Abstract
Objective
To describe a novel process and present results of formative research to develop a pediatric office intervention that uses available systems of care for addressing parental smoking.
Methodology
The scientific development of the intervention occurred in three stages. In stage one, we designed an office system for parental tobacco control in the pediatric outpatient setting based on complementary conceptual frameworks of preventive services delivery, conceptualized for the child healthcare setting through a process of key interviews with leaders in the field of implementing practice change; existing Public Health Service guidelines that had been shown effective in adult practices; and adaptation of an evidenced-based adult office system for tobacco control. This was an iterative process that yielded a theoretically framed intervention prototype. In stage two, we performed focus group testing in pediatric practices with pediatricians, nurses, clinical assistants, and key office staff. Using qualitative methods, we adapted the intervention prototype based on this feedback to include five key implementation steps for the child healthcare setting. In stage three, we presented the intervention to breakout groups at two national meetings of pediatric practitioners for further refinements.
Results
The main result was a theoretically grounded intervention that was responsive to the barriers and suggestions raised in the focus groups and at the national meetings. The CEASE intervention is designed to be flexible and adaptable to the particular practices' staffing, resources, and physical configuration. Practices can choose materials relevant to their own particular systems of care (www.ceasetobacco.org).
Conclusions
Conceptually-grounded and focus group tested strategies for parental tobacco control are now available for implementation in the pediatric outpatient setting. The tobacco control intervention development process might have particular relevance for other chronic pediatric conditions that have a strong evidence base and have available treatments or resources that are underused.
Keywords: smoking, tobacco, pediatrics, family practice, parent, smoking cessation, secondhand smoke, environmental tobacco smoke, tobacco control, quitline, telephone counseling
Introduction
In summarizing the past 20 years of research on the health effects of secondhand smoke (SHS), the 2006 Surgeon General's Report on the health consequences of involuntary exposure to tobacco smoke emphasizes that SHS is a major cause of disease, with no safe level of exposure.1 Exposure puts children at risk for asthma, bronchiolitis, sinusitis, bacterial respiratory infections, decreased lung growth, decreased exercise tolerance, cognitive deficits, and sudden infant death syndrome.2–4
Children are exposed to higher levels of SHS than adults.1 Even as a growing number of state regulations protect workers, regulations do not protect millions of non-smoking children from exposure to tobacco toxins in their own homes and vehicles, indicating that involuntary smoking will be a persistent and significant cause of morbidity and mortality in the years ahead. 1 While SHS exists in many different environments that children may frequent, nowhere is it more dangerous than in their own homes where they spend most of their time. Over 30% of children in the United States currently are exposed to SHS at home.2, 5, 6
In child healthcare settings, assisting smoking parents to quit can yield great benefit for the family. Quitting smoking adds an average of seven years to a parent's life,7 improves the health of all household members, eliminates the majority of SHS exposure of the children, reduces tobacco-related poor pregnancy outcomes, eliminates the greatest cause of house fire mortality, improves the financial resources of the family,8 and decreases teen smoking initiation. 9–11 Unfortunately, SHS exposure of children is assessed sporadically and almost never addressed with parents in an evidence-based fashion.3, 12–15
Finding appropriate and acceptable opportunities to intervene with parents who smoke is a challenge. Parents may lack health insurance and often lack a primary care clinician.16,17 Parental smokers often see their child's healthcare clinician more frequently than their own, with an average of over four visits per year, and 11 pediatric well-child visits in the first two years of a child's life.18, 19 Therefore, child healthcare offices are in a key position to influence, in a repeated and consistent manner, parents who are willing to address their smoking.20 However, not all parents are ready to make a quit attempt at any given visit to the pediatric office. The frequency of visits and the fact that many are for SHS exposure related problems creates numerous opportunities for tobacco control interventions, increasing the likelihood of a visit coinciding with high readiness to quit smoking, an important predictor of successful quitting.17 The tobacco policy of the Ambulatory Pediatrics Association states the critical importance of implementing smoking cessation evidence-based strategies for all family members in child healthcare settings.20
Despite the existence of national guidelines on the subject,21, 22 few effective smoking cessation interventions have used the pediatric outpatient setting to reach adult smokers. While some pediatric offices have systems to prompt clinicians to screen for parental tobacco use, none systematically employ current PHS treating tobacco use and dependence guidelines to treat parents. National rates of parental tobacco control service delivery are low within child healthcare settings. Only half of parents in a national survey about clinician involvement in this issue reported being asked whether or not they smoke and fewer than half were advised to quit.13 Fewer than 10% of parental smokers had cessation medication prescribed, and despite the availability of free quitlines in every state, fewer than 1% were enrolled in a quitline or any program to help them quit.14
No prior studies have used currently available systems of care to cue and support the major counseling and treatment components of the current PHS guideline. Successful efficacy trials have relied on external study staff to deliver the interventions.23–25 Implementation of parental tobacco control guidelines remains elusive in real-world pediatric practice.
The aim of this paper is to describe a novel process to develop a pediatric office intervention that uses available systems of care for addressing parental smoking. Specifically we explore the development of an intervention using currently available systems of care to address parental smoking in the child healthcare setting that employs, in combination, evidence-based brief smoking cessation counseling, proactive referral to free regional and national “quitlines”, and pharmacologic management of tobacco dependence. Furthermore, we aim to detail how, based on our focus group research, we mapped out five key implementation steps identified by practices, framed the intervention from the practice perspective, and designed a flexible implementation process that can be tailored to the needs of each practice. Finally, we hope that elucidating the steps involved in developing the intervention, known as CEASE, the Clinical Effort Against Secondhand Smoke Exposure, will help inform the development process of other systems change interventions for the pediatric setting. The office-based pediatric intervention development process described in this paper may also have specific relevance and generalizability to other chronic conditions such as attention deficit hyperactivity disorder, asthma, parental depression, and obesity.
Methods
Overview of intervention development
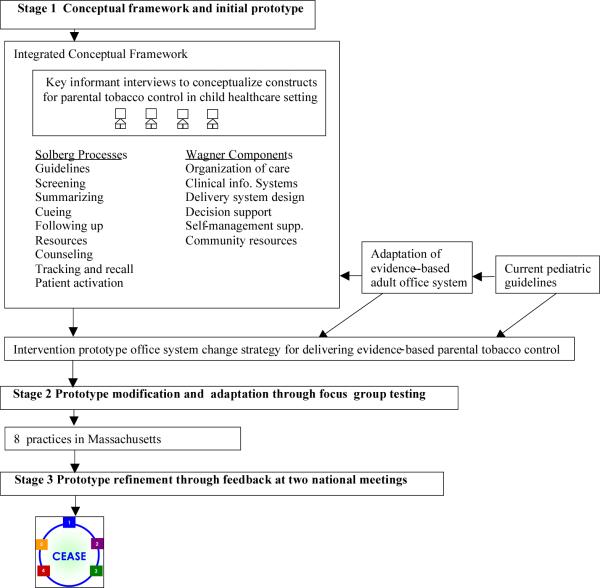
Our goal was to use existing conceptual frameworks, guidelines, and office-based tools for tobacco control in the adult setting and develop a conceptually driven pediatric office intervention for addressing parental smoking. As shown in Figure 1, the scientific development of the intervention occurred in three stages: stage one—conceptual framework and initial prototype; stage two—prototype modification and adaptation through focus group testing; stage three—prototype refinement through feedback at two national pediatric meetings.
Figure 1.
