SUMMARY
The goal of this study was to test the hypothesis that spectral indices of heart rate variability, such as high-frequency power (HFP), low-to-high frequency power (LHR), and their respiration-adjusted counterparts (HFPra, LHRra) are correlated with severity of sleep-disordered breathing (SDB), as quantified by the respiratory disturbance index (RDI). A total of 436 subjects, non-smoking, normotensive, and free of cardiovascular disease and diabetes were selected from the Sleep Heart Health Study (SHHS). Of these, 288 records with sufficiently high quality electrocardiogram signals were selected for further analysis [males / females: 221 / 67; age: 46.1 to 74.9 years; body mass index (BMI): 21.5 to 46.4 kg m−2; 0.3 < RDI < 85.0−1]. From each polysomnogram, the respiration channels (thoracic and abdominal) and R-R interval (RRI) derived from the electrocardiogram were subjected to spectral analysis and autoregressive moving average modeling in consecutive 5-min segments. After adjusting for age and BMI, mean RRI was found to be negatively correlated with RDI in men in all sleep-wake states (all P < 0.001). HFP and HFPra were negatively correlated with RDI in men only during wakefulness (all P < 0.01). In women, LHR and LHRra were not correlated with RDI during wakefulness, but were positively correlated during non-rapid eye movement Stage 1 and 2 sleep (all P < 0.01). These findings suggest that the indices of cardiac autonomic control are correlated with SDB severity, but gender and state affect the nature of these correlations. In both genders, however, vagal modulation of heart rate increases while sympathetic modulation decreases from wakefulness to sleep.
Keywords: autonomic function, heart rate variability, obstructive sleep apnea, power spectral analysis
INTRODUCTION
It is now well recognized that sleep-disordered breathing (SDB) can lead to adverse effects on the heart and vasculature, resulting in cardiovascular diseases that include pulmonary and systemic hypertension, ischemic heart disease and myocardial infarction (Leung and Bradley, 2001; Parish and Shepard, 1990). The evidence for this view in humans has come primarily from large epidemiologic studies. For instance, the Wisconsin Sleep Cohort Study with over 700 subjects demonstrated that the adjusted odds ratio of incident systemic hypertension in SDB subjects with respiratory disturbance index (RDI) >15 was 2.89 compared with subjects without SDB (Peppard et al., 2000). Shahar et al., (2001) found in the Sleep Heart Health Study (SHHS) cohort of 6132 subjects that SDB is strongly correlated with prevalent coronary heart disease, heart failure, and stroke. In another report from the Sleep Heart Health Study Research Group, the odds ratio of hypertension was found to increase with severity of SDB, as quantified by RDI, even after adjustment of possible confounders (Nieto et al., 2000).
A key factor in the causal link between SDB with cardiovascular disease may be abnormal autonomic control (Somers et al., 1995). Evidence of sympathetic overactivity has been demonstrated in the form of elevated plasma and urinary catecholamine levels in patients with SDB (Fletcher et al., 1987) as well as in direct measurements of sympathetic activity from peroneal microneurography (Somers et al., 1995). Treatment of SDB by nocturnal application of continuous positive airway pressure over several months has been found to reduce muscle sympathetic nerve activity and plasma catecholamine levels (Hedner et al., 1995; Narkiewicz et al., 1999). Assuming that SDB leads to autonomic nervous system (ANS) dysfunction which, in turn, predisposes to cardiovascular disease, we hypothesize that after discounting for confounding variables, measures of ANS dysfunction should be correlated with severity of SDB.
Although there are more direct measures of autonomic activity, heart rate variability (HRV) has become a widely employed tool because of its non-invasiveness and ease of application. In addition to the mean R-R interval (mRRI) derived from the electrocardiogram (ECG), the power spectrum of HRV time-series provides information about parasympathetic (vagal) and sympathetic modulation of heart rate (Task Force, 1996). High-frequency power (HFP) (0.15–0.40 Hz) is believed to be parasympathetic in origin, while low-frequency power (LFP) (0.04–0.15 Hz) reflects a combination of both sympathetic and parasympathetic activities. As such, the ratio of low-to-high frequency power (LHR = LFP / HFP) has been widely used to represent the balance between sympathetic and vagal activities (‘sympath-ovagal balance’) (Task Force, 1996). However, caution needs to be exercised when changes in these HRV spectral indices are interpreted as representing changes in vagal and sympathetic activities (Eckberg, 1998). For instance, an increase in LHR that occurs concomitantly with a reduction in HFP could be because of a simultaneous increase in sympathetic activity and decrease in vagal activity, or simply a decrease in vagal activity. On the other hand, an increase in LHR that occurs without a corresponding reduction in HFP would suggest an increase in cardiac sympathetic activity.
High-frequency power is closely associated with the respiratory modulation of heart rate, commonly known as respiratory sinus arrhythmia (RSA). The fact that both tidal volume and breathing frequency can be highly variable within a given individual at different times introduces some complications into the interpretation of HFP as a measure of parasympathetic activity. The traditional means by which researchers have sought to circumvent the confounding influence of respiration on RSA magnitude has been to control the breathing pattern (Grossman et al., 1991). Typically, breathing is voluntarily controlled at a given rate and / or depth, while the responses in RSA to various pharmacologic or posture-related interventions which affect sympathovagal balance are measured. However, this allows changes in RSA to be determined only at the controlled respiratory frequency and volume. Moreover, this kind of voluntary control of the breathing pattern is not possible during sleep when the level of ventilation and breathing pattern can change considerably with various sleep stages. In subjects with SDB, ventilatory pattern can go through even larger temporal changes, as breathing alternates between regular tidal respiration, and episodes of apnea and hyperpnea during night. We have demonstrated previously (Khoo et al., 1999) that these non-uniform ventilatory patterns can substantially influence LFP and HFP, and also introduce substantial power in the very-low frequency (VLF, <0.04 Hz) region. These effects can seriously complicate the interpretation of the traditional spectral measures of HFP and LHR as indices of autonomic regulation. To be able to extract useful information about the ANS from indices of HRV, it is thus important for the effects of respiration to be discounted when these measures are being computed.
To test our hypothesis that autonomic control, as measured from HRV, is affected by severity of SDB, we computed five ANS power spectral indices (mRRI, LHR, HFP, LHRra, HFPra) from a large number of overnight polysomnographic studies and tested each of these indices for possible correlation with RDI. The latter two indices were employed to adjust for the effects of differences in respiratory pattern and ventilation within and between subjects.
