Abstract
Background
To fully utilize a schizophrenia endophenotype in gene search and subsequent neurobiological studies, it’s critical that the precise underlying physiological deficit is identified. Abnormality in smooth pursuit eye movements is one of the endophenotypes of schizophrenia. The precise nature of the abnormality is unknown. Previous work has shown a reduced predictive pursuit response to a briefly masked (i.e. invisible) moving object in schizophrenia. However, the overt awareness of target removal can confound the measurement.
Methods
The current study employed a novel method that covertly stabilized the moving target image onto the fovea. The foveal stabilization was implemented after the target on a monitor had oscillated at least for one cycle, and near the change of direction when the eye velocity momentarily reached zero. Thus, the subsequent pursuit eye movements were completely predictive and internally driven. Eye velocity during this foveally stabilized smooth pursuit was compared among schizophrenia patients (n=45), their unaffected 1st degree relatives (n=42), and healthy comparison subjects (n=22).
Results
Schizophrenia patients and their unaffected relatives performed similarly and both had substantially reduced predictive pursuit acceleration and velocity under the foveally stabilized condition.
Conclusions
These findings show that inability to maintain internal representation of the target motion, or integration of such information into a predictive response, may be the specific brain deficit indexed by the smooth pursuit endophenotype in schizophrenia. Similar performance between patients and unaffected relatives suggested that the refined predictive pursuit measure may index a less complex genetic origin of the eyetracking deficits in schizophrenia families.
Introduction
Identifying disease-related genetic effects is important in schizophrenia in order to define the etio-pathophysiology of the disorder leading to development of novel treatments. This endeavor has proven more difficult than initially appreciated due to the complex nature of the disorder. The clinical diagnosis of schizophrenia is now thought to encompass a varying collection of stable specific physiological deficits that are heritable and likely reflect the effects of a small subset of genes, perhaps interacting with the environmental insults. These specific physiological deficits, termed endophenotypes, are measured externally using tools such as cognitive, neurophysiological, or imaging studies. The preciseness to which an external laboratory measure capture/s the specific physiological deficit related to the schizophrenia liability determines the utility of that measure in genetic and etio-physiological studies of schizophrenia (1).
Abnormal smooth pursuit eye movement is a consistently observed neurophysiological deficit in schizophrenia patients and their relatives, and is a recognized schizophrenia endophenotype. However, the traditional laboratory measures of smooth pursuit eye movement examine a widely distributed neurophysiological function. Recent research efforts have been focused on identifying the underlying neural mechanism (2–6). Smooth pursuit eye movements are initiated by the motion of an object’s image across the retina, which creates velocity errors between the image and the fovea. This velocity error is a powerful stimulus for generating smooth pursuit, which functions to minimize retinal error by keeping the moving image on the fovea (7). Paradoxically, accurate pursuit reduces the velocity error to near zero, making it not possible to rely on retinal error to accurately maintain pursuit. Instead, smooth pursuit eye movements are carried out based on the prediction of target motion with minor corrections based on retinal errors. Therefore, motion information for predictive pursuit is not derived from the current retinal events but from internal sources and thus termed extraretinal. This extraretinal component is represented by neuronal firings independent of immediate retinal error (8).
The contribution of extraretinal motion can be estimated when the retinal error is removed by briefly masking the target or by stabilization of the moving image onto the fovea to artificially set the retinal error to zero (8–10). Previous work based on target masking has shown reduced predictive pursuit in schizophrenia patients, suggesting a deficit in processing extraretinal motion (2;11). However, target-masking is not ideal since the response is partly dependent on the expectation of the continuation of the target trajectory. Loss of target during masking is also a signal for stopping pursuit making it difficult to evaluate predictive pursuit longer than few hundred milliseconds. The foveal stabilization procedure keeps moving image on the fovea no matter what the eye does. It removes retinal error thereby isolating the brain’s internal pursuit mechanism. This procedure maintains visual feedback as the target is visible, minimizing potential confounds caused by target disappearance and provides a means to definitively verify predictive pursuit abnormality in schizophrenia.
Methods and Materials
Subjects
Forty-five schizophrenia patient probands (proband is defined as the first patient from a family that entered the study), 42 first-degree relatives without schizophrenia, 7 first-degree relatives with schizophrenia, and 22 unrelated normal controls were included. Inclusion criteria limited ages between 16–58 years due to age effect on eye movements outside of this range (12–14). Medical conditions likely to affect eye movements were excluded, including neurological conditions, substance dependence within the past 6 months or current substance abuse. All patients were recruited through our outpatient programs. Patients were individuals with DSM-IV schizophrenia based on The Structured Clinical Interview for DSM-IV (SCID-IV). Three patients were on first-generation antipsychotic medications, 47 on second generation antipsychotic medications, and 2 on no antipsychotic medications. Clinical symptoms were assessed using the Brief Psychiatric Rating Scale (BPRS). Ten schizophrenia patients were retested after one month to examine the reliability of the new measures. The enrolled relatives were from 25 probands’ families. None of the families had participated in previous eyetracking studies (2;5).
