Abstract
Zebrafish, one of the preferred study species of geneticists, is gaining increasing popularity in behavioral neuroscience. This small and prolific species may be an excellent tool with which the biological mechanisms of vertebrate brain function and behavior are investigated. Zebrafish has been proposed as a model organism in the analysis of fear responses and human anxiety disorders. Species-specific cues signaling the presence of predators have been successfully utilized in such research. Zebrafish has been shown to respond to its natural alarm substance with species-typical fear reactions. However, the extraction of this alarm substance and ascertaining its consistent dosing has been problematic. A synthetic substance with a known chemical identity and molecular weight would allow precise dosing and experimental control. Previously, the chemical component, hypoxanthine 3-N-oxide, common to several fish alarm substances has been identified and has been shown to elicit alarm reactions in fish species belonging to the Osteriophysan superorder. In the current study we investigate the effect of hypoxanthine 3-N-oxide by exposing zebrafish to three different concentrations of this synthetic substance. Our results show that the substance efficaciously induces species-typical fear reactions increasing the number of erratic movement episodes and jumps in zebrafish. We discuss the translational relevance of our findings and conclude that hypoxanthine 3-N-oxide will have utility to elicit fear responses in the laboratory in a precisely controlled manner in zebrafish.
Keywords: alarm substance, anxiety, fear, H3NO, zebrafish
Introduction
Zebrafish have provided significant insights for developmental biologists who utilized numerous molecular methods to identify genes and biological mechanisms involved in embryonic development of vertebrates using this species as a model organism [19]. As a result of the past three decades of this research numerous genetic tools have been developed for zebrafish and these tools now are making this species an attractive study organism for other fields of biology as well. Behavioral brain research has benefited from genetic approaches using the mouse as a model organism and revealed fundamental biological mechanisms of brain function and behavior of vertebrates (e.g. [22]). The good understanding of the genetics of zebrafish is now making this species strongly preferred as a tool for behavioural brain reserach [16, 33].
This small and prolific species offers some advantages over traditional laboratory rodent species. Although a vertebrate with a sophisticated central nervous system, it is easy and inexpensive to keep in large numbers. Its prolific nature (200-300 eggs per spawning per female every other day) has made this species ideal for large scale screening purposes including mutagenesis screens (forward genetics) and drug screening [19]. From a behavioral brain research perspective, however, this species suffers from a drawback. Its behavioral responses are not as well characterized as those of laboratory rodents and the number of behavioral tests available for this species is also very limited [33, 28]. Given that the foundation of mutation and drug screening is the phenotypical testing paradigms, the above represents a significant bottleneck in zebrafish behavioral brain research. Briefly, development of behavioral test methods and procedures for zebrafish is of great importance.
Preclinical studies, i.e. translational research, can facilitate the understanding of the mechanisms of human disorders. Two of the most prevalent human neuropsychiatric diseases are the anxiety disorders and phobias [35, 8]. Although numerous medications have been developed for these disorders, they still represent a large unmet medical need [12]. This is mainly because the biological mechanisms of these disorders are not well understood. Nevertheless, some, including us, argue that these disorders are likely the result of abnormally functioning neurobiological mechanisms that have originally evolved to serve the function of avoidance of danger, e.g. predators in nature [21, 25, 13]. Consistent with this line of argument, it is also proposed that animal research that utilizes naturalistic approaches and, for example, employs species-specific fear inducing stimuli may have the highest translational relevance [2, 27, 28]. In our laboratory, we have developed a number of behavioral paradigms to induce fear with the use of naturalistic cues, including the sight of a sympatric predator of zebrafish [1, 13] and the natural alarm substance of zebrafish [34]. The latter is particularly noteworthy as one, in principle, could parametrically manipulate the level of fear induced by controlling the concentration and/or length of exposure to the alarm substance. However, the exact chemical identity of the zebrafish alarm substance is not known and in the past we [34] and others [20, 36] had to resort to extracting this substance from the skin of zebrafish and employ the extract and its dilutions without knowing exactly what and how much was in the extracted “cocktail”. Briefly, a synthetic alarm substance with a known chemical identity and concentration in the water when administered would facilitate precision and experimental control.
