Abstract
The urban environment presents new and different challenges to wildlife, but also potential opportunities depending on the species. As urban encroachment onto native habitats continues, understanding the impact of this expansion on native species is vital to conservation. A key physiological indicator of environmental disturbance is the vertebrate stress response, involving increases in circulating glucocorticoids (i.e., corticosterone), which exert influence on numerous physiological parameters including energy storage, reproduction, and immunity. We examined how urbanization in Phoenix, Arizona influences corticosterone levels, blood parasitism, and innate immunity in populations of tree lizards (Urosaurus ornatus) to determine whether urbanization may be detrimental or beneficial to this species. Both baseline and stress-induced corticosterone concentrations were significantly lower in urban lizards relative to the rural ones, however, the magnitude of the increase in corticosterone with stress did not differ across populations. Urban lizards also had a lower ratio of heterophils to lymphocytes, but elevated overall leukocyte count, as compared to lizards from the natural site. Urban and rural lizards did not differ in their prevalence of the blood parasite, Plasmodium mexicanum. Taken together, these results suggest that urban tree lizards may have suppressed overall corticosterone concentrations possibly from down-regulation as a result of frequent exposure to stressors, or increased access to urban resources. Also, urban lizards may have bolstered immunocompetence possibly from increased immune challenges, such as wounding, in the urban environment, or from greater energetic reserves being available as a result of access to urban resources.
Keywords: disturbance, corticosterone, leukocytes, urbanization, parasites
Introduction
As human-dominated landscapes encroach on native habitats, the ability of animals to adapt to these novel environments will become an increasingly important factor for their long-term persistence. In cities animals contend with repeated, yet highly variable anthropogenic disturbances, as well as drastic changes to their native habitats (Shochat et al. 2006). Although urbanization is usually regarded as detrimental to animal populations, recent research into urban ecology has demonstrated organismal responses as variable and species specific (Hostetler 1999; Hostetler and Knowles-Yanez 2003). Cities present novel “stressors” to wildlife including: human disturbance (Fernandez-Juricic 2002), vehicle traffic (Bautista et al. 2004), non-native predators (Koenig et al. 2002; Woods et al. 2003; Baker et al. 2005), exposure to pollutants (Eens et al. 1999; Burger et al. 2004), and warmer ambient temperatures (McLean et al. 2005). Despite these factors some species persist in cities which may provide advantages to species equipped to exploit them (Ficetola et al. 2007; Bonier et al. 2007b). These benefits may include: abundant exotic and anthropogenic food resources (Parris and Hazell 2005; Anderies et al. 2007), refuge from solar exposure in the form of ornamental trees (Braman et al. 2000), and year-round water resources (Fokidis et al. 2008). The latter may be especially important in arid regions, where water is the predominant factor influencing the timing of life-history events in all desert flora and fauna (Beck et al. 1973; Giuliano et al. 1995; Vleck 1984). Urban water sources may also harbor parasite-transmitting vectors (Fokidis et al. 2008) which have aquatic life-stages (Marquardt et al. 2000). Parasitic infection can negatively influence the overall health of urban wildlife, and may interfere with colonization success (Garamszegi et al. 2007). The ability of a species to avoid the costs and reap the benefits of city environments may be an important determinant of the relative success of acclimatization to urbanization.
Although studies have documented demographic changes in wildlife resulting from urban development, only recently have the physiological consequences of urbanization been examined. In vertebrates, environmental stressors are usually detected as increases in circulating glucocorticoids, such as corticosterone (CORT) result from the activation of the hypothalamic-pituitary-adrenal (HPA) axis (Sapolsky et al. 2000). The HPA axis functions to mobilize nutrient stores, and modify behaviors during stressful situations and activation is adaptive in the short-term, but detrimental if long-term (Sapolsky et al. 2000). The ability to modulate HPA functioning allows normal life-history processes to continue despite of environmental change (Wingfield et al. 1998; Astheimer et al. 1995; Boonstra et al. 2001). To date all studies of urbanization and HPA functioning in wildlife have been done on birds. These studies have demonstrated population-level variation in stress-responsiveness associated with urbanization, with urban birds either showing suppressed (Partecke et al. 2006) or increased CORT production (Schoech et al. 2004; Fokidis et al. in review) in response to an acute stressor compared to rural birds. Thus, urban-associated variation in stress-responsiveness is species- (Fokidis et al. in review) and likely sex-specific (Bonier et al. 2007a).