Scientific development of the Intervention. In stage one, we designed an office system for parental tobacco control in the pediatric outpatient setting based on complementary conceptual frameworks of preventive services delivery that were conceptualized for the child healthcare setting through a process of key interviews with leaders in the field of implementing practice change; existing Public Health Service guidelines including the 5As framework21 that had been shown to be effective in adult practices; and adaptation of an evidenced-based adult office system39 for tobacco control. This was an iterative process that yielded a theoretically grounded intervention prototype. In stage two, we performed focus group testing in pediatric practices with pediatricians, nurses, clinical assistants, and key office staff. Using qualitative methods, we adapted the intervention prototype based on this feedback to include five key implementation steps for the child healthcare setting. In stage three, we presented the intervention to breakout groups at two national meetings of pediatric practitioners for further refinements.
Stage one. Conceptual framework and initial prototype
Integrating conceptual frameworks to address parental tobacco control in child healthcare settings
Many theoretical models have aided in understanding how to implement office systems in diverse practice settings.26–33 Still, implementing best-practice guidelines has been an ongoing challenge in primary care practices, especially for services outside those provided for symptom-driven diagnoses.34–36 While no single model adequately combines system characteristics, patient-care processes, and techniques for achieving change,37 the Solberg and Wagner models form a complementary, comprehensive, and comprehensible framework for outpatient pediatric office systems change. Wagner's Chronic Care Model includes characteristics of community and organizational domains to improve preventive care and Solberg's Prevention System focuses on specific processes of care delivered to patients within an identified practice. The Chronic Care Model describes the elements of an effective clinical system for ensuring effective patient and clinician interactions, while the Prevention System fills out the types of behaviors that should occur between patient and clinician.
The Solberg and Wagner models are consistent with the recommendations of current Public Health Service Guidelines and helped drive the development of our intervention through a series of key informant interviews with leaders in the field of implementing practice change.38 Using an iterative process of key informant interviews with members of the American Academy of Pediatrics Tobacco Consortium, we systematically adapted each construct of the Solberg and Wagner frameworks for parental tobacco control in child healthcare settings. Approximately 20 interviews were conducted by one of the team members (JPW). Key informants were asked how the constructs of the Solberg and Wagner frameworks needed to be adapted for tobacco control in child healthcare settings. After suggested adaptations were made, these adapted constructs were presented back to the key informants for an informal face-validity review. The key informants had only minor editorial suggestions during the face-validity review of the integrated framework.
Current Pediatric Guidelines
Previous work has given child healthcare clinicians specific guidelines for evidence-based parental tobacco control intervention but did not address how to implement those recommendations.3 We used these same current guidelines and presented specific office strategies for implementation within child healthcare settings. The 5As (Ask, Advise, Assess, Assist, Arrange) remained at the core of our evidence-based intervention prototype, however, significant reframing occurred as a result of focus group testing. (Stage 2)
Adaptation of evidence-based adult office system for tobacco control
In 2001, health plans in Massachusetts collaborated with the Massachusetts Department of Public Health to create and promote jointly a new quitline initiative for adult primary care, QuitWorks.39 Components of the adult office system that we needed to modify for our pediatric prototype intervention included 1) the smoking status screening questionnaire, which needed to change so that it identified not just the patient's smoking status, but all household sources of SHS exposure; 2) the method for documenting SHS exposure in the patient's chart, which needed to change so that documentation could be separated from vital signs and recorded in the chart of each child in the household; 3) method for inviting the accompanying parent's self-assessment (of readiness to quit, interest in pharmacotherapy, and/or enrollment in QuitWorks), which we wanted to be routine without imposing undue interpersonal strain between the parent and clinician; 4) method for prescribing pharmacotherapy, which we needed to streamline to include standard dosing options; and 5) educational brochures, which we needed to revise to accommodate low-literacy readers and to include SHS messaging for parents.
Stage 2. Prototype modification and adaptation through focus group testing
Eight practices in Massachusetts
Using a series of 8 pediatric practice focus groups, we elicited practicing pediatrician and key staff responses to our proposed office system change strategy (our intervention prototype) in order to refine the components for a range of varied outpatient pediatric office settings. We designed the focus groups and a semi-structured interview guide based on well described qualitative research methods, 40, 53 incorporating a discussion of constructs (e.g. screening, counseling, referral system) that have been shown to be important components of office systems in other settings. 31–33, 41–43 Just as in other qualitative studies,40 the hope was that the focus groups would enrich our understanding of key themes, in this case what the key implementation steps were, how they needed to be operationalized, and how to present them to practices.
Recruitment
In the Spring of 2004, we identified pediatric practices located within a 20-mile radius of Boston from a list of practices available from the Department of Public Health. Further eligibility requirements were that the practice had to have at least one pediatrician with greater than six months of full-time experience and agreed to have at least one practice pediatrician participate in the focus group. Recruitment involved a three-step process: 1) a telephone call, 2) email message, and 3) follow-up letter. Of the 23 eligible practices, 8 (35%) agreed to participate in the focus groups within a 2-week enrollment window, at which point target enrollment was reached and closed.
Participants and Setting
Focus groups were conducted at each practice site and included pediatricians (one to three per practice) and other key clinical and office staff as identified by the pediatrician team. Office staff included the office manager, nurse practitioners, clinical assistants, and secretaries. We welcomed office staff with different roles so that we could get a complete picture of the office workings and potential intervention feasibility. Having a wide variety of positions represented maximized our opportunity to create flexible strategies for a range of pediatric practice situations and staffing environments.
Data Collection
Focus groups lasted for approximately 90 minutes; all groups were audiotaped. Participants were given no monetary payment for their time and participation but were provided lunch. All procedures and data collection forms were approved by the Massachusetts General Hospital Institutional Review Board. A semi-structured interview guide was developed based on current guidelines and proposed theoretical underpinnings. The guide was piloted at a Boston-based practice to assess content, length, and understandability and then finalized and used for all group interviews. The guide contained questions about a) present individual and clinic-wide practice patterns for parental tobacco control; b) perceived barriers for individual and clinic-wide delivery of cessation services for parental smokers; c) strategies for implementing each element of the proposed intervention prototype and d) recommendations for modifications and refinements of the proposed intervention prototype. Groups were co-facilitated by a pediatrician (JW) and health educator (AB). Participants were asked to be frank and were assured that the purpose was not to achieve consensus but rather gather data to illustrate all of their impressions. Facilitators used probes to obtain comprehensive collection and to clarify responses.
Data Analysis
All session tapes were transcribed. Consistent with our goals for the focus groups, we conducted thematic content analysis using two research assistants under the supervision of our qualitative methodologist (EP).44 The two coders separately reviewed transcripts and entered data into a Microsoft Access© database. We coded transcriptions for key words, refined the content and parameters of the codes, and once thematic saturation was reached, coded categories within each descriptive theme relating to practice patterns, perceived barriers, implementation strategies, and specific modifications and refinements of the proposed intervention. 45 Reviewers also coded for frequency, intensity, and extensiveness.46, 47 At each analysis phase, the two coders compared their results and resolved discrepancies. Statements characteristic of the sentiment of the group were highlighted by the coders and selected by facilitators. An expert review of the data was conducted (JW, EP). No systematic differences were noted between the urban and suburban sites, and so we combined their responses.