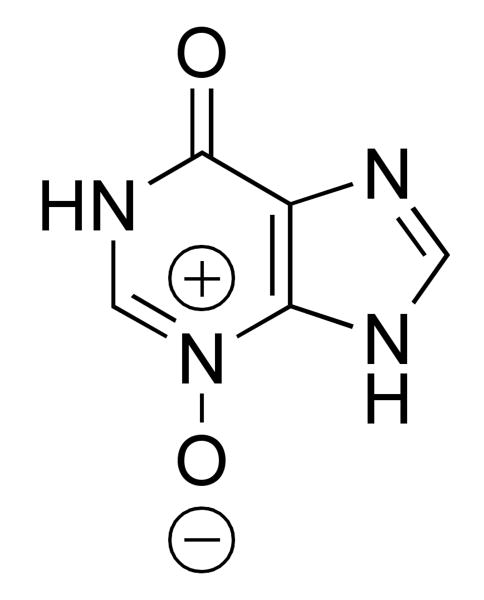
Previously, alarm substances from species of the Osteriophysan superorder of fishes were identified [30, 31] and a chemical compound common to these substances was found [24, 4, 7]. This compound is hypoxanthine 3-N-oxide (H3NO), a purine derivative oxidized at the 3-position (figure 1.). Hypoxanthine 3-N-oxide has been shown to induce alarm responses in a number of fish species particularly those that belong to the Osteriophysan superorder [30, 31, 4, 5, 6, 7]. Given that zebrafish belongs to this superorder it is possible that this species too is responsive to this synthetic alarm substance. This assumption is reasonable, if one considers that being too selective about the taxonomic origin of this odor cue would be maladaptive: determining the presence of a hunting predator should not depend on what species of prey it has caught.
Figure 1.
Hypoxanthine 3-N-oxide (H3NO).
Therefore, we hypothesized that zebrafish would be responsive to the synthetic alarm substance and would respond to its presence in the water by species-specific alarm or fear reactions. Previously, we found one of the most typical features of fear responses of zebrafish is the increase of erratic movements performed [34]. This behavior was seen in response to the sight of a sympatric predator [1, 13] as well as in response to the natural alarm substance of zebrafish [34]. Another typical response under fear inducing conditions is the increase of the number jumps zebrafish perform [1]. Under some fear inducing conditions, activity level of zebrafish may decrease and immobility (freezing) may increase. Also, zebrafish may spend increasing amount of time on the bottom, a response seen in other fish species (e.g. paradise fish, [17]) under aversive conditions. In the current study, we exposed zebrafish to three concentrations of hypoxanthine 3-N-oxide and quantified the above behavioral reactions.
Methods
Animals and Housing
Sexually mature and fully grown (6-12 month old, approximately 4 cm long) zebrafish (Danio rerio) of the SFWT (short fin wild type) population bred in our facility (University of Toronto Mississauga Vivarium, Mississauga ON, Canada) were used (one hundred fish, approximately 50-50% females and males). The fish were the second filial generation of parental fish obtained from a local pet store (Big Al's Aquarium Warehouse outlets Inc, Mississauga, Ontario, Canada). We have used SFWT in a number of studies including those that analyzed predator avoidance [1], shoaling [29, 32], and/or the effect of alcohol exposure [14] because we argued that, unlike more inbred standard zebrafish strains, these genetically heterogeneous fish may better resemble those from natural wild populations of zebrafish. Briefly, we expect SFWT fish to be more representative of the species and to possess fewer idiosyncratic features that may have developed during the inbreeding process of laboratory strains.
