Abstract
Background
Immunologic monitoring of pediatric transplant (Tx) recipients, who are at increased risk of EBV-driven post-transplant lymphoproliferative disease (PTLD), is an important goal in clinical transplantation. Here we investigated the impact of EBV load on T cell immunity from pediatric Tx recipients, using clinically applicable tests for improved assessment of T cell immune competence.
Methods
Thirty-five asymptomatic pediatric thoracic Tx patients were categorized according to their EBV load into: undetectable viral load (UVL); chronic low viral load (LVL); and chronic high viral load (HVL). Global and EBV-specific T cell immunity were assessed by ATP release using Cylex® Immuknow® and T Cell Memory™ assays.
Results
UVL patients exhibited normal ATP release to ConA and PHA (190 ± 86 ng/ml, 328 ± 163 ng/ml), and detectable EBV-specific (37 ± 34 ng/ml) ATP responses. LVL patients displayed significantly stronger responses to ConA (373 ± 174 ng/ml), PHA (498 ± 196 ng/ml) and EBV (152 ± 179 ng/ml), as compared to UVL or to HVL patients (ConA 185 ± 114 ng/ml, PHA 318 ± 173 ng/ml, and EBV 33 ± 42 ng/ml). Moreover, HVL patients displayed significant inverse correlation between CD4+ T cell ATP levels and EBV loads.
Conclusions
Evaluation of global and EBV-specific T cell immunity provides a rapid assessment of patients' immune competence. It is still unclear whether selective over-suppressed ATP release by CD4+ T cells reflects HVL patients at risk of PTLD. Further longitudinal studies will determine the importance of Immuknow® test in identifying asymptomatic HVL patients vulnerable to EBV complications.
Keywords: solid organ transplantation, EBV, pediatric, T cell immunity, Cylex® Immuknow®
Introduction
Post-transplant lymphoproliferative disease (PTLD) is a life-threatening complication of solid organ transplantation (Tx), caused most commonly, by EBV infection and the use of chronic non-specific immunosuppression (1-3). Overall, the incidence of PTLD ranges between 1-20%, and a wide number of risk factors have been incriminated (1). Negative EBV serostatus and lack of EBV memory T lymphocytes at Tx, often seen with pediatric recipients, is the strongest risk factor for the development of EBV-driven complications post-Tx (1, 2). Symptomatic PTLD is usually accompanied by an elevated EBV load in peripheral blood and suppressed CD4+ and CD8+ T cell immunity. Routine long-term post-Tx EBV load monitoring by PCR at our center has identified three groups of asymptomatic pediatric thoracic Tx patients who carry different levels of EBV loads: undetectable viral load (UVL), chronic low viral load (LVL), or chronic high viral load (HVL) (4, 5). In some patients, high EBV loads may persist for months to years after primary post-Tx EBV infection, and approximately 45% of these chronic HVL carriers will progress to late onset PTLD, including diffuse large B cell, Hodgkin's and Burkitt's lymphomas (6). The relationship between chronic high EBV load, T cell immune-surveillance and the risk of progression towards PTLD is still poorly understood. To date, the clinically available PCR-based analysis that measures EBV loads in peripheral blood is the only test used to monitor EBV infection in Tx patients. The test proved highly sensitive, but has a poor predictive value for progression towards PTLD (7). Therefore implementation of additional tests with clinical applicability, such as measuring T cell immunity, to complement EBV load monitoring in peripheral blood, may help identify early asymptomatic HVL patients with suppressed immunity at risk of progression towards PTLD.
The FDA approved ImmuKnow® test quantifies the CD4+ T cell immune competence by measuring the concentration of ATP released following PHA stimulation (8-13). Previous reports have shown that Tx recipients who exhibit low ImmuKnow® ATP values are over-immunosuppressed, and at risk for the development of bacterial or viral infections such as EBV, CMV and BKV (8, 10, 11, 14). Moreover, patients with PTLD have consistently displayed suppressed ImmuKnow® ATP values. (10, 15). These patients required reduction of immunosuppression as first line of intervention for the recovery of the immune responses, and close monitoring to prevent allograft rejection (8, 10, 11). In addition to global immune competence, monitoring of Ag-specific T cell memory responses by ATP release using the T cell Memory™ test, may serve as additional valuable assay to guide post-Tx patient care. Monitoring of CMV immunity post-lung Tx with T cell Memory™ test is such an example (9).