To assure the trustworthiness of our findings, many steps were taken to maximize dependability (consistency) and credibility (the truth of findings). We incorporated the process of triangulation by involving a multidisciplinary research team (investigator triangulation), including different types of practice sites (data triangulation), and comparing our findings to the conceptual framework models (theory triangulation).48 The facilitators used a standardized interview guide and discussed their impressions and debriefed with the research team following each session; co-facilitators took notes at each session to record interactions, nonverbal language, and environmental factors. The two coders thoroughly reviewed, separately and then together, all of the transcribed data. A facilitator reviewed every transcript to assure that the interview content was complete and accurate. Coders carefully examined data that seemed discrepant, unexpected, or unclear and compared all coded data to the transcript text, undergoing an iterative evaluation process until agreement was reached.
Stage 3. Prototype refinement through feedback at two national pediatric meetings
At two national pediatric meetings in 2004, we performed 60-minute small-group breakout sessions with a total of 50 pediatricians at each meeting to get national practitioner key informant reactions to our revised intervention prototype. After introducing the intervention and materials in structured fashion, the facilitator recorded session feedback that included clinician reactions and suggestions for improving the revised intervention prototype. A formal qualitative analysis of this key informant national practitioner step was beyond the scope of this study.
Results
Stage 1. Initial prototype development
The main result from our work in stage one was a conceptually framed prototype intervention for delivering evidence-based parental tobacco control and lessons learned from the development process. In developing the intervention we consistently went back to the conceptual framework to guide our decisions. Table 1 presents the framework for preventive services delivery as conceptualized for parental tobacco control in pediatric settings. A key example of this process was in the adaptation of the proactive quitline enrollment form to function within child healthcare settings. (Figure 2) At least three essential modifications in the areas of screening, counseling, clinical information systems were made to the form based on our framework. First, we had to change the “patient information” because the smokers enrolled from the child healthcare offices would not be the patients. Second, we had to adjust the second A “advice to quit” to include an empowering smokefree home and car message. Third, we needed to create a space to indicate the smoker's relationship to patient.
Table 1.
Conceptual framework for parental tobacco control in child healthcare settings*
| Solberg Processes “IMPROVE Model” 31, 42, 43 | Wagner Components “Chronic Illness Care Model” 32, 33, 41, 88 |
|---|---|
| Providing Guidelines. | Organization of Care. |
| • Providing the current PHS guideline that the American Academy of Pediatrics Tobacco Consortium has endorsed8, 21 | • Making tobacco preventive services a key goal of the organization |
| • Suggesting office-specific benchmarks for guideline adherence that the practice can endorse | • Ensuring that leadership is committed and visibly involved with parental tobacco control |
| • Encouraging periodic measurement of key intervention components | |
| Screening. | Clinical Information Systems. |
| • Screening for parents' smoking status, and for rules prohibiting smoking in the home and car | • Implementing a universal screening system (see Solberg Screening) |
| • Delivering proactive care to those who screen positive for tobacco use | |
| • Following individual's and practice's progress over time | |
| Summarizing. | Delivery System Design. |
| • Organizing and updating the information obtained in the screening process so that it is all in one place and easily reviewable by those needing to know the current tobacco-use prevention status of a particular parent | • Ensuring that the composition of the practice team89 can handle the key components of parental tobacco control and that every team member knows their responsibilities |
| Cueing. | Decision Support. |
| • Systematically cueing clinic staff and clinicians to address parental tobacco control and how to do it | • Providing evidence-based support for all aspects of the recommended intervention (see Solberg Providing Guidelines) |
| • Establishing a prompting system to increase adherence to those guidelines(see Solberg Cueing)58 | |
| Following-up. | Self-Management Support. |
| • Communicating back to the practices results of preventive services delivered to parents by quitlines, along with the appropriate information and recommendations for follow-up visits with parents | • Offering parental smokers educational resources, skills training, and psychosocial support |
| Resources. | Community Resources. |
| • Organizing and maintaining parent education materials and enrollment forms needed by both patients and clinic personnel | • Improving performance of child healthcare systems by establishing linkages with effective parental tobacco control in the community and at the state level (quitlines)90 |
| Counseling. | |
| • Assisting parents to make needed changes in their behavior through very brief and focused messaging to parents who smoke | |
| • Specifying messages needed to address teachable moments relevant to the parent-child dyad, such as using child health characteristics such as ear infections and asthma exacerbations as part of the longitudinal messaging process | |
| Tracking and Recall. | |
| • Documenting tobacco control services delivery to enable provision of patient centered follow-up counseling at subsequent visits | |
| Patient Activation. | |
| • Encouraging parents to take greater responsibility for their own smoking behavior particularly in the context of the child's well-being and specific health concerns | |
The Solberg and Wagner models provided a rich framework for our adaptation of current state-of-the-art tobacco control strategies available for adult practices into the pediatric office setting. Personal behavior change theory can be thought of in the context of the Counseling process of Solberg's framework and the Self-management support component of Wagner's model. These behavior change constructs are derived and often operationalized in terms of conceptual models of the smoking cessation process, including the stages of change model, motivations for smoking cessation, and self-efficacy theory.91, 92,93, 94,95,49 The 5As framework in the current Public Health Service Guideline, adapted for pediatrics as part of our intervention, also addresses elements of systems, clinician, and personal behavior change.21 The 5As have been used previously in conjunction with Wagner's model to optimize the care of other chronic conditions.96, 97
Figure 2.
Massachusetts example of quitline enrollment form adapted for child healthcare settings. Initially conceived for the Massachusetts “QuitWorks” program, the CEASE enrollment form now has national applicability due to the universal availability of quitline resources in the United States. State-specific CEASE forms reflect state-specific quitline names and enrollment methods. (attached to submission as a PDF)
A main lesson learned was to budget enough time for initial intervention development. Our prototype took over one year to create due to busy senior leaders in the field who needed time to evaluate iterations of our conceptual framework and creation of materials for the prototype that had the look and feel of a useable office intervention. Another important lesson learned in stage one was that intervention development is non-linear. New research, innovative approaches, and expert input can come in at any time. We chose to follow a relatively unconstrained path toward intervention creation that led to redoing materials many times over before we ever got to formal focus group testing. A finding demonstrating low rates of counseling for SHS exposure of children in cars led to the inclusion in the prototype of a novel parent counseling brochure that we had not anticipated including.
Stage 2. Main focus group findings
In total, eight practices with 6 to 10 participants each (64 individuals total) participated in the focus groups. This included 21 clinicians, 21 clinical assistants, 6 practice managers, and 16 administrative staff. Focus groups helped us understand 1) the lack of existing tobacco control systems within practices; 2) barriers to intervention implementation that needed to be addressed in the next version of the intervention; 3) how to conceptualize a series of implementation steps for the next version of the intervention; 4) how to document and follow parental smoking from visit to visit—a critical piece, unspecified in our prototype intervention. The main product from the focus group was a revised intervention prototype.
Existing systems. We asked focus group participants if there was a systematic method for documenting and monitoring parental smoking but we found that no office had such a system.
Barriers. When queried about barriers for individual and clinic-wide delivery of cessation services for parental smokers, the following issues emerged: parent is not the patient; time constraints; lack of counseling skills; no reimbursement for this service; lack of skill in medication prescription for smoking cessation; belief that addressing parental smoking may harm the therapeutic relationship with parents.
Implementation framework. When presented with the evidence base for smoking cessation and asked about how to implement it, the following framework emerged that mapped to the 5As themselves. Participants grouped the evidence-based intervention activities into five key implementation steps: 1. Identification and self-assessment of readiness to quit, willingness to use medication, and enroll in the quitline 2. Counseling 3. Referral 4. Medications and 5. Follow-up. Importantly, these steps are how pediatric offices conceptualized the operationalization of the implementation of the 5As in their practices. The five implementation steps for pediatric practice map nicely to the content of the 5As themselves (see Table 2)
Table 2.