Using public databases, we conducted random telephone contact to recruit a control by matching the age (± 3 years), gender, ethnicity, and zip code of a patient. Controls had no DSM-IV Axis I psychotic disorders, no Cluster A personality diagnoses and no family history of psychotic illness based on Family History Research Diagnostic Criteria considering three generations. After complete description of the study to the subjects, written informed consent was obtained.
Experimental procedures
Testing was conducted in a room with controlled illuminance of 2 lux. Stimuli were displayed on a 22′ flat screen monitor (Compaq P1220) set to 120 Hz, placed 60 cm in front of the subject. Eye data were collected using an EyeLink II eyetracker (SR Research Ltd., Canada) sampling at 500 Hz. Head movements were constrained using chin rest and forehead abutments. The target (a cross in a 0.25° × 0.25° box) had a photometric contrast at 2.1 log units. Calibration used a target at −12°, 0°, and +12° of visual angles until the error between target and eyes was less than 0.1°. A trial started with calibration steps (±12°), followed by 1–3 seconds of center fixation. The target started from the center position using a unpredictable initial target step in the opposite direction of subsequent target motion, which crossed the center point after 133 ms of motion (15), then traversed horizontally at a predictable constant speed of 10.0°/s or 18.7°/s at 24 ° of visual angle (a ramp). After 1–3 ramps, a virtual “zero velocity window” for foveal stabilization opened.
While following a target, the eye would stop moving momentarily, i.e., has zero velocity, each time it changed direction. This was when the software covertly switched the driver of the target from the computer to the eye when certain conditions were met. Since the eye did not always stop precisely at the turn of the target, a set of rules was applied for the foveal stabilization to be initiated. This switch occurred only if 1) the eye speed was near zero (−0.8°/s to +0.8°/s); and 2) the target was within − 50ms to + 200ms of the change in direction (see Figure 1 for experimental setup and Figure 2 for actual target and eye movements). These criteria constrained the eye as close to zero velocity and to the target turning point as possible when the stabilization could be triggered. If these criteria were not met then the foveal stabilization would not occur and the target would continue onto the next ramp. Up to 3 windows in 3 consecutive turning points were presented within a trial. Using these rules, some trials were automatically excluded, i.e., no foveal stabilization was triggered in these trials (see Results). If conditions were met, the target image was stabilized, i.e., the eye was now driving the target, for 1 second.
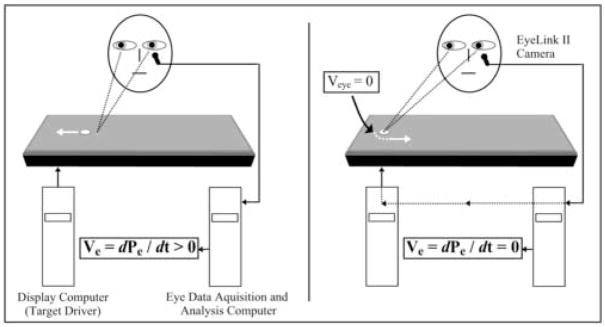
Figure 1.
Foveally stabilized smooth pursuit. The left panel shows the regular pursuit condition, where the target is driven by programs under the control of the display computer; while the eye position is recorded and eye velocity continuously calculated by the acquisition computer. Typically the eyes slightly lag behind the target, creating a positional error (Pe). Here the velocity error (Ve), or the difference in velocities between the target and the eyes, is larger than zero. The right panel shows the foveally stabilized pursuit. When eye velocity reaches about zero at the turn (arrow), the driver of the target is handed over to the acquisition computer. The target position is continuously placed on the eye (fovea) position with minimal delay, resulting in Pe ≈ 0 and thus Ve ≈ 0. At the end of the foveal stabilization window, the display computer again drives the target. Figure 2 demonstrates the outputs of these processes.
Figure 2.
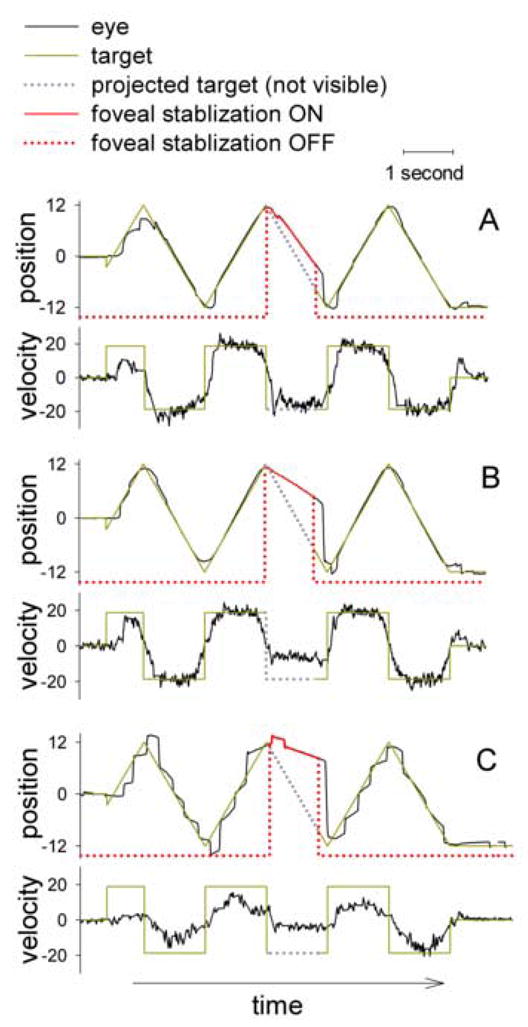
Examples of foveal stabilization trials representing a wide range of predictive pursuit performance.