The developing fry were raised in 1.3 liter nursery tanks (zebrafish nursery rack of Aquaneering Inc, San Diego California, USA) in system water (deionized and UV-light sterilized water supplemented with 60mg/l Instant Ocean Sea Salt [Big Al's Pet Store, Mississauga, Ontario, Canada]). The developing fish were fed Larval Artificial Plankton 100 (particle size below 100 μm, ZeiglerBros.Inc., Gardners, Pennsylvania, USA). At three weeks of age the small fish were moved into 2.8 liter rearing tanks (20 fish per tank) of a high density rack system (Aquaneering Inc, San Diego California, USA) designed specifically for zebrafish. This system had a multistage filtration that contained a mechanical filter, a fluidized glass bed biological filter, an activated carbon filter, and a fluorescent UV-light sterilizing unit. Every day 10% of the water was automatically replaced with fresh system water on the rack. The water temperature in the holding tanks was maintained at 27 °C. Illumination was provided by fluorescent light tubes from the ceiling with lights turned on at 0800h and off at 1900 h. While the fish were in the high density racks they received a mixture of dried fish food (4 parts of Nelson Silver Cup, Aquaneering Inc, San Diego California, USA) and powered spirulina (1 part, Jehmco Inc, Lambertville, New Jersey, USA) three times a day. Behavioral experiments were conducted after the fish reached 6 months of age (fully developed sexually mature young adults).
Apparatus and Procedure
The general experimental set up and procedure was similar to that described in detail elsewhere [1, 34]. Briefly, the observation tank (50.8cm × 25.5 cm × 30.5cm, length × width × height) was placed in a compartment that provided visual isolation from all directions except the front. The tank was illuminated from above by a fluorescent light tube (Eclipse 13W) placed directly above the tank. Its walls, except the front glass, were covered with a white plastic sheet to increase contrast for later viewing of video-recordings. The water temperature in the observation tank was held at 27 °C. The observation tank was filled with fresh system water identical to what was used for the maintenance of zebrafish (reverse osmosis purified salt reconstituted oxygenated water). A video-camera (JVC Everio GZ-MG50, JVC Corporation) was positioned in front of the tank. All experiments were recorded onto the hard drive of the camera and subsequently the recordings were downloaded onto DVD (Maxell DVD+R) discs and replayed later for analysis. The experimenter was not visible to the fish during the recording.
Experimental fish were placed singly into the test tank (of 35 L system water) using a net and a cup so that even while being transferred the experimental fish was not exposed to atmospheric air. The experimental fish received a 22 min habituation session in the test tank during which no treatment was employed and the fish was left undisturbed. After the habituation session, a 7 minute recording session was conducted during which the behavior of the experimental fish was video-recorded. The recording session was started with the delivery of the alarm substance in the form of a 2.5 mL solution.
The hypoxanthine 3-N-oxide (H3NO) was prepared in two steps from 6-chloropurine (purchased from Aldrich and used without purification) in a 68% overall yield [18, 23]. All spectroscopic characterization data were consistent with previously reported values [7]. The H3NO is precipitated from water at a pH of about 3-4, the compound is dried in a vacuum desiccator, at ambient temperature, for 24 hours followed by combustion analysis of an analytical of 15 mg (conducted by Analytical Microlabs of Norcross, Georgia, USA) to determine a formula weight of 173.76 g/mole, which corresponds to a formula of C5H4N4O2•1.2 H2O. For behavioral experimentation three concentrations of H3NO and a freshwater control were employed. For the high dose, 2.4mg H3NO was dissolved in 200mL distilled water (stock solution). The medium dose was made by removing 45mL of the stock (high H3NO dose) and transferring it to a beaker containing 105mL distilled water. The low H3NO dose was made by removing 20mL of the stock H3NO solution, and transferring it to a beaker containing 180mL of distilled water. In between experiments, all H3NO solutions were stored in a -20 °C freezer.
