer AJ. Neural regeneration in the chick retina. Prog Retin Eye Res. 2005;24(2):161–182. doi: 10.1016/j.preteyeres.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Dierks BD, Reh TA. Exogenous growth factors induce the production of ganglion cells at the retinal margin. Development. 2002a;129(9):2283–2291. doi: 10.1242/dev.129.9.2283. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, McGuire CR, Dierks BD, Reh TA. Insulin and fibroblast growth factor 2 activate a neurogenic program in Muller glia of the chicken retina. J Neurosci. 2002b;22(21):9387–9398. doi: 10.1523/JNEUROSCI.22-21-09387.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer AJ, Omar G, Eubanks J, McGuire CR, Dierks BD, Reh TA. Different aspects of gliosis in retinal Muller glia can be induced by CNTF, insulin and FGF2 in the absence of damage. Molecular Vision. 2004a;10:973–986. [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Identification of a proliferating marginal zone of retinal progenitors in postnatal chickens. Dev Biol. 2000;220(2):197–210. doi: 10.1006/dbio.2000.9640. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Reh TA. Growth factors induce neurogenesis in the ciliary body. Dev Biol. 2003;259(2):225–240. doi: 10.1016/s0012-1606(03)00178-7. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Seltner RL, Poon J, Stell WK. Immunocytochemical characterization of quisqualic acid- and N-methyl-D-aspartate-induced excitotoxicity in the retina of chicks. J Comp Neurol. 1998;393(1):1–15. [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Aloisio G, Hoy H, Stell WK. Heterogeneity of horizontal cells in the chicken retina. J Comp Neurol. 2007a;500(6):1154–1171. doi: 10.1002/cne.21236. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stanke JJ, Ghai K, Scott M, Omar G. Development of bullwhip neurons in the embryonic chicken retina. J Comp Neurol. 2007b;503(4):538–549. doi: 10.1002/cne.21404. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Stell WK. Nitric oxide synthase-containing cells in the retina, pigmented epithelium, choroid, and sclera of the chick eye. J Comp Neurol. 1999;405(1):1–14. doi: 10.1002/(sici)1096-9861(19990301)405:1<1::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wallman J, Mertz JR, Stell WK. Localization of retinoid binding proteins, retinoid receptors, and retinaldehyde dehydrogenase in the chick eye. J Neurocytol. 1999;28(7):597–609. doi: 10.1023/a:1007071406746. [DOI] [PubMed] [Google Scholar]
- Fischer AJ, Wang SZ, Reh TA. NeuroD induces the expression of visinin and calretinin by proliferating cells derived from toxin-damaged chicken retina. Dev Dyn. 2004b;229(3):555–563. doi: 10.1002/dvdy.10438. [DOI] [PubMed] [Google Scholar]
- Fuhrmann S, Kirsch M, Heller S, Rohrer H, Hofmann HD. Differential regulation of ciliary neurotrophic factor receptor-alpha expression in all major neuronal cell classes during development of the chick retina. J Comp Neurol. 1998;400(2):244–254. [PubMed] [Google Scholar]
- Galli-Resta L, Resta G, Tan SS, Reese BE. Mosaics of islet-1-expressing amacrine cells assembled by short-range cellular interactions. J Neurosci. 1997;17(20):7831–7838. doi: 10.1523/JNEUROSCI.17-20-07831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai K, Stanke JJ, Fischer AJ. Patterning of the circumferential marginal zone of progenitors in the chicken retina. Brain Res. 2007 doi: 10.1016/j.brainres.2007.01.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackam AS, Bradford RL, Bakhru RN, Shah RM, Farkas R, Zack DJ, Adler R. Gene discovery in the embryonic chick retina. Mol Vis. 2003;9:262–276. [PubMed] [Google Scholar]
- Hendrickson A, Hicks D. Distribution and density of medium- and short-wavelength selective cones in the domestic pig retina. Exp Eye Res. 2002;74(4):435–444. doi: 10.1006/exer.2002.1181. [DOI] [PubMed] [Google Scholar]
- Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23(2):183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Huttl S, Michalakis S, Seeliger M, Luo DG, Acar N, Geiger H, Hudl K, Mader R, Haverkamp S, Moser M, Pfeifer A, Gerstner A, Yau KW, Biel M. Impaired channel targeting and retinal degeneration in mice lacking the cyclic nucleotide-gated channel subunit CNGB1. J Neurosci. 2005;25(1):130–138. doi: 10.1523/JNEUROSCI.3764-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Sun L, Li Q, Guo L, Stanley EF. Long splice variant N type calcium channels are clustered at presynaptic transmitter release sites without modular adaptor proteins. Neuroscience. 2006;138(4):1115–1125. doi: 10.1016/j.neuroscience.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Kong L, Li F, Soleman CE, Li S, Elias RV, Zhou X, Lewis DA, McGinnis JF, Cao W. Bright cyclic light accelerates photoreceptor cell degeneration in tubby mice. Neurobiol Dis. 2006;21(3):468–477. doi: 10.1016/j.nbd.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Michalakis S, Geiger H, Haverkamp S, Hofmann F, Gerstner A, Biel M. Impaired opsin targeting and cone photoreceptor migration in the retina of mice lacking the cyclic nucleotide-gated channel CNGA3. Invest Ophthalmol Vis Sci. 2005;46(4):1516–1524. doi: 10.1167/iovs.04-1503. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Karavanov AA, Curtiss PE, Ault KT, Sugimoto N, Watabe T, Shiokawa K, Jamrich M, Cho KW, Dawid IB, Taira M. Xlim-1 and LIM domain binding protein 1 cooperate with various transcription factors in the regulation of the goosecoid promoter. Dev Biol. 2000;224(2):470–485. doi: 10.1006/dbio.2000.9778. [DOI] [PubMed] [Google Scholar]
- Montiani-Ferreira F, Fischer A, Cernuda-Cernuda R, Kiupel M, DeGrip WJ, Sherry D, Cho SS, Shaw GC, Evans MG, Hocking PM, Petersen-Jones SM. Detailed histopathologic characterization of the retinopathy, globe enlarged (rge) chick phenotype. Mol Vis. 2005;11:11–27. [PubMed] [Google Scholar]
- Morino P, Buchstaller A, Giger R, Sonderegger P, Rager G. Differential expression of the mRNAs of the axonal glycoproteins axonin-1 and NgCAM in the developing chick retina. Brain Res Dev Brain Res. 1996;91(2):252–259. doi: 10.1016/0165-3806(95)00184-0. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Furukawa T, Cepko CL. Vertebrate photoreceptor cell development and disease. Trends Cell Biol. 1998;8(9):353–358. doi: 10.1016/s0962-8924(98)01341-5. [DOI] [PubMed] [Google Scholar]
- Moshiri A, McGuire CR, Reh TA. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev Dyn. 2005 doi: 10.1002/dvdy.20299. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Valtschanoff J, Allison DW, Sala C, Kim E, Craig AM, Weinberg RJ, Sheng M. Interaction of the postsynaptic density-95/guanylate kinase domain-associated protein complex with a light chain of myosin-V and dynein. J Neurosci. 2000;20(12):4524–4534. doi: 10.1523/JNEUROSCI.20-12-04524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Agarwal N. Arrestin mRNA expression, biosynthesis, and localization in degenerating photoreceptors of mutant rds mice retinas. J Comp Neurol. 1991;308(1):1–10. doi: 10.1002/cne.903080102. [DOI] [PubMed] [Google Scholar]
- Ostendorff HP, Peirano RI, Peters MA, Schluter A, Bossenz M, Scheffner M, Bach I. Ubiquitination-dependent cofactor exchange on LIM homeodomain transcription factors. Nature. 2002;416(6876):99–103. doi: 10.1038/416099a. [DOI] [PubMed] [Google Scholar]
- Pak W, Hindges R, Lim YS, Pfaff SL, O'Leary DD. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell. 2004;119(4):567–578. doi: 10.1016/j.cell.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990;5(1):1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- Perrin FE, Rathjen FG, Stoeckli ET. Distinct subpopulations of sensory afferents require F11 or axonin-1 for growth to their target layers within the spinal cord of the chick. Neuron. 2001;30(3):707–723. doi: 10.1016/s0896-6273(01)00315-4. [DOI] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and Temporal Patterns of Neurogenesis in the Chick Retina. Eur J Neurosci. 1991;3(6):559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. Retinal regeneration: common principles but a diversity of mechanisms. Adv Neurol. 1997;72:171–184. [PubMed] [Google Scholar]
