Abstract
The African trypanosome, Trypanosoma brucei, can gauge its environment by sensing nutrient availability. For example, procyclic form (PF) trypanosomes monitor changes in glucose levels to regulate surface molecule expression, which is important for survival in the tsetse fly vector. The molecular connection between glycolysis and surface molecule expression is unknown. Here we partially characterize T. brucei homologs of the β and γ subunits of the AMP-activated protein kinase (AMPK), and determine their roles in regulating surface molecule expression. Using flow cytometry and mass spectrometry, we found that TbAMPKβ or TbAMPKγ-deficient parasites express both of the major surface molecules, EP- and GPEET-procyclin, with the latter being a form that is expressed when glucose is low such as in the tsetse fly. Last, we have found that the putative scaffold component of the complex, TbAMPKβ, fractionates with organellar components and colocalizes in part with a glycosomal marker as well as the flagellum of PF parasites.
Keywords: Trypanosoma brucei, African trypanosome, nutrient sensing, procyclins, mass spectrometry
Trypanosoma brucei, the protozoan parasite that causes African sleeping sickness, is a hemoflagellate that is transmitted to its mammalian host by the bite of an infected tsetse fly. In the mammalian infection, trypanosomes proliferate within the blood as long slender forms and can differentiate into short stumpy forms that are pre-adapted for life in the insect. When the insect ingests short stumpy trypanosomes during a blood meal, the parasites differentiate into procyclic form parasites (PF1) in the fly midgut (Vickerman, 1985). After about 3 weeks, they are found in the salivary glands where they develop into metacyclic forms that are infectious to mammals.
The PF parasites are covered by a coat consisting of members of the procyclin family of glycoproteins (Matthews and Gull, 1994, Roditi, et al., 1989, Ziegelbauer, et al., 1990). The GPI-anchored procyclins, EP-procyclins and GPEET-procyclin, have at their C-terminus 22–30 Glu-Pro repeats (EP-procyclin) or five or six Gly-Pro-Glu-Glu-Thr repeats followed by three EP repeats (GPEET-procyclin) (Mowatt and Clayton, 1987, Mowatt, et al., 1989, Richardson, et al., 1988, Roditi, et al., 1987). The different isoforms of procyclins have different features. For example, EP1-1, EP1-2, and EP3 are N-glycosylated with a homogeneous Man5GlcNAc2 while EP2 is unglycosylated (Acosta-Serrano, et al., 1999, Hwa, et al., 1999, Treumann, et al., 1997). GPEET is unglycosylated but is phosphorylated at the threonine residues (Butikofer, et al., 1999, Mehlert, et al., 1999). Expression of procyclins can be influenced by culture conditions in vitro. Glycerol in the culture medium triggers GPEET expression, as can growth in glucose-depleted (~ 0.03 mM) medium (Butikofer, et al., 1997, Morris, et al., 2002, Treumann, et al., 1997). Early in the in vitro differentiation of bloodstream forms to PFs, both types of procyclins are expressed (Vassella, et al., 2001). GPEET expression increases in the first 24 hours, to be replaced by glycosylated EP-procyclins later in development (Vassella, et al., 2001). A similar program of expression has been observed in parasites isolated from tsetse fly infections, though insufficient parasites were available for analysis prior to day 3 of the infection (Acosta-Serrano, et al., 2001, Vassella, et al., 2000). Although the function of the different procylin isoforms remains to be determined (Vassella, et al., 2009), it is suggested that its polyionic nature may help the parasite to resist attack by tsetse midgut proteases (Acosta-Serrano, et al., 2001).
Previously, we screened an RNAi-based genomic library for cells resistant to the lectin concanavalin A (Con A), which binds the N-glycan on EP-procyclins and induces cell death (Hwa, et al., 1999, Pearson, et al., 2000, Welburn, et al., 1996). We found that glycolysis (or the rate of glycolytic flux) was a regulator of procyclin expression. Under conditions in which the rate of glycolysis was reduced, EP-procyclin expression was repressed, while GPEET-procyclin expression was upregulated.
Complicating the connection between glucose metabolism and surface molecule expression is the compartmentalization of a majority of glycolysis in peroxisome-like organelles, the glycosomes. This compartmentalization suggests that some extra-glycosomal metabolic sensor may be involved in the transmission of information about glycolysis to the nucleus. To resolve the components that connect glucose metabolism and surface molecule expression, we have used reverse genetics to characterize genes that were predicted to be involved in glucose sensing based on observations from other systems. In other organisms, AMP-activated kinase (AMPK), a heterotrimeric enzyme complex consisting of α, β, and γ subunits, is a key regulator in nutrient sensing (reviewed in (Carling, 2004)). The α subunit contains a kinase domain as well as a regulatory domain that inhibits the enzyme in the absence of AMP (Crute, et al., 1998). The β subunit acts as a scaffold for the other components, while the γ subunit is thought to be involved in AMP binding (Cheung, et al., 2000).
AMPK is a member of the AMPK/SNF1 family, which has been described in many eukaryotes (Hardie, et al., 1998, Hardie and Hawley, 2001, Kemp, et al., 1999) and acts in nutrient sensing pathways in yeast and mammals. Under conditions that reduce glycolysis, the yeast SNF1 complex (a homolog of AMPK (Carlson, 1999)) is activated. This kinase modulates the cellular response to decreased glycolysis by phosphorylating (and activating) metabolic enzymes, including glycolytic enzymes such as hexokinase. Similarly, low glucose conditions activate mammalian AMPK (Salt, et al., 1998), which triggers changes in gene expression, including increase in expression of metabolic genes involved in glycolysis (reviewed in (Hardie and Hawley, 2001)).
While the α subunit remains elusive, we have identified homologous genes for the β and γ subunits (TbAMPKβ and TbAMPKγ, respectively) of the heterotrimeric components (α, β, and γ) of AMPK in T. brucei and have explored the role of these genes in the regulation of surface molecule expression in response to glucose levels. Silencing these genes triggers changes in surface molecule expression, while localization of the scaffold (β) subunit suggests positioning in the cell consistent with a role as an intermediary between surface molecule expression and glycolysis.
MATERIALS AND METHODS
Trypanosome growth and RNAi
PF 29-13 T. brucei, a 427 strain that expresses T7 RNA polymerase and the tetracycline repressor, were grown in SDM-79 as described (Wang, et al., 2000, Wirtz, et al., 1999). Low glucose SDM-79 was prepared with glucose-free RPMI 1640 replacing the liquid MEM. Also, additional glucose and glucosamine (normally present in SDM-79 at 1 mg/ml and 50 ng/ml, respectively) were omitted. This mixture was supplemented with normal FBS (10%), resulting in a final glucose concentration of ~ 0.5 mM.
RNAi constructs were generated by ligation of TbAMPKβ (276 bp, from nt 436 to 711 of the open reading frame) or TbAMPKγ (461 bp, from nt 895 to 1356) PCR products amplified from T. brucei strain 427 genomic DNA into XhoI/HindIII cut pZJM (Wang, et al., 2000). Parasites (1 × 108) were transformed with 10 µg NotI linearized pZJM(AMPKβ) or pZJM(AMPKγ) and stable integrants selected with phleomycin (2.5 µg/ml) as described (Wang, et al., 2000). RNAi was induced by the addition of tetracycline (tet, 1 µg/ml) to cultures.
Flow cytometry and surface glycoprotein labeling
Parasites were labeled with fluorescein-conjugated Con A as described (Morris, et al., 2002). Briefly, cells (1 × 106) were resuspended in 1 ml cytomix supplemented with 1 mM MnCl2 (cytoM (van den Hoff, et al., 1992)) containing 10 µg/ml FITC-labeled Con A (Sigma, St. Louis, MO). After 15 min at room temperature, trypanosomes were applied to a FACScan flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ) and 10,000 cells analyzed per sample. Antibody labeling of surface molecules was performed using 5 × 106 parasites that were washed in PBS, fixed in 4% paraformaldehyde/0.1% glutaraldehyde (12 hr, 4°C), and washed in 1% BSA in PBS (blocking solution). Antibodies to EP-procyclin (monoclonal antibody TBRP1/247, Cedarlane Laboratories, Ontario Canada (Richardson, et al., 1988, Richardson, et al., 1986)) or GPEET-procyclin (monoclonal 5H3 (Butikofer, et al., 1999)), which was a generous gift from Dr. Terry Pearson (University of British Columbia) were diluted (1:100) and incubated with cells (1hr, 4°C) in blocking solution. Parasites were washed, incubated in FITC-conjugated goat anti-mouse antibody (1 hr, 4°C in blocking solution), and then resuspended in sheath fluid for flow cytometry.