Mapping the CEASE implementation steps to the 5As
| Implementation Step | The A's |
|---|---|
| Step 1. Identification and self-assessment Identify smokers with the CEASE annual card during the office visit and document smoking status. Ask those who smoke to fill out the CEASE action sheet: self-assessment screener at each visit. Document no smoking policy in the home and car. | Ask and Assess |
| Step 2. Counseling Talk with smokers about tobacco use and establishing a strict no smoking policy in the home and car. For those who are not ready to quit, give a “Think About It” halflet. | Advise and Assist |
| Step 3. Referral Complete the Cease action sheet: quitline enrollment with smokers who are ready to quit. Give those who enroll a “Welcome” halflet. | Assist |
| Step 4. Medication Prescribe or recommend pharmacotherapy, if appropriate, for relief of withdrawal symptoms and to aid cessation. | Assist |
| Step 5. Follow-up File the CEASE action sheet and review before each visit. Talk with those who smoke about smoking at each visit until the family is smoke-free. | Arrange |
Suggested improvements. There was a lot of discussion about location of documentation of SHS exposure. For continuity of cessation support, participants decided that the documentation of smoking status should occur on the problem list. The research team had initially conceived of documentation of smoking status as a vital sign. However, we found out that vital signs are not done as part of every pediatric visit. Therefore, we changed to suggesting documentation on the problem list after the parent fills out an annual screener card. This documentation can be updated as the smoking status of family members changes. The other key observation was that problem list may be the only part of the medical record that is reviewed by other child healthcare clinicians in cross-coverage.
Stage 3. Feedback from national meetings
Approximately 50 pediatricians participated in breakout sessions at each of two pediatric national meetings. Pediatricians had the following main reactions and suggestions from the national meetings:
Fully endorsed the parental tobacco control project, idea of creating universal documentation of SHS exposure of children and parental smoking, and linking parental smokers to outside quitline resources for more extensive counseling support
Requested an implementation guide on a single page with suggested individuals who might perform key tasks
Felt that the clinician counseling burden in the office needed to be minimal (3 minutes) in order not to disrupt office operations
Requested that information on billing for services be incorporated into the intervention materials
Approximately half felt uneasy about prescribing medications to parents in the context of the child's care—thought that should be optional
A few expressed concerns that they would be sued for adverse outcomes if they prescribed medications for parental tobacco dependence
This step of soliciting reactions to our revised prototype also led to a change in how the intervention is presented to child healthcare clinicians. The program was initially billed as a method for getting parents to quit smoking. Pediatricians commented that their primary reason for wanting to adopt a tobacco control program was to protect their patients, the children, from secondhand smoke exposure. Therefore, we made a shift in how the program was presented and ended up with the Clinical Effort Against Secondhand Smoke Exposure (CEASE). Clinicians are introduced to the intervention in their reference frame rather than the preconceived reference frame of the research team.
Synthesis and description of CEASE intervention
The main result was a theoretically grounded intervention that was responsive to the barriers and suggestions raised in the focus groups and at the national meetings. We heard the persistent concern that a busy child healthcare clinician cannot spend the time to do a full motivational interviewing session,49 especially since the parent is not the actual patient. However, the behavior of the parental smoker does directly affect the health and well-being of the child healthcare clinician's patient. Therefore, a reasonable and agreed upon expectation included spending a couple of minutes on cessation messaging and trying to motivate the parental smoker to follow-up with an expert. Enrolling smokers in multi-session telephone counseling as an adjunct to office-based counseling ensures that smokers receive professional, evidence-based, ongoing counseling services that may not be possible otherwise.21, 50 Although the intervention focuses on referral of smokers to free quitlines, the training manual also encourages knowledge and use of local program resources. While young parental smokers may not often be available for such face-to-facecounseling, its inclusion for highly motivated individuals may be important.51
The clinician counseling component consists of very brief motivational messaging49 that is based on the parents' own concerns as well as potential teachable moments that may be cued by the child's illness. This approach has been well received by parental smokers in other studies.17 A large majority of smokers tend to give higher satisfaction ratings to pediatric clinicians who address their smoking and offer help.52–54 Most parents believe it is the responsibility of the pediatrician to counsel them on matters that affect their child's health, and that they should do more counseling regarding smoking cessation.52, 53, 55, 56 The intervention includes a focused library of “halflets” (A halflet is a two-sided sheet of paper larger than a bookmark, smaller than a pamphlet, used for messaging) for handing out to parents that address specific concerns that may arise during the child visit. Messaging elements may include brief collaborative goal setting, personal barriers to quitting, problem-solving strategies, and social support. 37, 57, 58 One addition to self-management for the parental smoker includes focused strategies for reducing SHS exposure of the child, such as implementation of rules prohibiting smoking inside the home and car.59
Interested clinicians can review the rationale for focused SHS messaging including how the institution of strict smoking bans in the home and car can help address the problem of parental smoking in at least three ways. First, by making smoking more difficult, bans may help the cessation process for parents who smoke.59–61 Second, bans may reduce smoking rates and cigarette consumption among youth.59, 62–66 Finally, bans have been recommended to reduce the SHS exposure of children and spouses from a parent's smoking.66–71 In reducing SHS exposure of children, recent studies using counseling and provision of written materials have proven successful.72–76 A strict household and car ban on smoking also reduces the amount of tobacco toxin exposure children receive from non-parent relatives and other visiting adults.77, 78
In addition, the CEASE intervention includes a direct-to-consumer marketing approach through the posting of pharmacotherapy options for tobacco dependence treatment. These posters cue the parental smoker to discuss with the clinician what type of pharmacotherapy might be right for them. On the poster is a dosing guide for the quick reference of those clinicians who wish to prescribe pharmacotherapy so that they may avoid the embarrassment and extra time required to look up the requested medication to treat the parent's tobacco dependence. Some of the concerns raised in the focus groups, such as fear of legal action if clinicians treat parents have been partially answered by the American Medical Association and are now emphasized in the CEASE materials. The American Medical Association amended its tobacco control policy to “…support efforts by any physician to identify and treat tobacco dependence in any individual, in the various clinical contexts in which they are encountered…” 79
The intervention developed in this study operationalizes the 5As in accordance with the most recent national guidelines3, 21 and the recommendations gathered from our focus groups and national pediatric meetings. Practices can choose materials relevant to their own particular systems of care. The CEASE intervention is designed to be flexible and adaptable to the particular practices' staffing, resources, and physical configuration. We therefore present one possible option for how a pediatric practice might operationalize some of the intervention materials. (Table 3)
Table 3.
