Abstract
Serum B-lymphocyte stimulator (BLyS) levels are elevated in a subset of non-Hodgkin lymphoma (NHL) patients, particularly those with a family history of B-cell malignancies or a polymorphism in the BLyS gene. BLyS promotes growth of malignant B-cells and increased serum BLyS levels are associated with a poor clinical outcome. In this study, BLyS levels were measured before and after 4 weekly doses of rituximab in 30 patients with previously untreated follicular Grade 1 NHL. A significant increase was seen in the serum levels of BLyS (P = 0.0001) after rituximab therapy. The increase was independent of genetic variability in the BLyS gene.
Introduction
B-lymphocyte stimulator, BLyS (also known as BAFF, TALL-1, THANK, and zTNF4), is a TNF-family molecule that functions as a key regulator of peripheral B-cell populations and promotes B-cell survival [1–4]. We previously reported that BLyS protects malignant B-cells from apoptosis [5,6] and that serum BLyS levels in large cell lymphoma patients correlate inversely with response to therapy and overall survival [6]. We have also found that patients with small lymphocytic lymphoma/chronic lymphocytic leukemia and a family history of B-cell malignancies have a higher incidence of elevated serum BLyS levels and this is associated with a polymorphism in the BLyS promoter region [7].
The anti-CD20 monoclonal antibody, rituximab, has been found to be effective therapy for patients with follicular NHL [8–11]. The use of this antibody has now been expanded to include patients with rheumatoid arthritis and there are reports of benefit in systemic lupus erythematosus, Sjogren's syndrome, and other autoimmune diseases. Recent reports have suggested that serum BLyS levels increase after rituximab therapy for autoimmune diseases and that BLyS modulates the repopulation of B-lymphocytes and may trigger disease relapse [12–15]. Rituximab therapy does not cure follicular lymphoma, and it is reasonable to ask whether BLyS levels following rituximab therapy may promote the proliferation of residual malignant B-cells.
The present study measured changes in serum BLyS levels in patients receiving single agent rituximab as initial therapy for follicular NHL, then correlated increases in serum BLyS levels with the likelihood of disease progression. We also examined the relationship between increases in serum BlyS levels and polymorphisms in the BLyS gene.
Results and Discussion
BLyS is critical for the maintenance of normal B cell development and homeostasis [1,16,17], and enhances the survival of malignant B cells [5,6,18] by activating the NF-κB pathway [19] and by regulating cell-cycle entry [20]. Recent reports have suggested that serum BLyS levels increase after rituximab therapy for autoimmune diseases [12–15] and that BLyS may contribute to the regeneration of B cell populations capable of triggering clinical relapse in these diseases. In this study, we determined whether serum BLyS levels increased after rituximab treatment in patients with follicular NHL who received four doses of the antibody and were then followed without treatment.
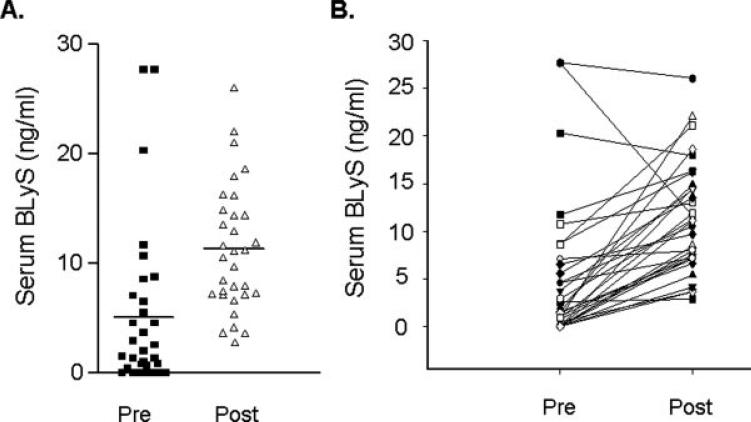
We found that there was a significant increase in the serum levels of BLyS measured 1 month after rituximab therapy (see Fig. 1). BLyS levels before therapy were low with a mean pretreatment serum level of 4.47 (±7.12) ng/ml. These pretreatment values were higher than healthy controls (n = 50) whose mean serum BLyS level was 2.71 (±3.82) ng/ml. After rituximab, serum BLyS increased to a mean post-treatment level of 10.75 (±5.5) ng/ml (P = 0.0001) and at this time point there was profound B-cell depletion with a median absolute CD19+ cell number of 8 cells/μL (range: undetectable − 43 cells/μL). The increase in serum BLyS was seen in all but three patients. In these three patients, BLyS levels remained unchanged or decreased slightly. Of note, these three patients had the highest serum levels before therapy.