In this study, by using the ImmuKnow® test, and the novel T cell Memory™ test, we assessed the global and the EBV-specific T cell immunity in asymptomatic pediatric thoracic Tx patients carrying different levels of EBV loads. We have further correlated the functional T cell responses to the levels of immunosuppression, patient age, and the EBV load measurements in peripheral blood for each patient. Our results suggest that implementation of ImmuKnow® and T cell Memory™ tests, in addition to the currently available EBV load monitoring by PCR, may help assess pediatric thoracic Tx patients' immune-activation status. In addition, ImmuKnow® assay may uniquely identify within the asymptomatic HVL carriers those patients with suppressed CD4+ T cell ATP levels that may be vulnerable to EBV complications. Future prospective longitudinal studies are required to confirm whether this important preliminary observation may endorse implementation of ImmuKnow® assay, in addition to EBV PCR, as a second clinical monitoring test to help guide therapeutic interventions in high risk pediatric Tx recipients.
Material and Methods
Patient population
Forty-three peripheral blood samples were obtained from 35 asymptomatic pediatric thoracic Tx recipients without signs of allograft rejection, EBV disease/PTLD, or other infectious complications. These comprised 28 heart recipients, 6 lung recipients and 1 heart-lung recipient. In addition, 2 pediatric thoracic Tx recipients with biopsy confirmed PTLD were studied at diagnosis, as positive controls. All patients were EBV positive at the time of analysis as assessed by serology, and by EBV PCR in peripheral blood. The samples were collected from April 2006 to July 2007 under IRB-approved protocols at Children's Hospital of Pittsburgh. All patients were tested once, while 8 patients had their ATP levels measured twice. PTLD patients were tested at the time of biopsy-proven diagnosis. Patients' demographics and immunosuppressive regimens are shown in Table 1. Ten patients (27%) had received induction therapy with polyclonal anti-T cell antibodies (Thymoglobulin® or ATGAM®). However, the ImmuKnow® and T cell Memory™ analyses were performed at least one year post-Tx, when the lymphocyte counts were back to normal values. Patients were divided into three groups according to their peripheral blood levels of EBV load as UVL (n = 12), LVL (n = 17), and HVL (n = 6) (see definition of EBV load below). Both PTLD patients were HVL at the time of assay.
Table 1. Patients' Demographics.
| UVL (n=12) | LVL (n=17) | HVL (n=6) | PTLD (n=2) | |
|---|---|---|---|---|
| Mean Age (years) (Range) |
12.9 (3.1-18.9) |
13.1 (5.6-18.3) |
7.27 (3.6-12.4) |
16.0 (10.4 -21.6) |
| Type of Transplant | 10 Heart 2 Lung | 14 Heart 2 Lung 1 Heart/Lung |
4 Heart 2 Lung | 2 Heart |
| Gender | 7M/ 5F | 7M/ 10F | 4M/ 2F | 2F |
| Induction Therapy | 8% | 23% | 67% | 50% |
| EBV status pre-Tx* | 58% seronegative | 39% seronegative | 100% seronegative | 100% seronegative |
| Mean Years post-TX | 5.1 | 6.9 | 4.6 | 5.2 |
| IS regimen** | 37.5% CNI | 22% CNI | 12.5% CNI | NA |
| 12.5% CNI/MMF | 43% CNI/MMF | 62.5% CNI/MMF | ||
| 50.0% CNI/MMF/Pred | 35% CNI/MMF/Pred | 25.0% CNI/MMF/Pred |
All patients were EBV sero-positive at time of analysis
IS= immunesuppressive regimen/ NA= non applicable
CNI=calcineurin inhibitor MMF= mycophenolate mofetil, in particular cases MMF was changed to azathioprine or rapamycin Pred= Prednisone
EBV serology by ELISA
Anti-EBV IgG and IgM titers against viral capsid (VCA) and anti-nuclear EBV (EBNA) antibodies were determined in serum/plasma by routine ELISA (INCSTAR Corporation, Stillwater, MN) in the Clinical Immunopathology, Central Laboratory Services Inc., University of Pittsburgh Medical Center. Patients were considered EBV seropositive if the antibodies titers were > 0.9 mg/ml.
