Abstract
Mammalian auditory sensation is mediated by the organ of Corti, a specialized sensory epithelium found in the cochlea of the inner ear. Proper auditory function requires that the many different cell types found in the sensory epithelium be precisely ordered within an exquisitely patterned cellular mosaic. The development of this mosaic depends on a series of cell fate decisions that transform the initially nearly uniform cochlear epithelium into the complex structure of the mature organ of Corti. The prosensory domain, which contains the progenitors of both the mechanosensory hair cells and their associated supporting cells, first becomes distinct from both the neural and the non-sensory domains. Further cell fate decisions subdivide prosensory cells into populations of inner and outer hair cells, and several different types of supporting cells. A number of different signaling pathways and transcription factors are known to be necessary for these developmental processes; in this review we will summarize these results with an emphasis on recent findings.
Introduction
In mammals, the perception of sound is mediated through a specialized sensory epithelium, referred to as the organ of Corti, which extends along the length of the coiled cochlear duct located in the ventral region of the inner ear. The organ of Corti contains both mechanosensory hair cells, which act as the primary transducers of auditory stimuli, and surrounding supporting cells, which, as their name suggests, provide both structural and physiological support to the hair cells. One of the more remarkable aspects of the organ of Corti is the precise alignment of hair cells and supporting cells into highly ordered rows that extend along the length of the cochlear duct. Both hair cells and supporting cells are derived from progenitor cells that originate in the otocyst, a placodally-derived ectodermal invagination that arises adjacent to the developing hindbrain. In addition to hair cells and supporting cells, progenitor cells within the otocyst will give rise to a number of other cell types; this suggests that specific cellular and molecular interactions direct a subset of otocyst cells along a developmental pathway, culminating in the formation of a hair cell or a supporting cell. While the factors that dictate these fate choices remain incompletely understood, recent results have identified factors that both positively and negatively regulate these cell fate decisions.
Overview of inner ear development and cochlear anatomy
As discussed, all of the cells within the organ of Corti, as well as most other cell types within the membranous portion of the inner ear, are derived from the otocyst. The otocysts can first be visualized as bilateral thickenings (otic placodes) within the ectoderm located adjacent to the hindbrain. In mice, otic placodes first become evident at embryonic day 8.5 (E8.5), with fully invaginated and enclosed otocysts usually present by 24 hours later. Following enclosure, a population of neuroblasts delaminates from the ventral region of the otocyst. These cells migrate a short distance ventromedially and then coalesce to form the developing VIIIth cranial (statoacoustic) ganglion. Following neuroblast delamination, the spherical otocyst undergoes an extended and elaborate series of morphogenetic changes, which results in the formation of distinct dorsal vestibular and ventral cochlear regions. The vestibular region is characterized by the presence of the three semi-circular canals and the endolymphatic duct, while the ventral region contains the coiled cochlea. As the cochlear duct extends and coils, a subset of cells within its ventral aspect begins to develop as the sensory epithelium, also referred to as the organ of Corti. Development of the organ of Corti continues throughout the embryonic and early post-natal period, with an onset of hearing function around postnatal day 10 (P10)-P14 in mice.
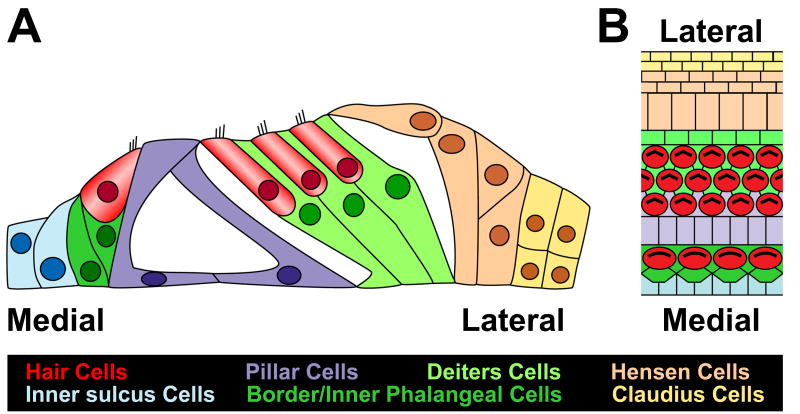
At the cellular level, the structure of the organ of Corti is striking (Fig. 1). Sound induced pressure waves are detected by small bundles of modified microvilli, referred to as stereocilia, located on the apical surfaces of mechanosensory hair cells. Two types of hair cells, inner hair cells (IHCs) and outer hair cells (OHCs), are arranged in ordered rows that extend along the length of the spiral. A single row of IHCs is located on the medial edge of the organ of Corti while three, or sometimes four, rows of OHCs are located on the lateral edge. IHCs and OHCs are morphologically and physiologically distinct from one another. Moreover, virtually all of the afferent neurons within the cochlea form synapses on IHCs, indicating that these are the predominant cells that respond to sounds, while OHCs are primarily innervated by efferent neurons, indicating that they act to modulate the response of the organ of Corti to a particular sound. In fact, OHCs have been shown to be electrically motile and to modulate hearing acuity.
Figure 1. Anatomy of the organ of Corti.
Cross-sectional (A) and lumenal surface (B) illustrations of the cellular anatomy of the organ of Corti. Medial and lateral sides are labeled for orientation. A single row of inner hair cells (red) is located on the medial side of the epithelium, while three rows of outer hair cells (red) are located more laterally. Inner hair cells are separated from one another by border cells and inner phalangeal cells (dark green), while outer hair cells are separated by Deiters cells (light green). The inner and outer hair cell regions are separated by the tunnel of Corti, a fluid filled structure that is bounded by single rows of inner and outer pillar cells (purple). Hensen and Claudius cells (beige, yellow) are located at the lateral edge.
In addition to hair cells, the organ of Corti also contains between five and seven distinct types of non-sensory cells, collectively referred to as supporting cells. The variation in the number of supporting cells arises not from differences among species, but as a result of the rather vague definition of a supporting cell as any cell that is associated with a hair cell but is not a hair cell. The bodies of most supporting cells are located adjacent to the basement membrane, but virtually all of these cells extend apical projections to the lumenal surface. Single border cells contact the medial and lateral sides of each IHC, while each IHC is separated from neighboring IHCs by the lumenal projection from a single inner phalangeal cell. Similarly, each OHC is separated from neighboring OHCs within the same row and in adjacent rows by a lumenal projection from a single Deiters cell. The single row of IHCs and the first row of OHCs are separated by single rows of inner and outer pillar cells. The two rows of pillar cells combine to form the tunnel of Corti, a triangular structure that is thought to play a role in stiffening of the organ of Corti. Finally, lateral to the outermost row of OHCs are several rows of cells referred to as the Cells of Hensen and Cladius, with Hensen's cells located closer to the OHCs. At this point it is not clear whether either Hensen's or Claudius' cells should or should not be considered as supporting cells.