- Raymond PA, Hitchcock PF. How the neural retina regenerates. Results Probl Cell Differ. 2000;31:197–218. doi: 10.1007/978-3-540-46826-4_11. [DOI] [PubMed] [Google Scholar]
- Reh TA, Fischer AJ. Stem cells in the vertebrate retina. Brain Behav Evol. 2001;58(5):296–305. doi: 10.1159/000057571. [DOI] [PubMed] [Google Scholar]
- Reh TA, Levine EM. Multipotential stem cells and progenitors in the vertebrate retina. J Neurobiol. 1998;36(2):206–220. [PubMed] [Google Scholar]
- Rogers JH. Two calcium-binding proteins mark many chick sensory neurons. Neuroscience. 1989;31(3):697–709. doi: 10.1016/0306-4522(89)90434-x. [DOI] [PubMed] [Google Scholar]
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65-/- mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46(10):3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- Sears S, Erickson A, Hendrickson A. The spatial and temporal expression of outer segment proteins during development of Macaca monkey cones. Invest Ophthalmol Vis Sci. 2000;41(5):971–979. [PubMed] [Google Scholar]
- Sonderegger P, Kunz S, Rader C, Buchstaller A, Berger P, Vogt L, Kozlov SV, Ziegler U, Kunz B, Fitzli D, Stoeckli ET. Discrete clusters of axonin-1 and NgCAM at neuronal contact sites: facts and speculations on the regulation of axonal fasciculation. Prog Brain Res. 1998;117:93–104. doi: 10.1016/s0079-6123(08)64010-8. [DOI] [PubMed] [Google Scholar]
- Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005;25(15):3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckli ET. Molecular mechanisms of commissural axon pathfinding. Prog Brain Res. 1998;117:105–114. doi: 10.1016/s0079-6123(08)64011-x. [DOI] [PubMed] [Google Scholar]
- Stoeckli ET, Landmesser LT. Axonin-1, Nr-CAM, and Ng-CAM play different roles in the in vivo guidance of chick commissural neurons. Neuron. 1995;14(6):1165–1179. doi: 10.1016/0896-6273(95)90264-3. [DOI] [PubMed] [Google Scholar]
- Sung CH, Tai AW. Rhodopsin trafficking and its role in retinal dystrophies. Int Rev Cytol. 2000;195:215–267. doi: 10.1016/s0074-7696(08)62706-0. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Koo SJ, Kania A, Lettieri K, Andrews S, Cox C, Jessell TM, Pfaff SL. A postmitotic role for Isl-class LIM homeodomain proteins in the assignment of visceral spinal motor neuron identity. Neuron. 2004;41(3):337–350. doi: 10.1016/s0896-6273(04)00011-x. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Lee SK, Jurata LW, Gill GN, Pfaff SL. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein-protein interactions. Cell. 2002;110(2):237–249. doi: 10.1016/s0092-8674(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Toy J, Norton JS, Jibodh SR, Adler R. Effects of homeobox genes on the differentiation of photoreceptor and nonphotoreceptor neurons. Invest Ophthalmol Vis Sci. 2002;43(11):3522–3529. [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Wahlin KJ, Adler R. Experimental Analysis of Photoreceptor Synaptogenesis: Cloning of Chicken Neuroligins, and Development of Bioassays for Their Functional Investigation. ARVO. 2007 4467. [Google Scholar]
- Xie HQ, Adler R. Green cone opsin and rhodopsin regulation by CNTF and staurosporine in cultured chick photoreceptors. Invest Ophthalmol Vis Sci. 2000;41(13):4317–4323. [PubMed] [Google Scholar]
- Yamagata K, Goto K, Kuo CH, Kondo H, Miki N. Visinin: a novel calcium binding protein expressed in retinal cone cells. Neuron. 1990;4(3):469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]