Tree lizards (Urosaurus ornatus) are a small phrynosomatid lizard species found throughout the southwestern United States and Northern Mexico and are abundant on boulders, trees, and shrubs throughout urban localities in the Sonoran Desert. This wide distribution encompassing numerous habitat types make tree lizards a useful model for examining the physiological consequences of urbanization. Additionally, the stress physiology, reproduction, behavior, and seasonal hormone profiles of this species have been extensively studied in both captive and wild populations (Moore et al., 1998; Moore et al., 1991; Thompson and Moore, 1992; Woodley and Moore, 2002). In tree lizards increases in CORT resulting from restraint stress depresses wound healing, a measure of innate immunity (French et al., 2006; French et al., 2007c). During energy-limiting conditions, wild female tree lizards trade off investment into immune function with that into reproduction(French et al., 2007a; French and Moore, 2008). This highlights the energetic cost of maintaining immunity and thus potentially “stressful” components of the urban environment may influence immune function, which may be particularly important during energetically taxing life-history stages.
We examined stress-responsiveness and innate immune function in tree lizards living within and around Phoenix, Arizona. Specifically, we attempt to differentiate between two hypotheses. The first hypothesis suggests that exposure to acute urban stressors and the presence of parasite-transmitting vectors in urban water resources will result in urban lizards having higher baseline CORT levels, impaired stress-responsiveness, increased blood parasitism, and lowered immune capacity (changes in number and distribution of leucocytes). An alternate hypothesis proposes that tree lizards in urban areas will acclimatize to repetitive and benign urban “stressors” and increased access to urban food and water resources may act to lower baseline CORT levels. Alternatively, studies in birds show that prolonged elevations in CORT levels that are associated with chronic stress can act to down-regulated HPA functioning resulting in decreased baseline CORT levels (Cyr et al., 2007; Rich and Romero, 2005). In addition, acclimatization to benign urban stressors may enable urban lizards to maintain a bolstered stress-response. In turn, urban lizards may show a strengthened immune capacity and lower levels of blood parasitism compared to rural conspecifics. This study is the first to examine the physiological consequences of urbanization in a non-avian species and provides preliminary insight into how endocrine and immunological mechanisms may interact to influence the colonization of novel habitats by native species.
Methods
Field sampling and study sites
Male and female tree lizards were sampled from April 18 – April 28 2007 between 1000–1400 hrs) at three sites located within and around Phoenix, Arizona. Two sites were centrally located in the Phoenix metropolitan area in close proximity to one another. The first was an urban site (Urban – U; 33° 26.12 N, 111° 55.83 W) which consisted of residential housing developments constructed in 1955 and contained both xeric and mesic (i.e. grass) landscaping. The second site, (Semi-natural – SN; 33° 26.19 N, 111° 55.87 W) was a protected desert remnant (Papago Park) located about 1km west of the urban site. Here the habitat consists of a heterogenous matrix of upland and lowland native Sonoran Desert vegetation, as well as both native and ornamental riparian plant species. We also sampled an undeveloped rural site located in the McDowell Mountain Sonoran Reserve (Natural – N; 33° 41.54 N, 111° 47.26 W) located near the Northern periphery of Phoenix, about 21 km from the other two sites. This site is composed of large tracts of typical native upland Sonoran Desert region flora. All animals included in the study were sexually mature, reproductive adults. Lizards were captured by noosing and a blood sample was taken, by rupturing the orbital sinus with a heparinized capillary tube, within 3 minutes of initial sighting. Upon capture lizards were bled immediately and no longer than 30 seconds was taken to obtain a blood sample. Lizards were then placed individually in opaque cloth bags for 10 minutes, after which a second blood sample was taken (hereafter “stress-induced”). This enabled us to assess change in CORT concentrations in response to the stress of capture and handling. Past studies in tree lizards showed a significant stress-related increase in CORT levels by 10 minutes with a maximum increase attained at approximately 60 minutes (Moore et al. 1991). Plotting the CORT trajectory, we can estimate the magnitude of the overall increase based on the 10 minute sample (Moore et al. 1991). Thus we examined two measures; the concentration of CORT after 10 minutes of capture/handling stress, and the rate of increase in CORT levels within 10 minutes with respect to baseline levels (i.e. magnitude of change in CORT). Blood was stored on ice until transported back to the laboratory. Thin blood smears were made in the field on glass microscope slides using about 5 µl of the initial blood sample (Bennett 1970; Walberg 2001; Fokidis et al. 2008). Smears were air-dried at ambient temperature and stored until fixation. We assessed reproductive condition of females by manually palpating the abdomen for the number and firmness of follicles/eggs, whereas all males were in reproductive condition during sampling (French and Moore, 2008). Males are known to be in breeding condition during the chosen sampling period based on previous studies which assessed testes size (French and Moore, 2008). We also recorded the presence of scars and injuries, including caudal autotomy (ie. tail breaks), which are common in many lizard species, and evidence of past stressor (Table 1). Lastly, we recorded snout-vent length (SVL) defined as the length from the tip of the animal’s snout to its cloacal vent. All handling and procedures were approved by Arizona State University’s Institutional Animal Care and Use Committee, protocol # 03-678R.
