Abstract
In many organisms, population-density sensing and sexual attraction rely on small-molecule-based signalling systems1,2. In the nematode Caenorhabditis elegans, population density is monitored through specific glycosides of the dideoxysugar ascarylose (the `ascarosides') that promote entry into an alternative larval stage, the non-feeding and highly persistent dauer stage3,4. In addition, adult C. elegans males are attracted to hermaphrodites by a previously unidentified small-molecule signal5,6. Here we show, by means of combinatorial activity-guided fractionation of the C. elegans metabolome, that the mating signal consists of a synergistic blend of three dauer-inducing ascarosides, which we call ascr#2, ascr#3 and ascr#4. This blend of ascarosides acts as a potent male attractant at very low concentrations, whereas at the higher concentrations required for dauer formation the compounds no longer attract males and instead deter hermaphrodites. The ascarosides ascr#2 and ascr#3 carry different, but overlapping, information, as ascr#3 is more potent as a male attractant than ascr#2, whereas ascr#2 is slightly more potent than ascr#3 in promoting dauer formation7. We demonstrate that ascr#2, ascr#3 and ascr#4 are strongly synergistic, and that two types of neuron, the amphid single-ciliated sensory neuron type K (ASK) and the male-specific cephalic companion neuron (CEM), are required for male attraction by ascr#3. On the basis of these results, male attraction and dauer formation in C. elegans appear as alternative behavioural responses to a common set of signalling molecules. The ascaroside signalling system thus connects reproductive and developmental pathways and represents a unique example of structure- and concentration-dependent differential activity of signalling molecules.
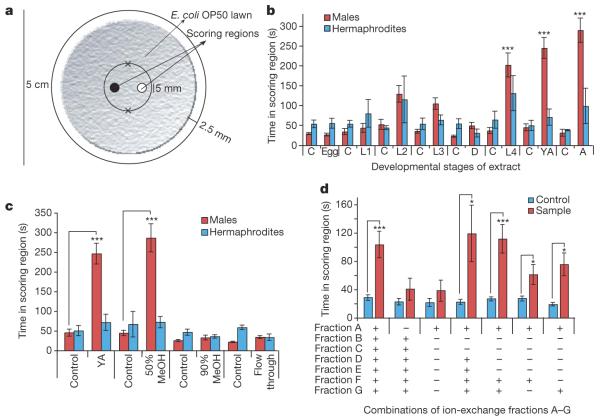
C. elegans hermaphrodites produce a chemical signal that strongly attracts males5,6. To identify this signal, we developed a new protocol for obtaining samples of secreted metabolites from different life stages of C. elegans (Supplementary Information). Biological activity of these samples was confirmed using a bioassay in which time spent by males or hermaphrodites within the vicinity of a chemical sample was measured (Fig. 1a, Supplementary Movie 1, Supplementary Methods). To determine the timing of mating pheromone release, samples from all life stages (egg, L1, dauer, L2, L3, L4, young adult and adult) were tested for biological activity on both males and hermaphrodites (Fig. 1b). C. elegans males were strongly attracted to L4, young adult and adult samples, whereas hermaphrodites were not attracted. Consistent with the observation that C. elegans reaches sexual maturity at the end of the L4 stage8 (Fig. 1b), these results indicated that L4, young adult and adult hermaphrodites secrete a chemical signal that specifically attracts males.
Figure 1. Activity-guided fractionation of worm metabolites.
a, Representation of the bioassay used to measure mating behaviour in worms. Crosses mark the initial positions of the assayed animals (see Supplementary Methods).b, Male and hermaphrodite responses to secreted metabolites produced by hermaphrodites at different developmental stages. L1-L4, the first four larval stages; D, dauer stage; YA, young adult stage; A, adult stage; C, control. n≥30 animals for each histogram. c, Assay results for C18-reversed-phase chromatography fractions of young adult metabolite extract. d, Assay results for combinations of ion-exchange fractions of the active fraction from c: A, neutral; B, 250 mM KCl anion; C, 250 mM KCl cation; D, 500 mM KCl anion; E, 500 mM KCl cation; F, 1,000 mM KCl cation; G, 1,000 mM KCl anion. Error bars, s.e.m.; *P<0.01, ***P<0.0001, unpaired t-test (see Supplementary Methods).