For illustration, trials selected were identical in starting direction, target velocity (18.7°/s), and the ramp on which foveal stabilization was triggered. These conditions were randomized in the experiment. In trial A, the healthy control subject tracked the target with a gain of nearly 1. All conditions for foveal stabilization were met 10 milliseconds after the turn of the ramp. The eye then maintained a high predictive gain of 0.9. Trials B and C were from 2 schizophrenia patients. In B, the subject also tracked the target with a gain of nearly 1; while the eye maintained a predictive gain of about 0.4. In C, the maintenance pursuit gain was about 0.6 and the pursuit trajectory was marked by frequent catch-up saccades. The condition for foveal stabilization was met 60 milliseconds after the turn of the ramp, and the eye maintained a predictive pursuit gain of about 0.2. Note the marked reduction in saccade frequency during foveal stabilization. This is likely due to the elimination of positional error information. In the graph saccades were removed from velocity traces and replaced by a straight line. Also note that even at low gain velocity during stabilized pursuit was relatively constant.
Three examples are shown in Figure 2. No subjects had reported any awareness of notable event in the target behavior when the stabilization was switched on. One exception may be when eye blinks occurred during stabilization. In such instances the EyeLink II camera temporarily lost pupil image. Even though no subject had reported this, the subject might perceive a target jitter when the system re-acquired the pupil at the end of the blinks, despite the fact that the subject was not aware of the underlying process and would continue pursuit. Such blinks were infrequent (mean ± SD: 0.08±0.11, 0.05±0.09, 0.06±0.11 per second of pursuit for patients, relatives, and controls, respectively; p>0.05). For this reason we gave subjects simple instructions that their task was to follow the target; occasionally there might be shakiness of the target but they should just keep following the target. Target speed and initial target motion direction were randomized between trials. Each session included 12 trials, half at 10.0°/s and half at 18.7°/s of target speed arranged in pseudorandom order. Each subject was tested for two 4-minute sessions. All subjects were naïve to the task.
The system digitally stabilized the target to the fovea within 7 ms of delay (3 ms system delay plus 4 ms for the calculation of eye velocity using 3 consecutive data point). The refreshing rate of the monitor was 120 Hz, or 8.3ms per frame, which introduced an obligated delay of 0 to 8.3ms. The now eye-driven target followed the eye with a < 3 ms delay. At 18.7°/s eye speed, 3 to 11.3 ms causes a delay of 5.6′ to 20′ arc of visual angle, well within the ~60′ arc diameter of the fovea, ensuring that the target is maintained within the fovea. This digitally executed method is comparable to mechanical/optical foveal stabilization methods (8;16;17), but has additional benefit of digital control, which allows control to be switched on and off at will. The eye data filtered at 20Hz lowpass filter were used for pursuit measures. Data filtered at 75Hz were used in saccade analyses. The scoring algorithm identified a saccade based on velocity (>35°/sec) and acceleration (>600°/sec2) criteria. All saccades and artifacts were identified as missing data points.
Once the eye reached zero velocity and the target was stabilized on the fovea, the eye accelerated without retinal error information (Figure 3). This was termed predictive acceleration, measured as the mean acceleration in the first 100ms of the stabilized pursuit. We also calculated the predictive pursuit gain averaged over the 1-second stabilization period. Pursuit gain is the averaged artifact-free (i.e., saccades and blinks removed) eye velocity divided by target velocity. Eye velocity at the adjacent ramp with regular pursuit target was used to calculate maintenance pursuit gain. To further describe the characteristics of smooth pursuit under the stabilization condition, the predictive pursuit eye velocities were averaged by 50 ms epochs starting from 50 ms before to 1000 ms after the onset of stabilization. For comparisons, maintenance pursuit gain from the adjacent same-direction ramp was also analyzed in 50 ms epochs starting from 50 ms before to 1000ms after the ramp turning point.
Figure 3.
Group averages of all smooth pursuit trials during foveal stabilization vs. unstabilized (regular) visual target, using best-fit polynomial regressions on the 50 ms pursuit epochs. Note that y-axes are not on the same scale but are scaled to the ranges of the data. Left panels show smooth pursuit behaviors during the 1-second foveal stabilization period, including a 50ms epoch prior to stabilization. For comparisons, the right panels show averages of unstabilized smooth pursuit for 1-second, taken from the same-direction ramp prior to the ramp of stabilization and starting 50ms prior to until 1-second after the turn of the ramp.