Table 1.
Mean snout-vent length (SVL) ± 1 standard error and prevalence of plasmodium (percentage infected with plasmodium), for female and male tree lizards (Urosaurus ornatus) sampled at three sites (see text) across an urban-rural gradient. n indicates sample size.
| Site | Sex | n | SVL (mm) | Plasmodium prevalence (%) |
% Wounded animals |
|---|---|---|---|---|---|
| U | female | 9 | 49.89 ± 1.53* | 22 | 33.3* |
| male | 7 | 51.79 ± 1.52 | 14 | 28.6* | |
| SN | female | 6 | 43.25 ± 1.36 | 33 | 0 |
| male | 9 | 52.22 ± 1.25 | 11 | 0 | |
| N | female | 5 | 43.60 ± 0.29 | 20 | 0 |
| male | 5 | 47.2 ± 2.44 | 0 | 0 |
Asterisks denote significant differences at α = 0.05.
Plasma samples and radioimmunoassay
In the laboratory plasma was separated from the blood via centrifugation (30min at 2500rpm) and stored at −20° C until assayed. Plasma samples were assayed for CORT in duplicate and within a single radioimmunoassay previously described in (Moore, 1986). In brief, samples were extracted using 30% ethyl acetate/isooctane extractions. The 30% phase was separated, dried, and resuspended in 90% ethanol. Samples were then washed with hexane to remove excess lipids and were resuspended in PBS buffer. Duplicate aliquots of these samples were then assayed for CORT. The intra-assay coefficient of variation was 9% for CORT.
Blood smears and analysis
Blood smears were fixed for 10 min in absolute methanol within 3 days of collection and subsequently Giemsa-stained (Bennett 1970). Stained smears were then dehydrated for one week under partial vacuum. Stained slides were then cleared using xylene, cover-slipped, and sealed using Cytoseal 60 (VWR, San Francisco, California) to facilitate long-term storage. Parasite prevalence, the percentage of infected individuals in a population (Bush et al. 1997) was determined by surveying blood smears at 250x magnification for 10 min and then at 400x magnification for 5 min using an Olympus BX60 light microscope (Olympus Optical Co., Tokyo, Japan).
To assess innate immunity we examined the ratio of heterophil leukocytes to lymphocytes (H:L). This measure is often used as an indicator of chronically elevated glucocorticoids resulting from “stressful” stimuli (Gross and Siegel 1986; Vleck et al. 2000; Bonier et al. 2007a), which can suppress lymphocyte numbers, in whole blood (Harmon 1998). We examined randomly selected and non-overlapping microscope fields in each smear under oil immersion (1000× magnification) and counted leukocytes until a total of 100 white blood cells was reached (Fokidis et al. 2008). We identified eosinophils, basophils, and monocytes, using the criteria of (Campbell 1996), however only heterophils and lymphocytes as a ratio were analyzed statistically. All slides were examined by a single observer (HBF) without knowledge of locality, sex, or date that the samples were collected.