EBV load by real time PCR
EBV load was determined as previously described (16). Briefly, DNA purified from 200μl whole blood was used as a template for TaqMan PCR amplification of the EBV DNA polymerase gene (BALF5) target sequence, in parallel to a pGEM-BALF6 plasmid standard control. Results were expressed as EBV copies/ml of blood. The assay's lower limit of detection is <100 EBV genomic copies/ml whole blood.
Definition of EBV load
Pediatric thoracic Tx patients were categorized into three groups according with their peripheral blood EBV load levels as follows: a) UVL carriers have no EBV load detected by PCR in more than 80% of determinations including the time of analysis; b) LVL carriers are carrying EBV loads ranging between 100-16,000 copies/ml blood, detected in more than 20% of measurements, including the time of analysis; and c) HVL carriers have EBV loads above 16,000 copies/ml blood, on at least 50% of determinations, and over a period of at least 6 months prior current immunologic analysis.
Cylex® ImmuKnow® and T Cell Memory™ (ATP release) assays
Global immune competence and EBV-specific immunity were measured by Cylex® Immuknow® and T Cell Memory™ assays according to manufacture's instructions. Briefly, whole blood was diluted with sample diluent, added to 96-well plates and incubated for 15-18h with PHA (Immuknow® plates), ConA or EBV-containing cell lysate (T cell Memory™ plates) in a 37°C, 5% CO2 incubator. In the following day, CD4+ (Immuknow®) or CD3+ (T Cell Memory™) T cells were positively selected by magnetic separation (Cylex Magnet tray 1050, Cylex Inc., Columbia, MD) using anti-human CD4 or CD3 monoclonal antibodies coated magnetic beads (Dynabeads, Dynal, Oslo, Norway). Cells were then washed to remove residual cells and lysed. The ATP release was measured using luciferin/luciferase and a luminometer (Berthold, Knoxville, TN or Tuner Biosystems, Sunnyvale, CA). The immune response was assessed by the concentration of ATP release (ng/ml) comparing stimulated to non-stimulated samples. A three fold increase over the background was considered a positive response.
Statistical analysis
Results were expressed as means ± standard deviation. Statistical analysis was performed using two-tail Student t test. P values ≤0.05 were considered statistically significant.
Results
Assessment of global T cell immunity from asymptomatic pediatric thoracic Tx patients carrying different levels of EBV load
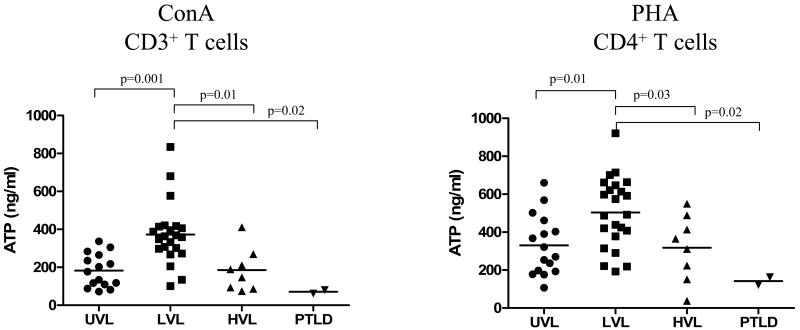
The global T cell immune competence was assessed by measuring the ATP release following ConA (CD3+) and PHA (CD4+) T cell stimulation of peripheral blood. As shown in Figure 1, UVL patients display normal ranges for ATP release by both ConA (190 ± 86 ng/ml) stimulation of CD3+ T cells and PHA (328 ± 163 ng/ml) stimulation of CD4+ T cells, as previously reported for healthy children and pediatric solid organ recipients (17). In contrast, LVL patients exhibited significantly elevated levels of ATP release following both ConA (373 ± 174 ng/ml p = 0.001) and PHA (498 ± 196 ng/ml p = 0.01) stimulation of CD3+ and CD4+ T cells, respectively, suggestive of global immune-activation. Interestingly, HVL carriers, although under chronic high EBV-Ag challenge, showed significantly lower levels of ATP release in response to ConA (185 ± 114 ng/ml p = 0.01) and PHA (318 ± 173 ng/ml p = 0.03) as compared to LVL patients, suggestive of a decline in their immunecompetence. However, most of these levels were still in the normal range of ATP release (17). Conversely, at diagnosis, PTLD patients exhibited very low levels of ATP upon ConA (71 ± 13 ng/ml) and PHA (120 ± 43 ng/ml) stimulation, confirming previous published data (10, 15), and suggestive of their global immunosuppression (Figure 1).