Analysis
The dependent measures were compared using a mixed model ANOVA (PROC MIXED in SAS, SAS, Inc), applying Kenward-Roger approximation to calculate the appropriate denominator degrees of freedom (18;19). The PROC MIXED model for unbalanced repeated measures ANOVA was done by given a family ID to each subject regardless whether there was more than one subjects recruited from a family, which toke account of within-family correlations among those families with multiple members in the study, without requiring that all families had more than one member. We included the relatives group in part to examine pursuit deficits that are not affected by psychotic symptoms or medications. For this reason the 7 relatives with schizophrenia were excluded from the relatives group during group comparisons. Velocity and ramp direction were within-subjects factors; group was the between-subjects factor. For the analysis of 50ms epochs, epoch was included as a within-subject factor. Age was included as a covariate. For significant effect or interaction, post hoc testing used the least squares estimated means for pair-wise comparisons and Tukey-Kramer method for adjustment of the t-test statistics. The 50 ms epoch data were subjected to principal component analysis (PCA) to extract latent components during foveally stabilized pursuit. PCA was performed by including all subjects. Principal components were defined by the eigenvectors with eigenvalues > 1 using a variance-maximizing (Varimax) rotation. Finally, the reliabilities of dependent measures over time were calculated by the intraclass correlation coefficient (ICC) and the 95% confidence interval (CI).
Results
There were no statistical differences in gender (female/male: 17/28, 25/17, and 10/12 in patients, relatives, and controls, respectively; χ2 =4.2, p=0.12), ethnicity (Caucacian:African American:others: 24:20:1, 18:21:3, and 15:5:2, respectively; χ2 =6.0, p=0.20), and years of education (mean ± SD: 11.5 ± 2.0, 15.3 ± 13.6, and 14.5 ± 1.9, respectively, F(2, 108) = 2.23, p = 0.11). Differences in age were marginally nonsignificant (38.7 ±12.6, 43.0 ± 11.0, and 35.8 ± 13.3, respectively; F(2, 108) = 2.83, p=0.06).
There was no significant effect of target direction on dependent measures; therefore data were collapsed across directions. Stabilized pursuit was not triggered in 2.4% of the trials, while in 88.2% of the trials stabilization was triggered in the first, 8.4% in the second, and 0.9% in the third “zero velocity window”. The percentages were nearly identical in the 3 groups (p > 0.05).
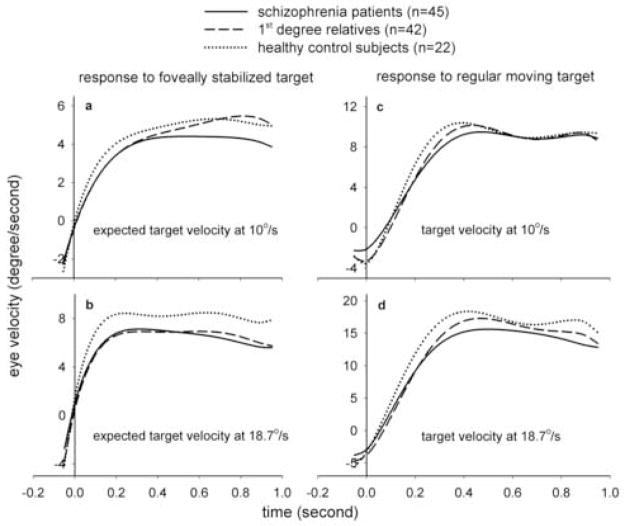
For predictive acceleration, there was a significant main effect of group (F(2, 129) = 5.43, p=0.006) and velocity (F(1, 135) = 74.92, p<0.0001), but no significant group by velocity interaction (p=0.25) or effect of age (p=0.84). Note that the effective degree of freedom was estimated by Kenward-Roger approximation in the mixed model to account for within family correlation. Post hoc tests showed that schizophrenia patients (p= 0.004) and non-schizophrenia relatives (p= 0.04) had reduced acceleration compared to the healthy controls (Figure 4). Patients and relatives were not significantly different (p=0.65). The differences were more prominent at (expected) target velocity of 18.7°/s (patients vs. controls, p=0.01; relatives vs. controls, p= 0.03) as compared to 10°/s (patients vs. controls, p=0.16; relatives vs. controls, p= 0.56). Removing age as a covariate did not change the significance levels (patients vs. controls, p=0.02; relatives vs. controls, p= 0.02 for 18.7°/s and patients vs. controls, p=0.61; relatives vs. controls, p= 0.93 for 10°/s).
Figure 4.
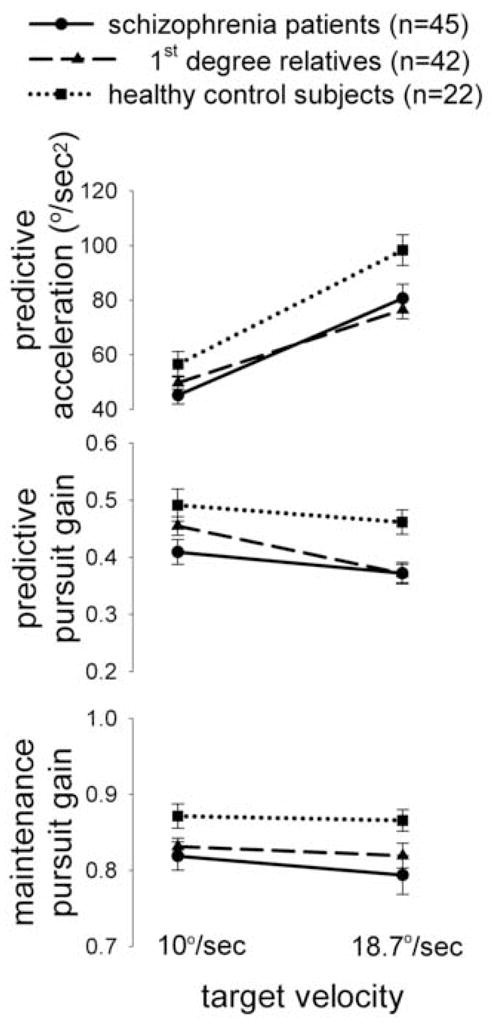
Comparisons of smooth pursuit measures (mean ± S.E.) in schizophrenia patients, non-schizophrenia 1st degree relatives, and healthy controls.