Another measure of innate immunity is the total number of circulating leukocytes, usually expressed as a total leukocyte count (TLC; (Godfrey et al. 1987; Gering and Atkinson 2004). To obtain the TLC, 25 randomly selected non-overlapping microscope fields containing non-overlapping single cell layers were digitized at 400x magnification. Image-Pro analysis software (v. 4.1, Media Cybernetics, Silver Springs, MD, USA) was then used to identify 10,000 erythrocytes per slide, which is the minimum recommended for accurate assessment of TLC (Gering and Atkinson 2004). Erythrocytes were identified based on morphological characteristics of their nucleus (aspect ratio, length, width, perimeter, roundness, relative brightness, and color) and were included in the counts if their entire nucleus was visible on the digitized image. Leukocytes as well as infected erythrocytes seen on these digitized images were manually counted and expressed as the number of leukocytes per 10,000 non-infected erythrocytes.
Statistical analyses
Initially, t-tests were performed to assess sex-biased differences for all continuous variables to determine whether or not sexes could be pooled for subsequent analysis. Hormonal and hematology data were log-transformed to satisfy the assumptions of normality and equal variances. Repeated measures analysis of variance (ANOVA) was used to assess site differences in circulating hormone concentrations. These data violated assumptions of sphericity and thus were Greenhouse-Geiser corrected, which conservatively adjusts the degrees of freedom. Separate one-way ANOVAs, with an α level adjusted using Bonferroni, were used to assess site differences in concentration and magnitude of changes in CORT, hematology and SVL. Tukey-Kramer honestly significant difference (HSD) corrected post-hoc comparisons allowed us to discern differences between sites. Parasite prevalence was compared with hierarchical log-linear analysis, which tests for associations between categorical variables in a manner similar to ANOVA (Fokidis et al. 2008). The significance level for all statistical tests was α = 0.05, unless otherwise stated. Data are presented as means ± 1 standard error (se). All statistical analyses were performed using JMP.IN (v. 5.1, SAS Institute Inc., Cary, NC, USA) and SPSS (v. 13, SPSS Inc., Chicago, IL, USA).
Results
Snout-vent length
Body size is usually a sexually dimorphic characteristic in tree lizards (French and Moore 2008), and therefore the sexes were analyzed separately for SVL. There was a significant difference in SVL among the different sites for females (F 2, 19 = 7.58, P < 0.01) but not males (F 2, 20 = 2.45, P = 0.11). The females at site U were significantly larger than females from the other sites (SN, N), according to post hoc comparisons (Table 1).
Corticosterone data
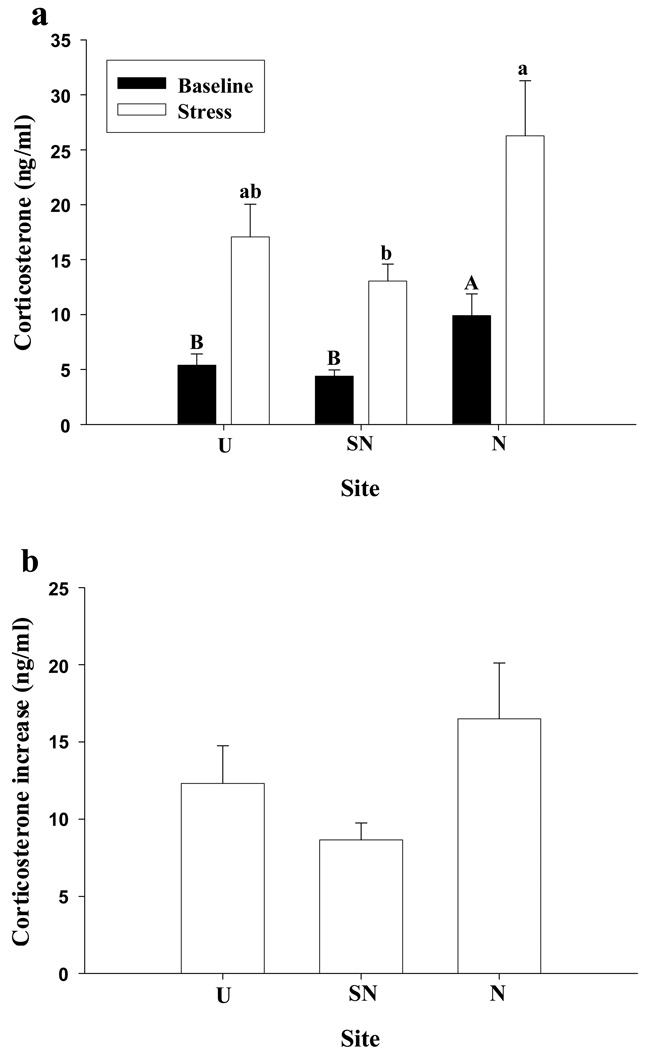
There was no significant sex difference in baseline (t = 0.45, df = 1, P = 0.65) or stress-induced increase (t = 0.51, df = 1, P = 0.61) in CORT concentrations, and therefore hormone data were pooled between the sexes. As predicted there were significant differences in baseline CORT concentrations (F 2, 36 = 5.54, P < 0.01; Fig 1a) among the sites, with higher circulating concentrations at the N site, relative to the other sites (SN, U). However, there was no difference in the change in CORT concentration over time (F 2, 36 = 0.25, P = 0.78; Fig 1b) among sites. This was also reflected by no significant difference in the magnitude of change in CORT concentrations among the populations (F 2, 38 = 2.58, P = 0.089; Fig 1b), although there was a trend for the N population having a greater change than the U and SN sites. A similar result was attained using the percentage increase above baseline CORT levels (F 2, 38 = 0.12, P = 0.88). Regarding stress-induced concentrations of CORT, the N animals > the U site lizards > SN animals.