MALDI-TOF-MS analysis of procyclins
For mass-spectrometry analysis, procyclins were purified from freeze-dried parasites (1 × 108) by sequential extraction with organic solvents (Acosta-Serrano, et al., 1999). To remove GPI anchors from polypeptides, dry butanolic extracts were dephosphorylated with 25 µl of 48% aqueous hydrofluoric acid (aq. HF) for 20 h at 0 °C. After treatment, samples were dried in a Speed-Vac and resuspended in 20 µl of 0.1% TFA. An aliquot (~ 0.5 µl; ~ 5 × 106 parasite equivalents) was co-crystallized with 0.5 µl 10 mg/ml sinapinnic acid in 70 % acetonitrile, 0.1 % TFA and analyzed by positive-ion mode. Data collection was in linear mode on a PerSeptive Biosystems Voyager-DE mass spectrometer (located at the Sir Henry Wellcome Functional Genomics Facility, Glasgow University). The accelerating voltage was 2500 V and the grid voltage was set at 91 % with an extraction time delay of 100 nsec. Data were collected manually at 200 shots per spectrum, with laser intensity set at 2800. To confirm assignments, HF-treated samples (~5 × 107 parasite equivalents) were submitted to mild acid hydrolysis with 40 mM TFA for 20 min at 100 °C, and an aliquot analyzed by MALDI-TOF-MS as described above (Acosta-Serrano, et al., 1999).
Digitonin fractionation and Hexokinase assays
Digitonin fractionation was performed using a method adapted from(Lorenz, et al., 1998). Parasites (1 × 107) were washed in cold PBS, followed by a wash in STE buffer (250 mM sucrose, 25 mM Tris, pH 7.4, 1 mM EDTA). Cells were resuspended in STE+N (150 mM NaCl) with 0.1 M PMSF, pelleted, and resuspended in STE+N with digitonin (0.2 mg/ml). The mixture was vortexed for 5 min and then incubated at 25°C for 4 min. Lysates were centrifuged (2 min, 15,000 × g, 4°C) and pellets resuspended in 0.1 M TEA, pH 7.5 (5 × 105 cell equivalents/ml).
Hexokinase assays were performed in a coupled assay as previously described on equal volumes of the resulting supernatant and pellet from the digitonin fractionation (Misset and Opperdoes, 1984, Morris, et al., 2002). Assays were performed in triplicate in 96-well microtiter plate format using a GENios spectrophotometer (Phenix Research Products, Hayward, CA).
Localization of TbAMPKβ subunit
To localize TbAMPKβ, PF parasites expressing eYFP fused to the T. brucei aldolase peroxoisomal targeting sequence 2 (PTS2, which targets proteins to glycosomes) were harvested by centrifugation at 800 × g, washed with Voorheis’s modified PBS (vPBS; 137 mM NaCl, 3 mM KCl, 16 mM Na2HPO4, 3 mM KH2PO4, 46 mM sucrose, 10 mM glucose, pH 7.6) and fixed in an equal volume of 6% paraformaldehyde and vPBS for 1 hr on ice. Cells were washed with vPBS and then allowed to settle on poly-lysine coated slides prior to permeabilization with 0.1% Triton X-100 in PBS (10 min, RT) and then washed in excess PBS three times before the addition of block (1% BSA and 0.25% Tween in PBS, 1 hr, RT). Primary affinity purified rabbit polyclonal antibodies for TbAMPKβ, which was purified using an antigen coupled AminoLink Coupling Resin (Thermo Scientific, Rockford Il) following the manufacture’s instructions, and a mouse monoclonal against GFP (3E6, Molecular Probes, Eugene, OR) were applied at a dilution of 1:10 and 1:100, respectively, for 1 hour. The slides were washed 3 times in excess PBS before the addition of the secondary antibodies (TexasRed-conjugated goat anti-rabbit and FITC-conjugated goat anti-mouse; Rockland, Gilbertsville, PA, both at 1:100). Slides were then washed 3 times in excess PBS and prepared for visualization by addition of Vectashield mounting media with DAPI. Images were taken on a Zeiss Axiovert 200M using Axiovision software version 4.6.3 for image analysis.
To express TbAMPKβ with a C-terminal fusion to GFP in live parasites the TbAMPKβopen reading frame was cloned into pLew111(2T7)GFPβ (the generous gift of Drs. Shawn Motyka and Paul Englund, Johns Hopkins School of Medicine). PF 29-13 strain trypanosomes were transformed with linearized DNA and selected as described above. Recombinant TbAMPKβ̃ GFP expression was induced for 4 days by addition of tet (1 µg/ml) and GFP expression monitored by microscopy.
RESULTS
Identification and silencing of AMPK subunit homologs in T. brucei
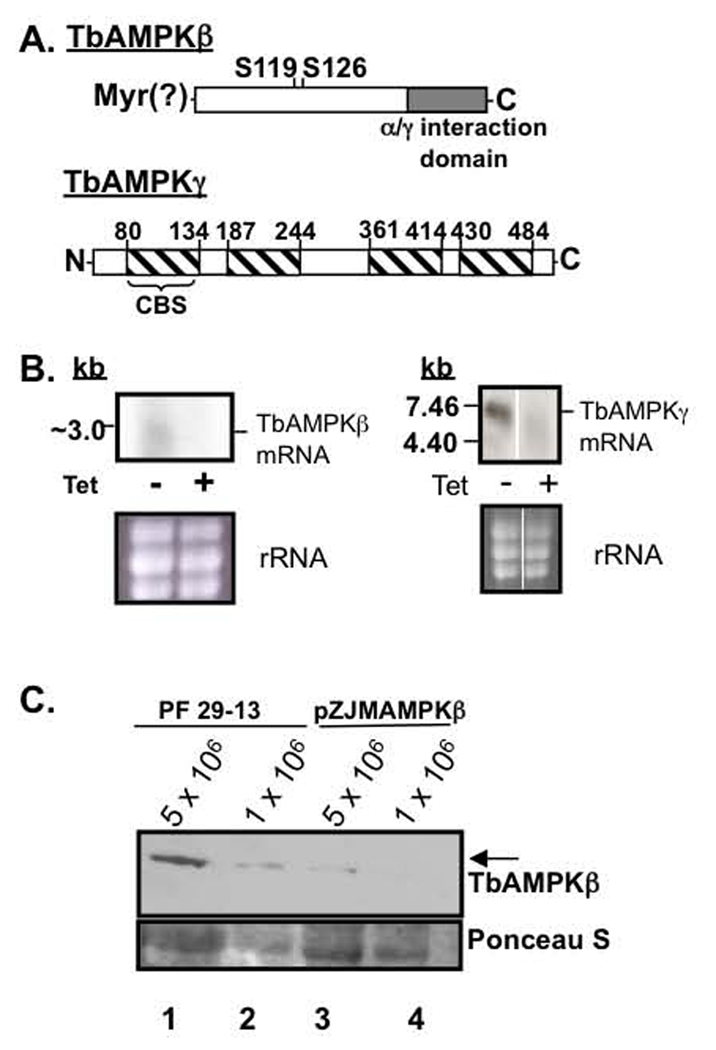
We have identified candidate single copy homologous genes for the β and γ subunits components of the heterotrimeric enzyme AMPK in the T. brucei genome database. The putative T. brucei AMPKβ subunit (TbAMPKβ̣ systematic name Tb927.8.2450) is similar to the rat AMPKβ1 protein (E value = 2.8 × 10−10), sharing 33% amino acid identity. TbAMPKβ is predicted to be a 34.4 kDa protein that contains a 5’ AMP-activated protein kinase β-subunit complex interacting region (α/γ interaction domain, residues 195–303) found in the β subunit of mammalian AMPK and yeast homologs of AMPK (Thornton, et al., 1998) (Fig. 1A). In other systems, this domain allows interaction with the kinase (α) subunit and is characteristic of β subunits. TbAMPKβ also shares with the rat AMPKβ1 protein a conserved putative N-myristolyation signal (Fig. 1A) and two (of four) conserved Ser (at residue 119 and 126) (Carling, 2004).
