Abstract
Melioidosis is endemic to Southeast Asia. The incidence of infection in visitors is not well known, especially for short visits. Thirteen (38%) of 34 previously unexposed U.S. Marines had positive serology after two weeks in Thailand, and one developed acute disseminated disease. Asymptomatic infection with Burkholderia pseudomallei may be common, even from brief exposures.
Keywords: Melioidosis, Burkholderia pseudomallei, military, Thailand, travel medicine
Melioidosis is a disease of protean manifestations caused by the saprophytic gram-negative bacteria, Burkholderia pseudomallei. Most disease occurs among residents of Southeast Asia and Australia, but cases have been reported in returning travelers.1,2 The degree of risk to travelers for brief visits is unknown. We report a case of melioidosis and the results of an epidemiologic survey among U.S. Marines returning from military exercises on the coast of Thailand.
Patients and methods
A joint multi-national military exercise was conducted on the coast of Thailand near Utapao in May of 2006. One U.S. Marine from a participating platoon was diagnosed with disseminated melioidosis and treated at our medical facility. The majority (n=38) of the other members of his platoon (n=47) were then evaluated by a history and physical examination, written epidemiologic survey regarding exposures, and serologic testing. They were also asked about symptoms during or after the exercise. The assessment was completed four months after return; no member travelled to other melioidosis endemic areas in this time period. Blood samples of 34 Marines were tested for antibody titers of IgG and IgM to Burkholderia pseudomallei via indirect fluorescence assay (IFA; Focus Diagnostics). IgM titers ≥ 1:40 and IgG titers ≥ 1:64 were considered positive.
Fifteen months after the exposure, electronic charts of the 38 Marines were examined for any interim healthcare visits which might suggest delayed onset melioidosis. Due to subsequent deployment of the members overseas, follow-up serologic testing was not performed. This study was not designed prior to deployment and thus predeployment serologies were not available. We compared military personnel with and without positive serologies by one-tailed Fishers exact and rank sum tests to identify potential risk factors for disease acquisition (STATA 10.0, College Station, TX).
Case report
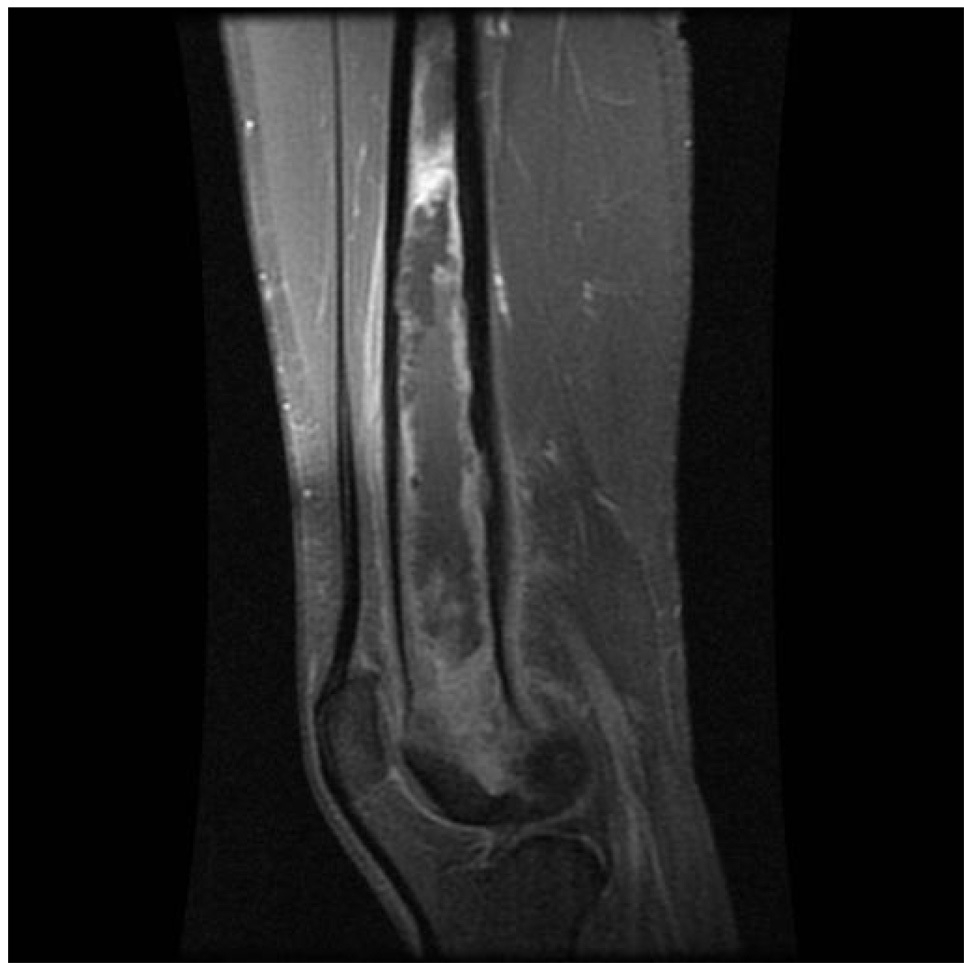