METHODS
Subject pool and measurements
A total of 436 participants in the second SHHS examination (1999–2004) who were free of known chronic cardiopulmonary disorders and were representative of the full range of SDB severity, as quantified by RDI (the number of hypopneic or apneic events occurring per hour overnight) were identified. Exclusionary criteria included history of lung disease (chronic obstructive pulmonary disease, asthma), cardiovascular disease, diabetes, hypertension (systolic blood pressure > 140 or diastolic pressure > 90 mmHg; subjects taking anti-hypertensive medications were also excluded) and current smoking. In addition, subjects were selected to achieve a sample of an equal number of subjects in each of four RDI categories (< 5, 5–15, 15–30, > 30 h−1), who also were of generally comparable age (±1 years), and roughly similar BMI (± 2 kg m−2). After detailed examination of the polysomnograms using an automated algorithm with manual checks, studies with long segments of extensive ectopic-beats [more than 6 b min−1(ectopic) overnight was considered pathological] (Dubin, 1998) and excessive artifact in the ECG signals (with RRI > 3 SDs after interpolation) were excluded from further analysis. If the majority of overnight 5-min ECG segments met our criteria (more than ¾ of overnight data length), the whole night data was used to avoid selection bias from using only the ‘clean’ segments. Overall, the analysis procedures that are described below were applied to only 288 subjects (males / females: 221 / 67, age: 62.6 ± 6.7 years; BMI: 30.3 ± 4.3 kg m−2; 0.3 < RDI < 85.0). The characteristics of these 288 subjects are summarized in Table 1. Although there was an attempt in the subject selection process to limit the variation of BMI across the different RDI groups, the male subjects with RDI < 5 h−1 were significantly lower in BMI compared with the other three RDI categories (Table 1). A comparison of the subject characteristics displayed in Table 1 with a corresponding table derived using all 436 subjects showed no significant differences. For instance, the distribution of the 288 subjects among the four RDI groups was relatively uniform, similar to the uniform distribution in the original sample of 436 subjects. Thus, the exclusion of bad-quality recordings did not lead to a selection bias in the final sample that was analyzed.
Table 1.
Demographics (statistically significant postulated relationships in bold)
| RDI categories (h−1) | ||||||
|---|---|---|---|---|---|---|
| Overall | 0–5 | 5–15 | 15–30 | 30+ | P-value* | |
| Men | 221 | 57 | 53 | 60 | 51 | – |
| RDI (h−1) | 19.1 (17.0) | 2.9 (1.4) | 9.4 (2.8) | 21.3 (4.1) | 44.5 (13.1) | < 0.001 |
| Age (years) | 62.2 (6.9) | 61.0 (7.2) | 62.8 (7.2) | 62.0 (6.7) | 63.1 (6.4) | 0.41 |
| BMI (kg m−2) | 29.5 (3.6) | 26.6 (2.5) | 30.4 (3.4) | 30.6 (3.5) | 30.4 (3.3) | < 0.001 |
| SBP (mmHg) | 121.1 (10.3) | 120.1 (10.6) | 124.0 (8.7) | 123.0 (10.4) | 121.3 (11.0) | 0.18 |
| DBP (mmHg) | 74.0 (7.0) | 72.5 (7.9) | 74.7 (7.1) | 73.2 (7.9) | 75.8 (7.5) | 0.11 |
| Women | 67 | 17 | 14 | 18 | 18 | – |
| RDI (h−1) | 20.0 (16.4) | 2.8 (1.5) | 10.5 (3.5) | 20.6 (3.6) | 43.0 (9.5) | < 0.001 |
| Age (years) | 64.0 (5.9) | 62.8 (6.2) | 63.4 (6.0) | 65.7 (4.7) | 63.7 (6.7) | 0.51 |
| BMI (kg m−2) | 33.1 (5.3) | 33.2 (3.9) | 32.4 (6.0) | 31 .5 (4.6) | 35.1 (6.0) | 0.20 |
| SBP (mmHg) | 119.8 (10.3) | 118.6 (10.8) | 117.1 (8.8) | 123.8 (11.0) | 119.0 (10.0) | 0.27 |
| DBP (mmHg) | 71.0 (6.4) | 74.3 (8.2) | 69.1 (6.4) | 70.7 (4.1) | 69.6 (5.7) | 0.08 |
RDI, respiratory disturbance index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure. Mean (SD) for continuous variables and counts for gender.
P-values are from one-way anova, and if it is significant, all pair-wise comparisons to control are significant (Holm–Sidak).
The technical details of the instrumentation and data collection techniques employed in the Sleep Heart Health Study Research Group, 1996 have been described elsewhere (Quan et al., 1997; Redline et al., 1998), but a brief summary of the main features is as follows: physiological data (e.g., ECG) of participants in SHHS were collected in single night, unattended polysomnography at home accompanied with body weight and blood pressure measurement; other anthropometric and medical history information was obtained from a parent study database (e.g., height) or by a standard questionnaire (e.g., smoking status). Apneas were scored by SHHS if complete or almost complete cessation (> 75% decrease from baseline amplitude) of airflow measured by a nasal-oral thermal sensor was observed and hypopneas were scored if the amplitude signals from the thoracic and respiratory inductance plethysmography signals or thermistry decreased by 25% or more. All events were required to last longer than 10 s and also associated with oxyhemoglobin desaturation of 4% or greater as compared with the baseline. All channels of selected polysomnograms were manually checked to be of reasonably high signal-to-noise ratio.
Signal processing and spectral analysis
QRS detection from the ECG was achieved by employing an automatic derivative-based algorithm with the threshold set at 0.3 times the maximum magnitude of the of the 5 min ECG segment (Kohler et al., 2002). Validation of correct processing by the automatic algorithm was performed using visual inspection of the ECG segment with superimposed R-wave markers. In the noisier recordings, the noisy segments were rejected altogether and only the shorter segments of sufficiently good quality were employed in subsequent analyses. RRI was computed on a beat-to-beat basis, but the resulting time-series was resampled at 2 Hz using the Berger algorithm (Berger et al., 1986) for subsequent spectral analysis. The abdominal and thoracic signals were also resampled at the same frequency to enable synchronization with the RRI time-series.