To characterize the mating signal, we subjected samples derived from young adults to a multistep fractionation scheme, starting with C18-reverse-phase solid-phase extraction chromatography. Strong male attraction was observed for one of the resulting fractions (Fig. 1c), which was further fractionated using coupled ion-exchange columns. Of the resulting seven fractions A to G, none was active at physiologically relevant concentrations when tested individually (data not shown), which suggested that male attraction depends on the synergy of two or more signalling molecules. To determine which fractions were required for activity, we assayed a series of combinations of fractions A-G; these assays showed that combination of fractions F or G with fraction A produced significant activity (Fig. 1d).
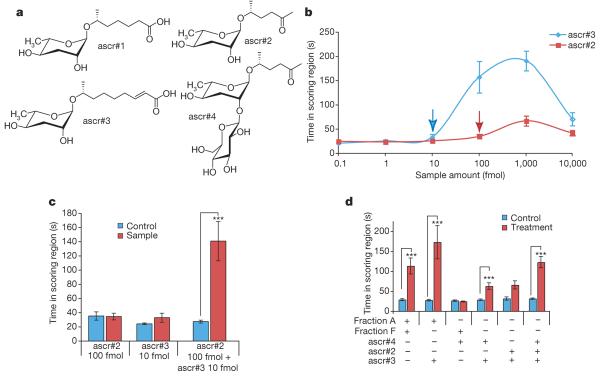
Because fraction A appeared to be required for full activity, we characterized it using nuclear magnetic resonance (NMR) spectroscopy9 and liquid chromatography-mass spectrometry (LC-MS) (Supplementary Figs 2 and 8 and Supplementary Table 1). Two-dimensional NMR spectroscopic analyses suggested that the major component of fraction A is a novel derivative of 5-O-ascarylosyl-5R-hydroxy-2-hexanone, or ascr#2 (according to proposed nomenclature for nematode compounds) (Fig. 2a, Supplementary Figs 3 and 4), which was recently shown to induce dauer formation in C. elegans7. Additional NMR spectroscopic analyses showed that the major component of fraction A features a β-glucosyl substituent attached to C2 of the ascarylose in ascr#2 (Supplementary Figs 5-7). These assignments were corroborated by LC-MS analyses that showed an m/z value of 426 to represent the ammonium adduct (M + NH44) of a compound with a nominal mass of 408 AMU and molecular formula C18H32O10. Comparison of these spectroscopic data with those of synthetic sample of 5-O-(2′-O-[β-D-glucosyl]-ascarylosyl)-5R-hydroxy-2-hexanone provided final proof for the identify of the major component of fraction A, which we named ascr#4 (Fig. 2a).
Figure 2. Synergy between ascr#2, ascr#3 and ascr#4.
a,Structures of ascr#1, ascr#2, ascr#3 and ascr#4. b, Dose-response curves of ascr#2 and ascr#3. c, Synergistic effects of ascr#2 and ascr#3 from points respectively indicated by red and blue arrows in b. d, Demonstration that the three synthetic compounds account for most of the mating activity. For all entries, ascr#4 was tested at 1 pmol and ascr#2 and ascr#3 were each tested at 20 fmol. n≥30 animals for each histogram. Error bars, s.e.m.; *P<0.01, ***P<0.0001, unpaired t-test.
The identification of ascr#4 in fraction A suggested that ascaro-sides might have a role as mating signals. Therefore, we analysed the fractions required for activity, A, F and G, for the presence of additional ascarosides. LC-MS analyses revealed the presence of ascr#3 in fractions F and G, as well as of small amounts of ascr#2 in fraction A (Supplementary Fig. 8). Next we tested synthetic samples of the three identified ascarosides, ascr#2, ascr#3 and ascr#4, for activity, using the assay described in Fig. 1a (Fig. 2a). Consistent with the assay results for fractions A, F and G, none of the three compounds was active at physiological concentrations when tested individually (data not shown). However, at higher concentrations, ascr#2 and ascr#3 were both active (Fig. 2b). The corresponding dose-response curves show a strongly biphasic activity profile (Fig. 2b), which is characteristic of many types of pheromone10. In contrast to ascr#2 and ascr#3, ascr#4 alone was not active in the male attraction assay, even at concentrations much greater than physiological levels. Because the assay results for the ion-exchange fractions (Fig. 1d) suggested that the mating signal consists of multiple compounds that act synergistically, we combined ascr#2 and ascr#3 in amounts that did not elicit significant male attraction when assayed individually (10 fmol ascr#3 and 100 fmol ascr#2; arrows in Fig. 2b). This mix produced strong male attraction, demonstrating synergism of ascr#2 and ascr#3 (Fig. 2c).