Specification of prosensory cells
As discussed, virtually all of the cell types within the membranous portion of the inner ear, including the afferent neurons, are derived from multipotent epithelial progenitor cells initially located in the otocyst. As a result, three main lineages are derived from otocyst cells, prosensory (cells that will develop as either hair cells or associated supporting cells), proneural (cells that will develop as auditory or vestibular neurons), and nonsensory (all other otocyst derived cells). Prosensory cells become localized to restricted and highly stereotyped regions of the inner ear, including a narrow stripe that extends along the length of the cochlear duct (Morsli et al., 1998; Morrison et al., 1999; Cole et al., 2000). Based on the nearly complete absence of hair cells or supporting cells outside of the prosensory regions, it has been suggested that cells within the prosensory lineage possess a unique ability to develop as hair cells or supporting cells. However, as will be discussed below, recent results should prompt a reconsideration of this aspect of the prosensory cell hypothesis.
Analysis of expression patterns in the otocyst identified three genes as candidates for markers of the prosensory domains: the Notch ligand, Jagged1 (Jag1), the Notch regulator, Lunatic fringe (Lfng), and the secreted signaling molecule, Bone morphogenetic protein 4 (Bmp4) (Morsli et al., 1998). However, Bmp4 expression does not persist in the prosensory regions of the cochlea, and deletion of Lfng or Bmp4 does not lead to loss of hair cells or supporting cells in the cochlea (Zhang et al., 2000; Chang et al., 2008). Although early cochlear expression of JAG1 does not correspond exactly to the eventual position of the organ of Corti (Kiernan et al., 2006), several studies suggest a crucial role for Jag1 in sensory development in the cochlea. Two different N-ethyl-N-nitrosourea (ENU)-induced mutations first implicated Jag1: mice heterozygous for the slalom and headturner mutations both showed a reduction in OHC number (Kiernan et al., 2001; Tsai et al., 2001). Moreover, in an inner ear-specific deletion of Jag1, no hair cells or supporting cells are present at the base of the cochlea, while a reduced number are present at the apex (Kiernan et al., 2006). Markers of the prosensory domain, such as the SRY-related high-mobility-group (HMG)-box transcription factor SOX2, and the cyclin-dependent kinase inhibitor, CDKN1B (also referred to as p27kip1), are greatly reduced. In humans, mutations in JAG1 are associated with Alagille syndrome, a syndrome that primarily consists of liver, eye, and cardiovascular defects, but hearing loss has also been reported in some cases (Le Caignec et al., 2002).
Since JAG1 is a Notch ligand, activation of the Notch signaling pathway should be required for prosensory development. Consistent with this hypothesis, in vitro inhibition of Notch signaling in cochlear explants with the γ-secretase inhibitor DAPT at early stages does reduce the number of hair and supporting cells that develop (Hayashi et al., 2008a). This result is in contrast to the well-established role for Notch signaling later in hair cell specification (discussed below). Moreover, based on expression, the same study identified the Notch effectors, Hey1 and Hey2 (sometimes referred to as Hesr1 and Hesr2), as likely mediators of Notch signaling at this stage, but presumed functional redundancy and early embryonic lethality precluded definitive loss-of-function analysis. Another Notch signaling mediator, Hes1, is broadly expressed at early otocyst stages (Li et al., 2008) and could also be involved in defining the prosensory domain. Gain-of-function studies using the constitutively active, intracellular domain of Notch1, NICD, induced expression of prosensory markers when over-expressed in the embryonic mouse cochlea (Dabdoub et al., 2008), and caused the formation of ectopic sensory patches in embryonic chickens (Daudet and Lewis, 2005). These studies support an inductive role for Notch signaling in the formation of prosensory patches within the inner ear, in addition to a subsequent role in lateral inhibitory signaling that determines hair cell versus supporting cell fates later in development.
As mentioned above, the transcription factor SOX2 is another marker of the prosensory region, although not a specific one, as it is also expressed in the inner ear proneural domain. In developing CNS tissues, SOX2 is associated with progenitor and stem cell populations (for review, see Ellis et al., 2004); the same appears to be true for the sensory progenitors of the cochlea. SOX2 is widely expressed in the otocyst, but as the inner ear develops and proneural cells delaminate, its expression becomes restricted to presumptive prosensory domains, though it does not entirely overlap with expression of JAG1, which is found more medially within the cochlea (Fig. 2). Mice carrying mutations in regulatory regions of Sox2 that specifically reduce (Sox2Ysb) or nearly ablate (Sox2Lcc) Sox2 expression in the inner ear also show loss of both hair cells and supporting cells, confirming the requirement for Sox2 in prosensory development (Kiernan et al., 2005b). Sensorineural hearing loss has also been reported in humans with mutations in SOX2, along with eye, hypothalamus, pituitary, and brain defects (Hagstrom et al., 2005; Kelberman et al., 2006).
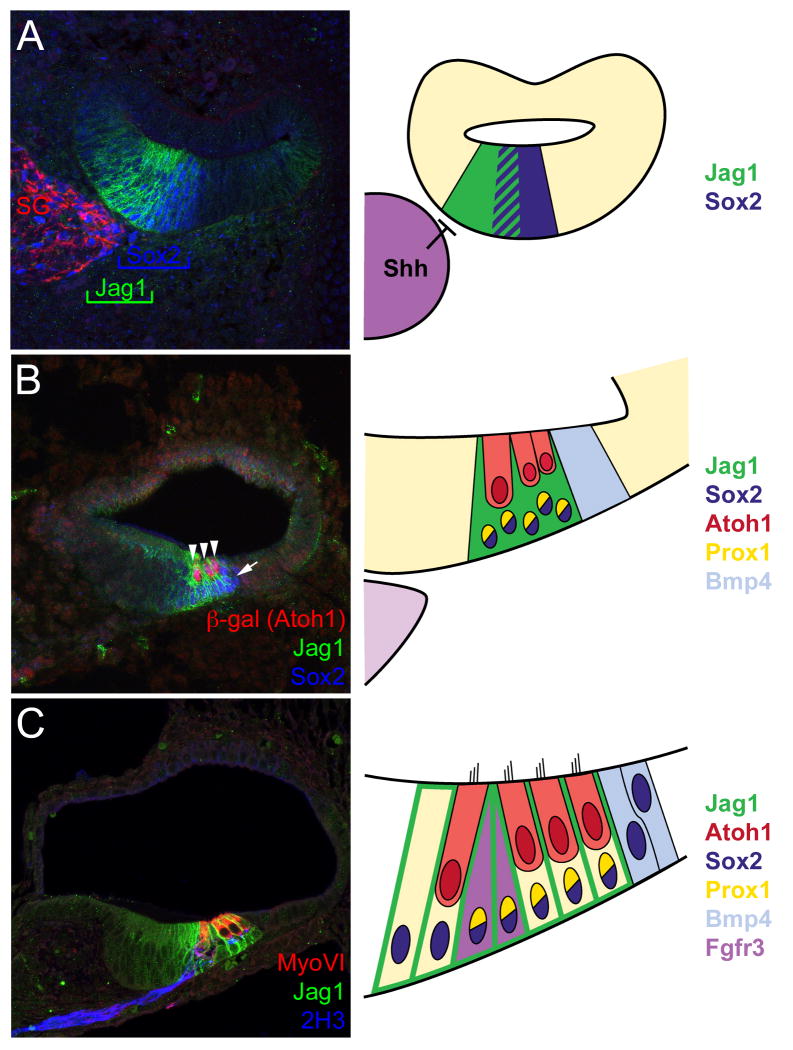
Figure 2. Development of the organ of Corti.