Figure 1. Profiles of global T cell immunity in different cohorts of pediatric thoracic Tx patients.
T Cell Memory™ and Cylex® Immuknow® assays were used to measure global immunity in peripheral blood of UVL, LVL, HVL and acute PTLD patients. ATP responses were measured after 18 hrs stimulation of CD3+ T cells or CD4+ T cells with ConA and PHA. Each dot represents the ATP (ng/ml) release of one patient's determination, and horizontal lines are of mean ATP values for each group.
To have a better understanding of global immunity distribution in each category of patients we stratified the mitogen responses into categories based on cut-off levels as (i) low ≤ 200 ng/ml, (ii) intermediate 200-400 ng/ml (200-500 ng/ml for PHA), and (iii) strong ≥ 400ng/ml (≥ 500 ng/ml for PHA) (Table 2). The LVL group had > 90% of the ATP determinations in the intermediate and strong ranges, while the other two groups exhibited >80% of responses in the low and intermediate ranges (Table 2).
Table 2. Distribution of ATP zones in response to mitogen stimulation.
| ConA (CD3) |
UVL (n=14) |
LVL (n=19) |
HVL (n=8) |
|---|---|---|---|
| Low ≤ 200 ng/ml | 50% | 10% | 62.5% |
| Intermediate 200-400 ng/ml | 50% | 58% | 25% |
| Strong ≥ 400 ng/ml | 0% | 32% | 12.5% |
|
PHA (CD4) |
UVL (n=15) |
LVL (n=20) |
HVL (n=8) |
| Low ≤ 200 ng/ml | 33% | 5% | 25% |
| Intermediate 200-500 ng/ml | 34% | 60% | 62.5% |
| Strong ≥ 500 ng/ml | 33% | 35% | 12.5% |
Furthermore, we have evaluated whether the level of immunosuppression or the age of the patient had any impact on the difference seen with T cell responses. The level of ATP release in individual patients did not correlate with the tacrolimus trough level at the time of the assay (ConA r = 0.298, p = 0.11; PHA r = 0.246, p = 0.18). Results also showed no significant difference in the mean levels of ATP obtained from patients under or over 12 years of age (ConA 271 ng/ml vs. 277 ng/ml and PHA 376 ng/ml vs. 439 ng/ml, respectively), and this was in contrast to what was described by Hooper et al (17).
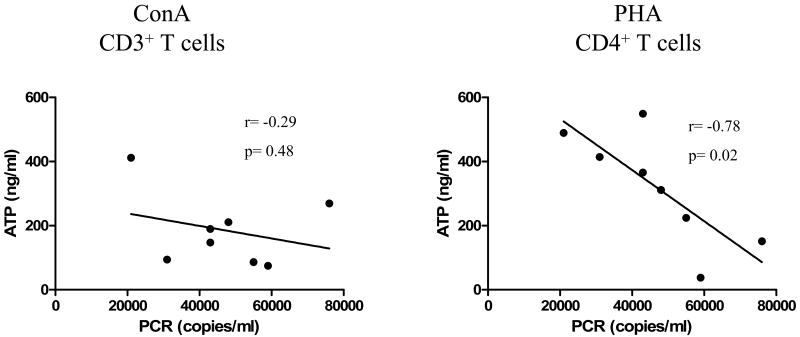
Next, we evaluated the relationship between global immunecompetence and EBV load of each group of pediatric Tx patients. HVL patients exhibited a significant inverse correlation between ImmuKnow® ATP values by CD4+ T cells and EBV loads (r = -0.78, p = 0.02) (Figure 2). For ConA stimulation (CD3+ T cells) however, although the trend was similar to PHA stimulation, no significant inverse correlation was achieved between these two variables (r = -0.29; p = 0.48) (Figure 2). In addition, no significant correlation between ConA or PHA-triggered ATP release and EBV loads were observed for UVL or LVL patients (data not shown).