Fig. 1.
Targeting TbAMPKβ and TbAMPKγ by RNAi impacts transcript and protein levels. (A) TbAMPK β and γ diagram. TbAMPKβ contains a putative N-myristoylation sequence (MGNTSAE), two conserved Ser residues (corresponding to Ser101 and Ser108 from rat AMPKβ 1), and a β/γ interaction domain. TbAMPKγ contains 4 CBS domains that have been implicated in AMP binding (Cheung, et al., 2000, Daniel and Carling, 2002). (B) Analysis of RNAi of TbAMPKβ and TbAMPKγ by northern blot. Total RNA from parental (−) and tetracycline induced (+) (for 40 h) cells containing the construct pZJM(TbAMPKβ) or pZJM(TbAMPKγ) was purified from 5 × 107 parasites and electrophoresed on a formaldehyde 1.5% agarose gel. Ribosomal RNA levels were estimated by ethidium bromide staining to ensure equal loading of RNA. (C.) Western blot performed on 5 × 106 or 1 × 106 cell equivalents of PF 29-13 (lanes 1–2) or cells induced 4 days to silence TbAMPKβ with pZJMAMPKβ (lanes 3–4) were resolved by SDS-PAGE, transferred to nitrocellulose and probed with affinity purified TbAMPKβ antibodies. The bottom panel is a Ponceau S-stained band used to estimate loading.
The putative T. brucei AMPKγ subunit (TbAMPKγ, systematic name Tb10.70.3670) is predicted to be a 55.2 kDa protein that is most similar to human AMPKγ 3 (E value = 1.1 × 10−11, 27% identical at the amino acid level). The trypanosome protein is predicted to have 4 cystathionine-β-synthase (CBS) domains (residues 80–134, 187–244, 361–414, 430–484), which have been found in other γ subunits (but are not limited to AMPKγ subunits) (Fig. 1A). The CBS domains have been implicated to function in AMP binding (Cheung, et al., 2000, Daniel and Carling, 2002).
To initiate analysis of the function of TbAMPK subunits, we silenced the putative subunits using the pZJM RNAi vector. The impact of RNAi of either subunit on cell viability was minimal, causing ~2-fold increase in doubling time (not shown). Induction of RNAi for 40 hours led to a dramatic decrease in TbAMPKβ and TbAMPKγ transcript abundance (Fig. 1B) (Wang, et al., 2000). Due to low transcript abundance, the northern blots of TbAMPKβ were particularly difficult to assess. Since RNAi ultimately leads to protein depletion, we used western blotting to confirm the TbAMPKβ knock down using polyclonal antibodies to recombinant TbAMPKβ protein after RNAi (Fig. 1C). The ~ 34 kDa polypeptide was detectable by western blot from 1 × 106 parental 29–13 cell equivalents, while silencing TbAMPKβ led to a ~5-fold reduction in detectable protein with 5 × 106 cell equivalents (Fig. 1C, lanes 3 and 4).
Bioinformatics-based approaches suggested there were 14 active members of the CAMK group of kinases in the T. brucei genome, which include the AMPKα subunit (Parsons, et al., 2005). Of these potential α subunits, a candidate T. brucei gene that clustered closely with yeast SNF1 and human AMPKα 1, Tb10.70.1760. RNAi of Tb10.70.1760 failed to yield a discernable Con A binding phenotype (see below), suggesting that we had either identified and tested the incorrect homolog, or that functional redundancy was obscuring the resolution of the α subunit. For these reasons, we have focused on the single-copy subunits for the remainder of this manuscript.
RNAi of TbAMPKβ or TbAMPKγ impacts surface molecule expression
Previously, we found through an RNAi-based genomic library screen that glycolysis modulated surface glycoprotein expression in PF parasites (Morris, et al., 2002). Therefore, we reasoned that knockdown of proteins that play a role in the connection of glycolysis to surface molecule expression may yield cells with similar dis-regulation of surface molecule expression.
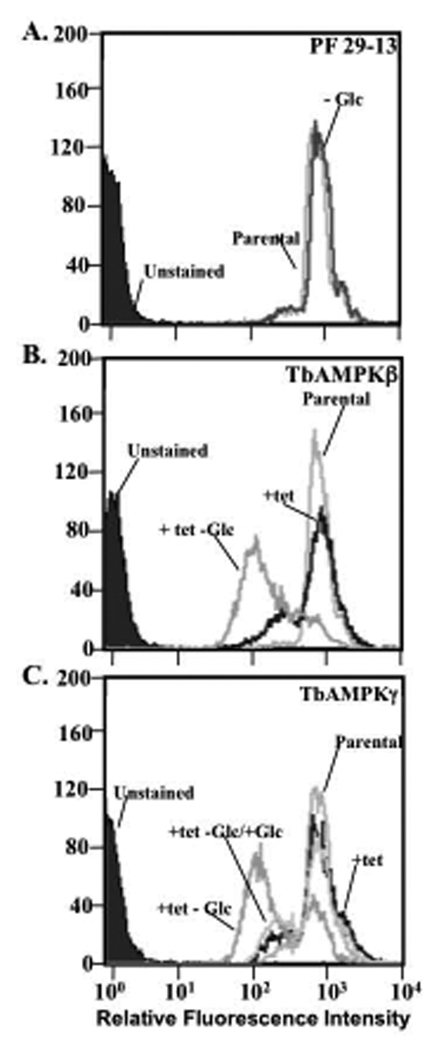
To determine if TbAMPK subunit silencing leads to this phenotype, we first stained TbAMPKβ and TbAMPKγ-deficient cells with the lectin Con A, which predominantly binds to glycosylated EP-procyclins but not to GPEET on PF trypanosomes (Pearson, et al., 2000) (Fig. 2). We have used this approach to previously characterize the impact of silencing glycolytic enzymes on surface molecule expression (Morris, et al., 2002). The impact of silencing TbAMPKβ and TbAMPKγ on Con A binding was assessed in cells grown in standard SDM-79 medium or in reduced glucose (~ 0.5 mM) medium for 7 days. While growth in reduced glucose medium did not alter parental PF 29-13 cell Con A-FITC binding (Fig. 2A, light gray line compared to dark grey line, and (Morris, et al., 2002)), culturing of TbAMPKβ or TbAMPKγ-deficient cells in normal medium caused a subtle reduction in Con A binding while growth in low glucose medium for 7 days caused a dramatic reduction in Con A binding (Fig. 2B and 2C, black line compared to grey line).
Fig. 2.
Silencing TbAMPKβ or γ causes a change in surface molecule expression that is enhanced by growth in reduced glucose medium. (A.) Living PF 29-13 trypanosomes were cultured for two weeks in normal SDM-79 (parental, light grey line) or reduced glucose medium (−Glc, dark grey line) and then incubated with 10 µg/ml fluorescein-conjugated Con A for 15 min at room temperature in cytoM. Parasites were then analyzed by flow cytometry (10,000 cells/assay). (B.) Cell line PF 29-13 (parental, light grey line), cells induced to silence TbAMPKβ for 7 days (+tet, black line) and cells induced to silence TbAMPKβ for 7 days grown in reduced glucose medium for 7 days (+tet −Glc, grey line) were analyzed after Con A-FITC staining. Laser intensity was adjusted to yield autofluorescence from unstained cells (purple shade) of ~3 fluorescence intensity units. (C.) Cells in which TbAMPKγ was silenced for 7 days (+tet, black line), trypanosomes induced to silence TbAMPKγ for 7 days grown in reduced glucose medium (+tet, −Glc, grey line), and cells induced to silence TbAMPKγ for 7 days grown in reduced glucose with glucose supplemented (5 mM) (+ tet, −Glc/+Glc, grey line) for 7 days were analyzed.