Reduced eye velocity in patients and relatives emerged early in the acceleration phase (Figure 3a & 3b). This was confirmed using the 50 ms epoch data. For 18.7°/s, group differences emerged at the 50–100ms epoch after stabilization began, with mean eye velocities at 3.3°/s, 2.8°/s, and 4.7°/s for patients, relatives, and controls, respectively. Both the patients (p=0.01) and relatives (p=0.001) were significantly different from the controls. For 10°/s, group differences again emerged at 50 –100 ms, however only the patients (p=0.02), but not the relatives (p=0.08) were significantly different from the controls.
For the predictive pursuit gain, there was a significant main effect of group (F(2, 131) = 4.31, p=0.01) and velocity (F(1, 136) = 14.58, p=0.0002), but no significant velocity by group interaction (p=0.17) or effect of age (p=0.62). Schizophrenia patients (p= 0.01) and relatives (p= 0.05) had reduced gain compared to healthy controls (Figure 4). The patients and relatives were not significant different (p=0.75). Again, the differences were more prominent at 18.7°/s (patients vs. controls, p=0.01; relatives vs. controls, p= 0.007) as compared to 10°/s (patients vs. controls, p=0.06; relatives vs. controls, p= 0.46). Removing age as a covariate did not change the significance levels.
Frequencies of saccades during the 1-second stabilized pursuit window were not statistically different (mean ± SD: 1.92±0.76, 2.04±0.71, 1.84±0.79 for patients, relatives, and controls, respectively, p>0.05).
Eye velocities during the twenty 50 ms-epochs were evaluated by means of PCA. It yielded two clearly separated principal components: an initial component consisting of the first 6 (equivalent to the first 300ms at 10.0°/s) or 2 (first 100ms at 18.7°/s) epochs and a second component consisting the subsequent epochs. These two latent components approximately correspond to the acceleration and steady state phases of the stabilized pursuit. Patients and relatives had significantly reduced eye velocity in both principal components at 18.7°/s, but not at 10.0°/s (Table 1).
Table 1.
Group comparisons of mean eye velocity (mean ± SD) during foveally stabilized pursuit among components derived from principle component analysis.
| Target velocity (°/sec) | Principle components | Mean stabilized pursuit velocity (°/sec) |
Group comparisons |
|||
|---|---|---|---|---|---|---|
| Schizophrenia patients | Non-schizophrenia relatives | Healthy controls | Patients vs. controls | Relatives vs. controls | ||
| 10.0 | 1st component | 2.1±1.1 | 2.1±1.0 | 2.6±1.3 | p=0.18 | p=0.20 |
| 2nd component | 4.3±1.8 | 4.9±1.3 | 5.1±1.3 | p=0.10 | p=0.89 | |
|
| ||||||
| 18.7 | 1st component | 1.8±1.3 | 1.3±1.2 | 2.7±1.6 | p=0.03 | p=0.002 |
| 2nd component | 6.5±2.4 | 6.6±1.7 | 8.2±1.9 | p=0.007 | p=0.01 | |
For maintenance pursuit gain, there was a significant main effect of group (F(2, 135) = 6.18, p=0.003) and velocity (p=0.04), but no significant effect of age (p=0.27) or group by velocity interaction (p=0.58). The patients had significant reduction in maintenance pursuit gain compared to the controls in 18.7°/s (p=0.04) but not 10.0°/s (p=0.06). The relatives did not differ from the controls in 10.0°/s (p=0.41) or 18.7°/s (p=0.20) (Figure 4). Removing age as a covariate did not change the significance levels.
Test-retest a month apart in 10 schizophrenia patients suggested high reliabilities for predictive acceleration [ICC(95% CI): 0.82 (0.19–0.96), p=0.01, at 10.0 °/s and 0.81(0.14–0.96), p=0.02, at 18.7 °/s] and predictive pursuit gain [0.84 (0.34–0.96), p=0.006, at 10.0 °/s and 0.87(0.46–0.97), p=0.003, at 18.7 °/s]. The patients’ mean (+ SD) BPRS total scores were 33.6 ± 9.0, which were not correlated with pursuit measures in any target velocities (n=52, all p>0.1).
Discussion
This study used a covert foveal stabilization procedure to demonstrate that predictive pursuit acceleration in the absence of corresponding retinal motion was abnormally low in schizophrenia. The patients and unaffected relatives also maintained predictive pursuit at a lower eye velocity than the controls. The findings provided confirmatory evidence of impaired predictive pursuit in schizophrenia, which was first observed using a target masking procedure (2;11), now in different subject cohorts and by a different laboratory method.