Figure 2. Correlation of ATP release in response to ConA or PHA stimulation and EBV load in HVL patients.
ATP (ng/ml) released by peripheral blood CD3+ or CD4+ T cells in response to ConA or PHA stimulation, respectively, from HVL patients were compared to peripheral blood EBV load levels (genomic copies/ml) measured by PCR on the day of analysis. Each dot represents the measurement of each individual patient.
Evaluation of EBV-specific T cell responses in asymptomatic pediatric thoracic Tx patients carrying different levels of EBV load
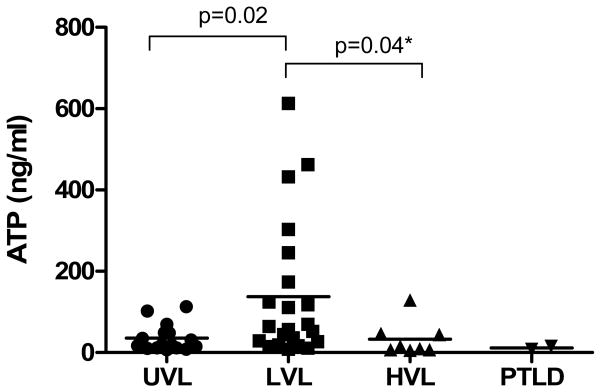
The pattern of T cell responses to EBV stimulation among the three groups of patients was similar to that of mitogen stimulation. Specifically, results shown in Figure 3 demonstrate that LVL patients showed significant higher levels of ATP release in response to EBV stimulation as compared to the UVL patients (152 ± 178 ng/ml vs. 37 ± 34 ng/ml p = 0.02), suggesting their EBV-specific memory T cell activated state. In contrast, although patients with HVL presented on average at least two orders of magnitude higher EBV loads in peripheral blood than LVL patients, they exhibited significant lower ATP levels in response to EBV stimulation (33 ± 42 ng/ml vs. 152 ± 178 ng/ml, p = 0.04). These results are suggestive of EBV-specific partial T cell exhaustion, possible due to the chronic high EBV-Ag challenge.
Figure 3. EBV-specific immunity in different cohorts of pediatric thoracic Tx patients.
T Cell Memory™ assay was used to measure EBV immunity in peripheral blood of UVL, LVL, HVL and PTLD patients. ATP responses were measured after 18 hrs stimulation of CD3+ T cells with EBV+-cell lysate. Each dot represents one individual determination, and the horizontal lines are of mean ATP values for each group. * indicates one-tail Student t test analysis.
Both patients with PTLD presented very low levels of ATP release in response to EBV (12 ± 5 ng/ml) as compared to all other groups (Figure 3) suggesting their profound EBV-specific immune deficiency. In addition, there was no significant correlation between ATP release in response to EBV-specific stimulation and EBV loads in any of the Tx patients studied (data not shown).
Discussion
Serial EBV load monitoring by PCR of pediatric heart Tx patients has identified a cohort of asymptomatic recipients with chronic HVL (≥16.000 copies/ml), that are at significant high risk of developing aggressive late-onset PTLD (1, 6). While clinical monitoring of EBV load by PCR has helped identify asymptomatic HVL patients, the assay alone did not prove to have a strong positive predictive value of patients' progression towards PTLD (6, 7, 18-20). In an effort to increase its positive predictive value, complementary assays such as cytokine gene polymorphism, tetramer staining or IFNγ ELISPOT have been implemented by different groups (7, 19-21). However, these tests are expensive, laborious, requiring large amounts of blood, and therefore not optimal for clinical monitoring of patients (14, 19, 21).