Possible Operationalization of the CEASE Intervention Materials* in Practice
| • A parent and child arrive at the practice and check in, if smoking status is unknown, a CEASE annual card with attached CEASE Sticker serves as the brief screener |
| • If the smoker is indeed present at the visit, then the parent fills out the self-assessment portion of the CEASE Action Sheet and returns it to the receptionist (See below for when smoker is not present) |
| • The receptionist places the self-assessment portion of the CEASE Action Sheet on the chart and puts the CEASE Sticker on the problem list |
| • The clinician quickly reviews the self-assessment portion of the CEASE Action Sheet and turns it over for the clinician portion of the CEASE Action Sheet |
| • The clinician briefly documents the tobacco control counseling delivered |
| • The clinician hands the parent an appropriate CEASE “halflet” about tobacco use for the parent's situation. |
| • The clinician offers to enroll the parent into the proactive state quitline (where available), using the enrollment portion of the CEASE Action Sheet. Where proactive quitlines are not available parent will be referred to quitline without direct fax. |
| • The clinician hands the enrolled parent a CEASE Welcome halflet, which reinforces the parent's decision to quit and reminds the parent that the proactive quitline will be calling |
| • For the parent resistant to quitting smoking or enrolling in the quitline, the clinician offers them a CEASE Think About It halflet, which details the contact information of the state quitline. The CEASE Think About It halflet is also suitable to give to a non-smoking parent to take home to the smoking parent as it encourages the smoker to attend the child's next clinic visit to obtain further help with addressing smoking. |
| • If desired and appropriate, the clinician offers the parent a prescription for NRT or more information about NRT using the CEASE pre-printed NRT pads |
The menu of available intervention materials include (1) a questionnaire that screens parents of pediatric patients for smoking status of the patients' household members; (2) a label that affixes to the child's problem list for documenting parent smoking status and indicating the child's SHS exposure, encouraging continuity of cessation support in cross-coverage situations; (3) a three-item self-assessment of the smoker's readiness to quit, interest in pharmacotherapy, and willingness to enroll in quitline counseling. The parent's own self-assessment helps guide the clinician's approach, thereby reducing the offering of unwanted services and increasing the clinician's confidence that they will not risk harming the therapeutic relationship with the parents of their patients; (4) decision support for clinicians that prompts a brief motivational messaging approach and exposure-reduction counseling, thus increasing systematic adherence to guidelines; 58 (5) a HIPAA-compliant form for enrolling the smoker in the telephone quitline (6) pre-printed, practice embossed prescription pads for prescribing over-the-counter NRT when desired by the smoker; (7) posters for exam rooms to activate parents of patients and cue clinicians for tobacco dependence treatment; (8) low literacy written information to support smoking cessation and SHS exposure reduction (9) a simple one-page implementation guide to support integrating the parent, clinician, and practice levels of the intervention. (See www.ceasetobacco.org for most recent version) A more detailed training manual discusses additional topics such as how an office can bill and obtain reimbursement for tobacco control services rendered, and how to establish initial contact with the quitline while the parent is still in the office (in places where faxed enrollment is not yet available). The manual includes research demonstrating high parent satisfaction with addressing parental smoking as part of the child healthcare visit.
The CEASE intervention is available from the website www.ceasetobacco.org and can be used by offices from any state in the United States. The state-of-the-art CEASE materials are updated as new research advances the field of tobacco control. Rather than reproducing the current set of materials in this paper, the most up-to-date materials will be found on the website.
Discussion
In this paper, we presented the conceptual framework and development of the CEASE intervention, a program that is now available for use in child healthcare settings nationally. Successful tobacco control interventions in the child healthcare setting have usually relied on study staff to deliver the intervention. We have previously demonstrated the feasibility of engaging parents in a smoking cessation intervention at the time of a child's clinic visit.17 High rates of program enrollment (63%), use of NRT(34%), and receipt of telephone quitline counseling (42%) in this prior study supported the hypothesis that a child's clinic visit is a teachable moment to address parental smoking cessation. Other prior studies have examined the efficacy and feasibility of specific tobacco control interventions with parents in child healthcare settings.17, 23–25, 72–76, 80–87 Studies in these settings have tried to improve parental smoking cessation rates primarily through the use of counseling and provision of written materials with varied results.
Most recently, in a randomized trial of 303 parents seen at pediatric clinics, Curry et al.25 conclusively demonstrated efficacy of an intervention to help parents quit smoking. The intervention consisted of brief cessation advice given by the pediatrician (usually lasting 1 to 5 minutes), a parent-tailored quit smoking guide distributed by the pediatrician, a 10-minute intervention with a practice nurse or health educator after the child's visit and up to 3 subsequent telephone calls from the practice nurse or health educator. At 12-month follow-up, 13.5% of the intervention group abstained as compared to 6.9% of the control group, resulting in an adjusted odds ratio of 2.77 (CI, 1.24–6.60), demonstrating that office-based and telephone counseling can be effective in increasing the quit rates of parents who smoke. However, external study staff were used to deliver the office-based and telephone counseling. The CEASE intervention employs currently available systems of care, quitlines, and office personnel to deliver the intervention without hiring additional office staff.
Specific features of the CEASE intervention that have been associated with improved tobacco control outcomes in adults include: materials that prompt delivery of the 5A's (Ask, Advise, Assess, Assist, Arrange), systematic and proactive enrollment of parental smokers in telephone counseling that will follow-up on clinician's advice to quit; explicit counseling of parents on the importance of strictly enforced smoking prohibitions within the home and car; and prescription of nicotine replacement therapy (NRT) for parental smokers in the context of the child's healthcare visit. The CEASE intervention follows the Public Health Service Guidelines by incorporating these evidence-based tobacco control treatments and practices.21 The intervention is unique in using the child healthcare setting to cue and support all of these elements for parents who smoke.
Many components of the CEASE intervention could be implemented with the use of an electronic medical record, i.e. documentation of parental smoking could be entered directly into the electronic problem list. Electronic medical records are not used by the majority of pediatric practices in 2007; however, practices still need to implement evidence-based parental tobacco control. Fortunately, effective clinical information systems can begin as adaptations of currently employed office systems and later be incorporated into fully developed electronic systems as they become available. The CEASE intervention addresses the need for a universal screening system, the ability to proactively deliver care to those who screen positive for smoking, and to follow the individual's progress. The placement on the chart of a filled-out self-assessment portion of the CEASE action sheet prompts the clinician to deliver the remaining four A's, including enrollment in telephone quitlines. In certain states, faxing the enrollment form to the state quitline will create a follow-up and tracking mechanism for the parental smoker that will yield a report back to the clinician about how the parent did in the program. The clinician will get a quarterly report listing how each smoker has done with the telephone counseling. All of these pieces together make up a clinical information system that holds promise for intervention with every smoking parent within a child healthcare practice.
In summary, articulating the steps involved in developing this intervention may help the development process of other systems change interventions for the pediatric setting. The tobacco control intervention development process might have particular relevance for other chronic pediatric conditions that have a strong evidence base and have available treatments or resources that are underused. Conceptually-grounded and focus group tested strategies for parental tobacco control are now available for implementation in the pediatric outpatient setting. Planned process evaluation of CEASE at the clinician level, the patient behavior level, and practice level will add substantially to the compilation of essential elements in the national tobacco control strategy for child healthcare settings.
Acknowledgments
Dr. Winickoff was supported by grants from the Flight Attendant Medical Research Institute (#024032) and NCI (K07 CA100213 A 01). Dr. Rigotti was supported by K24 HL0440. Funding for the development of CEASE has also come from the American Legacy Foundation and the Robert Wood Johnson Foundation. We gratefully acknowledge the support of the Massachusetts Quitworks program, Donna Warner, the New York Quitline, and Smokefree Homes.