The predictive acceleration deficit associated with schizophrenia is a novel finding. Primate’s ability to accelerate and maintain pursuit based on anticipated target velocity is well known (20;21). During predictive acceleration subjects initiated pursuit in the absence of corresponding retinal error. Thus, the predictive acceleration may not be based on processing of the retinal motion by motion processing regions such as the medial temporal area (MT) (8). Using a foveally stabilized target, Gottlieb et al have shown that stimulation of the pursuit-related FEF region in monkeys causes the eyes to accelerate (22). The observed predictive acceleration deficit in schizophrenia may reflect a deficit in the strength of the FEF discharges. In support of this view, several imaging studies have shown reduced FEF activation in schizophrenia patients or their relatives during SPEM (23–25). In addition, transcranial magnetic stimulation of FEFs at the time of change in pursuit direction results in acceleration of the eye in the expected target direction (26), suggesting that poor predictive acceleration may be subjected to behavioral improvement using interventions to enhance FEF function. Zaksas and Pasternak (27) illustrated a close interaction between FEF and MT in maintaining oculomotor working memory, which may provide a new framework to unify the findings of abnormal motion perception (4;6) and abnormal predictive pursuit in schizophrenia.
Other brain regions implicated in processing extraretinal motion include the medial superior temporal regions (MST) (8), the supplementary eye fields (SEF) (28), the posterior parietal lobe (29;30), and the cerebellum (31). How these regions interact in generating predictive pursuit is unclear. Further work is needed to understand the interaction of these regions and how schizophrenia-related disease process may disrupt the network generating predictive pursuit.
Another question is which component of the extraretinal motion system is abnormal in schizophrenia. Several neural sources of extraretinal motion have been proposed. Historically, extraretinal motion was conceived as efferent signals derived from either the proprioception or a corollary discharge of the motor command (32–35). Another internal source of the target motion information is the online encoding of the previous retinal motion (36;37). To start to address this question, we examined the predictive pursuit response when the eye velocity was momentarily at zero and thus the real-time motor command was at or near zero. PCA suggested two distinct components of predictive pursuit response under this manipulation. The first component included eye velocity immediately after the point in time when the eye velocity was momentarily near zero (thus setting the real-time proprioception and corollary oculomotor feedback commands to near zero). In addition, retinal error was set to zero by foveal stabilization. Thus we argue that this predictive acceleration component represented responses based on the internally stored motion percept and its anticipatory discharge. An abnormality here could not have primarily originated from a deficit in the real-time proprioceptive or motor feedback, but likely an abnormality based on internally stored motion percept and its discharge. Once the eye started moving at a stable speed, efferent signals contributed to the predictive pursuit response. We argue that this was the second component identified by the PCA.
Using foveal stabilization starting from zero velocity at the turning point as opposed to during the middle of the ramp allowed us to address the issue of predictive pursuit without real-time motor input. During the ramp other factors including the momentum of the eye movement and the real-time motor command would also contribute to the predictive pursuit output (2). Another important consideration using zero velocity was based on findings from previous target masking experiments which showed that predictive pursuit starting from the turning point was a measure with high heritability in families of schizophrenia patients (5). In comparison, predictive pursuit measured during the ramp had a lower heritability and a smaller effect size, and was not always significantly different between patients and controls (2;5;38).
Early foveal stabilization methods showed large individual variability when subjects were asked to voluntarily move their eyes with the stabilized target (39). The current method eliminated the problem by covertly stabilizing the target for a brief period, and returned the stabilized target to regular pursuit target without the subject’s awareness, a method made possible using electronic image stabilization.
The largest group difference between relatives vs. controls during stabilized pursuit was at 18.7°/s. This is consistent to previous observations in that predictive pursuit deficits in schizophrenia patients and their relatives during target-masking were primarily in higher target speed (2;11). Conceptually, impaired extraretinal motion may be a cognitive deficit in maintaining a working memory of the velocity information (37). The higher target velocity might have more severely taxed this component of the pursuit system.
To compare results from target masking procedure, we noted that effect size obtained in the target masking method was 0.33 across different conditions (2;5). Specifically, effect size for predictive pursuit measured during the ramp was low (effect size = 0.23 for 18.7°/s between relatives vs. controls); the effect size was higher (0.49) when measured at the beginning of the ramp (2). This current experiment could be viewed as a re-measurement the predictive pursuit during the beginning of the ramp under a covert condition. At 18.7°/s, the effect size for predictive pursuit was 0.87 between relatives and controls using the stabilization method. Therefore, the combined data from the previous target masking and the current foveal stabilization empirically suggest that a larger effect size was achieved by measuring the predictive pursuit right after the turn of the target; which could be further refined by the covert foveal stabilization method.
Many independent studies have documented the presence of eyetracking deficits in non-schizophrenia 1st degree relatives of patients (40–43). Typically, eyetracking measured by the traditional pursuit target in the non-schizophrenia relatives was between patients and controls. Our finding of similar performance between patients and the unaffected relatives is therefore intriguing. Age cannot explain the effect size because in the analysis age served as the covariate and there were no significant correlations between age and dependent measures in combined or individual groups (all p ≥ 0.20). Since unaffected relatives carried a high liability to develop schizophrenia but had no psychosis, this abnormality was not a confound of acute psychosis state or medication treatment. Rather, the result suggests that the refined eyetracking technique increased the sensitivity to detect pursuit abnormality.