Figure 1.
(a) Circulating concentrations of corticosterone in tree lizards (Urosaurus ornatus) before and after handling stress at the urban (U), semi-natural (SN), and natural (N) sites. Upper case letters denote significant differences of baseline levels and lower case letters denote significant differences of stress-induced levels of corticosterone among the different sites (α = 0.05 level). Additionally, stress-induced CORT concentrations were significantly greater relative to baseline CORT concentrations for all sites (α = 0.05 level). (b) The magnitude of the change in corticosterone, at three sites sampled across the urban-rural gradient of Phoenix, Arizona. (Note: a similar result was found using percentage increase above baseline). Error bars represent 1 standard error.
Blood parasites
Only a single blood parasite, Plasmodium mexicanum was detected at relatively low intensity (6–14 infected cells per 10,000 erythrocytes) during the study. This blood parasite only infected erythrocytes and was present in 8 of the 41 individuals (19.5% total). Parasite prevalence did not differ between sexes (X2= 0.98, df = 2, P = 0.53; Table 1) or across sites (X2 = 0.47, df = 3, P = 0.82; Table 1). Infected lizards did not differ in SVL from non-infected lizards (t = 0.83, df = 1, P = 0.49).
Leukocyte counts
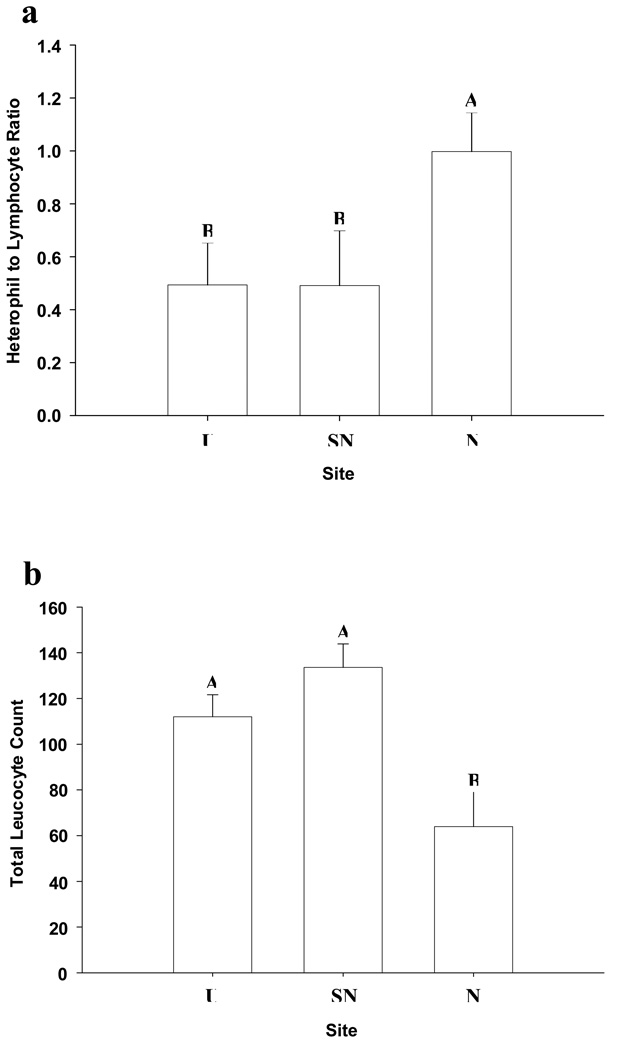
There was no difference in H:L (t = 0.182, df = 1, P = 0.857) or TLC (t = 0.169, df = 1, P = 0.866) between the sexes and thus data were pooled for comparison across sampling sites. Lizards sampled from the N site had a greater H:L ratio compared to lizards from either SN or U sites (F 2, 38 = 30.91, P < 0.01; Fig 2a). However, H:L ratio did not differ between the SN and U sites (P > 0.9). Lizards from the N site also had a lower TLC compared to both the SN or U sites (F 2, 38 = 9.06, P = 0.006; Fig 2b) but, lizards again did not differ with respect to TLC between these sites (P = 0.308). Neither, TLC nor H:L ratio were correlated to SVL (TLC: r = 0.095, N = 41, P = 0.553; H:L ratio: r = −0.282, N = 41, P = 0.074). Infected lizards did not differ in H:L ratio or TLC from non-infected lizards (H:L ratio: t = 0.71, P = 0.83; TLC: t = 1.03, P = 0.091;).
Figure 2.
(a) The ratio of heterophil to lymphocyte (H:L) white blood cells at the urban (U), semi-natural (SN), and natural (N) sites, and (b) the total leukocyte count (TLC), expressed number of leukocytes per 10,000 red blood cells, for tree lizards (Urosaurus ornatus) sampled at three sites across the urban-rural gradient of Phoenix, Arizona. Error bars represent 1 standard error. Letters denote significant differences among groups at the α = 0.05 level.
Discussion
The stress of city living in the tree lizard
This study is the first to investigate how physiology can vary with respect to urbanization in a reptile species. Overall, baseline and stress-induced CORT concentrations were elevated in lizards from the natural site, as compared to counterparts from urban and semi-natural locations. However, there was no difference in magnitude of CORT increase among the populations. This decrease in baseline CORT in urban and semi-natural lizards supports the hypothesis that urban lizards either: 1) acclimatized to repeated stressors in