Calculation of ‘respiration-adjusted’ HRV spectral indices
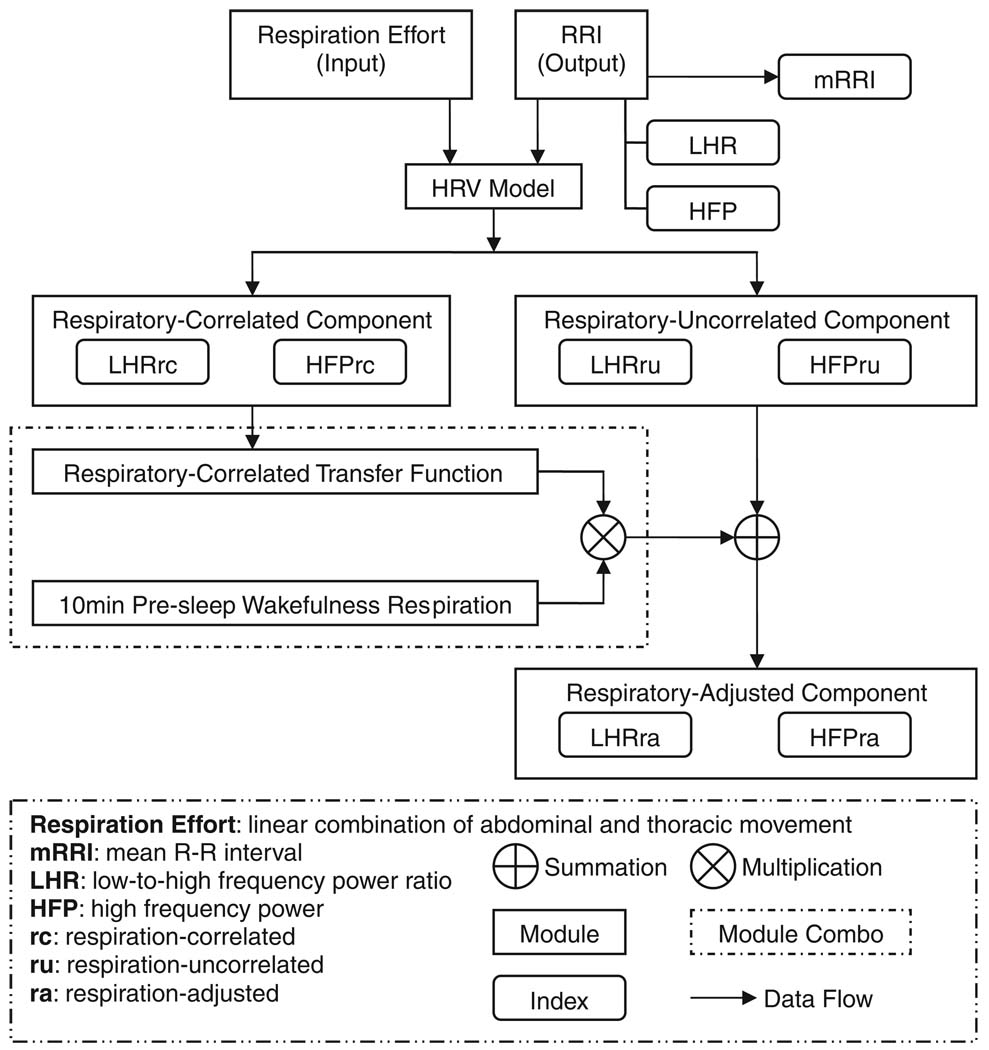
The algorithm employed to produce these indices has been summarized schematically in Fig. 1. To every consecutive 5-min segment of each polysomnogram, the modeling technique introduced by Khoo et al. (1999) was applied to establish the temporal relationship (shown in Fig.1 as ‘HRV model’) between respiration and fluctuations in RRI during the segment of time in question. This relationship (or ‘transfer function’, in engineering parlance) enabled the delineation of the respiratory-correlated component of HRV from the respiratory-uncorrelated component. The transfer function was also used to predict what the time-course of the RRI fluctuations would have been if the ventilatory pattern had remained the same as what was measured during the period of wakefulness prior to sleep onset, i.e., the ‘respiration-adjusted RRI’ time-series. A detailed account of this algorithm is given in the Appendix.
Figure 1.
Schematic representation of analysis procedures.
The spectra of the original RRI time-series and the ‘respiration adjusted’ RRI time-series were computed using the Welch method with Hanning windowing (Tompkins, 1993). The respective areas in the appropriate frequency bands (low-frequency: 0.04–0.15 Hz; high-frequency: 0.15–0.4 Hz) under each RRI spectrum were calculated to yield the HRV spectral indices, LFP, and HFP. Subsequently, LFP was divided by HFP to yield LHR. Analogous calculations were performed to arrive at the corresponding ‘respiration adjusted’ indices: LFPra, HFPra, and LHRra.
Statistical analyses
Using the aforementioned methods, HRV spectral indices were computed based on consecutive 5-min segments of ECG and respiration. As pointed out by the 1996 Task Force report on HRV measurement (Task Force., 1996), a duration of 5 min is probably the longest period over which stationarity in the ECG time-series can be assumed and standard spectral analysis algorithms applied. Moreover, as spectral analysis was applied to data segments that were only 5 min long, estimation of the power of the VLF < 0.04 Hz component was likely to be inaccurate (Task Force., 1996). Because of this and other reasons (see Discussion), estimates of VLF power are not reported here.
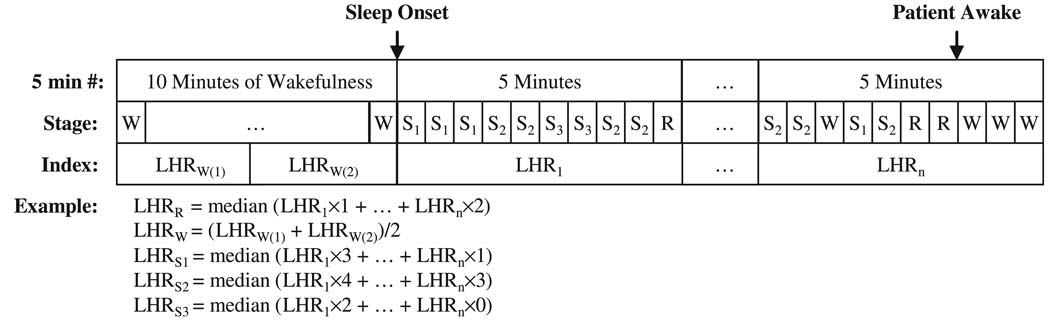
Scoring of sleep-wake state was carried out in consecutive segments of 30 s (epochs). To deduce the median HRV spectral index for each sleep state, the algorithm illustrated in Fig. 2 was employed. As each 5-minute segment of data was used to produce one value of each HRV spectral index, the segment was divided up into equal-length (30 s) sub-segments carrying the same HRV spectral index. The median for each parameter at a given sleep-wake state was deduced from all 30-s epochs in the overnight study. Figure 2 displays an example of the computations used to determine the median value of LHR representative of each sleep-wake state in each individual subject. Similarly, each of the other HRV spectral indices was represented by the median of all the overnight 5-min segment values for each sleep state. Medians were adopted instead of means to exclude outlier values that were produced primarily by ectopic events. Although there were periods after the start of each overnight study that were classified as ‘wake’, we employed only the last 10 min of quiet wakefulness prior to sleep onset to represent ‘true wakefulness’. In other words, the wakefulness stage in this study represents only the 10-min period before sleep onset; none of indices computed from periods classified as ‘wake’ after sleep onset were taken into consideration.
Figure 2.
Schematic illustration of the method used to compute the ‘median’ value of the heart rate variability spectral index representative of each sleep-wake state. LHR, low-to-high frequency power ratio; R, REM; W, wakefulness; S1, S2, S3: sleep Stage 1, 2, 3).