(A) Cross section through the cochlear duct at Embryonic day 13 (E13). Medial is to the left. The prosensory domain is marked by expression of Jag1 (green) and Sox2 (blue) in partially overlapping domains in the floor of the duct. Neurons within the developing spiral ganglion (SG) are labeled in red. The cartoon to the left indicates that spiral ganglion neurons act as a source of sonic hedgehog (Shh) that may act to inhibit sensory formation in the medial half of the duct. (B) Similar view of the duct at E15. Note that the prosensory domain is now located on the lateral half of the floor of the duct. Atoh1-positive hair cells (red) are present within the Jag1/Sox2-positive prosensory domain. A zoomed-in view of the prosensory domain is illustrated in the cartoon. In addition, the patterns of expression for Bmp4 (blue) and Prox1 (gold) are illustrated. (C) Cross-section at E18. Hair cells now express MyosinVI (red). Surrounding supporting cells are still positive for Jag1 (green). Innervating spiral ganglion neurites are labeled in blue. Cartoon summarizes expression patterns for most of the factors discussed in the text.
While both JAG1 and SOX2 were identified as candidate inducers of prosensory fate based on their expression patterns, and confirmed by loss-of-function studies in mice, the transcriptional co-activator gene, Eyes absent homolog 1 (Eya1), was identified based on its association with Branchio-oto-renal (BOR) syndrome in humans (Abdelhak et al., 1997). Eya1 is widely expressed in the otocyst, similarly to SOX2, and their overlap in prosensory expression continues until late stages, when Eya1 is found in hair cells, while SOX2 is restricted to supporting cells (Dabdoub et al., 2008; Zou et al., 2008). Total deletion of Eya1 results in arrest of cochlear development at the otocyst stage, precluding analysis of all but the earliest markers (Xu et al., 1999), but a more recent study used an allelic series of hypomorphs to address the role of Eya1 in sensory development (Zou et al., 2008). The number of sensory cells decreased in proportion to the level of Eya1 expression, and expression of prosensory markers, such as Bmp4 and Lfng, was also reduced. SOX2 was still present, though reduced, even in Eya1 null otocysts, suggesting that at least the initial expression of Eya1 and SOX2 may be independently regulated.
As development continues, prosensory cells within the cochlear duct up-regulate CDKN1B (Chen and Segil, 1999). The timing of CDKN1B expression (beginning at E13) correlates with terminal mitosis within the prosensory domain, and deletion of Cdkn1b leads to prolonged proliferation. As discussed, CDKN1B expression is dependent on expression of JAG1, and is similarly decreased in Sox2 mutants. The onset of CDKN1B expression closely precedes the first signs of hair cell differentiation, making it perhaps the most definitive marker of the prosensory domain, at least in the cochlea. However, hair cells and supporting cells are present in Cdkn1b mutants, indicating that it is not required for prosensory specification. Finally, as cells within the IHC region are beginning to differentiate (approximately E14), prosensory cells located lateral to the developing IHCs become positive for the transcription factor, PROX1 (Bermingham-McDonogh et al., 2006; Kirjavainen et al., 2008). As is the case for SOX2, developing OHCs down-regulate PROX1 while pillar cells and Deiters cells maintain expression. PROX1 and its Drosophila homolog, prospero, play key roles in development of a number of systems, including lymphatic vasculature, neural retina, and the CNS (reviewed in Cook, 2003; Hong and Detmar, 2003; Karcavich, 2005). The specific pattern of PROX1 expression in the organ of Corti suggests it is important in this system as well. However, experiments that test this hypothesis have not yet been reported.
Restriction of the prosensory domain
The studies discussed above have identified a few of the genes necessary for prosensory formation, but relatively little is known about the factors that act to refine the position and extent of the prosensory domain within the cochlear epithelium. Recent results suggest that the Hedgehog signaling pathway, already known to be necessary for early otic patterning (Riccomagno et al., 2002), may play such a role. Truncating mutations in the zinc-finger transcription factor, GLI3, a downstream effector of Hedgehog signaling, cause Pallister-Hall syndrome (PHS) in humans (Kang et al., 1997). Along with the previously described characteristics of PHS, hearing loss was recently reported (Driver et al., 2008). A mouse model of PHS, with a targeted deletion of Gli3 that mimics the truncations found in affected PHS individuals, also showed defects: shortened cochleae, expanded sensory regions, and ectopic sensory patches in Kölliker's Organ, a region of non-sensory otocyst-derived cells located adjacent to the organ of Corti (Driver et al., 2008). Because the truncated GLI3 protein is thought to act as a repressor of Hedgehog signaling, the expansion of sensory cells within the cochlea suggests that Hedgehog signaling normally acts to restrict prosensory cell fate. Sonic hedgehog (Shh) is expressed in the spiral ganglion (SG) underlying Kölliker's Organ, which may be a source of the repressive signal (Fig. 2). Exogenous SHH added to cochlear explants in vitro does repress sensory cell formation, and expansion of prosensory marker expression in Gli3 mutants suggests that Hedgehog signaling acts upstream of JAG1/Notch.
In other tissues, most famously the neural tube, but also the developing otocyst (Bok et al., 2007), BMP and/or canonical Wnt signaling often antagonize Shh signaling. It is tempting to speculate that the sensory-repressive, medial SHH signal from the spiral ganglion in the cochlea may be opposed by another, sensory-inducing signaling center on the opposite side of the organ of Corti. As mentioned previously, Bmp4 is expressed in some prosensory domains early in ear development, but in the cochlea is restricted to the lateral side of the organ of Corti, where the Hensen's and Claudius' cells will develop (Morsli et al., 1998). While experiments modulating BMP signaling with exogenous BMP4 protein or the BMP inhibitor, Noggin, in the chicken have produced conflicting results (Li et al., 2005; Pujades et al., 2006), loss-of-function analysis in the mouse has shown that Bmp4 is not required for sensory patterning in the cochlea (Chang et al., 2008). However, other experiments modulating BMP signaling in mouse cochlear explants, but beginning after the prosensory domain is formed, did show that BMP4 induced hair cells, whereas Noggin inhibited hair cell formation (Puligilla et al., 2007). These results suggest that BMP and SHH could act in opposing fashions across the cochlear epithelium, but given the difference in timing of the experiments, it remains to be seen whether this is in fact the case.