anthropogenically modified habitats, 2) lower overall “stress” as a function of increased availability of food/water resources in the U and SN sites, or 3) increased chronic activation of the HPA axis, possibly resulting from higher predation in urban areas. However, based on the data collected in this study we are unable to distinguish between these potential underlying causes for the observed variation in CORT in tree lizards. All scenarios would manifest as a decrease in overall circulating CORT concentrations. This type of response may be adaptive as chronic elevations in CORT are known to be detrimental to the health and fitness of many organisms (Dhabhar and McEwen 1999; Moore and Jessop 2003). However, we did not observe any modulation of the stress response in tree lizards across an urban-natural comparison, which has been found in many avian studies. Many species modulate their stress-response by decreasing HPA activity in response to repeated stressors, seasonally, or during energetically-demanding life-history events such as reproduction (Romero 2008; Wang 2006; Boonstra 2004; Wada 2004). We sampled lizards only during the spring and conceivably modulation of the stress-response may occur during other seasons or life-history stages.
Several different potential stressors for tree lizards exist in urban habitat as compared to rural areas, including increased predation risk from nonnative species (Woods et al. 2003; Baker et al. 2005). In our study, about 30% of the animals sampled at the U site had a regenerated tail or a scar of some type, whereas none of the animals sampled at the other sites had any noticeable scars or injuries. This suggests increased predation risks in the urban environment, possibly from the numerous domestic cats (Felis silvestris catus) in the area. Domestic cats are known to predate heavily on lizards in cities (Koenig et al. 2002; Gillies and Clout 2003). These wounds could result in increased CORT in urban lizards, and a blunted CORT response by urban lizards may mediate shifts in allocation of energy to various processes, including wound healing, which are known to limit resources available to other important physiological processes such as reproduction (French et al., 2007b). This is consistent with our observation that U lizards have lower CORT levels than counterparts from the SN or N sites. Decreased CORT levels in urban lizards may also result from lowered predation from natural enemies, such as snakes which occur at lower densities in urban environments (Fearn et al. 2001; Reed and Shine 2002). This is consistent with the “predator-release” hypothesis, which suggests that urban areas may provide refuges for species where population dynamics are heavily impacted by predation pressure from native animals (Sorace 2002; Jokimaki et al. 2005). However, increased predation from non-native urban species must also be considered in the context of the predator-release hypothesis, and may explain lowered baseline CORT levels in the U site lizards. Similarly, overall lizard diversity decreases in urban areas (Germaine and Wakeling 2001) and this may lower competition from native lizard species. These factors may also act to lower baseline CORT levels in urban populations of tree lizards.