Preliminary analysis of the data indicated that there were significant gender differences (for LHR, LHRra, mRRI, P < 0.0001) based on three-way repeated measures anova on RDI levels, gender, and sleep-wake state. Thus, our subsequent analyses considered male and female subjects separately. Our preliminary tests (Wang et al., 2006) also suggested significant confounding effects from age and BMI. Thus, multiple linear regression was applied with BMI and age as continuous independent variables and the log-transformed value of each HRV index as the dependent variable. Following linear adjustment for the effects of age and BMI, the HRV indices were subjected to two-way repeated measures anova, with sleep-wake state [Wake, rapid eye movement (REM), non-rapid eye movement (NREM) Stage 1, NREM Stage 2, NREM Stage 3–4] being the repeated factor and RDI category (Normal: RDI < 5, Mild SDB: 5 < RDI < 15, Moderate SDB: 15 < RDI < 30, Severe SDB: RDI > 30 h−1) as the unrepeated factor. Post hoc multiple pair-wise comparisons (Holm–Sidak test) were carried out if statistical significance was indicated.
To determine whether there were significant correlations between each HRV index and RDI in the different sleep-wake states, we subsequently extended the multiple linear regression model to include RDI, BMI, and age as continuous independent variables, following stratification of the data according to gender and sleep-wake state.
All statistical analyses were conducted using sigmastat®3.11 (Systat Software Inc., San Jose, CA, USA). A significance level of 0.05 was assumed.
RESULTS
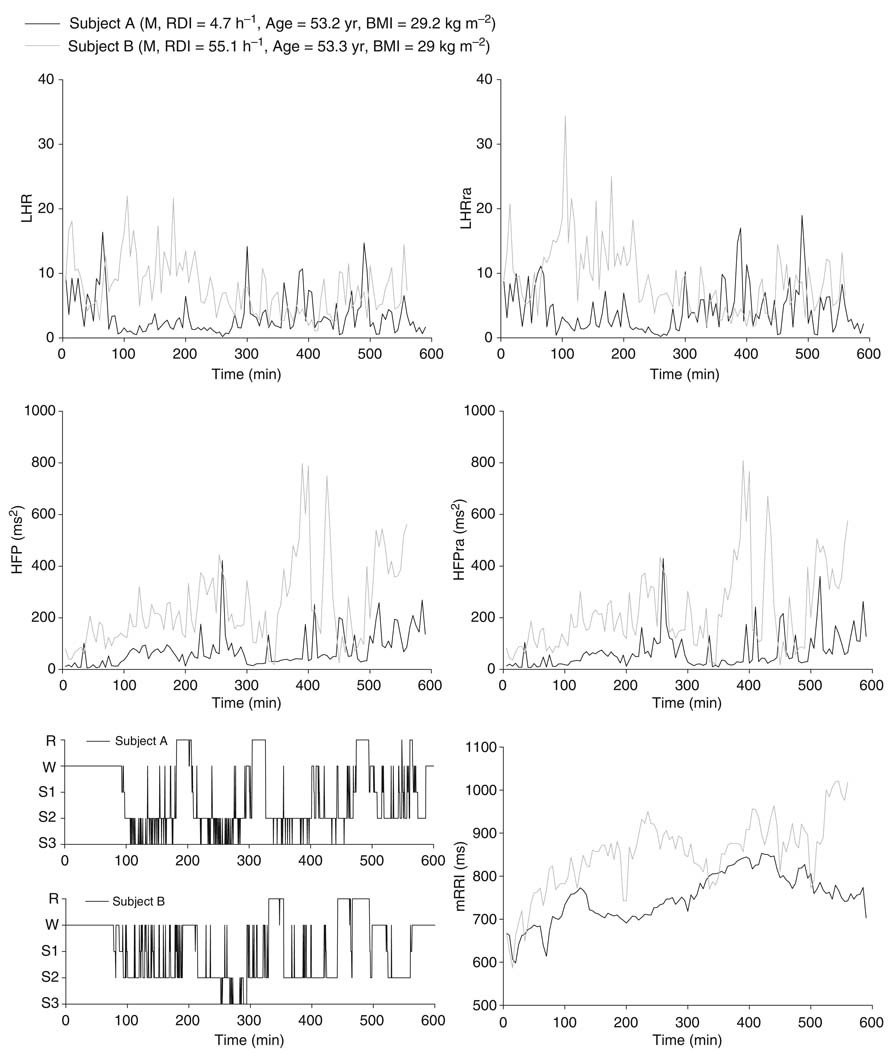
Figure 3 illustrates examples of the time-series for HFP, HFPra, LHR, and LHRra, along with corresponding hypnograms for two male subjects: a subject without evident SDB (RDI = 4.7 h−1) and a subject with SDB (RDI = 55.1 h−1). Both are of similar age (53 year) and BMI (29 kg m−2). Although the time courses of the respiration-adjusted spectral indices (HFPra, LHRra) are quite similar in form to the corresponding ‘raw’ spectral indices (HFP, LHR), there are noticeable differences in magnitude between the plots, particularly between LHR and LHRra in the subject with high RDI. These differences reflect the effect of non-uniform ventilatory patterns (e.g., alternating episodes of apnea and hyperpnea) on HRV during sleep, and thus, they are expected to be larger as RDI increases. In the comparison of this particular pair of individuals, it turned out that mRRI was generally higher in the SDB subject versus the subject without SDB. However, as demonstrated in the results to follow, we found the opposite to be true when statistical analysis was applied to all datasets.
Figure 3.
Examples of overnight time courses of heart rate variability indices and hypnograms: normal versus sleep-disordered breathing subject.