The ectopic patches of hair cells seen in Kölliker's Organ in the Gli3 mutants suggest that Hedgehog signaling acts to repress formation of sensory cells in this region. Moreover, in vitro experiments demonstrated that the formation of these ectopic cells is dependent on activation of Notch signaling (Driver et al., 2008). If Notch signaling is normally inhibited in this region, thus restricting the prosensory domain, then loss of endogenously expressed Notch inhibitors might result in formation of ectopic hair cells. In fact, in Hes5 mutants, scattered ectopic hair cells were observed in this region (Zine et al., 2001). Previous studies have shown that Kölliker's Organ cells readily become both hair cells and supporting cells (Zheng and Gao, 2000; Woods et al., 2004), so the development of only a few ectopic hair cells in this region in Hes5 mutants may be a result of functional redundancy of the various Hes and Hey genes expressed in the cochlea. It will be interesting to see if genetic manipulations allowing for inner-ear specific deletions of multiple Hes and Hey genes uncover more roles for these genes in patterning the developing cochlear epithelium.
Specification of individual cell types
Following the specification of the prosensory domain, individual cells within the domain are thought to become broadly specified to develop as either hair cells or supporting cells. Studies examining the early expression of specific morphological markers indicated that hair cells become specified prior to supporting cells, suggesting that the hair cell fate represents the primary fate within the prosensory domain. This conclusion is supported by hair cell ablation studies in explants of the embryonic organ of Corti which demonstrated that hair cell removal results in the differentiation of replacement hair cells from surrounding uncommitted progenitors that would normally have developed as supporting cells (Kelley et al., 1995). Similar results have been demonstrated in hair cell regeneration paradigms in the chicken cochlea (reviewed in Stone and Cotanche, 2007). The results of these studies are also consistent with the presence of a lateral inhibitory mechanism in which developing or mature hair cells prevent neighboring cells from adopting the same fate.
Lateral inhibitory interactions, such as the one described above for hair cells and supporting cells, are often mediated through the Notch signaling pathway (reviewed in D'Souza et al., 2008). In fact, a series of publications over the last 10 years has confirmed a crucial role for Notch in formation of the mosaic of hair cells and supporting cells (Lanford et al., 1999; Kiernan et al., 2005a; Brooker et al., 2006). Notch1 is broadly expressed throughout the cochlear duct, while Jagged2 (Jag2), Delta-like 1 (Dll1) and Delta-like 3 (Dll3) are observed only in developing hair cells (Lanford et al., 1999; Morrison et al., 1999; Hartman et al., 2007). In addition, several Notch-target genes, including Hes1, Hes5, Hey1, Hey2, and Heyl (also referred to as Hesr3), have been reported to be expressed in supporting cells and the expression of at least some of these genes has been shown to be dependent on Notch activation (Lanford et al., 2000; Zheng et al., 2000; Zine et al., 2001; Murata et al., 2006; Hayashi et al., 2008a; Li et al., 2008; Doetzlhofer et al., 2009). Moreover, as would be predicted, deletion of any of the implicated genes leads to a variable over-production of hair cells. For instance, a conditional inner-ear deletion of Notch1 leads to more than a two-fold increase in the number of hair cells, with a concomitant decrease in supporting cells. These results are consistent with the demonstrated lateral inhibitory interactions between developing hair cells and surrounding undifferentiated progenitors; they suggest that, with the exception of two specific subtypes of supporting cells to be discussed below, virtually all cochlear prosensory cells will develop as hair cells if Notch signaling is eliminated.
The initial demonstration of an important role for Notch signaling in the inhibition of hair cell fate also provided valuable insight into the identification of other genes that might be important for hair cell specification. In particular, studies in both vertebrates and invertebrates had demonstrated that members of the basic-Helix-Loop-Helix (bHLH) family of transcription factors were often negatively regulated by Notch signaling (reviewed in Cornell and Eisen, 2005). An examination of bHLH family member expression in the developing cochlea identified the atonal homolog, Atoh1, as a likely candidate, and a concomitant analysis of Atoh1 mutant mice confirmed the necessity of this gene for hair cell formation (Bermingham et al., 1999; Lanford et al., 2000). The onset of Atoh1 promoter activity and mRNA expression precedes that of Dll1 and Jag2, and deletion of Atoh1 results in a complete absence of hair cells. Moreover, forced expression of Atoh1, either within the prosensory domain, or even outside of the prosensory domain, is sufficient to induce a hair cell fate (Zheng and Gao, 2000; Woods et al., 2004; Jones et al., 2006). To date, Atoh1 is the only factor that has been shown to be sufficient for hair cell formation and the timing of its onset suggests that it is one of, if not the earliest gene to be expressed within the hair cell lineage.
Given that disruption of Notch signaling leads to an increase in the number of cells that develop as hair cells, and the demonstration of the key role of Atoh1 in hair cell formation, these results together suggest that Notch acts to inhibit hair cell fate through inhibition of Atoh1 expression. This hypothesis implies that Atoh1 is initially expressed in a broad group of prosensory cells and that subsequent activation of Notch signaling leads to down-regulation of Atoh1 in those cells that will develop as hair cells. Consistent with this idea, inhibition of Notch signaling does lead to an increase in the number of Atoh1-positive hair cells (Lanford et al., 2000; Takebayashi et al., 2007; Doetzlhofer et al., 2009). However, it has been difficult to determine the full extent of early Atoh1 expression within the cochlea. Different methods of assaying Atoh1 expression, including promoter activity, mRNA expression, and protein expression, have given different results, in some cases suggesting broad expression and in others observing expression that is restricted to committed hair cells (Lanford et al., 2000; Chen et al., 2002; Woods et al., 2004). Careful lineage studies are clearly required to determine the extent of Atoh1 expression in cochlear prosensory cells.