It is notable that H3NO has been found sensitive to acidic conditions. Brown et al. [5] found that even under mildly acidic conditions (pH 5-6) the behavioral response to the alarm substance H3NO disappeared in the studied Ostariophysan fishes. This was due to a covalent change in the H3NO, resulting in the loss of the N-oxide functional group, which has been implicated as the “molecular trigger.” This covalent chemical change is non-reversible. Others have also demonstrated that when H3NO is reacted with 3M HCl (pH ≪ 5) four new compounds can be isolated, all of which have lost the N-oxide functional group and in one case the purine ring system is disrupted [23]. All of these compounds are inactive as alarm substances. However, H3NO is very stable in aqueous solution under neutral or slightly alkaline conditions (pH = 7-8) [5]. Given that our system water is derived from reverse osmosis purified water with salts added that maintain and in fact buffer for neutral pH, we infer that the synthetic alarm substance was stable and remained active for the short duration of our behavioral experiments.
Using a syringe connected to fine polyethylene tubing (internal diameter 0.58 mm) 2.5 mL of one of the above four solutions of H3NO was delivered to each corresponding experimental fish making the final concentration in the experimental tank 0 nM (control freshwater), 0.5 nM (low dose of H3NO), 1.5 nM (medium dose of H3NO), or 5 nM (high dose of H3NO). These concentrations were chosen based upon our own preliminary studies and also on publications using other fish species. For example, fathead minnows were exposed to H3NO at concentrations of 6.7 to 0.1 nM in order to determine the minimum stimulus threshold and the nature of the response curve [6]. In this latter study significant increases in anti-predatory behavior (compared to distilled water controls) were found at concentrations as low as 0.4 nM but the response intensity were found not to change in the studied dose range, i.e. between 6.7 to 0.4 nM.
In our current study, the alarm substance delivery tube was mounted onto the inside of the experimental tank so that its opening end was positioned precisely at the water line. Due to this positioning, and to the slow and careful injection, the confounding effect of disturbance (waves, splashes, or bubbles) could be avoided. The 100 experimental fish were randomly assigned to one of the four treatment groups (the four concentrations of H3NO; for each group n = 25), i.e. all fish were tested only once and with a single H3NO dose, a between subject design. The behavior of experimental fish was monitored after delivery of the solution for 7 minutes. At the conclusion of the recording session the experimental zebrafish was returned to its home tank and was kept for future use. The experimental tank was emptied and thoroughly rinsed and filled with system water for the subsequent recording session. The experiments were conducted between 09:00-17:00h. All fish were experimentally naïve, previously untested, and the order of their testing was randomized according to treatment condition. The experimenter was unaware of the group designation of the fish until the completion of the data analyses.
Quantification of behavior
The digital video-recordings stored on DVDs were replayed and the behavior of fish was quantified using the Observer ColorPro event recorder software (Noldus, Wageningen, The Netherlands). This software application essentially turns the computer into a multichannel stopwatch and allows the human observer to precisely measure the time devoted to particular predefined behaviors and the number of occurrences of these behaviors (for more detailed description and comparison of this method with other behavior quantification techniques for zebrafish, see [3]). In the current study we focused our attention to motor patterns and behaviors that have been known to change in response to alarm substance or other fear inducing stimuli [13, 34, 1, 17]. The behavioral responses measured were as follows. Erratic movement is a characteristic zig-zagging response associated with fast (over 3 cm/sec) swimming and quick direction changes [3]. Jumping is a single forceful leap using the caudal fin [3]. The number of occurrences, i.e. frequency, of these two behaviors was quantified because they represent stereotypical and short movement patterns. Immobility, or freezing, was also measured. During the freezing episode complete cessation of movement is observed and only the gills and occasionally the eyes of the fish may move. Creeping is a very slow (less then 0.5 cm/sec) movement during which the caudal fin is not used. These behaviors were previously found to be associated with fear or pain inducing stimuli in other fish species (e.g. [9], [15] and references therein). The duration of time the fish were immobile or performed creeping was measured and values are expressed as percent of the total session length because these behaviors vary in terms of how long they are exhibited. In addition to these measures, we also recorded the vertical position of fish. We divided the experimental tank into four equal imaginary layers and quantified the percent of time the fish spent in the bottom layer. Swimming to the bottom was seen in zebrafish in response to novelty and it was assumed to indicate increased fear [26]. Last, we measured the total number of crosses from one layer to another and summed all the crosses and this way estimated vertical locomotory activity.