At the concentrations of ascr#2 and ascr#3 found in fractions A and F, a mixture of these two compounds also produced significant activity, but was less potent than the combination of fractions A and F (Fig. 2d), suggesting synergy with a third component, perhaps ascr#4. Additional tests using ternary mixtures of synthetic ascr#2, ascr#3 and ascr#4 confirmed this hypothesis. A physiological mixture of 20 fmol ascr#2, 20 fmol ascr#3 and 1 pmol ascr#4 reproduced the activity in fractions A and F and elicited significantly stronger male attraction than a mixture of 20 fmol ascr#2 and 20 fmol ascr#3 alone (Fig. 2d). The same mixture of ascr#2, ascr#3 and ascr#4 was then tested on different species of the Caenorhabditis genus. Caenorhabditis brenneri and Caenorhabditis remanei males responded to the mixture in a way very similar to C. elegans, whereas Caenorhabditis briggsae and Caenorhabditis japonica males responded only weakly (Supplementary Fig. 9)11.
We then tested whether attraction by the identified pheromone components is sex specific. Ascr#3, tested at the concentration that elicits maximal male attraction, was significantly less attractive to hermaphrodites (Supplementary Fig. 10a). Similarly, ascr#2 showed little or no activity for hermaphrodites (Supplementary Fig. 10a). At higher concentrations of ascr#2 and ascr#3, hermaphrodites were strongly deterred whereas males showed neither attraction nor deterrence (Supplementary Fig. 10a). These results demonstrate that the identified ascarosides are sex-specific attractants.
These results indicate that ascarosides regulate both dauer formation and male attraction in C. elegans. To determine how these signalling molecules elicit such different biological responses, we compared their activity profiles with regard to both dauer formation and male attraction. We found that ascr#3 elicited the strongest response for male attraction. Males spent approximately ~6.6 times longer in the ascr#3-spotted region than in the control region, whereas ascr#2 elicited a maximum ~2.8-fold increase (Fig. 2b and Supplementary Fig. 10a). However, ascr#4 and the first-published dauer-inducing ascaroside, ascr#1 (ref. 12), the second of which we did not detect in any of our active fractions, were not active in the attraction assay at the range of concentrations tested (data not shown). In the dauer formation assay, ascr#4 was weakly active and ascr#2 and ascr#3 showed strong dauer-inducing activity at the concentrations previously reported (Supplementary Fig. 11a-c). Ascr#3 is much more potent as a male attractant than ascr#2, whereas in the dauer assay ascr#2 is slightly more potent than ascr#3. Together with the observed synergy, these activity profiles suggest that ascr#2 and ascr#3, and possibly ascr#4, act by means of different molecular mechanisms13,14.
To further investigate the connection between dauer and mating signals, we analysed media extracts of daf-22 mutants, which are known not to contain the dauer pheromone15. We found that neither daf-22 media extracts nor daf-22 worm pellet contained ascr#2, ascr#3 or ascr#4, and that daf-22 media extracts did not attract males at the range of dilutions tested (Supplementary Fig. 12). Additional analyses of extracts from Escherichia coli cultures (HB101 and OP50) by LC-MS and NMR spectroscopy confirmed that E. coli do not produce ascr#2, ascr#3 or ascr#4 (Supplementary Figs 13-15); however, it is conceivable that bacterial food sources contribute a precursor to the biosynthesis of these compounds.