Identification of the factors that regulate the onset of Atoh1 expression has been challenging. Jagged1 and Sox2 are required for Atoh1 expression, but reciprocal experiments to examine sufficiency have either not been done (Jagged1) or have yielded negative results (Sox2; Dabdoub et al., 2008). For Sox2, at least, this suggests that the prosensory domain may represent an environment that is permissive, rather than instructive, for Atoh1 expression, and that other factors may actually induce Atoh1 within this domain. Candidate Atoh1-inducing factors are limited, but a recent study has demonstrated a possible role for fibroblast growth factor (Fgf) signaling. The Fgf signaling pathway is crucial for early development and patterning of the otocyst and inner ear in most vertebrates (Leger and Brand, 2002; Maroon et al., 2002; Wright and Mansour, 2003; Wright et al., 2004; Ladher et al., 2005). In addition, Fgf receptor1 (Fgfr1) is uniquely required for the formation of both hair cells and supporting cells within the cochlea (Pirvola et al., 2002). Cochleae from Fgfr1 hypomorphs or animals with a conditional otocyst deletion of Fgfr1 have sparse, mis-patterned sensory patches containing only inner hair cells. While the prosensory domain was reported to still be present in these cochleae, a dose dependent decrease in Atoh1 was observed, suggesting that FGFR1 acts within the prosensory domain to regulate Atoh1. The ligand for FGFR1 in the cochlea has not been determined, but a recent study generated an Fgfr1 mutant-like phenotype in cochlear explants using function-blocking antibodies against FGF20 (Hayashi et al., 2008b). These results suggest that binding of FGF20 to FGFR1 is a necessary step for Atoh1 expression within the cochlea; however, whether this regulation is direct or indirect remains to be determined.
While the importance of Notch signaling in preventing the formation of supernumerary hair cells is clear, the factors that regulate which and how many progenitors will develop as hair cells remain unclear. As discussed, Dll1 and Jag2 appear to only be expressed in cells that have already become committed to a hair cell fate. This observation suggests that other factors must regulate the selection of the hair cell population. Although the mechanisms of this regulation remain unknown, several candidate factors have been identified. The first of these are the IDs (inhibitors of differentiation), a family of four HLH gene products that act to antagonize bHLH molecules, such as ATOH1, through direct competition for a common dimerization partner (reviewed in Norton, 2000). Briefly, prior to DNA binding, bHLH proteins, such as ATOH1, must form heterodimers with a ubiquitously expressed bHLH, such as E47, or the products of the E2A gene. IDs lack the basic DNA binding domain of bHLH proteins, but contain the HLH dimerization domain. As a result, IDs can compete with bHLH molecules for the common dimerization partner with the consequence of ID binding being sequestration of the dimerization partner. Three of the four Id genes, Id1, Id2 and Id3, are expressed throughout the developing prosensory domain prior to hair cell development (Jones et al., 2006). However, a specific down-regulation of all three Ids was observed to occur at about the onset of hair cell formation. Finally, forced expression of ID3 in prosensory cells strongly inhibited hair cell formation.
A second gene that has recently been shown to influence Atoh1 activity is Sox2. As discussed above, Sox2 plays a key role in specification of the prosensory domain, but the subsequent down-regulation of SOX2 in developing hair cells led to the suggestion that Sox2 might also act as an Atoh1 antagonist. This hypothesis was supported by co-transfection experiments, which demonstrated that Sox2 can inhibit the ability of Atoh1 to induce hair cell formation (Dabdoub et al., 2008). Moreover, animals with reduced, but not completely absent, Sox2 expression actually show an increased number of hair cells in the organ of Corti, supporting the idea that Sox2 acts as an Atoh1 antagonist. Prox1, a homeodomain transcription factor that is expressed in most prosensory cells, is absent in Sox2 mutants and is induced in Sox2-transfected cochlear cells, and also inhibits Atoh1 activity. Finally, as would be expected, at least two Notch target genes, Hes1 and Hey2, also inhibit Atoh1-induced hair cell formation in co-transfection assays (Zheng and Gao, 2000; Doetzlhofer et al., 2009).
To summarize, Atoh1 acts as a key inducer of hair cell formation. The factors that regulate the onset of Atoh1 expression in the cochlea have not been fully determined, nor has the size of the initial Atoh1-positive population. In contrast, a number of factors that act to antagonize Atoh1 signaling in the cochlea have been identified. Modulation of these signaling factors or pathways leads to varying numbers of supernumerary hair cells, suggesting that multiple pathways act in concert to strictly regulate hair cell number. Considering the largely invariant pattern of hair cells within the normal organ of Corti, a clear challenge will be to determine how these different pathways are regulated to specify the correct number of hair cells.
Specification of supporting cells
In contrast to hair cells, the specification of supporting cells is rather poorly understood. While a focus on hair cells as the primary transducer of mechanosensory stimuli has contributed to the limited work on supporting cells, a greater impediment has been the lack of definitive markers for specific supporting cell types. As discussed earlier in the section on Specification of prosensory cells, most of the mRNA or protein markers that become restricted to supporting cells are initially expressed in prosensory cells. As a result, it has been challenging to discriminate between changes in supporting cell populations and modulation in the size of the prosensory domain. The ability to create ectopic hair cells has provided at least one method to assay for the creation of supporting cells. At present, in every case in which it has been examined, ectopic hair cells appear to be surrounded by ectopic supporting cells (Woods et al., 2004; Driver et al., 2008). These cells typically express the supporting cell/prosensory cell markers, JAG1 and SOX2, but in a least one instance, ectopic supporting cells were also shown to express Otogelin, a supporting cell marker that is not expressed in prosensory cells (El-Amraoui et al., 2001; Woods et al., 2004). These results suggest that hair cells employ specific inductive mechanisms to recruit surrounding cells to develop as supporting cells. The mechanisms for this induction are unknown. As discussed, the Notch pathway is activated between hair cells and neighboring cells that will ultimately develop as supporting cells, but whether Notch signaling could act as a direct inducer of supporting cell fate has not been examined.
The organ of Corti contains several different supporting cell types, including the inner and outer pillar cells. Pillar cells are only present in mammalian auditory sensory epithelia and have a unique morphology. Recent results have suggested that the regulation of pillar cell fate is also unique. Beginning at approximately E16.5 the Fgf receptor, Fgfr3, is upregulated in a population of progenitor cells that appears to include cells that will develop as pillar cells, outer hair cells, and Deiters cells (Mueller et al., 2002; Jacques et al., 2007). However, as development proceeds, expression of Fgfr3 becomes largely restricted to developing pillar cells. At the same time point, developing inner hair cells, located at the medial edge of the Fgfr3 domain, begin to express Fgf8, a known ligand for Fgfr3 (Zhang et al., 2006; Jacques et al., 2007). Deletion of either Fgfr3 or Fgf8 in the inner ear results in similar phenotypes, in which pillar cell development is specifically disrupted (Colvin et al., 1996; Hayashi et al., 2007; Jacques et al., 2007; Puligilla et al., 2007). These results are consistent with an inductive interaction in which FGF8 arising in inner hair cells binds to and activates FGFR3 in adjacent progenitor cells, leading to the formation of pillar cells. Consistent with this hypothesis, increased activation of FGFR3, either through the addition of increased amounts of ligand or deletion of the FGFR antagonist, Sprouty2, leads to an increase in the number of cells that develop as pillar cells (Mueller et al., 2002; Shim et al., 2005; Jacques et al., 2007). In the absence of Fgfr3, not only do pillar cells fail to develop, but additional outer hair cells are also observed within the organ of Corti, suggesting that prosensory cells that would have developed as pillar cells have undergone a fate change (Hayashi et al., 2007; Puligilla et al., 2007). These results indicate that FGFR3 signaling acts to both inhibit a hair cell fate and to induce a pillar cell fate. A recent study has identified a least one important factor in the inhibition of hair cell formation in the pillar cell region. Deletion of Notch1 within the inner ear results in a nearly complete loss of supporting cells within the organ of Corti. However, a key exception is the population of pillar cells, which appear to be unaffected, suggesting that some aspect of the Notch signaling pathway may still be active in pillar cells, even in the absence of Notch1. Based on this result, Doetzlhofer and colleagues (2009) examined the expression of known Notch target genes in the presence of Notch or Fgfr antagonists or agonists. They found that Hey2, which is expressed specifically in pillar cells, is activated by FGFs and that deletion of Hey2, along with inhibition of Notch signaling, leads to a loss of pillar cells. These results suggest that within the organ of Corti, Hey2 is regulated by the Fgf pathway, rather than Notch signaling, and that this pathway is used to specifically regulate pillar cell development. The reasons for this change are unknown, but may be related to the selective pressures driving the evolution of pillar cells and the tunnel of Corti. Further experiments are clearly required to determine the mechanisms that allow the Fgf pathway to regulate Hey2 and the evolutionary changes that contributed to this change in molecular signaling.