Statistical analysis
Data were analyzed using SPSS (version 14.1) for the PC. Univariate variance analysis (ANOVA) was used to investigate the effect of sex and of alarm substance treatment. Sexes did not differ and thus the data were pooled and only the alarm substance effect is presented in the analysis. In case of significant effects, post hoc Tukey Honestly significant difference (HSD) tests were performed.
Results
The number of erratic movement episodes increased with increasing alarm substance concentrations (figure 2). ANOVA confirmed this observation and found the alarm substance treatment effect significant (F(3, 90) = 3.242, p < 0.05). Post hoc Tukey HSD test showed that the two highest alarm substance concentrations (1.5 and 5 nM) elicited significantly (p < 0.05) larger number of erratic movement episodes compared to freshwater control, while fish in the smallest concentration (0.5 nM) group did not significantly (p > 0.05) differ from the freshwater control or the other groups treated with the higher concentrations.
Figure 2.
The number of Erratic Movement episodes zebrafish performed is increased by H3NO. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. The results of Tukey HSD post hoc analysis are indicated by the small letters above the bars. Bars that do not share the same letter designation are significantly (p < 0.05) different. For further details of statistical analyses see Results.
The number of jumps performed also appeared to increase in response to the alarm substance treatment (figure 3). Confirming this observation, ANOVA revealed a significant treatment effect (F(3, 90) = 3.289, p < 0.05) and the post hoc Tukey HSD test showed that despite the apparent trend, only the highest alarm substance concentration was significantly (p < 0.05) effective in elevating the number of jumps.
Figure 3.
The number times zebrafish jumped is increased by H3NO. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. The results of Tukey HSD post hoc analysis are indicated by the small letters above the bars. Bars that do not share the same letter designation are significantly (p < 0.05) different. For further details of statistical analyses see Results.
The percent of time creeping showed a similar dose response profile (figure 4), with fish in the highest alarm substance dose showing the largest amount of this behavior. However, ANOVA found the differences among treatment groups non-significant (F(3, 90) = 2.151, p > 0.05).
Figure 4.
The percent of time Creeping is performed by zebrafish appears increased by H3NO but the effect is non-significant. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. For results of statistical analyses see Results.
Immobility was not affected by the different doses of the alarm substance (figure 5). ANOVA found no significant treatment effect (F(3, 90) = 0.309, p > 0.80). Similarly, neither the time spent in the bottom layer (figure 6; ANOVA F(3, 90) = 0.308, p > 0.80) nor the activity score (figure 7; ANOVA F(3, 90) = 0.792, p > 0.50) showed significant alarm substance treatment effects. Last, the alarm substance treated fish showed no abnormal or unusual motor or posture patterns.
Figure 5.
The percent of time Freezing (immobility) is performed by zebrafish is not affected by H3NO. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. For results of statistical analyses see Results.
Figure 6.
The percent of time zebrafish spent in the lowest layer (bottom) of the tank is not affected by H3NO. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. For results of statistical analyses see Results.
Figure 7.
Vertical activity (ambulation score) of zebrafish is not affected by H3NO. Mean ± S.E.M. are shown. Sample sizes are n = 25 for each concentration group. For methodological details see Methods. For results of statistical analyses see Results.
Discussion
The results demonstrated that zebrafish respond to the synthetic alarm substance, H3NO in a dose dependent manner and the responses include two characteristic fear reactions, increased number of erratic movements and jumps. This is notable as it supports the notion that zebrafish, similarly to other species belonging to the Osteriophysan superorder of mostly shoaling fish species [30, 7] are also sensitive to the chemical element common to known natural alarm substances of these species. Although empirically not tested, the alarm substances (natural and the synthetic as well) are currently viewed as external olfactory stimuli rather than centrally acting drugs that directly modify the functioning of the central nervous system [30, 7].