Figure 1.
Changes in serum BLyS after rituximab treatment (A) Serum levels before (pre) and 1 month after (post) four doses of rituximab in patients with follicular Grade 1 lymphoma receiving rituximab as their initial therapy. Dark bars are the mean value for the group. (B) Change in serum BLyS level for each individual patient before (pre) and after (post) rituximab treatment.
In previous work [7,18], we found that single nucleotide polymorphisms (SNP) in the BLyS promoter and the BLyS gene (TNFSF13B) resulted in increased BLyS transcription and higher serum BLyS levels, suggesting that genetic variation in TNFSF13B may influence its expression. We therefore investigated whether the increased BLyS levels in most patients and the lack of increase in serum BLyS seen in three patients was related to SNPs in TNFSF13B. We focused on the four SNPs that we had previously shown to be associated with an increase in serum BLyS (rs12428930, rs12583006, rs1224141, rs9514828). Twenty-three (64%) patients had at least one of the four SNPs in TNFSF13B. In this study, however, we did not find a significant association between SNPs in TNFSF13B and pretreatment BLyS levels or the subsequent change in serum BLyS.
As previously reported [10], the overall response rate in the patients treated with rituximab in this study was 72% (CI: 57−84%). The median TTP is 2.2 years (95% CI: 1.2−3.3 years) and the 5-year overall survival is 77% (95% CI: 64−92%). The pretreatment serum BLyS level however was not associated with the overall response to rituximab (P = 0.39) or with the complete response rate (P = 0.4). However, patients with higher BLyS levels post-rituximab were more likely to have had a complete response to treatment (P = 0.01). Serum BLyS before or after rituximab did not significantly correlate with the TTP (P = 0.26 and P = 0.12, respectively). Differences in OS could not be determined because of the fact that only eight patients enrolled in the study have died.
In this study, we found that serum BLyS levels increase significantly in follicular lymphoma patients after rituximab treatment. Initial pretreatment serum BLyS levels in these follicular lymphoma patients were low, similar to previous data in follicular lymphoma [6], but were still slightly elevated when compared to healthy controls. This is somewhat different to the findings in chronic lymphocytic leukemia where serum BLyS levels are significantly lower than healthy controls [21–23], or large cell lymphoma where serum BLyS levels are commonly higher [6]. In this study, serum BLyS levels subsequently more than doubled after B-cell depletion with rituximab therapy. The magnitude of the increase in serum BLyS was similar to that seen in patients with autoimmune diseases treated with rituximab [12]. Furthermore, the increase in BLyS seemed to be independent of polymorphisms in the BLyS gene. Although we hypothesized that elevated serum BLyS levels would predispose to increased malignant B-cell growth and subsequently to a shorter TTP, neither the pretreatment nor post-treatment serum BLyS levels correlated with response to therapy or subsequent relapse in this group of patients. The lack of association between serum BLyS levels and clinical outcome may however be due to the small number of patients analyzed in this study and further studies in a larger patient cohort may be necessary.
The mechanism that accounts for the increase in BLyS is not known. It is possible that rituximab-induced depletion of both normal and malignant B-cells results in a dramatic decrease in available BLyS receptors, and that the lack of BLyS binding to B-cells increases serum BLyS levels. It is also possible that the increased serum BLyS levels is a physiological compensatory mechanism for the depletion of B-cells by rituximab. Furthermore, binding of rituximab to malignant B-cells may activate BLyS-producing immune cells resulting in increased BLyS levels. Because rituximab is not a curative therapy for follicular lymphoma, further studies will be necessary to determine whether increased serum BLyS levels promote proliferation of residual malignant B-cells and treatment combinations that deplete B-cells but also inhibit BLyS may need to be considered.