The Cylex® Immuknow® assay has recently become a FDA approved assay for assessment of CD4+ T cell-mediated immunity and management of Tx recipients at risk of opportunistic infections (11). We, and others, have shown that Tx patients who develop infectious complications exhibit much suppressed global immune reactivity (8, 10, 11, 14, 15). In addition, Tx recipients with PTLD display profoundly suppressed ATP levels of ImmuKnow® (10, 15). In the current study, we characterized immune reactivity of pediatric thoracic Tx recipients exposed to different levels of EBV viremia by analyzing, in addition to CD4+ T cell reactivity (by ImmuKnow® kit), the CD3+ T cell global and EBV-specific immunity (using the T Cell Memory™ kit). To our knowledge, this is the first study to assess the global- and EBV-specific T cell immunity in quiescent pediatric thoracic Tx patients carrying different levels of EBV load.
In the case of EBV infection, CD8+ T cells play the pivotal role in controlling both lytic and latent EBV life cycles, while CD4+ T cells are essential for providing “help” to CD8+ T cells during T cell priming and memory maintenance (22-24). Therefore, monitoring immune responses to EBV should encompass both CD8+ and CD4+ T cell immunity. In addition to ImmuKnow® test, the novel T Cell Memory™ assay allows for assessing global CD3+ T cell responses (CD4+ and CD8+). T Cell Memory™ assay kit also allows screening of EBV-specific immunity by stimulation of CD3+ T cells with EBV lysates obtained from EBV-infected cell lines. We found comparable patterns of immune reactivity in response to PHA and ConA stimulation within each category of Tx patients (Figure 1). In addition, our results suggested that, unlike reported previously, global immune reactivity was not influenced by patients' age (17), but rather by patients' levels of EBV load (Figure 1). Accordingly, the distribution of ATP values in each patient cohort showed normal, low or elevated ranges that matched well with the levels of circulating chronic EBV antigenic load (Table 2). Interestingly, UVL recipients had normal distribution of PHA ATP responses in the low to intermediate immune response zones, as previously established by Hooper et al for pediatric transplant recipients age <12 yr (Table 2) (17). In contrast, LVL group exhibited strong ATP responses to PHA that were situated in the intermediate and high immune response zones, similar to those of non-transplant normal adults (11, 17). Recently, Ben-Youssef et al have also reported unexpected high ImmuKnow® ATP levels in pediatric kidney Tx recipients with detectable EBV viremia (25). According to our results, the ATP values were not influenced by tacrolimus trough levels, and this is in good agreement with previous reports by Sampson et al (26). They concluded that monitoring patients only for immunosuppressive drug levels fail to predict T-cell reactivity in pediatric liver or kidney Tx recipients (26).
Most of HVL recipients displayed mitogen ATP responses similar to UVL patients, but significantly lower than those of LVL recipients (Figure 1). A significant inverse correlation between global ATP release by CD4+ T cells and EBV load for HVL patients was also established, suggesting that selected patients with dangerously high EBV loads that display levels of ATP below the normal expected “threshold” may be over immunosuppressed, and at high risk of EBV complications (Figure 2). Similar to our findings, Lee et al reported the increased probability of high viral loads to occur in pediatric liver Tx patients with mitogen ATP levels less than 125 ng/ml (15). Although Lee's study was performed on a small cohort of pediatric liver Tx patients, they suggested that the Cylex® assay may be used to guide immunosuppressive management in high risk pediatric liver Tx patients (15).
The ATP release in response to EBV-specific stimulation, reflective of EBV memory T cell responses, complemented the assessment of global CD4+ and CD3+ T cell immune competence observed in UVL, LVL and HVL patients (Figure 3). A recent report has shown the use of T Cell Memory™ assay to identify CMV-specific memory T cells in adult lung Tx recipients (9). The report emphasized the importance of immunologic CMV monitoring by Cylex® technology, in addition to viral monitoring, for successful clinical management and improved outcomes. However, our preliminary results show that only the Immuknow® CD4+ T cell ATP values inversely correlated with EBV loads in HVL patients, suggesting that measuring CD4+ T cell help is most sensitive for detecting EBV immunosuppression. To demonstrate this, and to endorse combined immunologic and virologic clinical monitoring of HVL patients, future prospective studies with follow-up testing are required.