Given that these substances are released when a piscivore damages the skin of its prey [30, 11], the evolutionary/adaptive significance of the above finding is clear: individuals that are capable of sensing and responding to alarm substances, irrespective of their species origin, have a higher likelihood of being able to successfully avoid predation. Selectivity of responding only to the alarm substance of one's own species would be disadvantageous, after all the ability to detect the presence of a nearby predator should not be dependent upon whether this predator caught a zebrafish or a fish of another species. Our prior prediction, essentially using the above argument [34] is thus confirmed.
Although alarm reactions and fear responses have not been described in zebrafish in their natural habitat, based on aquarium observations, the adaptive function of these behaviors has been hypothesized. Erratic movement is often observed on the bottom of the experimental tank. Given that zebrafish has been found in sediment rich slowly moving streams and ponds [10] in its geographic origin, erratic movement, if performed in these habitats, is expected to lead to the disturbance of the debris, in essence resulting in the creation of a cloud of “dust” under which zebrafish could hide from visually hunting predators. In addition, both jumping and erratic movement are expected to confuse the predator due to the high speed and unexpected swim direction changes associated with these motor patterns.
Irrespective of the actual function of these behaviors, the fact that they have been observed under fear inducing circumstances, e.g. in response to live or animated images of predators [1, 13] and in response to the natural alarm pheromone of zebrafish [34], suggests that they indeed represent alarm reactions that are likely correlate with the level of fear zebrafish experiences. If this argument is correct, the current results have an important practical implication. The chemically synthesized alarm substance, H3NO, can now be used to induce fear under controlled laboratory conditions. The amount of alarm substance, and thus the level of fear, is no longer a matter of guess work. The dose and the timing of delivery of the alarm substance can now be precisely determined, unlike in the case of a natural alarm substance extract, which is likely to vary in its concentration, chemical content, and thus potency. Briefly, induction of fear responses is now feasible under controlled laboratory conditions in zebrafish. This is important as appropriate behavioral paradigms are crucial for forward genetic screening of mutants, e.g. if one wants to identify a genetic alteration that affects fear responses. Similarly, pharmacological screens may also employ this fear paradigm allowing one to identify chemical substances, “small molecules” that influence (enhance or decrease) the fear responses. However, before the usefulness of H3NO induced fear reactions in forward genetic and pharmacological screens can be claimed, genetic and/or pharmacological validation may be necessary. Comparison of zebrafish strains with known differences in fear responses could be tested with H3NO. The effects of known anxiolytic and/or anxiogenic compounds could also be ascertained in this context.
It is also important to characterize the H3NO induced fear responses using automated behavior quantification methods, e.g. video-tracking [3]. Predator image induced fear reactions have been successfully quantified using such approaches [13]. In fact the video-tracking method allowed the authors of this latter study to reveal numerous differences in speed, turn angle, and location of zebrafish and in the intra-individual temporal variability of these measures in a manner that was not possible to observe with the naked eye. Briefly, the automated video-tracking method may detect behavioral characteristics that are more sensitive to H3NO treatment than the observed motor patterns we report here.