Epidemiological studies point to a polygenic inheritance of schizophrenia, whereby multiple genes contribute to the risk of the illness. Single or smaller numbers of susceptibility genes may not express as psychosis, but carriers of the gene(s) in non-psychotic relatives may still have subclinical phenotypes or endophenotype expressed by these genes. Use of endophenotype in genetic studies is an alternative approach to identify genes with small effects on the clinical disease, although these genes may have a large effect on the endophenotype (1). In this context, if an endophenotypic measure was well refined so that it captured an elementary biological dysfunction under narrow genetic control, the measure could show similar effect in patients and relatives if they share one or few susceptibility gene(s). It is tempting to speculate that the current foveal stabilized pursuit measure may be sensitive enough so that the measure is indexing a well-defined genetic origin of the eyetracking deficits in schizophrenia families. This is consistent with our previous finding that the predictive pursuit measure is a specific component of the eyetracking system that may have the highest heritability (5).
In conclusion, foveal stabilization appeared an important technique to examine smooth pursuit response affected by brain diseases. The procedure can be performed by experimentally naïve subjects. It was brief, covert, simple to perform, and required minimal instructions. The findings permit a major refinement of the model regarding extraretinal motion deficit in schizophrenia, implicating impaired storage of the motion percept, or its integration into a predictive pursuit response, in schizophrenia.
Acknowledgments
Support was received from NIMH grants MH-67014, MH-68282, MH-68580, MH-49826, and MH-70644; the National Alliance for Research on Schizophrenia and Depression; General Clinical Research Center grant M01-RR16500; and the VA Capitol Health Care Network (VISN 5) Mental Illness Research, Education, and Clinical Center (MIRECC)
Footnotes
Financial Disclosures
All authors report no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Thaker GK, Ross DE, Cassady SL, Adami HM, LaPorte D, Medoff DR, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, et al. Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biol Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci USA. 1999;96:4724–4729. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong LE, Mitchell BD, Avila M, McMahon RP, Adami H, Thaker GK. Familial aggregation of eye tracking endophenotypes in families of schizophrenic patients. Arch Gen Psychiatry. 2006;63:1–6. doi: 10.1001/archpsyc.63.3.259. [DOI] [PubMed] [Google Scholar]
- 6.Tadin D, Kim J, Doop ML, Gibson C, Lappin JS, Blake R, et al. Weakened center-surround interactions in visual motion processing in schizophrenia. J Neurosci. 2006;26:11403–11412. doi: 10.1523/JNEUROSCI.2592-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lisberger SG, Westbrook LE. Properties of visual inputs that initiate horizontal smooth pursuit eye movements in monkeys. J Neurosci. 1985;5:1662–1673. doi: 10.1523/JNEUROSCI.05-06-01662.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 9.van den Berg AV. Human smooth pursuit during transient perturbations of predictable and unpredictable target movement. Experimental Brain Research. 1988;72:95–108. doi: 10.1007/BF00248504. [DOI] [PubMed] [Google Scholar]
- 10.Barnes GR, Asselman PT. Pursuit of intermittently illuminated moving targets in the human. J Physiol. 1992;445:617–637. doi: 10.1113/jphysiol.1992.sp018943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaker GK, Ross DE, Buchanan RW, Adami HM, Medoff DR. Smooth pursuit eye movements to extraretinal motion signals: Deficits in patients with schizophrenia. Psychiatry Res. 1999;88:209–219. doi: 10.1016/s0165-1781(99)00084-0. [DOI] [PubMed] [Google Scholar]
- 12.Hutton JT, Nagel JA, Loewenson RB. Variables affecting eye tracking performance. Electroencephalogr Clin Neurophysiol. 1983;56:414–419. doi: 10.1016/0013-4694(83)90223-7. [DOI] [PubMed] [Google Scholar]
- 13.Ross RG, Olincy A, Harris JG, Radant A, Adler LE, Compagnon N, et al. The effects of age on a smooth pursuit tracking task in adults with schizophrenia and normal subjects. Biol Psychiatry. 1999;46:383–391. doi: 10.1016/s0006-3223(98)00369-2. [DOI] [PubMed] [Google Scholar]
- 14.Katsanis J, Iacono WG, Harris M. Development of oculomotor functioning in preadolescence, adolescence, and adulthood. Psychophysiology. 1998;35:64–72. [PubMed] [Google Scholar]
- 15.RASHBASS C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dursteler MR, Wurtz RH, Newsome WT. Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey. J Neurophysiol. 1987;57:1262–1287. doi: 10.1152/jn.1987.57.5.1262. [DOI] [PubMed] [Google Scholar]
- 17.Heidenreich SM, Turano KA. Speed discrimination under stabilized and normal viewing conditions. Vision Res. 1996;36:1819–1825. doi: 10.1016/0042-6989(95)00270-7. [DOI] [PubMed] [Google Scholar]