Conclusions
The organ of Corti represents a remarkable achievement in the regulation of cell fate and patterning. At least seven different cell types are specified in specific ratios to one another and then arranged in a rigorous mosaic pattern. While overall understanding of the mechanisms that are required to create and refine this structure remains limited, significant progress has been made (see summary diagram in Fig. 3). The progenitor cells that will give rise to the organ of Corti are specified as a population of prosensory cells that originate at the otocyst stage of inner ear development. The Notch signaling pathway and the transcription factor, SOX2, along with others, play key roles in specifying prosensory identity, while other pathways, including Hedgehog signaling, appear to inhibit prosensory formation in other regions of the inner ear. Once prosensory cells are established, expression of the transcription factor, Atoh1, possibly induced by Fgfr1 signaling, initiates a genetic program that is sufficient for formation of mechanosensory hair cells. Once specified, hair cells influence neighboring prosensory cells through both Notch-mediated inhibitory actions and undefined inductive signals that combine to recruit a population supporting cells. The factors that regulate specification of individual supporting cell types are largely unknown, with the exception of pillar cells, which are formed through an inductive interaction between FGFR3 and FGF8. Concomitant with cell fate specification, prosensory cells must become patterned into ordered rows. Only now, with a good baseline understanding of how the primary cell types are specified, is it possible to begin to examine these patterning events. Recent results have identified the nuclear protein, SOBP1, the Myosin II motor protein, and members of the planar cell polarity pathway as regulators of cellular patterning (Montcouquiol et al., 2003; Wang et al., 2005; Chen et al., 2008; Yamamoto et al., 2009). Hopefully, future experiments will demonstrate how cell fate specification and cellular patterning pathways are coordinated during cochlear formation.
Figure 3. Summary of factors that direct and/or are expressed in otocyst cells along the prosensory, hair cell, and supporting cell lineages.
Lineages are indicated with black arrows, inductive interactions are marked with green arrows, and inhibitory interactions are marked in red. Briefly, the Notch signaling pathway, along with Sox2 and Eya1, is believed to direct otocyst cells towards a prosensory fate. Next, an undetermined number of prosensory cells become positive for Atoh1, possibly through activation of Fgfr1. Sox2 and Id1, 2, and 3 act to limit the number of cells that become Atoh1 positive. Cells that have begun to develop as hair cells express Jag2, Dll1, and Dll3, leading to activation of Notch1 and the downstream targets Hes1, Hes5, Hey1, Hey2, and Heyl in neighboring prosensory cells. Notch activation inhibits these cells from developing as hair cells. At the same time, hair cells also generate largely unknown inductive signals that recruit neighboring cells to develop as supporting cells. Pillar cell formation is dependent on an inductive interaction between Fgf8, expressed in inner hair cells, and Fgfr3, expressed in progenitor cells, possibly acting through Hey2. Finally, activation of the Hedgehog pathway, possibly mediated through expression of Shh in neuroblasts, acts to inhibit non-sensory cells from moving into the prosensory lineage.
Acknowledgments
The authors' research is supported by the Intramural Program of the National Institute on Deafness and Other Communication Disorders at National Institutes of Health. The authors wish to apologize to any of their colleagues whose work was unavoidably excluded from this review due to space constraints.
References
- Abdelhak S, Kalatzis V, Heilig R, et al. A Human Homologue of the Drosophila Eyes Absent Gene Underlies Branchio-Oto-Renal (Bor) Syndrome and Identifies a Novel Gene Family. Nat Genet. 1997;15(2):157–164. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, et al. Math1: An Essential Gene for the Generation of Inner Ear Hair Cells. Science. 1999;284(5421):1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O, Oesterle EC, Stone JS, et al. Expression of Prox1 During Mouse Cochlear Development. J Comp Neurol. 2006;496(2):172–186. doi: 10.1002/cne.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J, Dolson DK, Hill P, et al. Opposing Gradients of Gli Repressor and Activators Mediate Shh Signaling Along the Dorsoventral Axis of the Inner Ear. Development. 2007;134(9):1713–1722. doi: 10.1242/dev.000760. [DOI] [PubMed] [Google Scholar]
- Brooker R, Hozumi K, Lewis J. Notch Ligands with Contrasting Functions: Jagged1 and Delta1 in the Mouse Inner Ear. Development. 2006;133(7):1277–1286. doi: 10.1242/dev.02284. [DOI] [PubMed] [Google Scholar]
- Chang W, Lin Z, Kulessa H, et al. Bmp4 Is Essential for the Formation of the Vestibular Apparatus That Detects Angular Head Movements. PLoS Genet. 2008;4(4):e1000050. doi: 10.1371/journal.pgen.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Johnson JE, Zoghbi HY, et al. The Role of Math1 in Inner Ear Development: Uncoupling the Establishment of the Sensory Primordium from Hair Cell Fate Determination. Development. 2002;129(10):2495–2505. doi: 10.1242/dev.129.10.2495. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) Links Cell Proliferation to Morphogenesis in the Developing Organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen Z, Montcouquiol M, Calderon R, et al. Jxc1/Sobp, Encoding a Nuclear Zinc Finger Protein, Is Critical for Cochlear Growth, Cell Fate, and Patterning of the Organ of Corti. J Neurosci. 2008;28(26):6633–6641. doi: 10.1523/JNEUROSCI.1280-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LK, Le Roux I, Nunes F, et al. Sensory Organ Generation in the Chicken Inner Ear: Contributions of Bone Morphogenetic Protein 4, Serrate1, and Lunatic Fringe. J Comp Neurol. 2000;424(3):509–520. doi: 10.1002/1096-9861(20000828)424:3<509::aid-cne8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, et al. Skeletal Overgrowth and Deafness in Mice Lacking Fibroblast Growth Factor Receptor 3. Nat Genet. 1996;12(4):390–397. doi: 10.1038/ng0496-390. [DOI] [PubMed] [Google Scholar]