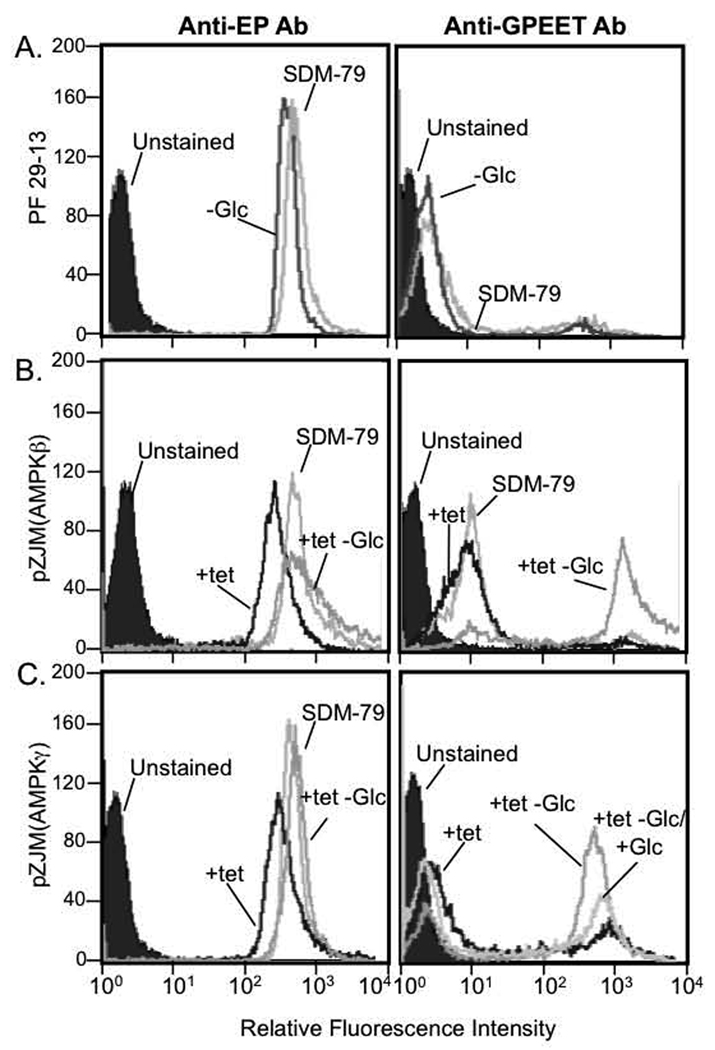
To further characterize the nature of the surface molecule change, we used antibodies to the major surface glycoproteins to assess expression by flow cytometry. We found that cells deficient in either TbAMPKβ or TbAMPKγ have a similar glucose-dependent RNAi phenotype that is not observed in the parental cell line. Parental 29-13 PF trypanosomes predominantly expressed EP-procyclin (Fig. 3A, left panel) with little detectable GPEET-procyclin (Fig. 3A, right panel). These findings are in agreement with the procyclin repertoire that has been described from PF 29-13 parasites (Acosta-Serrano, et al., 1999, Morris, et al., 2002).
Fig. 3.
Trypanosomes deficient in either TbAMPKβ or TbAMPKγ express GPEET-procyclin. Parasites were grown in normal SDM-79 (light grey line) or reduced glucose medium (−Glc, dark grey line), and cells induced to silence TbAMPK subunits for 7 days (+tet). Induced cells were also grown in reduced glucose medium (+tet −Glc), or in media with glucose supplemented (5 mM) (+ tet, −Glc/+Glc). Parasites (5 × 106) were fixed and stained with antibodies to EP-procyclin (TBRP1/247) or GPEET-procyclin (monoclonal 5H3). Primary antibodies were detected with FITC-conjugated anti-mouse secondary antibody and cells (10,000) analyzed by flow cytometry.
Like parental PF 29-13 cells, TbAMPKβ-deficient parasites expressed EP-procyclin (Fig. 3B, left panel) and expressed very little GPEET-procyclin (Fig. 3B, right panel). However, if parasites deficient in TbAMPKβ were grown under reduced glucose (~ 0.5 mM) conditions, a greater proportion expressed GPEET-procyclin (Fig. 3B, right panel, +tet, −glc). These cells continued to express EP-procyclin (Fig. 3B, left panel).
Cells with TbAMPKγ silenced also expressed EP-procyclin (Fig. 3C, left panel) and demonstrated a subtle increase in the surface expression of GPEET-procyclin (Fig. 3C, right panel). Under reduced glucose (~ 0.5 mM) conditions, a greater proportion expressed GPEET-procyclin (Fig. 3C, right panel, +tet, −glc). Again, these cells continued to express EP-procyclin (Fig. 3C, left panel), with both procyclins expressed for the duration of these experiments (~14 days). Addition of glucose to these cells blocked the increased expression of GPEET-procyclin (Fig. 3C, right panel, +tet – glc/+glc).
MALDI-TOF mass spectrometry confirms that normally glycosylated EP-procyclin, in addition to GPEET-procyclin, is expressed after RNAi of TbAMPKβ or TbAMPKγ
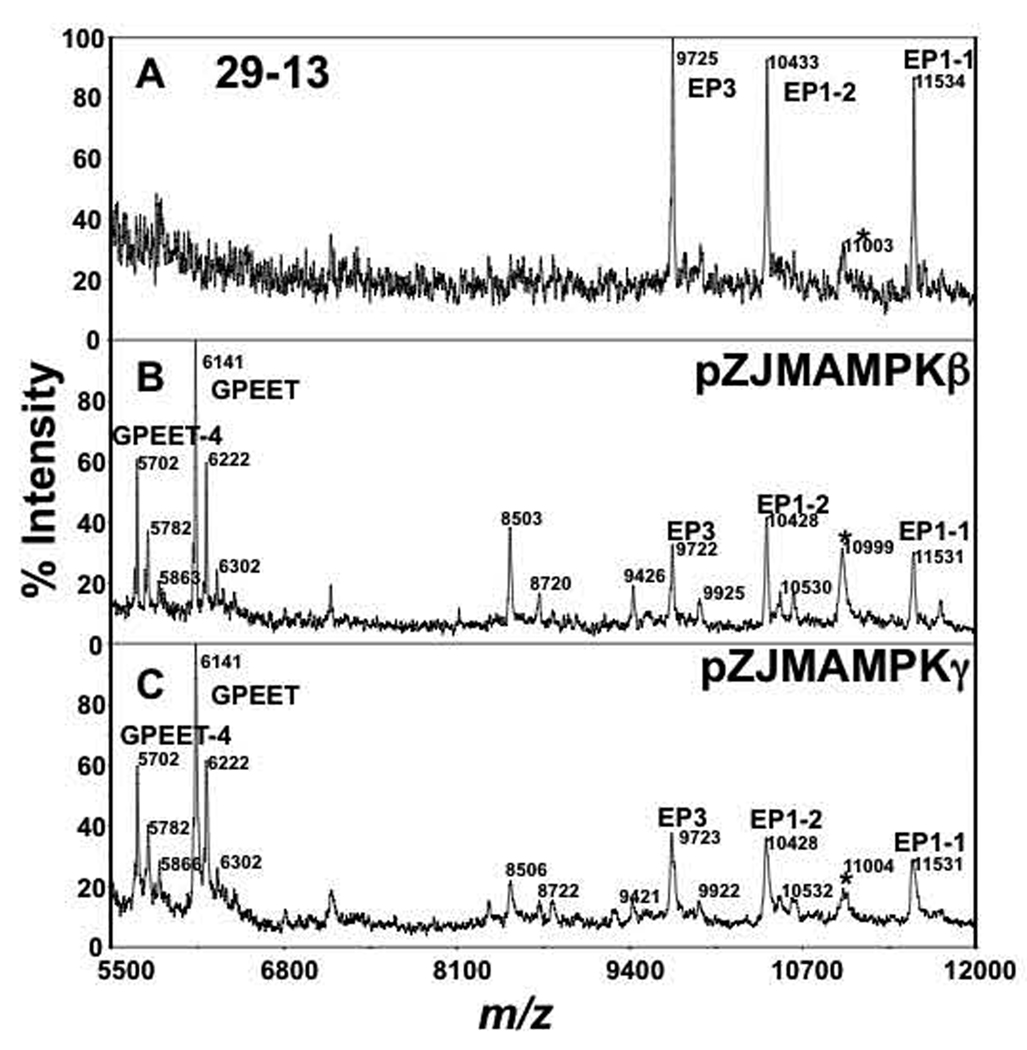
Silencing either TbAMPKβ or TbAMPKγ subunits triggered a loss of Con A binding, while antibody staining and flow cytometry analysis of these cells indicated that EP-procyclin was expressed on the surface. These observations seem to conflict, as Con A binds to the N-glycan of EP-procyclin. How is there a reduction in Con A binding while the protein to which Con A binds is still expressed? These data suggested that GPEET-procyclin could be masking the N-glycan of EP-procyclin. Alternatively, EP-procyclins may not be properly N-glycosylated in the TbAMPKβ or TbAMPKγ-deficient cells, or EP-2 (a naturally occurring unglycosylated EP-procyclin) could be the dominant EP-procyclin in these cells. To resolve these possibilities, we performed MALDI-TOF mass spectrometry on partially purified procyclins from parental PF 29-13, TbAMPKβ-deficient, or TbAMPKγ-deficient trypanosomes grown in low glucose medium (Fig. 4).