In conclusion, this study shows that Cylex® Immuknow® and T Cell Memory™ assays, in addition to EBV load monitoring by PCR, help define the immune status of pediatric thoracic Tx patients. Furthermore, those HVL pediatric thoracic Tx recipients who display suppressed CD4+ T cell immunity to PHA (ATP< 200ng/ml) while carrying dangerous high levels of EBV in peripheral blood, may be at increased risk of developing PTLD, and therefore closely monitored.
Acknowledgments
This work was supported by a SCCOR grant from National Institutes of Health (5P50HL074732).
Abbreviations
- HVL
high viral load
- LVL
low viral load
- PTLD
post-transplant lymphoproliferative disease
- Tx
transplant
- UVL
undetectable viral load
Footnotes
Camila Macedo: designed and performed experiments, collected and analyzed data, and wrote the manuscript. No conflict of interest. 200 Lothrop St. E1513, Pittsburgh PA 15213
Adriana Zeevi: designed research, contributed to scientific writing. No conflict of interest. 200 Lothrop St. W1551, Pittsburgh PA 15213
Carol Bentlejewski: performed experiments. No conflict of interest. 200 Lothrop St. E1511, Pittsburgh PA 15213
Iulia Popescu: performed experiments. No conflict of interest. 200 Lothrop St. E1513, Pittsburgh PA 15213
Michael Green: performed clinical assessment of patients. No conflict of interest. 3520 Fifth Avenue, Pittsburgh PA 15213
David Rowe: performed measurement of EBV loads. No conflict of interest. 130 DeSoto St., Pittsburgh PA 15261
Louise Smith: recorded clinical data, and coordinated collection of blood samples. No conflict of interest. 3705 Fifth Avenue, Pittsburgh PA 15213
Steven Webber: provided access to patients, performed clinical assessment, and contributed to scientific writing. No conflict of interest. 3705 Fifth Avenue, Pittsburgh PA 15213
Diana Metes: designed research, analyzed data, and contributed to scientific writing. No conflict of interest. 200 Lothrop St. E1549, Pittsburgh PA 15213
References
- 1.Webber SA, Naftel DC, Fricker FJ, et al. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367(9506):233. doi: 10.1016/S0140-6736(06)67933-6. [DOI] [PubMed] [Google Scholar]
- 2.Russo LM, Webber SA. Pediatric heart transplantation: immunosuppression and its complications. Curr Opin Cardiol. 2004;19(2):104. doi: 10.1097/00001573-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Webber SA, McCurry K, Zeevi A. Heart and lung transplantation in children. Lancet. 2006;368(9529):53. doi: 10.1016/S0140-6736(06)68969-1. [DOI] [PubMed] [Google Scholar]
- 4.Green M, Webber SA. Persistent increased Epstein-Barr virus loads after solid organ transplantation: truth and consequences? Liver Transpl. 2007;13(3):321. doi: 10.1002/lt.21015. [DOI] [PubMed] [Google Scholar]
- 5.Qu L, Green M, Webber S, Reyes J, Ellis D, Rowe D. Epstein-Barr virus gene expression in the peripheral blood of transplant recipients with persistent circulating virus loads. J Infect Dis. 2000;182(4):1013. doi: 10.1086/315828. [DOI] [PubMed] [Google Scholar]
- 6.Bingler MA, Feingold B, Miller SA, et al. Chronic high Epstein-Barr viral load state and risk for late-onset posttransplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8(2):442. doi: 10.1111/j.1600-6143.2007.02080.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsai DE, Douglas L, Andreadis C, et al. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8(5):1016. doi: 10.1111/j.1600-6143.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 8.Batal I, Zeevi A, Heider A, et al. Measurements of global cell-mediated immunity in renal transplant recipients with BK virus reactivation. Am J Clin Pathol. 2008;129(4):587. doi: 10.1309/23YGPB1E758ECCFP. [DOI] [PubMed] [Google Scholar]
- 9.Zeevi A, Husain S, Spichty KJ, et al. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7(2):471. doi: 10.1111/j.1600-6143.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 10.Thai NL, Blisard D, Tom K, et al. Pancreas transplantation under alemtuzumab (Campath-1H) and tacrolimus: Correlation between low T-cell responses and infection. Transplantation. 2006;82(12):1649. doi: 10.1097/01.tp.0000250655.14026.5c. [DOI] [PubMed] [Google Scholar]