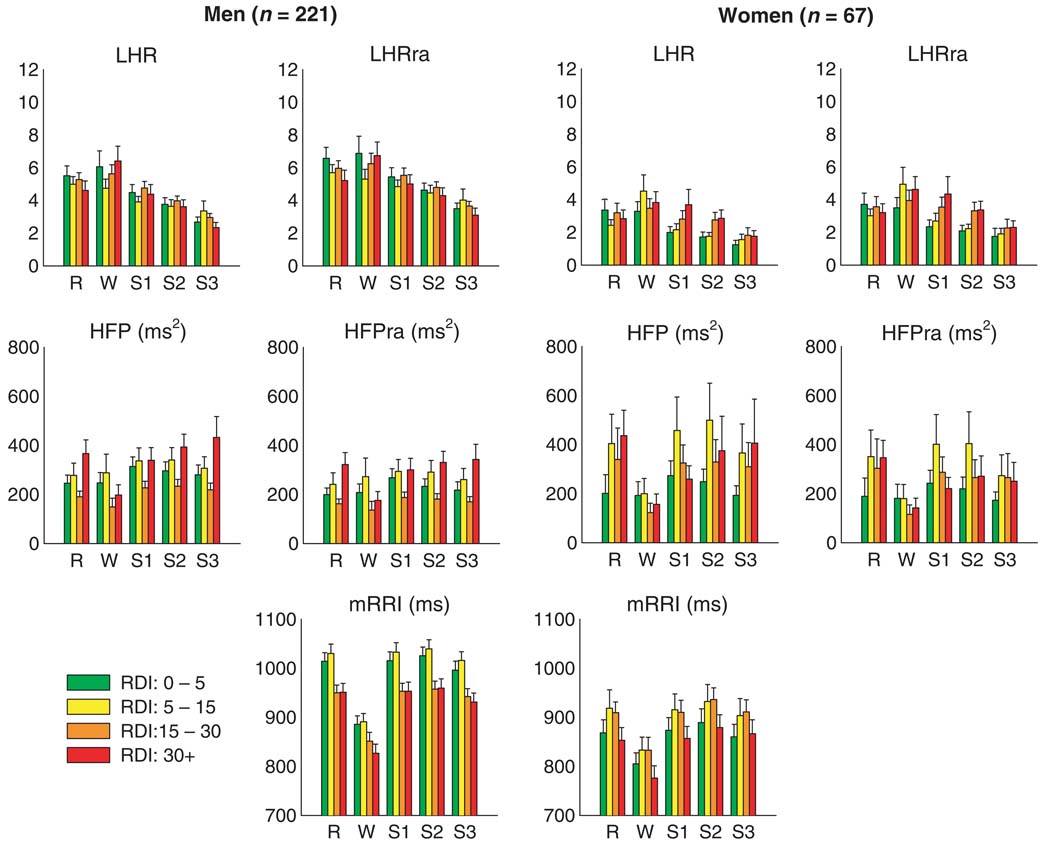
Figure 4 summarizes the results computed from all 288 studies for the ‘raw’ and respiration-adjusted HRV spectral indices as well as mRRI, following adjustment for age and BMI. Because of significant differences in trends between the male and female subjects, the findings for each gender have been displayed separately. For the same sleep-wake state and RDI group, the female subjects tended to have lower mRRI (or equivalently, higher heart rate), LHR, and LHRra than their male counterparts. There were no gender differences in HFP and HFPra.
Figure 4.
Median heart rate variability spectral indices across different levels of respiratory disturbance index and sleep-wake state, after adjusting for age and body mass index.
As expected, in both gender groups, LHR and LHRra tended to decrease as sleep-wake state changed from wakefulness to REM and to NREM Stages 1–3 / 4. HFP and HFPra also tended to increase from wakefulness to REM to NREM sleep, although without further increasing as the depth of NREM sleep increased from Stage 1 to Stage 3 / 4. This strong dependence on sleep-wake state is confirmed by the results of the two-way repeated measures anova performed on the autonomic indices following adjustment for age and BMI (Table 2). In addition, mRRI was lower (i.e., average heart rate was higher) in wakefulness compared with all sleep states. The male subjects showed a significant negative relationship between RDI and mRRI (P < 0.001) independent of sleep-wake state: as severity of SDB increased from normal or mild (apnea-hypopnea index, RDI: 0–15) to moderate and severe (RDI > 15), mRRI decreased. Men also displayed a significant interaction of HFPra between RDI and sleep / wake state (Table 2, P = 0.018), which could be readily recognized by comparing the trend of HFPra as RDI level changes between wakefulness and other sleep states. During wakefulness, HFPra was lower in those with RDI ≥15 than in those with RDI < 15. During sleep, however, the relation of HFPra with RDI was not monotonic, being highest in those with RDI ≥30 but lowest in those with RDI of 15–30. In women, there was some tendency for mRRI to be higher in the subjects with mild and moderate SDB relative to the normals and those subjects with severe SDB, but these differences were not significant.
Table 2.
P-values from two-way repeated measures anova on HRV indices after adjusting for age and BMI (statistically significant postulated relationships in bold)
| Men (n = 221) | Women (n = 67) | |||||
|---|---|---|---|---|---|---|
| Indices | RDI | SS | Interaction | RDI | SS | Interaction |
| LHR | 0.910 | < 0.001 | 0.322 | 0.636 | < 0.001 | 0.126 |
| LHRra | 0.823 | < 0.001 | 0.466 | 0.503 | < 0.001 | 0.244 |
| HFP | 0.098 | < 0.001 | 0.039 | 0.577 | < 0.001 | 0.397 |
| HFPra | 0.070 | 0.005 | 0.018 | 0.647 | < 0.001 | 0.465 |
| mRRI | < 0.001 | < 0.001 | 0.198 | 0.317 | < 0.001 | 0.958 |
HRV, heart rate variability; RDI, respiratory disturbance index; HFP, high-frequency power; mRRI, mean R-R interval; LHR, low-to-high frequency ratio; SS, sleep-wake state; HFPra, HFP respiration-adjusted counterparts; LHRra LFP respiration-adjusted counterparts; R, REM; W, wakefulness; BMI, body mass index. There are four levels (0–5, 5–15, 15–30, 30+) of RDI and five repeated levels (R, W, S1, S2, S3) of SS.
Careful inspection of Fig. 4 shows that in wakefulness, HFP and HFPra were noticeably lower in the subjects with moderate-to-severe RDI, relative to the normals and subjects with mild SDB. As well, in females during the light stages of NREM sleep, LHR and LHRra tended to increase with severity of SDB. Stratifying for sleep-wake state, the multiple regression analysis with RDI as one of the continuous independent variables provided further clarification of this issue. Table 3 shows the linear regression coefficients linking each log-transformed index to RDI for each sleep-wake state, following adjustment for the effects of age and BMI. Consistent with the findings displayed in Fig. 4, mRRI decreased with increasing RDI in men across all sleep stages (all P < 0.001). However, no significant association between RDI and mRRI among women was identified. Significant negative correlations between HFP and RDI (P = 0.004) and HFPra and RDI (P = 0.007) were also identified during wakefulness in men, but not in women. On the contrary, significant trends for LHR and LHRra to increase with RDI were identified during NREM Stages 1 and 2 (P < 0.01 in all cases) in women, but not in men.
Table 3.