Fig. 4.
Mass spectrometry analysis of procyclins. Butanol extracts of (A) PF 29-13, (B) TbAMPKβ, and (C) TbAMPKγ silenced cells grown in low glucose medium were dephosphorylated with aqueous HF and aliquots analyzed by MALDI-TOF-MS in positive mode. Some of the unlabelled ions in B and C correspond to fragments of EP-procyclins that lack 10 amino acids from the N-terminus (Acosta-Serrano, et al., 2000). Asterisks represent a contaminant that has been previously assigned as KMP-11 (Acosta-Serrano, et al., 1999).
Expression of GPEET was confirmed by positive-ion MALDI-TOF-MS on HF-treated procyclin polypeptides. Parental PF 29-13 expressed only glycosylated EP-procyclin species (i.e. EP1-1, EP1-2 and EP3) (Fig. 4A). Ions representing GPEET (m/z 6141) or EP2-procyclin (m/z 8345; the only naturally unglycosylated procyclin) were not detected, consistent with the Con A and antibody binding observed by flow cytometry. In contrast, silencing of either TbAMPKβ or TbAMPKγ yielded a reduction (but not complete ablation) of glycosylated EP-procyclins with a concurrent increase in expression of GPEET-procyclin. GPEET-procyclin expression is characterized by expression of full length polypeptides (m/z 6,141) and the presence of a truncated form lacking the N-terminus sequence VIVK (marked as "GPEET-4", Fig 4B and 4C) (Acosta-Serrano, et al., 2000). Assignments of the procyclins species present in all samples were confirmed by negative ion MALDI-TOF-MS analyses of the C-terminus after mild acid hydrolysis (not shown). Since the EP-species have the expected masses of polypeptides bearing the parental oligomannose N-glycan, the reduction in Con A binding observed in TbAMPKβ or TbAMPKγ-deficient cells is not likely due to alteration in N-glycosylation but rather is possibly due to masking of the glycan by GPEET-procyclin.
Localization of the TbAMPKβ subunit homolog
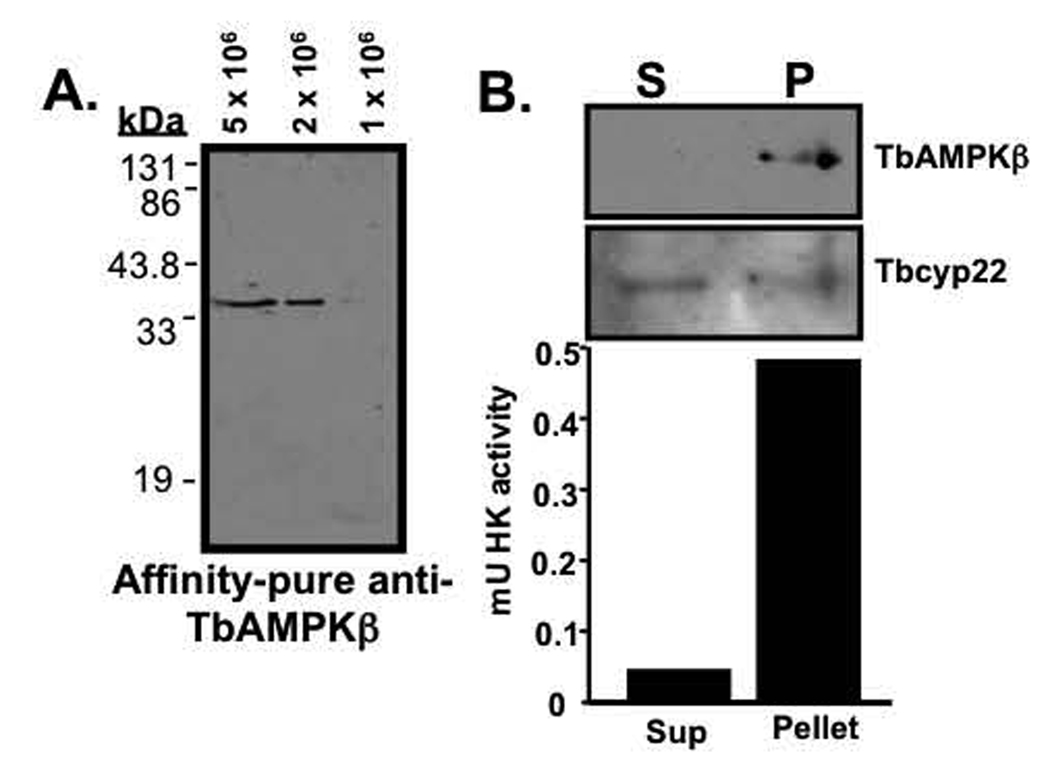
In mammals, the AMPK β-1 subunit localizes to extranuclear particulate structures that are neither mitochondria nor endoplasmic reticulum (Warden, et al., 2001). The localization and activity of the AMPK complex are altered if the AMPK β-1 subunit N-myristolyation site is mutated, suggesting that this component of the complex directs the subcellular localization of the complex. To explore the localization of TbAMPKβ, affinity-purified antibodies were generated against recombinant TbAMPKβ. These antibodies recognized a single polypeptide (that does not react with pre-immune sera) in western blots of whole trypanosomal lysates (Fig. 5A and data not shown).
Fig. 5.
TbAMPKβ has an organellar digitonin fractionation. (A.) Affinity-purified antibodies raised against TbAMPKβ recognize a single polypeptide in total T. brucei cell lysates. (B.) TbAMPKβ partitions with the particulate fraction of digitonin permeablized cells. Following fractionation, equal volumes of supernatant and resuspended pellet (1 × 106 cell equivalents/lane) were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to either TbAMPKβ (1:100) or Tbcyp22 (1:100). Primary antibodies were detected with HRP-conjugated goat anti-mouse antibodies (1:10,000). To explore hexokinase activity, fractions were assayed in a coupled hexokinase reaction, with the conversion of NAD to NADH by glyceraldehyde-3-phosphate dehydrogenase monitored by spectrophotometer.
Digitonin fractionation of trypanosomes was used to separate soluble cytoplasmic proteins from organellar components. When this fraction was analyzed by western blot, TbAMPKβ was detected exclusively in the particulate fraction (Fig. 5B, P). As a control, the hexokinase activity of each of the fractions was determined and ~90% of the hexokinase activity was found associated with the particulate fraction (which should include intact glycosomes). Not all proteins fractionated with the particulate matter, as a putative cyclophilin-like protein (Tbcyp22) was found with near-equal distribution in both fractions (Fig. 5B).