Last, we comment on the behaviors we found non-responsive to H3NO treatment. We have measured numerous motor responses and no significant changes were found in any of these behaviors, only erratic movement and jumping frequency increased significantly in response to the synthetic alarm substance. This is notable because it suggests that the effect of the alarm substance is specific to the above fear behaviors and is unlikely to be due to generalized toxicity leading to compromised health. The substance is also unlikely to irritate the skin or gill of the fish. Although irritancy has not been characterized at the behavioral level in zebrafish, fish that are exposed to external parasites or chemicals that irritate their skin are expected to perform typical behaviors including rubbing against the surface of a solid object in the tank (e.g. heater, air stone or the glass wall) and or shaking their body and closing and opening their fin with high frequency (Gerlai personal observation). None of these behaviors were observed in any of our experimental fish. It is also notable that some behavioral changes that were previously found under fear inducing circumstances in zebrafish were not seen in the current study. For example, increased immobility and bottom dwell time were both previously found in response to fear inducing stimuli. Levin et al. [26] describe “diving”, i.e. swimming to the bottom in zebrafish, which is seen when the fish are placed in a novel environment, a situation presumed to be fear inducing. Freezing was found to be induced by novelty and by the sympatric predator in another fish species, paradise fish (Macropodus opercularis) [9, 17], and reduced amount of activity was found in zebrafish in response to presentation of the animated image of a sympatric predator [13]. However, in response to the natural (skin extracted) zebrafish alarm substance neither freezing, nor bottom dwell time were found to change significantly [34]. In the current study, we also did not find significant alarm substance induced changes in these behaviors. Given that both the natural [34] and the synthetic alarm substances (current study) induced changes in species-typical fear responses such as erratic movement and jumping, it is unlikely that the lack of effects in other behaviors was due to too low a concentration of these substances. It appears that alarm substances do not enhance bottom dwell time or duration of immobility, at least under the conditions these responses have been studied so far. It is possible that different fear reactions may be elicited by different fear inducing stimuli. In the empty and featureless laboratory tank that offers no escape routes or hiding places, a diffuse fear stimulus such as the smell of the alarm substance, may evoke active, rather than passive, fear reactions and thus may bias the responses towards erratic movements and jumps as opposed to performing freezing on the bottom. The validity of this speculation, along with many other parameters of the current fear paradigm will have to be further investigated in order for the test to be optimized for potential screening applications.
Nevertheless, our pilot data demonstrate that H3NO, the synthetic alarm substance, is an effective agent that induces significant fear reactions in zebrafish, a result that now opens the avenue towards the establishment of behavioral tests with which fear inducing and fear reducing properties of mutations and/or drugs may be investigated in the future.
Acknowledgments
This study was supported by NIAAA/NIH (#1R01AA015325-01A2) grant to RG
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bass SL, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Blaser R, Gerlai R. Behavioral phenotyping in Zebrafish: Comparison of three behavioral quantification methods. Behav Res Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 4.Brown GE, Adrian JC, Jr, Nabil TN, Mark CH, Jocelyn MK. Nitrogen-oxides elicit antipredator responses in juvenile channel catfish, but not convict cichlids or rainbow trout: conservation of the Ostariophysan alarm pheromone. J Chem Ecol. 2003;29:1781–1796. doi: 10.1023/a:1024894026641. [DOI] [PubMed] [Google Scholar]
- 5.Brown GE, Adrian JC, Jr, Lewis MG, Tower JM. The Effects of Reduced pH on Chemical Alarm Signaling in Ostariophysan fishes. Canadian Journal of Fisheries and Aquatic Sciences. 2002;59:1331–1338. [Google Scholar]
- 6.Brown GE, Adrian JC, Jr, Shih ML. Behavioural Responses of Fathead Minnows (Pimephales promelas) to Hypoxanthine-3-N-oxide at Varying Concentrations. Journal of Fish Biology. 2001;58:1465–1470. [Google Scholar]