Linear regression analysis of HRV indices on RDI over REM, wakefulness and sleep Stage 1–3 after adjusting for age and BMI (statistically significant postulated relationships in bold)
| RDI | |||||||
|---|---|---|---|---|---|---|---|
| Men (n = 221) | Women (n = 67) | ||||||
| Indices | |||||||
| Sleep state | (log-transformed) | β* | (SE*) | P-value | β* | (SE*) | P-value |
| R | LHR | −0.18 | (0.14) | 0.190 | 0.06 | (0.27) | 0.237 |
| LHRra | −0.25 | (0.14) | 0.069 | 0.10 | (0.25) | 0.702 | |
| HFP | 0.26 | (0.20) | 0.200 | 0.76 | (0.47) | 0.109 | |
| HFPra | 0.31 | (0.20) | 0.128 | 0.73 | (0.47) | 0.124 | |
| mRRI | −0.09 | (0.02) | < 0.001 | −0.06 | (0.04) | 0.147 | |
| W | LHR | 0.21 | (0.17) | 0.212 | 0.03 | (0.27) | 0.927 |
| LHRra | 0.16 | (0.16) | 0.318 | 0.13 | (0.27) | 0.626 | |
| HFP | −0.67 | (0.23) | 0.004 | −0.20 | (0.40) | 0.627 | |
| HFPra | −0.62 | (0.23) | 0.007 | −0.22 | (0.41) | 0.583 | |
| mRRI | −0.10 | (0.03) | < 0.001 | −0.07 | (0.04) | 0.128 | |
| S1 | LHR | 0.03 | (0.13) | 0.825 | 0.74 | (0.26) | 0.005 |
| LHRra | −0.04 | (0.12) | 0.733 | 0.75 | (0.25) | 0.004 | |
| HFP | −0.15 | (0.19) | 0.419 | −0.18 | (0.42) | 0.660 | |
| HFPra | −0.11 | (0.19) | 0.553 | −0.17 | (0.42) | 0.688 | |
| mRRI | −0.09 | (0.02) | < 0.001 | −0.06 | (0.04) | 0.166 | |
| S2 | LHR | 0.05 | (0.14) | 0.703 | 0.66 | (0.24) | 0.007 |
| LHRra | −0.02 | (0.13) | 0.899 | 0.67 | (0.22) | 0.004 | |
| HFP | 0.01 | (0.19) | 0.953 | 0.10 | (0.43) | 0.812 | |
| HFPra | 0.06 | (0.19) | 0.755 | 0.09 | (0.42) | 0.834 | |
| mRRI | −0.10 | (0.02) | < 0.001 | −0.05 | (0.04) | 0.288 | |
| S3 | LHR | −0.08 | (0.16) | 0.615 | 0.39 | (0.28) | 0.164 |
| LHRra | −0.08 | (0.16) | 0.633 | 0.49 | (0.28) | 0.086 | |
| HFP | −0.07 | (0.21) | 0.752 | 0.45 | (0.44) | 0.310 | |
| HFPra | −0.02 | (0.21) | 0.913 | 0.27 | (0.43) | 0.534 | |
| mRRI | −0.11 | (0.02) | < 0.001 | −0.03 | (0.04) | 0.529 | |
BMI, body mass index; HRV, heart rate variability; RDI, respiratory disturbance index; HFP, high-frequency power; mRRI, mean R-R interval; LHR, low-to-high frequency ratio; HFPra, HFP respiration-adjusted counterparts; LHRra LFP respiration-adjusted counterparts; R, REM; W, wakefulness.
Values are scaled to 10−2.
DISCUSSION
Adjustment for non-uniform ventilatory patterns in SDB
The premise that spectral indices of HRV can provide useful information about autonomic control of the heart to a large extent is based on the original study of Katona and Jih (1975), which showed in an animal preparation that respiratory-related fluctuations in RRI were linearly related to cardiac vagal firing rates. In that study and the subsequent validation studies on humans that followed (Berntson et al., 1997; Grossman et al., 1991), respiration was either controlled or changed little, while vagal tone was altered using pharmacologic or physiologic interventions. Although some studies have underscored the important effect of respiratory rate and tidal volume on the HRV spectrum (Brown et al., 1993; Grossman et al., 1991), this confounding influence of respiration has been largely ignored in the HRV literature. Brown et al. (1993) showed that depending on breathing frequency, changes in respiration within a given individual can substantially alter estimates of both HFP and LFP of the RRI spectrum. We have previously demonstrated that the highly non-uniform ventilatory patterns associated with SDB during sleep can profoundly exaggerate this confounding influence of respiration on estimates of HRV spectral power (Khoo et al., 1999). For instance, the emergence of hyperpneic breaths following the termination of apnea can increase estimates of HFP, leading to the erroneous suggestion of an increase in vagal tone during these episodes. In this and our previous study (Khoo et al., 1999), we found that there was significant power in the VLF region, consistent with the ventilatory periodicities that ranged from 30 s to 1.5 min in SDB in the HRV spectra of data segments with episodic apnea or hypopnea. Respiratory entrainment of heart rate at such low frequencies has been demonstrated in experiments by Lorenzi-Filho et al. (1999), in which normal subjects were able to generate VLF oscillations in the HRV spectrum by simulating periodic breathing through voluntary control of the ventilatory pattern.
In the present study, we introduced the approach of ‘computationally correcting’ these respiratory-related distortions of the HRV spectrum in each dataset by first estimating the transfer function between respiration and HRV, and subsequently convolving this transfer function with the respiratory signal measured during wakefulness in the same subject to derive the ‘respiration-adjusted’ HRV spectral indices (i.e., HFPra and LHRra). This procedure also largely eliminated the HRV oscillations in the VLF region. Thus, analogous to the well-accepted statistical technique of ‘adjusting’ for confounding variables, our ‘respiration adjustment’ approach permits a more valid comparison of HRV spectral indices computed from different times during wakefulness and sleep within a given individual, as well as across individuals.
Differences from previous studies
Previous studies employing spectral analysis of HRV have generally reported significantly reduced HFP and enhanced LHR in awake SDB subjects relative to normal controls (Khoo et al., 1999; Narkiewicz et al., 1998; Wiklund et al., 2000). Similar findings on frequency-domain indices of HRV have appeared in studies of subjects with SDB conducted during sleep (Shiomi et al., 1996; Vanninen et al., 1996) or through Holter monitoring over 24 h (Aydin et al., 2004; Gula et al., 2003). However, the major limitations in all these studies were that sample size was relatively small and that the effects of potential confounding factors, such as subject gender, BMI, and differences in respiration were not carefully accounted for. Wiklund’s study (Wiklund et al., 2000) produced findings from a relatively large group of 117 subjects, but these were based on short periods of measurement (several minutes) conducted only during wakefulness. In contrast, the present study was based on data collected from close to 300 normotensive subjects drawn from a community-based cohort. Another important difference is that, in our study, the HRV spectral indices representing the various sleep-wake states in each of the subjects were determined from the entire overnight poly-somnographic recording and not from selected short segments of data. As well, close attention was paid in minimizing the effects of potential confounding variables or co-morbidities through subject selection or by computationally adjusting these effects during analysis.
Dependence of HRV indices on sleep/wake state
One important finding from this study that stood out among the others was the clear dependence of mRRI and the other indices of HRV on sleep-wake state among all subject groups. LHR and LHRra were generally highest in wakefulness but decreased with NREM sleep depth. mRRI, HFP, and HFPra tended to be lowest in wakefulness but increased during sleep. These findings are consistent with well-accepted observations in normals where sympathetic activity decreases and parasympathetic activity increases during sleep (Mancia, 1993; Zemaityte et al., 1984). However, the indices of parasympathetic activity (mRRI, HFP, and HFPra) did not increase with increasing depth of NREM sleep. It should also be noted that our methodology is limited by the underlying assumption that all sleep states of the same stage (e.g., NREM Stage 2) were the same and that there were no time of night or circadian effects. As well, another limitation is that each 5-min segment would contribute the same HRV index to all the sleep stages that appeared within that duration, in effect averaging out the influences of rapid changes in state. One alternative could have been to compute the HRV spectra over consecutive epochs of 30 s segments, each of which would be scored for sleep stage; however, this would have produced inaccurate estimates of LFP and even HFP. In the final analysis, it is not possible to completely dissociate the changes in ANS activity from the accompanying changes in physiology (i.e., changes in state and respiration) that occur during sleep in SDB as all these variables are by nature highly interconnected.