Abbreviations
- CEASE
Clinical Effort Against Secondhand Smoke Exposure
- SHS
secondhand smoke
- NRT
nicotine replacement medication
- PHS
Public Health Service
References
- 1.US Department of Health and Human Services The Health Consequences of Involuntary Tobacco Smoke: A Report of the Surgeon General. 2006 [Google Scholar]
- 2.Kum-Nji P, Meloy L, Herrod HG. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics. 2006 May;117(5):1745–1754. doi: 10.1542/peds.2005-1886. [DOI] [PubMed] [Google Scholar]
- 3.Winickoff JP, Berkowitz AB, Brooks K, et al. State-of-the-art interventions for office-based parental tobacco control. Pediatrics. 2005 Mar;115(3):750–760. doi: 10.1542/peds.2004-1055. [DOI] [PubMed] [Google Scholar]
- 4.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents. Environ Health Perspect. 2005 Jan;113(1):98–103. doi: 10.1289/ehp.7210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA. 1996;275(16):1233–1240. [PubMed] [Google Scholar]
- 6.Schuster MA, Franke T, Pham CB. Smoking patterns of household members and visitors in homes with children in the United States. Archives of Pediatrics & Adolescent Medicine. 2002;156(11):1094–1100. doi: 10.1001/archpedi.156.11.1094. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DH, Jr., Hasselblad V, Henley SJ, Thun MJ, Sloan FA. Benefits of smoking cessation for longevity. Am J Public Health. 2002;92(6):990–996. doi: 10.2105/ajph.92.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavanaugh M, McMillen RC, Pascoe JM, Hill Southward L, Winickoff JP, Weitzman M. The co- occurrence of maternal depressive symptoms and smoking in a national survey of mothers. Ambul Pediatr. 2005 Nov-Dec;5(6):341–348. doi: 10.1367/A04-207R.1. [DOI] [PubMed] [Google Scholar]
- 9.Farkas AJ, Distefan JM, Choi WS, Gilpin EA, Pierce JP. Does parental smoking cessation discourage adolescent smoking? Preventive Medicine. 1999;28(3):213–218. doi: 10.1006/pmed.1998.0451. [DOI] [PubMed] [Google Scholar]
- 10.Chassin L, Presson C, Rose J, Sherman SJ, Prost J. Parental smoking cessation and adolescent smoking. Journal of Pediatric Psychology. 2002;27(6):485–496. doi: 10.1093/jpepsy/27.6.485. [DOI] [PubMed] [Google Scholar]
- 11.Bricker JB, Leroux BG, Peterson AV, Jr., et al. Nine-year prospective relationship between parental smoking cessation and children's daily smoking. Addiction. 2003;98(5):585–593. doi: 10.1046/j.1360-0443.2003.00343.x. see comment. [DOI] [PubMed] [Google Scholar]
- 12.Tanski SE, Klein JD, Winickoff JP, Auinger P, Weitzman M. Tobacco counseling at well-child and tobacco-influenced illness visits: opportunities for improvement. Pediatrics. 2003 Feb;111(2):E162–167. doi: 10.1542/peds.111.2.e162. [DOI] [PubMed] [Google Scholar]
- 13.Winickoff JP, McMillen RC, Carroll BC, et al. Addressing parental smoking in pediatrics and family practice: a national survey of parents. Pediatrics. 2003 Nov;112(5):1146–1151. doi: 10.1542/peds.112.5.1146. [DOI] [PubMed] [Google Scholar]
- 14.Winickoff JP, Tanski SE, McMillen RC, Hipple BJ, Friebely J, Healey EA. A national survey of the acceptability of quitlines to help parents quit smoking. Pediatrics. 2006 Apr;117(4):e695–700. doi: 10.1542/peds.2005-1946. [DOI] [PubMed] [Google Scholar]
- 15.Winickoff JP, Tanski SE, McMillen RC, Klein JD, Rigotti NA, Weitzman M. Child health care clinicians' use of medications to help parents quit smoking: a national parent survey. Pediatrics. 2005 Apr;115(4):1013–1017. doi: 10.1542/peds.2004-1372. [DOI] [PubMed] [Google Scholar]
- 16.Rowland D, Lyons B, Salganicoff A, Long P. A profile of the uninsured in America. Health Aff (Millwood) 1994;13(2):283–287. doi: 10.1377/hlthaff.13.2.283. [DOI] [PubMed] [Google Scholar]
- 17.Winickoff JP, Buckley VJ, Palfrey JS, Perrin JM, Rigotti NA. Intervention with parental smokers in an outpatient pediatric clinic using counseling and nicotine replacement. Pediatrics. 2003 Nov;112(5):1127–1133. doi: 10.1542/peds.112.5.1127. [DOI] [PubMed] [Google Scholar]
- 18.Klein JD, Portilla M, Goldstein A, Leininger L. Training pediatric residents to prevent tobacco use. Pediatrics. 1995;96(2 Pt 1):326–330. [PubMed] [Google Scholar]
- 19.Newacheck PW, Stoddard JJ, Hughes DC, Pearl M. Health insurance and access to primary care for children. N Engl J Med. 1998;338(8):513–519. doi: 10.1056/NEJM199802193380806. [DOI] [PubMed] [Google Scholar]
- 20.Best D, Moss DA, Winickoff JP, Simpson L. Ambulatory Pediatric Association policy on tobacco. Ambul Pediatr. 2006 Nov-Dec;6(6):332–336. doi: 10.1016/j.ambp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Fiore MC, Bailey WC, Cohen SJ. Treating Tobacco Use and Dependence. US Department of Health and Human Services, Public Health Service; Rockville, MD: 2000. [Google Scholar]
- 22.Agency for Health Care Policy and Research . Smoking cessation clinical practice guideline no.18. Vol PHS 96-0692. US Department of Health and Human Services; Rockville, MD: 1996. [Google Scholar]
- 23.Wall MA, Severson HH, Andrews JA, Lichtenstein E, Zoref L. Pediatric office-based smoking intervention: impact on maternal smoking and relapse. Pediatrics. 1995;96(4 Pt 1):622–628. [PubMed] [Google Scholar]
- 24.Severson HH, Andrews JA, Lichtenstein E, Wall M, Akers L. Reducing maternal smoking and relapse: long-term evaluation of a pediatric intervention. Preventive Medicine. 1997;26(1):120–130. doi: 10.1006/pmed.1996.9983. [DOI] [PubMed] [Google Scholar]
- 25.Curry SJ, Ludman EJ, Graham E, Stout J, Grothaus L, Lozano P. Pediatric-based smoking cessation intervention for low-income women: a randomized trial. Archives of Pediatrics & Adolescent Medicine. 2003;157(3):295–302. doi: 10.1001/archpedi.157.3.295. comment. [DOI] [PubMed] [Google Scholar]
- 26.Carney PA, Dietrich AJ, Keller A, Landgraf J, O'Connor GT. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract. 1992;35(4):388–394. [PubMed] [Google Scholar]
- 27.Leininger LS, Finn L, Dickey L, et al. An office system for organizing preventive services: a report by the American Cancer Society Advisory Group on Preventive Health Care Reminder Systems. Arch Fam Med. 1996;5(2):108–115. doi: 10.1001/archfami.5.2.108. [DOI] [PubMed] [Google Scholar]
- 28.Pommerenke FA, Weed DL. Physician compliance: improving skills in preventive medicine practices. Am Fam Physician. 1991;43(2):560–568. [PubMed] [Google Scholar]
- 29.Thompson RS, Taplin SH, McAfee TA, Mandelson MT, Smith AE. Primary and secondary prevention services in clinical practice. Twenty years' experience in development, implementation, and evaluation. Jama. 1995;273(14):1130–1135. [PubMed] [Google Scholar]
- 30.Walsh JM, McPhee SJ. A systems model of clinical preventive care: an analysis of factors influencing patient and physician. Health Educ Q. 1992;19(2):157–175. doi: 10.1177/109019819201900202. [DOI] [PubMed] [Google Scholar]
- 31.Solberg LI, Kottke TE, Conn SA, Brekke ML, Calomeni CA, Conboy KS. Delivering clinical preventive services is a systems problem. Ann Behav Med. 1997;19(3):271–278. doi: 10.1007/BF02892291. [DOI] [PubMed] [Google Scholar]
- 32.Wagner EH, Austin BT, Von Korff M. Organizing care for patients with chronic illness. Milbank Q. 1996;74(4):511–544. [PubMed] [Google Scholar]
- 33.Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness. Manag Care Q. 1996;4(2):12–25. [PubMed] [Google Scholar]
- 34.Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? Milbank Q. 1998;76(4):517–563. doi: 10.1111/1468-0009.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Starfield B. Primary Care: Balancing Health Needs, Services, and Technology. Oxford University Press; New York: 1998. [Google Scholar]
- 36.US Department of Health and Human Services . Healthy People 2010: Understanding and Improving Health. Government Printing Office; Washington D.C.: 2000. [Google Scholar]
- 37.Glasgow RE, Orleans CT, Wagner EH. Does the chronic care model serve also as a template for improving prevention? Milbank Q. 2001;79(4):579–612. iv–v. doi: 10.1111/1468-0009.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berwick D, Bonomi A, Curry S, Homer C, Solberg L. [Google Scholar]
- 39. www.Quitworks.org. Accessed March 3, 2008.