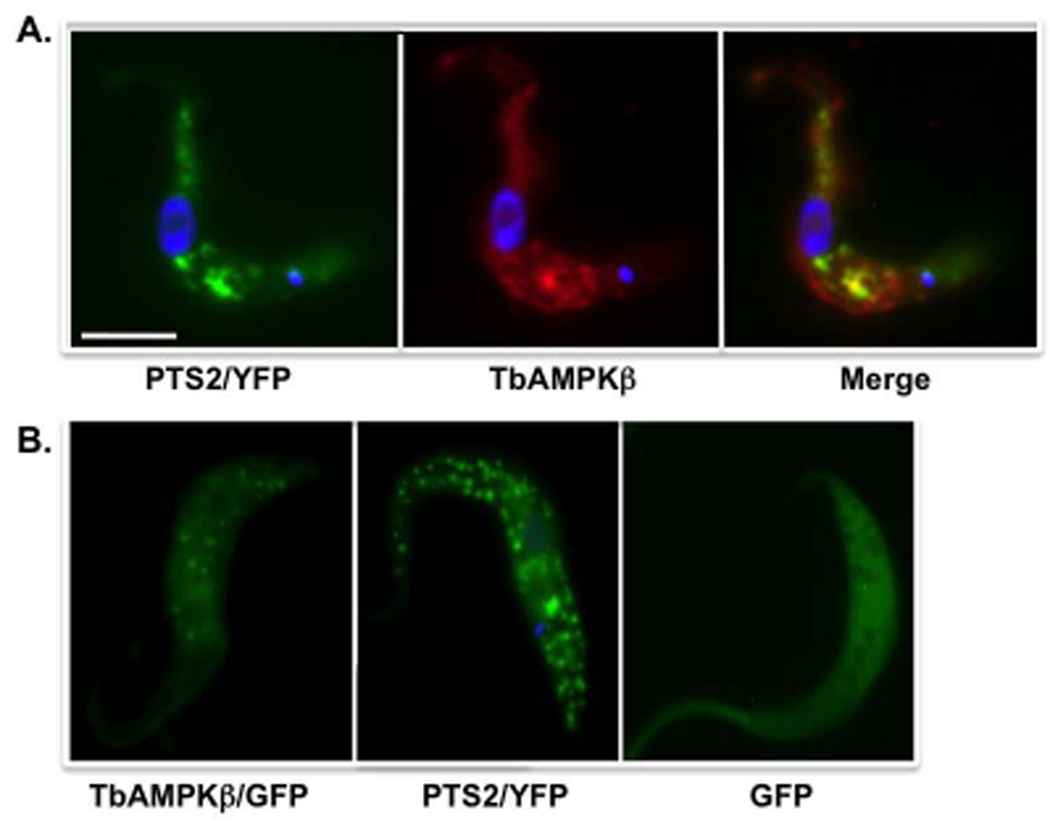
Immunofluorescence of parasites expressing yellow fluorescent protein (YFP) targeted to glycosomes (as a result of fusion with an N-terminal PTS2) was used to further assess the cellular localization of TbAMPKβ. These parasites had a TbAMPKβ distribution that overlapped with the glycosomal YFP marker but was not exclusively glycosomal (Fig. 6A). For example, signal was also detected in or near the flagellum. Similarly, live parasites expressing a fusion of TbAMPKβ with GFP yielded punctate fluorescence consistent with glycosomes, as well as faint flagellar signal (Fig. 6B). YFP bearing an N-terminal PTS2 (for glycosome targeting, Fig. 6B, left panel) localizes primarily in points in live cells, while expression of GFP without a targeting sequence yielded signal throughout the parasite (Fig. 5B, right panel).
Fig. 6.
TbAMPKβ is localized to punctate bodies that partially co-localize with glycosomes. (A.) Immunofluorescence using affinity-pure polyclonal sera raised against recombinant TbAMPβ. Transgenic PF 29-13 harboring pXS2(PTS2YFP) were fixed, permeabilzed , and stained with a mouse anti-GFP monoclonal antibody (left panel) or affinity-purified polyclonal anti-TbAMPKβ antibodies (center panel). Primary antibodies were detected with FITC-conjugated goat anti-mouse or mouse anti-rabbit antibodies, respectively. Scale bar = 5 µm. (B.) Expression of TbAMPKβ fused to GFP in live cell using pLew111(2T7)GFPβ (middle panel) Transformed trypanosomes were induced to express the recombinant fusion protein for 4 days by addition of tet (1 µg/ml). For comparison, live cells expressing glycosomally targeted YFP (left panel) or GFP without a targeting sequence (right panel) are included. Cells were washed, resuspended in PBS and spotted on slides after mixing with an equal volume of antifade reagent.
DISCUSSION
The African trypanosome inhabits two very different environments, requiring adaptation to conditions found in these different hosts. In the mammalian bloodstream, the parasite is bathed in its primary carbon source, glucose, while in the tsetse fly glucose is rapidly depleted during bloodmeal digestion. In the fly, the parasites persist by metabolism of amino acids, taking advantage of the abundance of proline produced by the fly to power its flight muscles.
Both PF and bloodstream parasites monitor glucose abundance to make developmental decisions. During differentiation from BSF to PF, parasites switch surface coats from VSG to procyclin in a process that can be triggered in vitro by growth in low glucose medium (Milne, et al., 1998). In the fly, PF parasites respond to changes in glucose concentrations by altering surface molecule expression. PF parasites very early in the differentiation progression express both EP- and GPEET- procyclins, but switch to express predominantly GPEET-procyclin shortly after initiation of the infection (Acosta-Serrano, et al., 2001, Vassella, et al., 2001, Vassella, et al., 2000). This switch is coincident with the fall of glucose levels during digestion of the bloodmeal. This progression can be recapitulated by growing parasites in very low (0.03 mM) glucose conditions or by using RNAi to silence glycolytic enzymes (Morris, et al., 2002). These observations suggest that glucose levels, presumably reflected in the rate of glycolysis, are being monitored to regulate GPEET-procyclin expression.
The molecular mechanisms that govern the expression of the procyclins likely differ, as both cis- and trans- factors have been identified (Vassella, et al., 2000, Walrad, et al., 2009). The 3’ UTR of GPEET-procyclin contain a glucose response element required for the upregulation of GPEET-procyclin expression in response to low glucose medium and during its development in the fly (Vassella, et al., 2004). EP-procyclin expression is likely not regulated by glucose, as culturing parasites in low glucose does not ablate EP-procyclin expression (Morris, et al., 2002). Furthermore, deletion of the TbGalE gene, which encodes an epimerase that interconverts UDP-Glc and UDP-Gal in glycosomes, leads to a 10-fold increase in the expression of EP- but not GPEET-procyclin, suggesting that Gal metabolism is also involved in controlling the expression and copy numbers of the procyclin molecules (Roper, et al., 2005).
We have demonstrated here that TbAMPK β and γ play a role in surface molecule expression, as silencing of the genes leads to upregulation of GPEET-procyclin expression. The change in procyclin expression is only detectable when RNAi cells were grown in low glucose media, suggesting that the incomplete penetrance of the RNAi of the AMPK subunits was not alone sufficient to trigger GPEET expression.