A 20-year-old previously healthy Marine developed fatigue, fevers, and night sweats while in Thailand. These symptoms continued to worsen, and four weeks after leaving Thailand he was admitted with fever of 104.7°F, night sweats, and a 12 pound weight loss. Laboratory evaluation showed a white blood cell count of 15,900 cells/mm3, an erythrocyte sedimentation rate of 99 mm/hr, and elevated transaminases (AST 302 IU/L and ALT 221 IU/L). Further evaluation revealed multiple bilateral pulmonary nodules and small abscesses in the liver, spleen, kidney and skin. A bone scan showed osteomyelitis of several bones, including the skull and wrist as well as extensive medullary osteomyelitis of his left distal femur extending for 17 cm (Figure). Cultures from both sputum and skin biopsies grew Burkholderia pseudomallei, and he was diagnosed with disseminated melioidosis. HIV serology was negative.
Figure.
T1 weighted MRI image showing extensive osteomyelitis of the distal left femur.
The patient was treated with ceftazidime, trimethoprim-sulfamethaxazole (TMP-SMX), and doxycycline for 14 weeks. He responded well clinically and repeat imaging showed resolution of the abscesses. Surgery was considered for the femur osteomyelitis, but due to the rapid clinical recovery and potential morbidity of the procedure, medical therapy alone was given. Because of the extensive bone involvement of the femur, a prolonged 12 month total course of therapy with doxycycline and TMP-SMX was prescribed, and he recovered without any physical limitations. He was cleared to return to full military duty and subsequently deployed to Iraq.
Results
Thirty-eight Marines completed the physician interview and survey, and serology was obtained among 34. All were male, and each reported that this was his first trip to Thailand or any other country with endemic melioidosis. The mean age was 23 years, with 71% non-Hispanic/white, 26% Hispanic/white, and 3% African Americans.
A total of 13 (38%) of the 34 Marines who had serologic testing were positive, including the case reported. Of the positives serologies, nine were IgM and four were IgG; none were positive for both IgG and IgM. In addition to the case above, four other members with positive serologies developed interval symptoms (Table 1), including one who had ongoing cough, facial pain, night sweats, and an unintentional 15 pound weight loss. This possible second clinical case was further evaluated with computed tomography, which showed a 4 mm right upper lobe nodule and pansinusitis. A nasal culture grew Staphylococcus aureus and treatment for sinusitis was initiated. His symptoms resolved, and he exited military service without further follow-up.
Table 1.