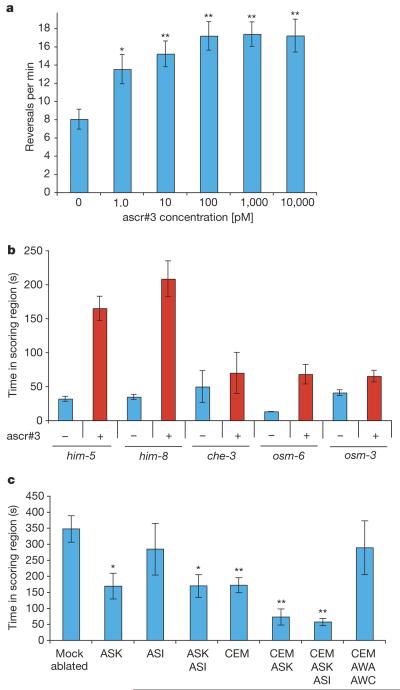
Given the nature of the attraction assay used in this study (Fig. 1a), the exact concentration of the compounds tested in the scoring region was not well defined. However, it seems likely that the test sample volume (1 μl), when added to the scoring region, was diluted by diffusion, suggesting that the actual concentrations of the assayed compounds on the plate were much lower than the original concentrations in the test sample volumes. Male C. elegans change direction of motion more frequently in the presence of an attractant, which correlates with an increase in time spent in the sample scoring region5, suggesting that reversal frequency could be used as a measure of pheromone perception. Thus, we monitored reversal frequency of males on agar plates with a range of concentrations of the most active pheromone components, ascr#2 and ascr#3, using the automated worm tracking system16. As shown in Fig. 3a, reversal frequency is increased by concentrations of ascr#3 as low as 1 pM. For ascr#2, only weak increases of reversal frequency were observed (Supplementary Fig. 10c, d). These results suggest that ascr#3 acts as a male attractant at concentrations more than 10,000 times lower than those required for dauer induction.
Figure 3. Neurons mediating response to ascr#3.
a,Dose-response curve for male reversal rates on plates with increasing concentrations of ascr#3. n≥15 animals for each histogram. Error bars, s.e.m.; *P<0.05, **P<0.01, one-factor analysis of variance with post test. b, Male attraction by ascr#3 in sensory-deficient mutants17-19. Error bars, s.e.m. c, General sensory neurons and sex-specific neurons mediate response to ascr#3. Ablation of neurons involved in volatile chemotaxis (amphid winged sensory neuron type A (AWA) and amphid winged sensory neuron type C (AWC)) together with the CEM neurons6 did not affect response to ascr#3, in comparison with animals lacking only CEM neurons. n≥15 animals for each ablation set. Error bars, s.e.m.;*P<0.05, **P<0.01, one-factor analysis of variance with post test.
General sensory mutants such as osm-6, which is expressed in all ciliated neurons in hermaphrodites and males17, are defective in response to ascr#3 (Fig. 3b). Mutants defective in osm-3, which is expressed in a subset of these ciliated sensory neurons and in the male-specific cephalic companion (CEM) neurons18,19, were also defective in response to ascr#3 (Fig. 3b). Two G-protein α-subunits (gpa-2 and gpa-3) responsible for sensing the dauer pheromone are expressed in the amphid single-ciliated sensory neuron type I (ASI), the amphid double-ciliated sensory neuron type L and the amphid single-ciliated sensory neuron type K (ASK)20. Ablation of the ASK neurons, but not the ASI neurons, partially affected response to ascr#3 (Fig. 3c). The male-specific CEM neurons have been implicated in sensing the mating signal along with other neurons6,11. Removal of the CEM neurons also resulted in partial insensitivity to ascr#3, but removal of the CEM and ASK neurons resulted in complete loss of sensitivity to ascr#3 (Fig. 3c), indicating that response to ascr#3 is mediated by both sex-specific and general sensory neurons. This finding provides a cellular mechanism by which males and hermaphrodites respond differentially to the ascarosides.
Both sexual reproduction and dauer formation, a population-control mechanism that increases larval lifespan and resilience, are major life-history traits. In many organisms, including C. elegans, both experimental manipulation and natural genetic variation often have opposite effects on fecundity and lifespan, suggesting a pervasive, inverse relationship between these two traits21-23. The discovery that largely overlapping families of small molecules regulate these traits (Supplementary Fig. 1) provides a direct linkage between the corresponding molecular pathways. Characterization of the ascaroside receptors and their downstream targets, as well as the elucidation of ascaroside biosynthesis and the molecular identity of daf-22, could provide further insights into how developmental and reproductive pathways are connected.