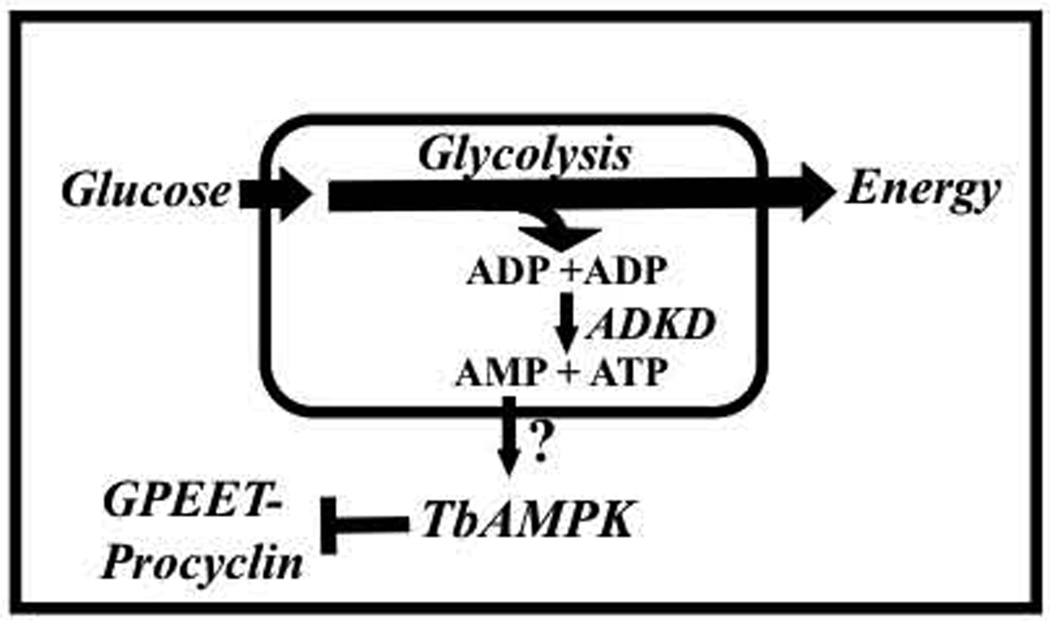
The physical connection between glucose and surface molecule expression is problematic, as the majority of glucose metabolism is compartmentalized in the glycosome – how is a signal transmitted from the glycosome to the rest of the cell? In other eukaryotes, AMPK plays a key role in responding to cellular glucose metabolism, being activated to trigger changes throughout the cell in response to changes in glycolysis (Hardie, 2008). AMP generated as a result of glucose metabolism could activate a T. brucei complex that is homologous to mammalian AMPK (Fig. 7). Hexokinase and phosphofructokinase both consume ATP, which may be regenerated by a glycosomal adenylate kinase (ADKD) (Ginger, et al., 2005). In other systems, this enzyme synthesizes ATP from ADP with the concomitant production of AMP and the cells use the ATP:AMP ratio as a marker for the status of energy levels (Hardie, 2008).
Fig. 7.
A schematic representation of the connection of TbAMPK to surface molecule expression. The pathway labeled with a question mark remains to be resolved, as extra-glycosomal AMP may function to activate TbAMPK. ADKD, glycosomal adenylate kinase.
Immunolocalization of TbAMPKβ indicates a widespread distribution, which could reflect the role of the subunit as part of a signaling complex. Although TbAMPKβ lacks a C-terminal tripeptide peroxisomal targeting sequence (PTS1) (Opperdoes, 1987) or a PTS2-type targeting sequence, the subunit partially co-localizes with glycosomes (Fig. 5A), where its activity may be influenced by ADKD (Ginger, et al., 2005). Additionally, the β subunit localizes to the flagellum, where it is positioned to interact with a previously described flagellar AK (Pullen, et al., 2004). TbAMPKβ subunit localization (and function) may be dependent on the protein’s myristoylated state, akin to mammalian AMPK-β1 (Resh, 1999, Warden, et al., 2001). It is also possible that the as of yet unidentified α subunit provides cellular targeting information to the complex. The partial localization of TbAMPKβ with glycosomes positions the complex to potentially monitor the status of glycolysis in the cell, which has been previously demonstrated to play a central role in the modulation of surface molecule regulation.
ACKNOWLEDGMENTS
This work was supported by US National Institutes of Health 1R15AI075326 to JCM. CSC was partially supported by a research grants from the Calhoun Honors College, Clemson University and from the HHMI/SCLIFE Undergraduate Research Program. AAS was supported by a Wellcome Trust Research Career Development Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: AMPK, AMP-activated kinase; Con A, concanavalin A; aq. HF, aqueous hydrofluoric acid; MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry; PF, procyclic form trypanosome; RNAi, RNA interference; TbAMPKβ , T. brucei homolog of AMP-dependent kinase β subunit; TbAMPKγ , T. brucei homolog of AMP-dependent kinase γ subunit; tet, tetracycline;
LITERATURE CITED
- 1.Acosta-Serrano A, Cole RN, Englund PT. Killing of Trypanosoma brucei by concanavalin A: structural basis of resistance in glycosylation mutants. Journal of Molecular Biology. 2000;304:633–644. doi: 10.1006/jmbi.2000.4246. [DOI] [PubMed] [Google Scholar]
- 2.Acosta-Serrano A, Cole RN, Mehlert A, Lee MG, Ferguson MA, Englund PT. The procyclin repertoire of Trypanosoma brucei. Identification and structural characterization of the Glu-Pro-rich polypeptides. Journal of Biological Chemistry. 1999;274:29763–29771. doi: 10.1074/jbc.274.42.29763. [DOI] [PubMed] [Google Scholar]
- 3.Acosta-Serrano A, Vassella E, Liniger M, Kunz Renggli C, Brun R, Roditi I, Englund PT. The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proceedings of the National Acadamey of Sciences U.S.A. 2001;98:1513–1518. doi: 10.1073/pnas.041611698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butikofer P, Ruepp S, Boschung M, Roditi I. 'GPEET' procyclin is the major surface protein of procyclic culture forms of Trypanosoma brucei brucei strain 427. Biochemical Journal. 1997;326:415–423. doi: 10.1042/bj3260415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butikofer P, Vassella E, Ruepp S, Boschung M, Civenni G, Seebeck T, Hemphill A, Mookherjee N, Pearson TW, Roditi I. Phosphorylation of a major GPI-anchored surface protein of Trypanosoma brucei during transport to the plasma membrane. Journal of Cell Science. 1999;112(Pt 11):1785–1795. doi: 10.1242/jcs.112.11.1785. [DOI] [PubMed] [Google Scholar]
- 6.Carling D. The AMP-activated protein kinase cascade--a unifying system for energy control. Trends in Biochemical Sciences. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Carlson M. Glucose repression in yeast. Current Opinions in Microbiology. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 8.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochemical Journal. 2000;346(Pt 3):659–669. [PMC free article] [PubMed] [Google Scholar]
- 9.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. Journal of Biological Chemistry. 1998;273:35347–35354. doi: 10.1074/jbc.273.52.35347. [DOI] [PubMed] [Google Scholar]
- 10.Daniel T, Carling D. Functional analysis of mutations in the gamma 2 subunit of AMP-activated protein kinase associated with cardiac hypertrophy and Wolff-Parkinson-White syndrome. Journal of Biological Chemistry. 2002;277:51017–51024. doi: 10.1074/jbc.M207093200. [DOI] [PubMed] [Google Scholar]
- 11.Ginger ML, Ngazoa ES, Pereira CA, Pullen TJ, Kabiri M, Becker K, Gull K, Steverding D. Intracellular positioning of isoforms explains an unusually large adenylate kinase gene family in the parasite Trypanosoma brucei. Journal of Biological Chemistry. 2005;280:11781–11789. doi: 10.1074/jbc.M413821200. [DOI] [PubMed] [Google Scholar]
- 12.Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Letters. 2008;582:81–89. doi: 10.1016/j.febslet.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annual Review of Biochemistry. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 14.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 15.Hwa KY, Acosta-Serrano A, Khoo KH, Pearson T, Englund PT. Protein glycosylation mutants of procyclic Trypanosoma brucei: defects in the asparagine-glycosylation pathway. Glycobiology. 1999;9:181–190. doi: 10.1093/glycob/9.2.181. [DOI] [PubMed] [Google Scholar]
- 16.Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends in Biochemical Sciences. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 17.Lorenz P, Maier AG, Baumgart E, Erdmann R, Clayton C. Elongation and clustering of glycosomes in Trypanosoma brucei overexpressing the glycosomal Pex11p. The EMBO Journal. 1998;17:3542–3555. doi: 10.1093/emboj/17.13.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthews KR, Gull K. Evidence for an interplay between cell cycle progression and the initiation of differentiation between life cycle forms of African trypanosomes. Journal of Cell Biology. 1994;125:1147–1156. doi: 10.1083/jcb.125.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehlert A, Treumann A, Ferguson MA. Trypanosoma brucei GPEET-PARP is phosphorylated on six out of seven threonine residues. Molecular and Biochemical Parasitology. 1999;98:291–296. doi: 10.1016/s0166-6851(98)00168-6. [DOI] [PubMed] [Google Scholar]
- 20.Milne KG, Prescott AR, Ferguson MA. Transformation of monomorphic Trypanosoma brucei bloodstream form trypomastigotes into procyclic forms at 37 degrees C by removing glucose from the culture medium. Molecular and Biochemical Parasitology. 1998;94:99–112. doi: 10.1016/s0166-6851(98)00055-3. [DOI] [PubMed] [Google Scholar]