Patients and Methods
Patient selection
The specimens used in this study were obtained from patients treated in a North Central Cancer Treatment Group (NCCTG) clinical trial [10]. In this trial, eligible patients with untreated follicular Grade 1 NHL were treated with rituximab 375 mg/m2 intravenous weekly for four doses; no maintenance therapy was given. Thirty-seven patients were enrolled between July 1999 and May 2001, 36 of whom were evaluable for response. Patient characteristics are shown in Table I. Serum specimens (n = 30) and genomic DNA from peripheral blood mononuclear cells (n = 36) were obtained before and after 4 weeks of rituximab treatment. The specimens used in this study were retrospectively analyzed for BLyS levels and were only available at these two time points. Additional specimens at later time points were not collected as part of the initial study. Although the limited number of time points limited the assessment of BLyS kinetics after rituximab therapy, the significantly long follow up of patients allowed a correlation between increases in serum BLyS and the time to progression. The Institutional Review Board of the Mayo Clinic approved the study.
TABLE I.
Patient Characteristics
| Characteristic | Number (n = 36) | % |
|---|---|---|
| Sex | ||
| Male | 15 | 42 |
| Female | 21 | 58 |
| International prognostic index score | ||
| Low | 14 | 39 |
| Low-intermediate | 16 | 44 |
| High-intermediate | 6 | 17 |
| Age group | ||
| ≤65 years | 26 | 72 |
| >65 years | 10 | 28 |
| Stage | ||
| IIIA | 11 | 31 |
| IVA | 25 | 69 |
| Bone marrow | ||
| Negative | 14 | 39 |
| Positive | 22 | 61 |
| Lactate dehydrogenase | ||
| Normal | 30 | 83 |
| Elevated | 6 | 17 |
| Performance status | ||
| 0 | 32 | 89 |
| 1 | 4 | 11 |
| Median serum BLyS (ng/ml) | ||
| Pre-rituximab | 1.48 | (range: undetectable −27.73) |
| Post-rituximab | 10.12 | (range: 2.88−26.06) |
| BLyS polymorphism | ||
| rs12428930 | 12 | 40 |
| rs12583006 | 12 | 40 |
| rs1224141 | 14 | 47 |
| rs9514828 | 9 | 30 |
BLyS ELISA
ELISA plates were coated with 1 μg/ml anti-BLyS mAb (ZymoGenetics, Seattle, WA) and BLyS was detected with 1 μg/ml biotinylated anti-BLyS mAb (ZymoGenetics) followed by strepavidin-HRP and TMB substrate [6]. Patient serum samples were diluted 1:5 and BLyS serum levels were calculated from a standard curve generated using recombinant human BLyS (ZymoGenetics) in 20% human sera. All testing was done in triplicate and the ELISA was repeated in a random subset of the specimens with similar results. The detection limit of purified BLyS was 300 pg/ml.
Sequencing of BLyS polymorphisms
Genomic DNA from the patients in this study was isolated from peripheral blood mononuclear cells frozen in dimethylsulfoxide cryopreservation media using a DNA isolation kit from PUREGENE (Gentra Systems, Minneapolis, MN). Purified DNA was amplified by polymerase chain reaction (PCR) using primer pairs that spanned the regions of the BLyS promoter and the BLyS gene previously shown to be associated with increased serum BLyS levels [7,18].
Statistical analysis
Comparisons between patient groups were based on chi-square tests for nominal variables; the Wilcoxon rank-sum test or the Kruskal-Wallis test was used for continuous variables. The Wilcoxon signed rank test was used to evaluate differences between paired values. Overall survival (OS) and time to progression (TTP) of all patients were estimated using the Kaplan-Meier method. The univariate associations between individual clinical features and survival were determined with the two-tailed log-rank test.
Acknowledgments
Contract grant sponsor: National Institutes of Health; Contract grant number: CA121569; Contract grant sponsor: ZymoGenetics, Inc; Contract grant number: CA25224; Contract grant sponsors: Merck Serono International, S.A. (Merck KGaA, Darmstadt, Germany) and Leukemia and Lymphoma Society.
Footnotes
Conflict of interest: S.R. Dillon is employed by a company whose potential product (BLyS ELISA) was used in the present work.