Gender differences
Consistent with previous studies (Antelmi et al., 2004; Evans et al., 2001; Kuo et al., 1999; Liao et al., 1995; Ryan et al., 1994) on normal subjects, we found significant gender differences in LHR: men had higher LHR values than women. Also consistent with previous studies (Evans et al., 2001; Liao et al., 1995; Ryan et al., 1994), HFP was generally not different between genders. However, there is a key finding from this study that is relatively new: the substantial difference between men and women in the relationship between SDB severity and the HRV indices. Sympathetic modulation (as reflected by LHR, LHRra) and mRRI in women were consistently lower than corresponding levels in men across all RDI and sleep levels. Compared with men, parasympathetic modulation in women during sleep was higher in the groups with mild and moderate SDB severity. However, these gender differences were not apparent in wakefulness. In men, mRRI was significantly and negatively associated with RDI in both wakefulness and sleep. As well, during wakefulness, HFP and HFPra were each negatively correlated with RDI, suggesting that as SDB severity increases, waking parasympathetic modulation of heart rate becomes progressively reduced. In contrast, in women during wakefulness, we found no relationship between RDI and high-frequency modulation of HRV. Instead, in sleep Stages 1 and 2, both indices of sympathovagal balance (LHR and LHRra) were positively correlated with RDI. These findings suggest that in men, SDB is associated with impaired cardiac autonomic control primarily through a reduction in vagal modulation of heart rate, whereas in women, the association appears to be different, with sympathetic modulation becoming more important with increased SDB severity during the light stages of NREM sleep. It appears that these effects tend to be quite subtle in otherwise healthy individuals, as they are easily obscured by the presence of other confounding factors. The impact becomes most visible only at the lowest levels of vagal tone (i.e., during wakefulness) in men or in states of low sympathetic tone (i.e., during NREM sleep) in women. However, the aforementioned findings should include the caveat that the statistical power of our computations for male subjects is probably substantially higher than that for the female subjects because of the smaller sample size of the latter.
Elimination of confounding influences
Although initial efforts were taken to limit the effects of confounding variables, precise matching for age and BMI across RDI groups was problematic. As it is known that autonomic function can be affected by obesity (Grassi et al., 1995) and aging (Brandenberger et al., 2003), we employed multiple linear regression to statistically adjust for the effects of these influences on the HRV indices. It was only after adjustment for age and BMI that the correlation between SDB severity and the HRV indices in certain sleep-wake states became visible.
CONCLUSIONS
In summary, we conducted a statistical analysis of the HRV spectral indices derived from 288 polysomnograms of middle-to-old aged subjects drawn from the SHHS cohort, in which computational adjustments were made to attenuate the confounding influences from differences in respiration, BMI, and age. Consistent with previous findings from others, we found that changes in state from wakefulness to sleep led to increased parasympathetic modulation and decreased sympathetic modulation of heart rate. Our findings point to an association between SDB severity and cardiac autonomic control, but the nature of the association appears to be gender-dependent. In men, mRRI and the indices representing HFP of HRV decrease with increasing RDI during wakefulness, suggesting a progressive reduction in vagal modulation of heart rate as SDB becomes more severe. In women, however, mRRI and the indices representing high-frequency HRV power are not correlated with RDI during wakefulness; instead, both LHR and LHRra are positively correlated with RDI during the light stages of NREM sleep. These findings suggest that there are subtle but significant gender differences in the way in which SDB severity and changes in sleep-wake state affect cardiac autonomic control. These newly unravelled gender differences are as yet unexplained and require further confirmation using other tools for assessing ANS activity.
ACKNOWLEDGEMENTS
This study was supported by NIH Grants HL-076375, EB-001978, HL-63463, and HL53941.
APPENDIX: DETAILED METHODOLOGY FOR COMPUTING RESPIRATION-ADJUSTED HRV SPECTRAL INDICES
To adjust for temporal variations in the respiratory contribution to the HRV spectral indices in each subject, we employed an extension of ARX input modeling approach published previously (Khoo et al., 1999). In Khoo’s original method, esophageal pressure (Khoo et al., 1999) or respiratory airflow (Khoo et al., 2001) was used as the ‘input’ to the model while RRI was treated as the ‘output’. In the SHHS studies, however, respiration was monitored in the form of uncalibrated thoracic and abdominal inductance plethysmography. Therefore, two data channels were used as inputs to the model: the thoracic component and the abdominal component of the respiration signal.
Thus, the ARX model relating (uncalibrated) changes in lung volume, x(n), to changes in RRI, y(n), at time point n was:
| (1) |
where N represents the total number of data points and e(n) is the difference between the actual and predicted RRI. The uncalibrated lung volume change was assumed to be the weighted sum of the abdominal [xa(n)] and thoracic [xt(n)] excursions:
| (2) |
with an unknown coefficient γ, representing the relative contribution of the thoracic excursion to overall respiratory signal. γ and the other unknown parameters in the ARX model {p, q, ai (1 ≤ i ≤ p) and bk(0 ≤ k ≤ q)} were estimated for each successive 5-min segment of data by least-squares optimization. The ‘optimal’ model was selected by searching for a model candidate (with maximum model order 10) that produced the lowest value of the criterion known as the ‘minimum description length’ (Rissanen, 1982).
Equation 1 allows y(n) to be partitioned into a component that is correlated with respiration, yrc(n), and a respiration-uncorrelated component, yru(n):
| (3) |
where
| (4) |
and
| (5) |
where q−m represents a backward shift of m time-steps.
In Equation 4, the respiration-correlated component of HRV can change considerably even in the same subject if the magnitude and pattern of respiration, x(n), undergo significant change, such as what occurs during different sleep / wake stages. To enable comparisons of autonomic activity to be made across different times during the night in a given subject, we computed the ‘respiration-adjusted’ ΔRRI time-series (yra) for each consecutive data segment. The ‘respiration-adjusted’ ΔRRI time-series is defined as:
| (6) |
where x0(n) is the respiration time-series during the wakefulness period prior to sleep onset. Thus, for each given data segment during sleep, the respiration-correlated component was first dissociated from the respiration-uncorrelated component. From the respiration-correlated component, the transfer function between respiration and RRI was estimated. This transfer function was next used to predict what the respiration-correlated component of RRI would be if the respiration time-series during that interval of time were to take the same form as the respiration time-series measured during the short period of wakefulness immediately prior to the start of the sleep study. The predicted respiration-correlated component was subsequently added to the respiration-uncorrelated component to generate the ‘respiration-adjustedi’ RRI time-series.