- Cook T. Cell Diversity in the Retina: More Than Meets the Eye. Bioessays. 2003;25(10):921–925. doi: 10.1002/bies.10356. [DOI] [PubMed] [Google Scholar]
- Cornell RA, Eisen JS. Notch in the Pathway: The Roles of Notch Signaling in Neural Crest Development. Semin Cell Dev Biol. 2005;16(6):663–672. doi: 10.1016/j.semcdb.2005.06.009. [DOI] [PubMed] [Google Scholar]
- D'Souza B, Miyamoto A, Weinmaster G. The Many Facets of Notch Ligands. Oncogene. 2008;27(38):5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, et al. Sox2 Signaling in Prosensory Domain Specification and Subsequent Hair Cell Differentiation in the Developing Cochlea. Proc Natl Acad Sci U S A. 2008;105(47):18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudet N, Lewis J. Two Contrasting Roles for Notch Activity in Chick Inner Ear Development: Specification of Prosensory Patches and Lateral Inhibition of Hair-Cell Differentiation. Development. 2005;132(3):541–551. doi: 10.1242/dev.01589. [DOI] [PubMed] [Google Scholar]
- Doetzlhofer A, Basch ML, Ohyama T, et al. Hey2 Regulation by Fgf Provides a Notch-Independent Mechanism for Maintaining Pillar Cell Fate in the Organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver EC, Pryor SP, Hill P, et al. Hedgehog Signaling Regulates Sensory Cell Formation and Auditory Function in Mice and Humans. J Neurosci. 2008;28(29):7350–7358. doi: 10.1523/JNEUROSCI.0312-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Amraoui A, Cohen-Salmon M, Petit C, et al. Spatiotemporal Expression of Otogelin in the Developing and Adult Mouse Inner Ear. Hear Res. 2001;158(1-2):151–159. doi: 10.1016/s0378-5955(01)00312-4. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, et al. Sox2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev Neurosci. 2004;26(2-4):148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, Pauer GJ, Reid J, et al. Sox2 Mutation Causes Anophthalmia, Hearing Loss, and Brain Anomalies. Am J Med Genet A. 2005;138A(2):95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- Hartman BH, Hayashi T, Nelson BR, et al. Dll3 Is Expressed in Developing Hair Cells in the Mammalian Cochlea. Dev Dyn. 2007;236(10):2875–2883. doi: 10.1002/dvdy.21307. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Cunningham D, Bermingham-McDonogh O. Loss of Fgfr3 Leads to Excess Hair Cell Development in the Mouse Organ of Corti. Dev Dyn. 2007;236(2):525–533. doi: 10.1002/dvdy.21026. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Kokubo H, Hartman BH, et al. Hesr1 and Hesr2 May Act as Early Effectors of Notch Signaling in the Developing Cochlea. Dev Biol. 2008a;316(1):87–99. doi: 10.1016/j.ydbio.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ray CA, Bermingham-McDonogh O. Fgf20 Is Required for Sensory Epithelial Specification in the Developing Cochlea. J Neurosci. 2008b;28(23):5991–5999. doi: 10.1523/JNEUROSCI.1690-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Detmar M. Prox1, Master Regulator of the Lymphatic Vasculature Phenotype. Cell Tissue Res. 2003;314(1):85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- Jacques BE, Montcouquiol ME, Layman EM, et al. Fgf8 Induces Pillar Cell Fate and Regulates Cellular Patterning in the Mammalian Cochlea. Development. 2007;134(16):3021–3029. doi: 10.1242/dev.02874. [DOI] [PubMed] [Google Scholar]
- Jones JM, Montcouquiol M, Dabdoub A, et al. Inhibitors of Differentiation and DNA Binding (Ids) Regulate Math1 and Hair Cell Formation During the Development of the Organ of Corti. J Neurosci. 2006;26(2):550–558. doi: 10.1523/JNEUROSCI.3859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Graham JM, Jr, Olney AH, et al. Gli3 Frameshift Mutations Cause Autosomal Dominant Pallister-Hall Syndrome. Nat Genet. 1997;15(3):266–268. doi: 10.1038/ng0397-266. [DOI] [PubMed] [Google Scholar]
- Karcavich RE. Generating Neuronal Diversity in the Drosophila Central Nervous System: A View from the Ganglion Mother Cells. Dev Dyn. 2005;232(3):609–616. doi: 10.1002/dvdy.20273. [DOI] [PubMed] [Google Scholar]
- Kelberman D, Rizzoti K, Avilion A, et al. Mutations within Sox2/Sox2 Are Associated with Abnormalities in the Hypothalamo-Pituitary-Gonadal Axis in Mice and Humans. J Clin Invest. 2006;116(9):2442–2455. doi: 10.1172/JCI28658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Talreja DR, Corwin JT. Replacement of Hair Cells after Laser Microbeam Irradiation in Cultured Organs of Corti from Embryonic and Neonatal Mice. J Neurosci. 1995;15(4):3013–3026. doi: 10.1523/JNEUROSCI.15-04-03013.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, et al. The Notch Ligand Jagged1 Is Required for Inner Ear Sensory Development. Proc Natl Acad Sci U S A. 2001;98(7):3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, et al. The Notch Ligands Dll1 and Jag2 Act Synergistically to Regulate Hair Cell Development in the Mammalian Inner Ear. Development. 2005a;132(19):4353–4362. doi: 10.1242/dev.02002. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, et al. Sox2 Is Required for Sensory Organ Development in the Mammalian Inner Ear. Nature. 2005b;434(7036):1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Xu J, Gridley T. The Notch Ligand Jag1 Is Required for Sensory Progenitor Development in the Mammalian Inner Ear. PLoS Genet. 2006;2(1):e4. doi: 10.1371/journal.pgen.0020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen A, Sulg M, Heyd F, et al. Prox1 Interacts with Atoh1 and Gfi1, and Regulates Cellular Differentiation in the Inner Ear Sensory Epithelia. Dev Biol. 2008;322(1):33–45. doi: 10.1016/j.ydbio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Ladher RK, Wright TJ, Moon AM, et al. Fgf8 Initiates Inner Ear Induction in Chick and Mouse. Genes Dev. 2005;19(5):603–613. doi: 10.1101/gad.1273605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, et al. Notch Signalling Pathway Mediates Hair Cell Development in Mammalian Cochlea. Nat Genet. 1999;21(3):289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton CR, et al. Expression of Math1 and Hes5 in the Cochleae of Wildtype and Jag2 Mutant Mice. J Assoc Res Otolaryngol. 2000;1(2):161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Caignec C, Lefevre M, Schott JJ, et al. Familial Deafness, Congenital Heart Defects, and Posterior Embryotoxon Caused by Cysteine Substitution in the First Epidermal-Growth-Factor-Like Domain of Jagged 1. Am J Hum Genet. 2002;71(1):180–186. doi: 10.1086/341327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger S, Brand M. Fgf8 and Fgf3 Are Required for Zebrafish Ear Placode Induction, Maintenance and Inner Ear Patterning. Mech Dev. 2002;119(1):91–108. doi: 10.1016/s0925-4773(02)00343-x. [DOI] [PubMed] [Google Scholar]