Descriptive Characteristics, Laboratory Data, and Clinical Symptoms among Seropositive Melioidosis Cases
| Case | Age (years) |
Race/ethnicity | Type of antibody |
Titer | Rice paddy exposure |
Mud exposure |
Cuts or sores |
Clinical Illness/Symptoms |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | H | IgG | ≥1:1024 | Y | Y | Y | Disseminated melioidosis* |
| 2 | 23 | H | IgG | 1:256 | Y | Y | N | None |
| 3 | 31 | H | IgG | 1:128 | N | Y | Y | None |
| 4 | 23 | C | IgG | 1:64 | N | Y | Y | Night sweats, weight loss, sinus pain** |
| 5 | 21 | C | IgM | ≥1:640 | N | N | N | Sore throat |
| 6 | 25 | H | IgM | 1:320 | Y | Y | N | None |
| 7 | 22 | H | IgM | 1:160 | N | N | N | None |
| 8 | 20 | C | IgM | 1:160 | N | Y | Y | Cough, rash on thighs |
| 9 | 23 | C | IgM | 1:80 | N | Y | N | None |
| 10 | 22 | C | IgM | 1:80 | N | N | N | NR |
| 11 | 20 | C | IgM | 1:40 | N | Y | N | None |
| 12 | 24 | C | IgM | 1:40 | N | Y | N | None |
| 13 | 22 | C | IgM | 1:40 | N | Y | N | Fatigue, fever, headache for one day |
Case reported above
Symptoms at time of survey
N, no; NR, not reported; Y, yes; H, Hispanic; C, Caucasian
Epidemiologic data were compared between the seropositive and seronegative groups. Characteristics of the seropositive patients are shown in the Table 1. Fifty-six percent of Hispanics were seropositive compared to 32% of non-Hispanics, but this was not statistically significant (p=0.25). There was no difference in age between the groups. Only four of the Marines recalled wading through rice fields; three of these four had positive serology (relative risk (RR) of 2.25 (p= 0.15)). Eighty five percent of the seropositive Marines recalled being exposed to mud compared to 62% of seronegative Marines (RR of 2.39, p= 0.14). There were no differences in skin cuts or lesions, illnesses, swimming, or exposures to water or dust between the groups.
Discussion
Melioidosis is a disease endemic to a small part of the world, but can be seen worldwide in returning travelers. The majority of disease occurs in Southeast Asia and northern Australia, where it is the most common cause of severe community-acquired pneumonia.3 Mortality rates range from 20% to 50%.4 Exposure is ubiquitous in many areas; Burkholderia pseudomallei has been isolated from >50% of rice field samples, and serostudies suggest that 80% children in northeast Thailand are exposed by four years of age.5,6,7
Most cases in western countries occur in persons returning from endemic regions, but the degree of risk is less well characterized. Risk estimates are complicated by the organism’s ability to lay dormant for years before the disease becomes symptomatic; for instance one case occurred 62 years after exposure.8 This led to concern about a “medical time bomb” in Vietnam veterans in which recrudescent disease would appear years later.9,10 Serologic studies found positive titers in 8 to 21% of returning military personnel.11,12 In the early 1970s, many patients were seen with delayed presentation, but few have been reported among Vietnam veterans since this time.13 As the ease of global travel has increased, the concern today focuses on the risk faced by short term travelers.
Our study is unique in that it evaluated the risk of a group, who had been an endemic area for only two weeks, an exposure duration similar to that of many travelers. One U.S. Marine developed severe melioidosis. Of the 34 others for whom serology was obtained, 38% were seropositive. This is higher than previous reports, even for more extensive exposures during the war in Vietnam11,12 and may be due in part to the serologic test used. Most previous studies have used indirect hemagglutination (IHA) which has been shown to be less sensitive than the IFA used in the current study.14 Another important difference is the timing of the serologic study. In this study, all patients had serology performed four months after a known, short exposure period. This may explain the high number of positive IgM results. While cross-reactivity to Legionella has been shown, false positives were infrequent in early studies and the specificity of IgM IFA has been reported at 99%.14 There are very little published data on the sensitivity and specificity of the IFA method since then. No predeployment serology was available so it cannot be definitively determined if the seropositive results were due to true seroconversions or were false positives. It is possible that the seropositive Marines seen in this study demonstrate true infection in young, healthy individuals who may remain asymptomatic or have a mild self-limited illness. Due to war-related deployments, most members of the group were not available for later evaluations, but may be at risk for delayed presentation of disease.
Melioidosis is usually acquired via cutaneous inoculation or inhalation, and rice farmers are often thought to become infected while wading through fields via small cuts in their feet. In our study group, 70% recalled significant mud exposure and 40% recalled skin cuts or sores. A greater percentage of the seropositive group recalled mud exposure or wading through rice fields, but this was not statistically significant in this small cohort.
The activities of this group of U.S. Marines differ from typical tourists, but perhaps not from those of adventure or occupational travelers. Conventional tourists may more closely resemble American diplomats living in Bangkok, who were found to have a 2.7% seropositivity rate using IHA testing.15 This study, however, is limited by an ill-defined exposure period and the decreased sensitivity of IHA. Our study is limited by the lack of predeployment serology and the relatively sparse data on the specificity of IFA serology. Nevertheless, it might be a better estimate for travelers with more extensive environmental exposures and those whose occupations require field work.