- 7.Brown GE, Adrian JC, Jr, Smyth E, Leet H, Brennan S. Ostariophysan alarm substances: Laboratory and field tests of the functional significance of nitrogen oxides. J Chem Ecol. 2000;26:139–154. [Google Scholar]
- 8.Choy Y, Fyer AJ, Goodwin RD. Specific phobia and comorbid depression: a closer look at the National Comorbidity Survey data. Comp Psychiatry. 2007;48:132–136. doi: 10.1016/j.comppsych.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Csányi V, Gerlai R. Open field behavior and the behavior genetic analysis of the paradise fish (Macropodus opercularis) J Comp Psychol. 1998;102:326–336. [Google Scholar]
- 10.Engeszer R, Patterson L, Rao A, Parichy D. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 11.von Frisch K. Uber einen schreckstoff der fischhaut und seine biologische Bedeutung. Z vergl Physiol. 1941;29:46–145. [Google Scholar]
- 12.Garner M, Baldwin D. How effective are current drug treatments for anxiety disorders, and how could they be improved? In: Blanchard RJ, Blanchard DC, Griebel G, Nutt D, editors. Handbook Behav Neurosci. 2008. pp. 395–411. [Google Scholar]
- 13.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: Towards the development of an automated aversive task. Behav Brain Res. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerlai R, Prajapati S, Ahmad F. Differences in acute alcohol induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlai R, Csányi V. Genotype environment interaction and the correlation structure of behavioral elements in paradise fish (Macropodus opercularis) Physiol Behav. 1990;47:343–356. doi: 10.1016/0031-9384(90)90153-u. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R. Zebra fish: An uncharted behavior genetic model. Behav Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 17.Gerlai R. Can paradise fish (Macropodus opercularis) recognize its natural predator? An ethological analysis. Ethology. 1993;94:127–136. [Google Scholar]
- 18.Giner-Sorolla A. Purine N-Oxides: XXXVII. Derivatives from 6-Chloropurine 3-Oxide. J Heterocycl Chem. 1971;8:651–655. [Google Scholar]
- 19.Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 20.Hall D, Suboski MD. Visual and olfactory stimuli in learned release of alarm reactions by zebra danio fish (Brachydanio rerio) Neurobiol Learn Mem. 1995;63:229–240. doi: 10.1006/nlme.1995.1027. [DOI] [PubMed] [Google Scholar]
- 21.Hendrie CA, Weiss SM, Eilam D. Exploration and predation models of anxiety: evidence from laboratory and wild species. Pharmacol Biochem Behav. 1996;54:13–20. doi: 10.1016/0091-3057(95)02176-0. [DOI] [PubMed] [Google Scholar]
- 22.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna F, MacDonald J, Carlen P, Abramow-Newerly W, Roder J. Enhanced LTP in the absence of GluR2. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 23.Kawashima H, Kumashiro I. Studies of Purine N-Oxides. III. The Synthesis of Purine 3-N-Oxides. Bull Chem Soc Jpn. 1969;42:750–755. [Google Scholar]
- 24.Kelly JM, Adrian JC, Jr, Brown GE. Can the ratio of aromatic skeletons explain cross-species responses within evolutionary conserved Ostariophysan alarm cues?: testing the purine-ratio hypothesis. Chemoecology. 2006;16:93–96. [Google Scholar]
- 25.Klein DF. Panic disorder and agoraphobia: hypothesis hothouse. J Clin Psychiatry. 1996;57 6:21–27. [PubMed] [Google Scholar]
- 26.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 27.McGregor IS, Schrama L, Ambermoon P, Dielenberg RA. Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats. Behav Brain Res. 2002;129:1–16. doi: 10.1016/s0166-4328(01)00324-2. [DOI] [PubMed] [Google Scholar]
- 28.Mikloski A, Andrew RJ. The zebrafish as a model for behavioural studies. Zebrafish. 2006;3:227–234. doi: 10.1089/zeb.2006.3.227. [DOI] [PubMed] [Google Scholar]
- 29.Miller N, Gerlai R. Quantification of Shoaling Behaviour in Zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer W. The distribution of fright reaction and alarm substance cells in fishes. Copeia. 1977;4:653–665. [Google Scholar]
- 31.Pfeiffer W, Riegelbauer G, Meier G, Scheibler B. Effect of hypoxanthine 3-N-oxide and hypoxanthine 1-N-oxide on central nervous excitation of the black tetra, Gymnocorymbus ternetzi (Characaidae, Ostariophysi, Pisces) indicated by dorsal light response. J Chem Ecol. 1985;11:507–523. doi: 10.1007/BF00989562. [DOI] [PubMed] [Google Scholar]
- 32.Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 34.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet. 2006;16:2156–2166. doi: 10.1016/S0140-6736(06)69865-6. [DOI] [PubMed] [Google Scholar]
- 36.Waldman B. Quantitative and developmental analysis of the alarm reaction in the zebra Danio, Brachydanio rerio. Copeia. 1982;1:1–9. [Google Scholar]