- 11.Kowalski RJ, Post DR, Mannon RB, et al. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82(5):663. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 12.Zeevi A, Britz JA, Bentlejewski CA, et al. Monitoring immune function during tacrolimus tapering in small bowel transplant recipients. Transpl Immunol. 2005;15(1):17. doi: 10.1016/j.trim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski R, Post D, Schneider MC, et al. Immune cell function testing: an adjunct to therapeutic drug monitoring in transplant patient management. Clin Transplant. 2003;17(2):77. doi: 10.1034/j.1399-0012.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhorade SM, Janata K, Vigneswaran WT, Alex CG, Garrity ER. Cylex ImmuKnow assay levels are lower in lung transplant recipients with infection. J Heart Lung Transplant. 2008;27(9):990. doi: 10.1016/j.healun.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Lee TC, Goss JA, Rooney CM, et al. Quantification of a low cellular immune response to aid in identification of pediatric liver transplant recipients at high-risk for EBV infection. Clin Transplant. 2006;20(6):689. doi: 10.1111/j.1399-0012.2006.00537.x. [DOI] [PubMed] [Google Scholar]
- 16.Wadowsky RM, Laus S, Green M, Webber SA, Rowe D. Measurement of Epstein-Barr virus DNA loads in whole blood and plasma by TaqMan PCR and in peripheral blood lymphocytes by competitive PCR. J Clin Microbiol. 2003;41(11):5245. doi: 10.1128/JCM.41.11.5245-5249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooper E, Hawkins DM, Kowalski RJ, et al. Establishing pediatric immune response zones using the Cylex ImmuKnow assay. Clin Transplant. 2005;19(6):834. doi: 10.1111/j.1399-0012.2005.00429.x. [DOI] [PubMed] [Google Scholar]
- 18.Allen UD, Farkas G, Hebert D, et al. Risk factors for post-transplant lymphoproliferative disorder in pediatric patients: a case-control study. Pediatr Transplant. 2005;9(4):450. doi: 10.1111/j.1399-3046.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee TC, Savoldo B, Barshes NR, et al. Use of cytokine polymorphisms and Epstein-Barr virus viral load to predict development of post-transplant lymphoproliferative disorder in paediatric liver transplant recipients. Clin Transplant. 2006;20(3):389. doi: 10.1111/j.1399-0012.2006.00498.x. [DOI] [PubMed] [Google Scholar]
- 20.Toyoda M, Moudgil A, Warady BA, Puliyanda DP, Jordan SC. Clinical significance of peripheral blood Epstein-Barr viral load monitoring using polymerase chain reaction in renal transplant recipients. Pediatr Transplant. 2008;12(7):778. doi: 10.1111/j.1399-3046.2008.00904.x. [DOI] [PubMed] [Google Scholar]
- 21.Smets F, Latinne D, Bazin H, et al. Ratio between Epstein-Barr viral load and anti-Epstein-Barr virus specific T-cell response as a predictive marker of posttransplant lymphoproliferative disease. Transplantation. 2002;73(10):1603. doi: 10.1097/00007890-200205270-00014. [DOI] [PubMed] [Google Scholar]
- 22.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 23.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 24.Thorley-Lawson DA. EBV the prototypical human tumor virus--just how bad is it? J Allergy Clin Immunol. 2005;116(2):251. doi: 10.1016/j.jaci.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 25.Ben-Youssef R, Baron PW, Sahney S, et al. The impact of intercurrent EBV infection on ATP levels in CD4+ T cells of pediatric kidney transplant recipients. Pediatr Transplant. 2008 doi: 10.1111/j.1399-3046.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 26.Sampson VB, Dunn SP, Rymeski B, et al. Failure of immunosuppressive drug levels to predict T-cell reactivity in pediatric transplant patients. J Pediatr Surg. 2008;43(6):1134. doi: 10.1016/j.jpedsurg.2008.02.044. [DOI] [PubMed] [Google Scholar]