METHODS SUMMARY
Synchronized C. elegans (N2 Bristol) were grown on S-complete medium supplemented with E. coli (strain HB101) to desired life stage, washed with M9 buffer to remove bacteria and incubated for 1 h in double-diluted water (ddH2O) to collect worm-secreted metabolites. Metabolite samples thus produced were tested for mating activity, chromatographically fractionated and analysed using NMR spectroscopy and mass spectrometry (see Methods and Supplementary Information for details).
Mating assays were performed as described previously5 but were population based. All assays were conducted on plates containing nematode growth medium with a thin film of E. coli (OP50) spread throughout the plate as a food source. The worms were given a choice of worm metabolite fraction (or synthetic ascaro-sides) and control water, and the amount of time spent in each region was measured (see Methods and Supplementary Methods for details). To analyse locomotory behaviour of worms in presence of the ascarosides, standard nematode-growth-medium plates were prepared with the different concentrations of the ascarosides, and worm movement was monitored using an automated tracker to calculate parameters of locomotion16.
Ascr#1, ascr#2 and ascr#3 were synthesized from L-rhamnose and (2R)-propylene oxide (ascr#1, ascr#3) or (2R,5R)-hexanediol (ascr#2) as described previously7, and ascr#4 was subsequently prepared from acetobromo-α-D-glucose and ascr#2 (see Supplementary Methods for details). For NMR spectroscopic comparisons of daf-22 and wild-type-derived metabolite mixtures, two-week-old liquid cultures of daf-22 or wild-type (N2) worms raised on E. coli (OP50) were extracted and the resulting metabolite samples directly prepared for NMR spectroscopic analyses by means of double-quantum filtered correlation spectroscopy as previously described7. For comparison of worm-derived and bacterial metabolites, E. coli (OP50) cultures were extracted and subsequently analysed by NMR spectroscopy using the same protocol.
METHODS
Collecting C. elegans-secreted metabolites
Synchronized C. elegans(N2 Bristol) with a worm density of 10,000 worms per millilitre was grown at 22 °C at 250 r.p.m. in an incubator shaker in S-complete medium supplemented with E. coli (strain HB101): 1% for L2, 2% for L3, 3% for L4, 3% for young adult, 4% for adult and 0% for L1, which was not fed. After worms reached the desired life stages, they were exposed to several wash and filtration (10-μm NITEX nylon filters) steps using M9 buffer to remove bacteria. The worms were collected between the washes either by gentle centrifugation at 121g for 30 s or by allowing the worms to settle for 10 min. To remove the bacteria in the gut of the worms, they were placed in M9 buffer in an incubator shaker for 30 min at 22 °C at 250 r.p.m., which was followed by three washes with ddH2O. Subsequently, C. elegans-secreted metabolites were collected by incubating in ddH2O in an incubator shaker for 1 h at 22 °C at 250 r.p.m. with a worm density of~30,000 worms per millilitre for L2, L3, young adult and adult;~15,000 worms per millilitre for L4; and ~100,000 worms per millilitre for L1. The worms were removed from conditioned water by gentle centrifugation at 121g for 10 s. The conditioned water was filtered through a 0.2-μm filter, lyophilized and stored at —80 °C. At least three independent experiments were done for each developmental stage. We developed a working unit called `worm equivalents' to keep track of relative concentrations of unknown compounds. One worm equivalent is the volume of worm water that contains the compounds secreted by one worm in 1 h.