- Li H, Corrales CE, Wang Z, et al. Bmp4 Signaling Is Involved in the Generation of Inner Ear Sensory Epithelia. BMC Dev Biol. 2005;5:16. doi: 10.1186/1471-213X-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mark S, Radde-Gallwitz K, et al. Hey2 Functions in Parallel with Hes1 and Hes5 for Mammalian Auditory Sensory Organ Development. BMC Developmental Biology. 2008;8(1):20. doi: 10.1186/1471-213X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroon H, Walshe J, Mahmood R, et al. Fgf3 and Fgf8 Are Required Together for Formation of the Otic Placode and Vesicle. Development. 2002;129(9):2099–2108. doi: 10.1242/dev.129.9.2099. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, et al. Identification of Vangl2 and Scrb1 as Planar Polarity Genes in Mammals. Nature. 2003;423(6936):173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, et al. Expression of Delta1 and Serrate1 (Jagged1) in the Mouse Inner Ear. Mech Dev. 1999;84(1-2):169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Morsli H, Choo D, Ryan A, et al. Development of the Mouse Inner Ear and Origin of Its Sensory Organs. J Neurosci. 1998;18(9):3327–3335. doi: 10.1523/JNEUROSCI.18-09-03327.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller KL, Jacques BE, Kelley MW. Fibroblast Growth Factor Signaling Regulates Pillar Cell Development in the Organ of Corti. J Neurosci. 2002;22(21):9368–9377. doi: 10.1523/JNEUROSCI.22-21-09368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J, Tokunaga A, Okano H, et al. Mapping of Notch Activation During Cochlear Development in Mice: Implications for Determination of Prosensory Domain and Cell Fate Diversification. J Comp Neurol. 2006;497(3):502–518. doi: 10.1002/cne.20997. [DOI] [PubMed] [Google Scholar]
- Norton JD. Id Helix-Loop-Helix Proteins in Cell Growth, Differentiation and Tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Trokovic R, et al. Fgfr1 Is Required for the Development of the Auditory Sensory Epithelium. Neuron. 2002;35(4):671–680. doi: 10.1016/s0896-6273(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Pujades C, Kamaid A, Alsina B, et al. Bmp-Signaling Regulates the Generation of Hair-Cells. Dev Biol. 2006;292(1):55–67. doi: 10.1016/j.ydbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Puligilla C, Feng F, Ishikawa K, et al. Disruption of Fibroblast Growth Factor Receptor 3 Signaling Results in Defects in Cellular Differentiation, Neuronal Patterning, and Hearing Impairment. Dev Dyn. 2007;236(7):1905–1917. doi: 10.1002/dvdy.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccomagno MM, Martinu L, Mulheisen M, et al. Specification of the Mammalian Cochlea Is Dependent on Sonic Hedgehog. Genes Dev. 2002;16(18):2365–2378. doi: 10.1101/gad.1013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim K, Minowada G, Coling DE, et al. Sprouty2, a Mouse Deafness Gene, Regulates Cell Fate Decisions in the Auditory Sensory Epithelium by Antagonizing Fgf Signaling. Dev Cell. 2005;8(4):553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Stone JS, Cotanche DA. Hair Cell Regeneration in the Avian Auditory Epithelium. Int J Dev Biol. 2007;51(6-7):633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- Takebayashi S, Yamamoto N, Yabe D, et al. Multiple Roles of Notch Signaling in Cochlear Development. Dev Biol. 2007;307(1):165–178. doi: 10.1016/j.ydbio.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Tsai H, Hardisty RE, Rhodes C, et al. The Mouse Slalom Mutant Demonstrates a Role for Jagged1 in Neuroepithelial Patterning in the Organ of Corti. Hum Mol Genet. 2001;10(5):507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, et al. Regulation of Polarized Extension and Planar Cell Polarity in the Cochlea by the Vertebrate Pcp Pathway. Nat Genet. 2005;37(9):980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C, Montcouquiol M, Kelley MW. Math1 Regulates Development of the Sensory Epithelium in the Mammalian Cochlea. Nat Neurosci. 2004;7(12):1310–1318. doi: 10.1038/nn1349. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Ladher R, McWhirter J, et al. Mouse Fgf15 Is the Ortholog of Human and Chick Fgf19, but Is Not Uniquely Required for Otic Induction. Dev Biol. 2004;269(1):264–275. doi: 10.1016/j.ydbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Wright TJ, Mansour SL. Fgf3 and Fgf10 Are Required for Mouse Otic Placode Induction. Development. 2003;130(15):3379–3390. doi: 10.1242/dev.00555. [DOI] [PubMed] [Google Scholar]
- Xu PX, Adams J, Peters H, et al. Eya1-Deficient Mice Lack Ears and Kidneys and Show Abnormal Apoptosis of Organ Primordia. Nat Genet. 1999;23(1):113–117. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Okano T, Ma X, et al. Myosin Ii Regulates Extension, Growth and Patterning in the Mammalian Cochlear Duct. Development. 2009 doi: 10.1242/dev.030718. dev.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Martin GV, Kelley MW, et al. A Mutation in the Lunatic Fringe Gene Suppresses the Effects of a Jagged2 Mutation on Inner Hair Cell Development in the Cochlea. Curr Biol. 2000;10(11):659–662. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ibrahimi OA, Olsen SK, et al. Receptor Specificity of the Fibroblast Growth Factor Family. The Complete Mammalian Fgf Family. J Biol Chem. 2006;281(23):15694–15700. doi: 10.1074/jbc.M601252200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 Induces Robust Production of Extra Hair Cells in Postnatal Rat Inner Ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, et al. Hes1 Is a Negative Regulator of Inner Ear Hair Cell Differentiation. Development. 2000;127(21):4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, et al. Hes1 and Hes5 Activities Are Required for the Normal Development of the Hair Cells in the Mammalian Inner Ear. J Neurosci. 2001;21(13):4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou D, Erickson C, Kim EH, et al. Eya1 Gene Dosage Critically Affects the Development of Sensory Epithelia in the Mammalian Inner Ear. Hum Mol Genet. 2008;17(21):3340–3356. doi: 10.1093/hmg/ddn229. [DOI] [PMC free article] [PubMed] [Google Scholar]