Tree lizards sampled from our natural site had higher baseline CORT levels than those from either modified habitat. Variation in CORT levels between our populations may result from factors unrelated to urbanization. Individual variation in baseline CORT may be influenced by both past and present conditions in the environment (Landys et al. 2006; Romero and Reed 2008). Population differences in baseline CORT have been reported in this species (M.C. Moore, personal observation) and may be related to variation associated with reproductive state (Wilson and Wingfield 1992; Moore and Mason 2001; Jessop et al. 2004) or diel patterns of CORT release (Romero and Remage-Healey 2000; Rodriguez et al. 2001). We sampled only adult lizards that were in active reproductive condition and all captures were made within a 10 day period from 1000–1400 hrs in an effort to minimize temporal variation in CORT. During this 10 day sampling period, the daily mean air temperature ranged from 21 to 27°C (daily max: 26 – 35°C; daily min: 8 – 15°C) and all days were characterized as having low cloud cover with little or no wind, and thus differences in weather likely do not account for the variation in baseline CORT observed between our sites. The predominant differences among our three sampling sites were the degree of anthropogenic development and overall habitat cover (N and SN sites have significantly greater amount of cover than the U site) and these factors likely influence, at least in part, baseline CORT levels (see methods for site description). This study is a preliminary examination and future studies should incorporate more sites and test specific components of urbanization that may influence baseline CORT levels.
Immunocompetence and the urban environment
Compatible with other studies in reptiles (Aguirre et al. 1995; Morici et al. 1997; Cartledge et al. 2005), we observed an increase in the ratio of heterophils to lymphocytes (H:L) that was consistent with population differences observed in baseline CORT. Saad and Elridi (1988) report that CORT acts to lyse lymphocytes in the lizard Chalcides ocellatus, resulting in depressed overall number of lymphocytes. Similarly, lymphocyte activity was highest in the turtle Mauremys caspica during periods of the year when steroids, including CORT were at lowest concentrations (Munoz et al. 2000). Decreases in baseline CORT in the tree lizards from the U and SN sites may increase lymphocytes, which are involved in specific responses to foreign antigens and ultimately strengthen immune response to infection. The urban and semi-natural lizards also had increased total numbers of leukocytes (i.e. TLC). These lizards may be in better overall condition enabling them to maintain a high reserve of leukocytes in preparation to fight potential infections. This condition-dependence of leukocyte numbers may potentially be mediated through concentrations in CORT.
Another factor that can influence the proportions and total numbers of leukocytes in peripheral blood is whether the organism is combating a current infection. We observed only a single blood parasite species, P. mexicanum in our study, however its prevalence did not vary with respect to site and infection was not associated with H:L ratio, TLC or SVL and was present at low intensity in all infected individuals. These findings suggest that the occurrence of vectors for P. mexicanum, such as the sandflies Lutzomyia vexator and L. stewarti, (Schall and Smith 2006) do not vary across the urban-rural gradient studied, despite differences in the amount of open standing water between habitats. Additionally, the low intensity of parasitic infection in our samples may be due to other factors, such as the ability of the immune system to eradicate or clear parasites from circulation. The clearance of parasites from circulation may be mediated by CORT (Hanley,K.A. 2002; Dunlap,K.D. 1995). Infection with blood parasites is typically associated with decreases in H:L ratio and overall increases in TLC, because of lymphocyte proliferation (Bonier et al. 2007a). The lack of a relationship between SVL and infection with P. mexicanum in the present study suggests that parasitism has minimal effects on tree lizard health, which are not sufficient to retard growth. Notably, we were unable to determine how long individuals had been infected and whether infections were new or the result of relapse in existing infection, perhaps in response to CORT. Little is understood concerning the relationship between habitat (natural or modified), parasitism, and immune function, particularly in arid regions. Future research may allow us to elucidate patterns, processes, and mechanisms that dictate long-term responses of species to modified habitats.