Mating (male-attraction) assay
We modified the single-worm response assay in C. elegans3 to test multiple worms. Standard nematode-growth-medium plates (5-cm diameter) were used for assaying biological activity of the worm-conditioned water. The assay plates consisted of a thin lawn of an E. coli OP50 culture, grown overnight, with a ~0.25-cm gap between the bacterial lawn and the edge of the plate to prevent the animals from escaping. Plates were stored at room temperature (20 °C) for two days before being used in trials. Two spots (5-mm diameter) were spotted 1.6 cm apart on a template and stuck to the bottom of the assay plate (Fig. 1a). 0.8 μl of the control and the worm metabolite were placed in the two circles and allowed to dry for approximately 30 s. To remove any bias, control and conditioned water spots were interchanged after every trial. Males and hermaphrodites were harvested daily at the L4 stage and stored at 20 °C overnight with, per plate, 50-60 worms of the same sex to be used as young adults the following day. Five worms were placed ~1.0 cm away from each spot (10 worms total) and allowed to acclimatize for 5 min. Trials were videotaped at 30 frames per second for 15 min using the Unibrain Fire-i software. For each sample, a minimum of four or five trials were conducted each day and each stage was tested on at least three different days.
Purification of mate-finding pheromones
Secreted metabolites were collected from 4,000,000 young adult worms using the method described above. The purification involved a series of fractionation steps guided by the male-attraction assay. Conditioned young adult water was lyophilized and the residue suspended in H2O. Reverse-phase solid-phase extraction was performed using Sep-Pak Plus C18 cartridges (Waters). The column was eluted sequentially with 50% and 90% MeOH. The active 50%-MeOH fraction was further fractionated by using a SAX anion-exchange column (Alltech) coupled to a SCX cation-exchange column (Alltech). After the neutral fraction was collected, the cation and anion columns were detached and eluted separately with 250, 500 and 1,000mM KCl. The 250-, 500- and 1,000-mM-KCl fractions were desalted using C18 columns (Waters). The neutral fraction (fraction A) was lyophilized and re-suspended in 6 μl of D2O containing 0.25 mM of the proton reference standard 3-(trimethylsilyl)propionic acid-D4, for characterization by means of two-dimensional NMR spectroscopy including double-quantum filtered correlation spectroscopy, total correlation spectroscopy, heteronuclear single-quantum coherence, heteronuclear multiple-bond correlation and nuclearÖverhauser enhancement spectroscopy. All NMR spectra were acquired at 27 °C using a 1-mm triple-resonance high-temperature superconducting probe and a 600-MHz Bruker Avance II spectrometer11 (Supplementary Figs 1-6). Total sample amount for these analyses corresponded to about 4,000,000 worm equivalents. Fractions A, F and G were analysed further by LC-MS (Supplementary Fig. 7). In addition, the peak corresponding to ascr#4 in fraction A was analysed by high-resolution mass spectrometry using an Agilent 6210 mass spectrometer: mass of sodium adduct of molecular ion [M + Na]+ calculated for C18H32O10 Na, 431.1888 AMU; found, 431.1907 AMU.
LC-MS analysis of ion-exchange fractions
A Thermo Finnigan LCQ Deca XP Max was used with electrospray ionization in positive or negative ion mode in the 50-1,000 AMU range (sheet gas, 25 arbitrary units; sweep gas, 5 arbitrary units; spray voltage, 5.00 kV; capillary temperature, 285 °C; capillary voltage, 3.0 V). Daughter ion spectra were obtained from a dependent scan of the most intense ion in a predefined mass range. The Thermo Separation spectra HPLC system consisted of a P4000 quaternary pump, an AS 3000 autosampler and a UV6000 diode array detector. The tertiary solvents are consisted of methanol with 0.05% formic acid (a), water with 10 mM ammonium formate (b) and 90% acetonitrile-10% water with 10 mM ammonium formate (c). With the column temperature maintained at 60 °C and a solvent flow of 1.0 ml min-1, the C18 column (ODS-AMQ, S-5 μm, 20 nm, 250×4.6 mm i.d., YMC) was eluted with a solvent composition starting with 4:90:6 (a:b:c) for 2 min followed by a gradient to 4:0:96 in 14 min and then kept at that composition for 5 min. Ultraviolet absorption was monitored at 190 to 400 nm and the solvent flow between the ultraviolet detector and the mass spectroscopy electrospray interface split 9:1 with a low-volume micro needle P450 splitter valve (Upchurch Scientific), making it possible to obtain spectra of eluted compounds and simultaneously collect 90% of the injected material for bioassays.