- 18.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. [PubMed] [Google Scholar]
- 19.Kowalchuk RK, Keselman HJ, Algina J, Wolfinger RD. The Analysis of Repeated Measurements with Mixed-Model Adjusted F Tests. Educational and Psychological Measurement. 2004;64:224–242. [Google Scholar]
- 20.Jarrett CB, Barnes GR. The volitional inhibition of anticipatory ocular pursuit using a stop signal. Brain Res Cogn Brain Res. 2003;17:759–769. doi: 10.1016/s0926-6410(03)00200-3. [DOI] [PubMed] [Google Scholar]
- 21.Collins CJ, Barnes GR. Scaling of smooth anticipatory eye velocity in response to sequences of discrete target movements in humans. Exp Brain Res. 2005;167:404–413. doi: 10.1007/s00221-005-0044-8. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. Journal of Neurophysiology. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- 23.O’Driscoll GA, Benkelfat C, Florencio PS, Wolff AL, Joober R, Lal S, et al. Neural correlates of eye tracking deficits in first-degree relatives of schizophrenic patients: a positron emission tomography study. Arch Gen Psychiatry. 1999;56:1127–1134. doi: 10.1001/archpsyc.56.12.1127. [DOI] [PubMed] [Google Scholar]
- 24.Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an FMRI study. Am J Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 25.Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Gagnon D, Paus T, Grosbras MH, Pike GB, O’Driscoll GA. Transcranial magnetic stimulation of frontal oculomotor regions during smooth pursuit. J Neurosci. 2006;26:458–466. doi: 10.1523/JNEUROSCI.2789-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaksas D, Pasternak T. Directional signals in the prefrontal cortex and in area MT during a working memroy for visual motion task. J Neurosci. 2006;45:11726–11742. doi: 10.1523/JNEUROSCI.3420-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol. 2004;92:1257–1262. doi: 10.1152/jn.01255.2003. [DOI] [PubMed] [Google Scholar]
- 29.Eskandar EN, Assad JA. Dissociation of visual, motor and predictive signals in parietal cortex during visual guidance. Nat Neurosci. 1999;2:88–93. doi: 10.1038/4594. [DOI] [PubMed] [Google Scholar]
- 30.Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, et al. Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. Eur J Neurosci. 2004;19:1430–1436. doi: 10.1111/j.1460-9568.2004.03229.x. [DOI] [PubMed] [Google Scholar]
- 31.Chou IH, Lisberger SG. The role of the frontal pursuit area in learning in smooth pursuit eye movements. J Neurosci. 2004;24:4124–4133. doi: 10.1523/JNEUROSCI.0172-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Helmholtz H. Treatise on Physiological Optics. New York: Dover; 1962. [Google Scholar]
- 33.Haarmeier T, Thier P, Repnow M, Petersen D. False perception of motion in a patient who cannot compensate for eye movements. Nature. 1997;389:849–852. doi: 10.1038/39872. [DOI] [PubMed] [Google Scholar]
- 34.Turano KA, Massof RW. Nonlinear contribution of eye velocity to motion perception. Vision Res. 2001;41:385–395. doi: 10.1016/s0042-6989(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 35.Souman JL, Hooge IT, Wertheim AH. Localization and motion perception during smooth pursuit eye movements. Exp Brain Res. 2006;171:448–458. doi: 10.1007/s00221-005-0287-4. [DOI] [PubMed] [Google Scholar]
- 36.Assad JA, Maunsell JH. Neuronal correlates of inferred motion in primate posterior parietal cortex. Nature. 1995;373:518–521. doi: 10.1038/373518a0. [DOI] [PubMed] [Google Scholar]
- 37.Wells SG, Barnes GR. Fast, anticipatory smooth-pursuit eye movements appear to depend on a short-term store. Exp Brain Res. 1998;120:129–133. doi: 10.1007/s002210050385. [DOI] [PubMed] [Google Scholar]
- 38.Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, et al. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: An fMRI study. NeuroImage. 2007;34:300–309. doi: 10.1016/j.neuroimage.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 39.Cushman WB, Tangney JF, Steinman RM, Ferguson JL. Characteristics of smooth eye movements with stabilized targets. Vision Res. 1984;24:1003–1009. doi: 10.1016/0042-6989(84)90077-4. [DOI] [PubMed] [Google Scholar]
- 40.Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1974;31:143–151. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- 41.Grove WM, Clementz BA, Iacono WG, Katsanis J. Smooth pursuit ocular motor dysfunction in schizophrenia: evidence for a major gene. Am J Psychiatry. 1992;149:1362–1368. doi: 10.1176/ajp.149.10.1362. [DOI] [PubMed] [Google Scholar]
- 42.Clementz BA, McDowell JE. Smooth pursuit in schizophrenia: abnormalities of open- and closed-loop responses. Psychophysiology. 1994;31:79–86. doi: 10.1111/j.1469-8986.1994.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 43.Lencer R, Malchow CP, Trillenberg-Krecker K, Schwinger E, Arolt V. Eye-tracking dysfunction (ETD) in families with sporadic and familial schizophrenia. Biol Psychiatry. 2000;47:391–401. doi: 10.1016/s0006-3223(99)00249-8. [DOI] [PubMed] [Google Scholar]