References
- 1.Mackay F, Browning JL. BAFF: A fundamental survival factor for B cells. Nat Rev Immunol. 2002;2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 2.Marsters SA, Yan M, Pitti RM, et al. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 3.Schneider P, Mackay F, Steiner V, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson JS, Bixler SA, Qian F, et al. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 5.Novak AJ, Bram RJ, Kay NE, Jelinek DF. Aberrant expression of B-lymphocyte stimulator by B chronic lymphocytic leukemia cells: A mechanism for survival. Blood. 2002;100:2973–2979. doi: 10.1182/blood-2002-02-0558. [DOI] [PubMed] [Google Scholar]
- 6.Novak AJ, Grote DM, Stenson M, et al. Expression of BLyS and its receptors in B-cell non-Hodgkin lymphoma: Correlation with disease activity and patient outcome. Blood. 2004;104:2247–2253. doi: 10.1182/blood-2004-02-0762. [DOI] [PubMed] [Google Scholar]
- 7.Novak AJ, Grote DM, Ziesmer SC, et al. Elevated serum B-lymphocyte stimulator levels in patients with familial lymphoproliferative disorders. J Clin Oncol. 2006;24:983–987. doi: 10.1200/JCO.2005.02.7938. [DOI] [PubMed] [Google Scholar]
- 8.Colombat P, Salles G, Brousse N, et al. Rituximab (anti-CD20 monoclonal antibody) as single first-line therapy for patients with follicular lymphoma with a low tumor burden: Clinical and molecular evaluation. Blood. 2001;97:101–106. doi: 10.1182/blood.v97.1.101. [DOI] [PubMed] [Google Scholar]
- 9.Hainsworth JD, Litchy S, Burris HA, III, et al. Rituximab as first-line and maintenance therapy for patients with indolent non-hodgkin's lymphoma. J Clin Oncol. 2002;20:4261–4267. doi: 10.1200/JCO.2002.08.674. [DOI] [PubMed] [Google Scholar]
- 10.Witzig TE, Vukov AM, Habermann TM, et al. Rituximab therapy for patients with newly diagnosed, advanced-stage follicular grade I non-Hodgkin lymphoma: A phase II trial in the North Central Cancer Treatment Group. J Clin Oncol. 2005;23:1103–1108. doi: 10.1200/JCO.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 11.Hainsworth JD. First-line and maintenance treatment with rituximab for patients with indolent non-Hodgkin's lymphoma. Semin Oncol. 2003;30(1 Suppl 2):9–15. doi: 10.1053/sonc.2003.50023. [DOI] [PubMed] [Google Scholar]
- 12.Lavie F, Miceli-Richard C, Ittah M, et al. Increase of B cell-activating factor of the TNF family (BAFF) after rituximab treatment: Insights into a new regulating system of BAFF production. Ann Rheum Dis. 2007;66:700–703. doi: 10.1136/ard.2006.060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vallerskog T, Heimburger M, Gunnarsson I, et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther. 2006;8:R167. doi: 10.1186/ar2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cambridge G, Stohl W, Leandro MJ, et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: Relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 15.Pers JO, Devauchelle V, Daridon C, et al. BAFF-modulated repopulation of B lymphocytes in the blood and salivary glands of rituximab-treated patients with Sjogren's syndrome. Arthritis Rheum. 2007;56:1464–1477. doi: 10.1002/art.22603. [DOI] [PubMed] [Google Scholar]
- 16.Gross JA, Johnston J, Mudri S, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 17.Gross JA, Dillon SR, Mudri S, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 18.Novak AJ, Slager SL, Ziesmer SC, et al. Polymorphisms in the BLyS gene are associated with an increased risk of developing B-Cell non-Hodgkin lymphoma. Blood. 2007;110:173A. [Abstract] [Google Scholar]
- 19.He B, Chadburn A, Jou E, et al. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–3279. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 20.Huang X, Di Liberto M, Cunningham AF, et al. Homeostatic cell-cycle control by BLyS: Induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27Kip1. Proc Natl Acad Sci USA. 2004;101:17789–17794. doi: 10.1073/pnas.0406111101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haiat S, Billard C, Quiney C, et al. Role of BAFF and APRIL in human B-cell chronic lymphocytic leukaemia. Immunology. 2006;118:281–292. doi: 10.1111/j.1365-2567.2006.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Planelles L, Castillo-Gutiérrez S, Medema JP, et al. APRIL but not BLyS serum levels are increased in chronic lymphocytic leukemia: Prognostic relevance of APRIL for survival. Haematologica. 2007;92:1284–1285. doi: 10.3324/haematol.10317. [DOI] [PubMed] [Google Scholar]
- 23.Molica S, Digiesi G, Mauro F, et al. Increased serum BAFF (B-cell activating factor of the TNF family) level is a peculiar feature associated with familial chronic lymphocytic leukemia. Leuk Res. 2008 Jun 13; doi: 10.1016/j.leukres.2008.05.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]