Supplementary Material
Acknowledgements
This work was supported by the Human Frontiers Science Program (A.S.E., P.W.S. and P.E.A.T.), a US National Institutes of Health grant (P41 GM079571) to F.C.S., and the Howard Hughes Medical Institute, of which J.S. is an associate and P.W.S. an investigator. NMR data were collected in the UF-AMRIS facility; we thank J. Rocca for assistance. We thank E. Peden and D. Xue for the ceh-30 strains, C. J. Cronin and A. Choe for advice on behavioural assays, L. R. Baugh for liquid-culture dauer formation assays, B. Fox for assistance with the synthesis of ascr#2, ascr#3 and ascr#4, and M. de Bono, A. Dossey and M. Stadler for discussions. E. Hallem, J. Bungert and D. Hutchinson provided comments on the manuscript.
Footnotes
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dulac C, Torello AT. Molecular detection of pheromone signals in mammals: from genes to behaviour. Nature Rev. Neurosci. 2003;4:551–562. doi: 10.1038/nrn1140. [DOI] [PubMed] [Google Scholar]
- 3.Golden JW, Riddle DL. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science. 1982;218:578–580. doi: 10.1126/science.6896933. [DOI] [PubMed] [Google Scholar]
- 4.Golden JW, Riddle DL. A pheromone-induced developmental switch in Caenorhabditis elegans: Temperature-sensitive mutants reveal a wild-type temperature-dependent process. Proc. Natl Acad. Sci. USA. 1984;81:819–823. doi: 10.1073/pnas.81.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon JM, Sternberg PW. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2002;99:1598–1603. doi: 10.1073/pnas.032225799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White JQ, et al. The sensory circuitry for sexual attraction in C. elegans males. Curr. Biol. 2007;17:1847–1857. doi: 10.1016/j.cub.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Butcher RA, Fujita M, Schroeder FC, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nature Chem. Biol. 2007;3:420–422. doi: 10.1038/nchembio.2007.3. [DOI] [PubMed] [Google Scholar]
- 8.Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev. Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- 9.Brey WW, et al. Design, construction, and validation of a 1-mm triple-resonance high-temperature-superconducting probe for NMR. J. Magn. Reson. 2006;179:290–293. doi: 10.1016/j.jmr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Carde RT, Elkinton JS. In: Field Trapping with Attractants: Methods and Interpretation. Hummel HE, Miller TA, editors. Springer; 1984. pp. 111–129. [Google Scholar]
- 11.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc. Natl Acad. Sci. USA. 2007;104:6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong PY, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 13.Gershenzon J, Dudareva N. The function of terpene natural products in the natural world. Nature Chem. Biol. 2007;3:408–414. doi: 10.1038/nchembio.2007.5. [DOI] [PubMed] [Google Scholar]
- 14.Lehar J, et al. Chemical combination effects predict connectivity in biological systems. Mol. Syst. Biol. 2007;3 doi: 10.1038/msb4100116. doi:10.1038/msb4100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golden JW, Riddle DL. A gene affecting production of the Caenorhabditis elegans dauer-inducing pheromone. Mol. Gen. Genet. 1985;198:534–536. doi: 10.1007/BF00332953. [DOI] [PubMed] [Google Scholar]
- 16.Cronin CJ, et al. An automated system for measuring parameters of nematode sinusoidal movement. BMC Genet. 2005;6 doi: 10.1186/1471-2156-6-5. doi:10.1186/1471-2156-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae YK, et al. General and cell-type specific mechanisms target TRPP2/PKD-2 to cilia. Development. 2006;133:3859–3870. doi: 10.1242/dev.02555. [DOI] [PubMed] [Google Scholar]
- 19.Tabish M, Siddiqui ZK, Nishikawa K, Siddiqui SS. Exclusive expression of C. elegans osm-3 kinesin gene in chemosensory neurons open to the external environment. J. Mol. Biol. 1995;247:377–389. doi: 10.1006/jmbi.1994.0146. [DOI] [PubMed] [Google Scholar]
- 20.Zwaal RR, Mendel JE, Sternberg PW, Plasterk RH. Two neuronal G proteins are involved in chemosensation of the Caenorhabditis elegans Dauer-inducing pheromone. Genetics. 1997;145:715–727. doi: 10.1093/genetics/145.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Naturea. 1996;379:723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- 22.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 23.Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.