- 40.Krueger RA. Focus Groups, A Practical Guide for Applied Research. 2nd edition Sage; Thousand Oaks: 1994. [Google Scholar]
- 41.Wagner EH, Davis C, Schaefer J, Von Korff M, Austin B. A survey of leading chronic disease management programs: are they consistent with the literature? Manag Care Q. 1999;7(3):56–66. [PubMed] [Google Scholar]
- 42.Solberg LI, Kottke TE, Brekke ML, Conn SA, Magnan S, Amundson G. The case of the missing clinical preventive services systems. Eff Clin Pract. 1998;1(1):33–38. [PubMed] [Google Scholar]
- 43.Solberg LI, Kottke TE, Brekke ML. Will primary care clinics organize themselves to improve the delivery of preventive services? A randomized controlled trial. Prev Med. 1998;27(4):623–631. doi: 10.1006/pmed.1998.0337. [DOI] [PubMed] [Google Scholar]
- 44.Miles M, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. 1994. Sage Publications; Thousand Oaks, CA: [Google Scholar]
- 45.Willms DG, Best JA, Taylor DW, et al. A systematic approach for using qualitative methods in primary prebention research. Medical Anthropology Quarterly. 1990;4(4):391–411. [Google Scholar]
- 46.Morgan D, Krueger R. The Focus Group Kit. Vol 1-6. Sage Publications; California: 1997. [Google Scholar]
- 47.Krueger R, Casey M. A Practical Guide for Applied Research. 3rd ed. Sage Publications; Thousand Oaks, CA: 2000. [Google Scholar]
- 48.Devers KJ. How will we know “good” qualitative research when we see it? Beginning the dialogue in health services research. Health Serv Res. 1999 Dec;34(5 Pt 2):1153–1188. [PMC free article] [PubMed] [Google Scholar]
- 49.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. Guildford Press; New York, NY: 1991. [Google Scholar]
- 50.Zhu SH, Stretch V, Balabanis M, Rosbrook B, Sadler G, Pierce JP. Telephone counseling for smoking cessation: effects of single-session and multiple-session interventions. Journal of Consulting & Clinical Psychology. 1996;64(1):202–211. doi: 10.1037//0022-006x.64.1.202. [DOI] [PubMed] [Google Scholar]
- 51.Winickoff JP, Hibberd PL, Case B, Sinha P, Rigotti NA. Child hospitalization: an opportunity for parental smoking intervention. Am J Prev Med. 2001;21(3):218–220. doi: 10.1016/s0749-3797(01)00355-5. [DOI] [PubMed] [Google Scholar]
- 52.Cluss PA, Moss D. Parent attitudes about pediatricians addressing parental smoking. Ambul Pediatr. 2002 Nov-Dec;2(6):485–488. doi: 10.1367/1539-4409(2002)002<0485:paapap>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 53.Frankowski BL, Weaver SO, Secker-Walker RH. Advising parents to stop smoking: pediatricians' and parents' attitudes. Pediatrics. 1993 Feb;91(2):296–300. [PubMed] [Google Scholar]
- 54.Groner J, Ahijevych K, Grossman L, Rich L. Smoking behaviors of women whose children attend an urban pediatric primary care clinic. Women & Health. 1998;28(2):19–32. doi: 10.1300/j013v28n02_02. [DOI] [PubMed] [Google Scholar]
- 55.Frankowski BL, Secker-Walker RH. Advising parents to stop smoking. Opportunities and barriers in pediatric practice. American Journal of Diseases of Children. 1989;143(9):1091–1094. doi: 10.1001/archpedi.1989.02150210127032. [DOI] [PubMed] [Google Scholar]
- 56.Moss D, Cluss PA, Mesiano M, Kip KE. Accessing adult smokers in the pediatric setting: What do parents think? Nicotine Tob Res. 2006 Feb;8(1):67–75. doi: 10.1080/14622200500431809. [DOI] [PubMed] [Google Scholar]
- 57.Orleans CT. Promoting the maintenance of health behavior change: recommendations for the next generation of research and practice. Health Psychol. 2000;19(1 Suppl):76–83. doi: 10.1037/0278-6133.19.suppl1.76. [DOI] [PubMed] [Google Scholar]
- 58.Ockene JK, Emmons KM, Mermelstein RJ, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19(1 Suppl):17–31. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- 59.Farkas AJ, Gilpin EA, White MM, Pierce JP. Association between household and workplace smoking restrictions and adolescent smoking. JAMA. 2000;284(6):717–722. doi: 10.1001/jama.284.6.717. [DOI] [PubMed] [Google Scholar]
- 60.Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. A prospective study of household smoking bans and subsequent cessation related behavior: the role of stage of change. Tobacco Control. 2004;13:23–28. doi: 10.1136/tc.2003.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farkas AJ, Gilpin EA, Distefan JM, Pierce JP. The effects of household and workplace smoking restrictions on quitting behaviours. Tobacco Control. 1999;8(3):261–265. doi: 10.1136/tc.8.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Proescholdbell RJ, Chassin L, MacKinnon DP. Home smoking restrictions and adolescent smoking. Nicotine Tob Res. 2000;2(2):159–167. doi: 10.1080/713688125. [DOI] [PubMed] [Google Scholar]
- 63.Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, Badcock N. Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med. 2000;19(3):188–192. doi: 10.1016/s0749-3797(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 64.Jackson C, Henriksen L. Do as I say: parent smoking, antismoking socialization, and smoking onset among children. Addict Behav. 1997;22(1):107–114. doi: 10.1016/0306-4603(95)00108-5. [DOI] [PubMed] [Google Scholar]
- 65.Gilpin EA, White MM, Farkas AJ, Pierce JP. Home smoking restrictions: which smokers have them and how they are associated with smoking behavior. Nicotine Tob Res. 1999;1(2):153–162. doi: 10.1080/14622299050011261. [DOI] [PubMed] [Google Scholar]
- 66.Pizacani BA, Martin DP, Stark MJ, Koepsell TD, Thompson B, Diehr P. Household smoking bans: which households have them and do they work? Preventive Medicine. 2003;36(1):99–107. doi: 10.1006/pmed.2002.1123. [DOI] [PubMed] [Google Scholar]
- 67.Biener L, Cullen D, Di ZX, Hammond SK. Household smoking restrictions and adolescents' exposure to environmental tobacco smoke. Prev Med. 1997;26(3):358–363. doi: 10.1006/pmed.1997.0152. [DOI] [PubMed] [Google Scholar]
- 68.Gehrman CA, Hovell MF. Protecting children from environmental tobacco smoke (ETS) exposure: a critical review. Nicotine & Tobacco Research. 2003;5(3):289–301. doi: 10.1080/1462220031000094231. [DOI] [PubMed] [Google Scholar]
- 69.Emmons KM, Hammond SK, Abrams DB. Smoking at home: the impact of smoking cessation on nonsmokers' exposure to environmental tobacco smoke. Health Psychology. 1994;13(6):516–520. doi: 10.1037//0278-6133.13.6.516. [DOI] [PubMed] [Google Scholar]