Our study suggests that healthy, young adults usually have a mild self-limited disease or remain completely asymptomatic. Severe, acute disease may reflect either a high inoculum or impaired immunity and appears to occur in a small percentage of infected, healthy adults. Our case patient had an open wound on his hand and waded in rice fields, and therefore may have developed disease due to a high inoculum. The majority of those infected will not develop clinically apparent, acute disease, but may develop late-onset disease, which can be precipitated by relatively minor disruptions to host defenses, such as surgery, trauma or the development of diabetes.13 In summary, our study suggests that up to 40% of high-risk short-term travelers may develop asymptomatic B. pseudomallei infections with travelers occasionally experiencing severe melioidosis..
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government.
References
- 1.Ezzedine K, Heenen M, Halvy D. Imported Cutaneous Melioidosis in Traveler, Belgium. EID. 2007;13:946–947. doi: 10.3201/eid1306.061460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yazdanpanah Y, Lemaire X, Senneville E, et al. Melioidotic Osteomyelitis of the Femur Occurring in a Traveler. J Travel Med. 2002;9:53–54. doi: 10.2310/7060.2002.23954. [DOI] [PubMed] [Google Scholar]
- 3.Currie BJ, Fisher DA, Howard DM, et al. Endemic Melioidosis in Tropical Northern Australia: A 10-Year Prospective Study and Review of the Literature. CID. 2000;31:981–986. doi: 10.1086/318116. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AC, Currie BJ. Melioidosis: Epidemiology, Pathophysiology, and Management. Clin Micr Rev. 2005;18:383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith MD, Wuthiekanun V, Walsh AL, et al. Quantitative Recovery of Burkholderia pseudomallei from Soil in Thailand. Trans R Soc Trop Med. 1995;89:488–490. doi: 10.1016/0035-9203(95)90078-0. [DOI] [PubMed] [Google Scholar]
- 6.Stone R. Racing to Defuse a Bacterial Time Bomb. Science. 2007;317:1022–1024. doi: 10.1126/science.317.5841.1022. [DOI] [PubMed] [Google Scholar]
- 7.Kanaphun P, Thirawattanasuk N, Supputtamongkol Y, et al. Serology and Carriage of Pseudomonas pseudomallei: A Prospective Study in 1000 Hospitalized Children in Northeast Thailand. JID. 1993;167:230–233. doi: 10.1093/infdis/167.1.230. [DOI] [PubMed] [Google Scholar]
- 8.Ngauy V, Lemeshev Y, Sadkowski L, et al. Cutaneous Melioidosis in a Man Who Was Taken as a Prisoner of War by the Japanese during World War II. J Clin Mic. 2005;43:970–972. doi: 10.1128/JCM.43.2.970-972.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg JH. Public Health Problems Relating to the Vietnam Returnee. JAMA. 1969;207:697–702. [PubMed] [Google Scholar]
- 10.Sanford JP, Moore WL. Recrudescent Melioidosis: A Southeast Asian Legacy. Am Rev Resp Dis. 1971;104:452–453. doi: 10.1164/arrd.1971.104.3.452. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto RA, Brown GL, Blair EB, et al. Melioidosis: Serologic Studies on US Army Personnel Returning from Southeast Asia. Mil Med. 1971;136:694–698. [PubMed] [Google Scholar]
- 12.Clayton AJ, Lisella RS, Martin DG. Melioidosis: A Serological Survey in Military Personnel. Mil Med. 1973;138:24–26. [PubMed] [Google Scholar]
- 13.Jackson AE, Moore WL, Sanford JP. Recrudescent Melioidosis Associated with Diabetic Ketoacidosis. Arch Int Med. 1972;130:268–271. [PubMed] [Google Scholar]
- 14.Ashdown LR, Johnson RW, Koehler JM, et al. Enzyme-Linked Immunosorbant Assay for the Diagnosis of Clinical and Subclinical Melioidosis. JID. 1989;160:253–260. doi: 10.1093/infdis/160.2.253. [DOI] [PubMed] [Google Scholar]
- 15.Riesland N, Simpsom AJH, Wilde H. Diplomats in Bangkok and Risk of Melioidosis. J Trav Med. 2001;8:146–147. doi: 10.2310/7060.2001.24377. [DOI] [PubMed] [Google Scholar]