- 21.Misset O, Opperdoes FR. Simultaneous purification of hexokinase, class-I fructose-bisphosphate aldolase, triosephosphate isomerase and phosphoglycerate kinase from Trypanosoma brucei. European Journal of Biochemistry. 1984;144:475–483. doi: 10.1111/j.1432-1033.1984.tb08490.x. [DOI] [PubMed] [Google Scholar]
- 22.Morris JC, Wang Z, Drew ME, Englund PT. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. The EMBO Journal. 2002;21:4429–4438. doi: 10.1093/emboj/cdf474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mowatt MR, Clayton CE. Developmental regulation of a novel repetitive protein of Trypanosoma brucei. Molecular and Cellular Biology. 1987;7:2838–2844. doi: 10.1128/mcb.7.8.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mowatt MR, Wisdom GS, Clayton CE. Variation of tandem repeats in the developmentally regulated procyclic acidic repetitive proteins of Trypanosoma brucei. Molecular and Cellular Biology. 1989;9:1332–1335. doi: 10.1128/mcb.9.3.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opperdoes FR. Compartmentation of carbohydrate metabolism in trypanosomes. Annual Review of Microbiology. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- 26.Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearson TW, Beecroft RP, Welburn SC, Ruepp S, Roditi I, Hwa KY, Englund PT, Wells CW, Murphy NB. The major cell surface glycoprotein procyclin is a receptor for induction of a novel form of cell death in African trypanosomes in vitro. Molecular and Biochemical Parasitology. 2000;111:333–349. doi: 10.1016/s0166-6851(00)00327-3. [DOI] [PubMed] [Google Scholar]
- 28.Pullen TJ, Ginger ML, Gaskell SJ, Gull K. Protein targeting of an unusual, evolutionarily conserved adenylate kinase to a eukaryotic flagellum. Molecular Biology of the Cell. 2004;15:3257–3265. doi: 10.1091/mbc.E04-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Resh MD. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochimica et Biophysica Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 30.Richardson JP, Beecroft RP, Tolson DL, Liu MK, Pearson TW. Procyclin: An unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Molecular and Biochemical Parasitology. 1988;31:203–216. doi: 10.1016/0166-6851(88)90150-8. [DOI] [PubMed] [Google Scholar]
- 31.Richardson JP, Jenni L, Beecroft RP, Pearson TW. Procyclic tsetse fly midgut forms and culture forms of African trypanosomes share stage-and species-specific surface antigens identified by monoclonal antibodies. Journal of Immunology. 1986;136:2259–2264. [PubMed] [Google Scholar]
- 32.Roditi I, Carrington M, Turner M. Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature. 1987;325:272–274. doi: 10.1038/325272a0. [DOI] [PubMed] [Google Scholar]
- 33.Roditi I, Schwarz H, Pearson TW, Beecroft RP, Liu MK, Richardson JP, Buhring HJ, Pleiss J, Bulow R, Williams RO, Overath P. Procyclin gene expression and loss of the variant surface glycoprotein during differentiation of Trypanosoma brucei. Journal of Cell Biology. 1989;108:737–746. doi: 10.1083/jcb.108.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roper JR, Guther ML, Macrae JI, Prescott AR, Hallyburton I, Acosta-Serrano A, Ferguson MA. The suppression of galactose metabolism in procylic form Trypanosoma brucei causes cessation of cell growth and alters procyclin glycoprotein structure and copy number. Journal of Biological Chemistry. 2005;280:19728–19736. doi: 10.1074/jbc.M502370200. [DOI] [PubMed] [Google Scholar]
- 35.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochemical Journal. 1998;335(Pt 3):533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thornton C, Snowden MA, Carling D. Identification of a novel AMP-activated protein kinase beta subunit isoform that is highly expressed in skeletal muscle. Journal of Biological Chemistry. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- 37.Treumann A, Zitzmann N, Hulsmeier A, Prescott AR, Almond A, Sheehan J, Ferguson MA. Structural characterisation of two forms of procyclic acidic repetitive protein expressed by procyclic forms of Trypanosoma brucei. Journal of Molecular Biology. 1997;269:529–547. doi: 10.1006/jmbi.1997.1066. [DOI] [PubMed] [Google Scholar]
- 38.van den Hoff MJ, Moorman AF, Lamers WH. Electroporation in 'intracellular' buffer increases cell survival. Nucleic Acids Research. 1992;20:2902. doi: 10.1093/nar/20.11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassella E, Acosta-Serrano A, Studer E, Lee SH, Englund PT, Roditi I. Multiple procyclin isoforms are expressed differentially during the development of insect forms of Trypanosoma brucei. Journal of Molecular Biology. 2001;312:597–607. doi: 10.1006/jmbi.2001.5004. [DOI] [PubMed] [Google Scholar]
- 40.Vassella E, Den Abbeele JV, Butikofer P, Renggli CK, Furger A, Brun R, Roditi I. A major surface glycoprotein of Trypanosoma brucei is expressed transiently during development and can be regulated post-transcriptionally by glycerol or hypoxia. Genes and Development. 2000;14:615–626. [PMC free article] [PubMed] [Google Scholar]
- 41.Vassella E, Oberle M, Urwyler S, Renggli CK, Studer E, Hemphill A, Fragoso C, Butikofer P, Brun R, Roditi I. Major surface glycoproteins of insect forms of Trypanosoma brucei are not essential for cyclical transmission by tsetse. PLoS ONE. 2009;4:e4493. doi: 10.1371/journal.pone.0004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vassella E, Probst M, Schneider A, Studer E, Renggli CK, Roditi I. Expression of a major surface protein of Trypanosoma brucei insect forms is controlled by the activity of mitochondrial enzymes. Molecular Biology of the Cell. 2004;15:3986–3993. doi: 10.1091/mbc.E04-04-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. British Medical Bulletin. 1985;41:105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- 44.Walrad P, Paterou A, Acosta-Serrano A, Matthews KR. Differential trypanosome surface coat regulation by a CCCH protein that coassociates with procyclin mRNA cis-elements. PLoS Pathogens. 2009;5:e1000317. doi: 10.1371/journal.ppat.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Morris JC, Drew ME, Englund PT. Inhibition of Trypanosoma brucei gene expression by RNA interference using an integratable vector with opposing T7 promoters. Journal of Biological Chemistry. 2000;275:40174–40179. doi: 10.1074/jbc.M008405200. [DOI] [PubMed] [Google Scholar]
- 46.Warden SM, Richardson C, O'Donnell J, Jr, Stapleton D, Kemp BE, Witters LA. Post-translational modifications of the beta-1 subunit of AMP-activated protein kinase affect enzyme activity and cellular localization. Biochemical Journal. 2001;354:275–283. doi: 10.1042/0264-6021:3540275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welburn SC, Dale C, Ellis D, Beecroft R, Pearson TW. Apoptosis in procyclic Trypanosoma brucei rhodesiense in vitro. Cell Death and Differentiation. 1996;3:229–236. [PubMed] [Google Scholar]
- 48.Wirtz E, Leal S, Ochatt C, Cross GA. A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Molecular and Biochemical Parasitology. 1999;99:89–101. doi: 10.1016/s0166-6851(99)00002-x. [DOI] [PubMed] [Google Scholar]
- 49.Ziegelbauer K, Quinten M, Schwarz H, Pearson TW, Overath P. Synchronous differentiation of Trypanosoma brucei from bloodstream to procyclic forms in vitro. Eupropean Journal of Biochemistry. 1990;192:373–378. doi: 10.1111/j.1432-1033.1990.tb19237.x. [DOI] [PubMed] [Google Scholar]