- 70.Berman BA, Wong GC, Bastani R, et al. Household smoking behavior and ETS exposure among children with asthma in low-income, minority households. Addictive Behaviors. 2003;28(1):111–128. doi: 10.1016/s0306-4603(01)00221-0. [DOI] [PubMed] [Google Scholar]
- 71.Blackburn C, Spencer N, Bonas S, Coe C, Dolan A, Moy R. Effect of strategies to reduce exposure of infants to environmental tobacco smoke in the home: cross sectional survey. BMJ. 2003;327(7409):257. doi: 10.1136/bmj.327.7409.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hovell MF, Meltzer SB, Zakarian JM, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: a controlled trial. Chest. 1994;106(2):440–446. doi: 10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- 73.Wahlgren DR, Hovell MF, Meltzer SB, Hofstetter CR, Zakarian JM. Reduction of environmental tobacco smoke exposure in asthmatic children. A 2-year follow-up. Chest. 1997;111(1):81–88. doi: 10.1378/chest.111.1.81. [DOI] [PubMed] [Google Scholar]
- 74.Hovell MF, Zakarian JM, Matt GE, Hofstetter CR, Bernert JT, Pirkle J. Effect of counselling mothers on their children's exposure to environmental tobacco smoke: randomised controlled trial. BMJ. 2000;321(7257):337–342. doi: 10.1136/bmj.321.7257.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Emmons KM, Hammond SK, Fava JL, Velicer WF, Evans JL, Monroe AD. A randomized trial to reduce passive smoke exposure in low-income households with young children. Pediatrics. 2001;108(1):18–24. doi: 10.1542/peds.108.1.18. [DOI] [PubMed] [Google Scholar]
- 76.Hovell MF, Meltzer SB, Wahlgren DR, et al. Asthma management and environmental tobacco smoke exposure reduction in Latino children: a controlled trial. Pediatrics. 2002;110(5):946–956. doi: 10.1542/peds.110.5.946. [DOI] [PubMed] [Google Scholar]
- 77.Johansson A, Hermansson G, Ludvigsson J. How should parents protect their children from environmental tobacco-smoke exposure in the home? Pediatrics. 2004;113(4):e291–295. doi: 10.1542/peds.113.4.e291. [DOI] [PubMed] [Google Scholar]
- 78.Spencer N, Blackburn C, Bonas S, Coe C, Dolan A. Parent reported home smoking bans and toddler (18-30 month) smoke exposure: a cross-sectional survey. Arch Dis Child. 2005 Jul;90(7):670–674. doi: 10.1136/adc.2004.054684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winickoff J, Davis R. [Google Scholar]
- 80.Winickoff JP, Hillis VJ, Palfrey JS, Perrin JM, Rigotti NA. A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145. doi: 10.1542/peds.111.1.140. [DOI] [PubMed] [Google Scholar]
- 81.Groner JA, Ahijevych K, Grossman LK, Rich LN. The impact of a brief intervention on maternal smoking behavior. Pediatrics. 2000;105(1 Pt 3):267–271. [PubMed] [Google Scholar]
- 82.Greenberg RA, Strecher VJ, Bauman KE, et al. Evaluation of a home-based intervention program to reduce infant passive smoking and lower respiratory illness. Journal of Behavioral Medicine. 1994;17(3):273–290. doi: 10.1007/BF01857953. [DOI] [PubMed] [Google Scholar]
- 83.Chilmonczyk BA, Palomaki GE, Knight GJ, Williams J, Haddow JE. An unsuccessful cotinine- assisted intervention strategy to reduce environmental tobacco smoke exposure during infancy. American Journal of Diseases of Children. 1992;146(3):357–360. doi: 10.1001/archpedi.1992.02160150097031. [DOI] [PubMed] [Google Scholar]
- 84.Woodward A, Owen N, Grgurinovich N, Griffith F, Linke H. Trial of an intervention to reduce passive smoking in infancy. Pediatr Pulmonol. 1987;3(3):173–178. doi: 10.1002/ppul.1950030311. [DOI] [PubMed] [Google Scholar]
- 85.McIntosh NA, Clark NM, Howatt WF. Reducing tobacco smoke in the environment of the child with asthma: a cotinine-assisted, minimal-contact intervention. Journal of Asthma. 1994;31(6):453–462. doi: 10.3109/02770909409089487. [DOI] [PubMed] [Google Scholar]
- 86.Irvine L, Crombie IK, Clark RA, et al. Advising parents of asthmatic children on passive smoking: randomised controlled trial. Bmj. 1999;318(7196):1456–1459. doi: 10.1136/bmj.318.7196.1456. see comments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eriksen W, Sorum K, Bruusgaard D. Effects of information on smoking behaviour in families with preschool children. Acta Paediatrica. 1996;85(2):209–212. doi: 10.1111/j.1651-2227.1996.tb13994.x. [DOI] [PubMed] [Google Scholar]
- 88.Wagner EH. Chronic disease management: what will it take to improve care for chronic illness? Eff Clin Pract. 1998;1(1):2–4. [PubMed] [Google Scholar]
- 89.Wagner EH. The role of patient care teams in chronic disease management. Bmj. 2000;320(7234):569–572. doi: 10.1136/bmj.320.7234.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Curry SJ, McBride CM. Relapse prevention for smoking cessation: review and evaluation of concepts and interventions. Annu Rev Public Health. 1994;15:345–366. doi: 10.1146/annurev.pu.15.050194.002021. [DOI] [PubMed] [Google Scholar]
- 91.Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977 Mar;84(2):191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 92.Strecher VJ, Bauman KE, Boat B, Fowler MG, Greenberg R, Stedman H. The role of outcome and efficacy expectations in an intervention designed to reduce infants' exposure to environmental tobacco smoke. Health Educ Res. 1993;8(1):137–143. doi: 10.1093/her/8.1.137. [DOI] [PubMed] [Google Scholar]
- 93.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol. 1983;51(3):390–395. doi: 10.1037//0022-006x.51.3.390. [DOI] [PubMed] [Google Scholar]
- 94.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. AmericanJournal of Health Promotion. 1997;12(1):38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 95.Prochaska J, DiClemente C. Toward a comprehensive model of change. In: Miller W, Heather N, editors. Treating Addictive Behaviors. Plenum Publishing Corp.; 1986. [Google Scholar]
- 96.Glasgow RE, Whitesides H, Nelson CC, King DK. Use of the Patient Assessment of Chronic Illness Care (PACIC) with diabetic patients: relationship to patient characteristics, receipt of care, and self-management. Diabetes Care. 2005 Nov;28(11):2655–2661. doi: 10.2337/diacare.28.11.2655. [DOI] [PubMed] [Google Scholar]
- 97.Glasgow RE, Nelson CC, Strycker LA, King DK. Using RE-AIM metrics to evaluate diabetes self-management support interventions. Am J Prev Med. 2006 Jan;30(1):67–73. doi: 10.1016/j.amepre.2005.08.037. [DOI] [PubMed] [Google Scholar]