The spectra of the original RRI time-series and the ‘respiration adjusted’ RRI time-series were computed using the Welch method with Hanning windowing (Tompkins, 1993). The respective areas in the appropriate frequency bands (low-frequency: 0.04–0.15 Hz; high-frequency: 0.15–0.4 Hz) under each RRI spectrum were calculated to yield the HRV spectral indices, LFP, and HFP. Subsequently, LFP was divided by HFP to yield LHR. Analogous calculations were performed to arrive at the corresponding ‘respiration adjusted’ indices: LFPra, HFPra, and LHRra.
Footnotes
DISCLOSURE STATEMENT
This was not an industry supported study. None of the authors have indicated any financial conflicts of interest.
REFERENCES
- Antelmi I, De Paula RS, Shinzato AR, Peres CA, Mansur AJ, Grupi CJ. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol. 2004;93:381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour Holter electrocardiograms. Tex. Heart Inst. J. 2004;31:132–136. [PMC free article] [PubMed] [Google Scholar]
- Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans. Biomed. Eng. 1986;33:900–904. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Brandenberger G, Viola AU, Ehrhart J, Charloux A, Geny B, Piquard F, Simon C. Age-related changes in cardiac autonomic control during sleep. J. Sleep Res. 2003;12:173–180. doi: 10.1046/j.1365-2869.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J. Appl. Physiol. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- Dubin D. Rapid Interpretation of EKG’s. 5th edn. Tampa, FL, USA: Cover Publishing Company; 1998. [Google Scholar]
- Eckberg DL. Sympathovagal balance: a critical appraisal. Reply. Circulation. 1998;98:2643–2644. [PubMed] [Google Scholar]
- Evans JM, Ziegler MG, Patwardhan AR, Ott JB, Kim CS, Leonelli FM, Knapp CF. Gender differences in autonomic cardiovascular regulation: spectral, hormonal, and hemodynamic indexes. J. Appl. Physiol. 2001;91:2611–2618. doi: 10.1152/jappl.2001.91.6.2611. [DOI] [PubMed] [Google Scholar]
- Fletcher EC, Miller J, Schaaf JW, Fletcher JG. Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep. 1987;10:35–44. doi: 10.1093/sleep/10.1.35. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25:560–563. doi: 10.1161/01.hyp.25.4.560. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Gula LJ, Krahn AD, Skanes A, et al. Heart rate variability in obstructive sleep apnea: a prospective study and frequency domain analysis. Ann. Noninvasive Electrocardiol. 2003;8:144–149. doi: 10.1046/j.1542-474X.2003.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedner J, Darpo B, Ejnell H, Carlson J, Caidahl K. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur. Respir. J. 1995;8:222–229. doi: 10.1183/09031936.95.08020222. [DOI] [PubMed] [Google Scholar]
- Katona PG, Jih F. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J. Appl. Physiol. 1975;39:801–805. doi: 10.1152/jappl.1975.39.5.801. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Kim TS, Berry RB. Spectral indices of cardiac autonomic function in obstructive sleep apnea. Sleep. 1999;22:443–451. doi: 10.1093/sleep/22.4.443. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Belozeroff V, Berry RB, Sassoon CS. Cardiac autonomic control in obstructive sleep apnea: effects of long-term CPAP therapy. Am. J. Respir. Crit. Care Med. 2001;164:807–812. doi: 10.1164/ajrccm.164.5.2010124. [DOI] [PubMed] [Google Scholar]
- Kohler BU, Hennig C, Orglmeister R. The principles of software QRS detection. IEEE Eng. Med. Biol. Mag. 2002;21:42–57. doi: 10.1109/51.993193. [DOI] [PubMed] [Google Scholar]
- Kuo TB, Lin T, Yang CC, Li CL, Chen CF, Chou P. Effect of aging on gender differences in neural control of heart rate. Am. J. Physiol. 1999;277:H2233–H2239. doi: 10.1152/ajpheart.1999.277.6.H2233. [DOI] [PubMed] [Google Scholar]
- Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am. J. Respir. Crit. Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- Liao D, Barnes RW, Chambless LE, Simpson RJ, Jr, Sorlie P, Heiss G. Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability – the ARIC study. Atherosclerosis Risk in Communities. Am. J. Cardiol. 1995;76:906–912. doi: 10.1016/s0002-9149(99)80260-4. [DOI] [PubMed] [Google Scholar]
- Lorenzi-Filho G, Dajani HR, Leung RST, Floras JS, Bradley TD. Entrainment of blood pressure and heart rate oscillations by periodic breathing. Am. J. Respir. Crit. Care Med. 1999;159:1147–1154. doi: 10.1164/ajrccm.159.4.9806081. [DOI] [PubMed] [Google Scholar]
- Mancia G. Autonomic modulation of the cardiovascular system during sleep. N. Engl. J. Med. 1993;328:347–349. doi: 10.1056/NEJM199302043280511. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Montano N, Cogliati C, Van De Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. doi: 10.1161/01.cir.100.23.2332. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Parish JM, Shepard JW., Jr Cardiovascular effects of sleep disorders. Chest. 1990;97:1220–1226. doi: 10.1378/chest.97.5.1220. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–1085. [PubMed] [Google Scholar]
- Redline S, Sanders MH, Lind BK, et al. Sleep Heart Health Research Group. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep. 1998;21:759–767. [PubMed] [Google Scholar]
- Rissanen J. Estimation of structure by minimum description length. Circ. Syst. Sig. Proc. 1982;1:395–406. [Google Scholar]
- Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J. Am. Coll. Cardiol. 1994;24:1700–1707. doi: 10.1016/0735-1097(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Shiomi T, Guilleminault C, Sasanabe R, Hirota I, Maekawa M, Kobayashi T. Augmented very low frequency component of heart rate variability during obstructive sleep apnea. Sleep. 1996;19:370–377. doi: 10.1093/sleep/19.5.370. [DOI] [PubMed] [Google Scholar]
- Sleep Heart Health Study Research Group. SHHS Coordinating Center. USA: Seattle, WA; 1996. Sleep Heart Health Study Manual of Operation. [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Task Force. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Tompkins WJ. Biomedical Digital Signal Processing: C Language Examples and Laboratory Experiments for the IBM PC. Englewood Cliffs, NJ USA: Prentice Hall; 1993. [Google Scholar]
- Vanninen E, Tuunainen A, Kansanen M, Uusitupa M, Lansimies E. Cardiac sympathovagal balance during sleep apnea episodes. Clin. Physiol. 1996;16:209–216. doi: 10.1111/j.1475-097x.1996.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Tretriluxana S, Redline S, Surovec S, Gottlieb DJ, Khoo MCK. Sleep-disordered breathing and cardiac autonomic function in a community-based sample: age-related differences. Proc. Am. Thorac. Soc. 2006;3:A197. [Google Scholar]
- Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin. Physiol. 2000;20:234–241. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- Zemaityte D, Varoneckas G, Sokolov E. Heart rhythm control during sleep. Psychophysiology. 1984;21:279–289. doi: 10.1111/j.1469-8986.1984.tb02935.x. [DOI] [PubMed] [Google Scholar]