Future directions in urban physiology
An individual’s ability to respond to environmental perturbations greatly influences its short-term survival and ultimate acclimatization to changing environments. Moreover, glucocorticoids that are involved in the mediation of the stress response, and often have permissive roles in regulating reproduction, metabolism, and resource distribution. Thus, it is critical to understand not only how organisms cope with environmental stressors, but also how different environments affect the stress response. This preliminary study examined a single species, yet it is likely that the environmental effects of the urban landscape on physiology vary depending on taxa. Furthermore, the fact that tree lizards readily persist under certain contexts in the urban landscape make them a useful model for comparison with other species less tolerant to urbanization such as Urosaurus graciosus or Sceloporus occidentalis. Comparative studies enable us to elucidate how pre-existing characteristics of species may enable their successful colonization of novel environments such as cities. Future studies should examine multiple cities, particularly ones from different regions containing different habitats. Future studies could also include additional measures of immunity, as different components of the immune system can vary in their response to the effects of urbanization. A greater understanding of the physiological implications of urbanization may enable us to mitigate the impact of growing cities on wildlife.
Acknowledgements
We’d like to thank Nataliya Emmert for help with field work, Pierre Deviche for use of his light microscope, and Jon Davis for use of his backyard and alleyway. We’d also like to thank PRG at ASU for critical feedback on this manuscript.
References
- Aguirre AA, Balazs GH, Spraker TR, Gross TS. Adrenal and hematological responses to stress in juvenile green turtles (Chelonia mydas) with and without fibropapillomas. Physiol Zool. 1995;68:831–854. [Google Scholar]
- Anderies JM, Katti M, Shochat E. Living in the city: Resource availability, predation, and bird population dynamics in urban areas. J Theor Biol. 2007;247:36–49. doi: 10.1016/j.jtbi.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Astheimer LB, Buttemer WA, Wingfield JC. Seasonal and acute changes in adrenocortical responsiveness in an arctic-breeding bird. Horm Behav. 1995;29:442–457. doi: 10.1006/hbeh.1995.1276. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Bentley AJ, Ansell RJ, Harris S. Impact of predation by domestic cats Felis catus in an urban area. Mamm Rev. 2005;35:302–312. [Google Scholar]
- Bautista LM, Garcia JT, Calmaestra RG, Palacin C, Martin CA, Morales MB, Bonal R, Vinuela J. Effect of weekend road traffic on the use of space by raptors. Cons Bio. 2004;18:726–732. [Google Scholar]
- Beck BB, Engen CW, Gelfand PW. Behavior and activity cycles of Gambels quail and raptorial birds at A sonoran-desert waterhole. Condor. 1973;75:466–470. [Google Scholar]
- Bennett GF. Simple techniques for making avian blood smears. Can J Zool. 1970;48:585. [Google Scholar]
- Bonier F, Martin PR, Sheldon KS, Jensen JP, Foltz SL, Wingfield JC. Sex-429 specific consequences of life in the city. Behav Ecol. 2007a;18:121–129. [Google Scholar]
- Bonier F, Martin PR, Wingfield JC. Urban birds have broader environmental tolerance. Biol Letters. 2007b;3:670–673. doi: 10.1098/rsbl.2007.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra R, McColl CJ, Karels TJ. Reproduction at all costs: The adaptive stress response of male arctic ground squirrels. Ecology. 2001;82:1930–1946. [Google Scholar]
- Boonstra R. Coping with changing northern environments: The role of the stress axis in birds and mammals. Integrat Comp Bio. 2004;44:95–108. doi: 10.1093/icb/44.2.95. [DOI] [PubMed] [Google Scholar]
- Braman SK, Latimer JG, Oetting RD, McQueen RD, Eckberg TB, Prinster M. Management strategy, shade, and landscape composition effects onurban landscape plant quality and arthropod abundance. J Econ Entomol. 2000;93:1464–1472. doi: 10.1603/0022-0493-93.5.1464. [DOI] [PubMed] [Google Scholar]
- Burger J, Bowman R, Woolfenden GE, Gochfeld M. Metal and metalloid concentrations in the eggs of threatened Florida scrub-jays in suburban habitat from south-central Florida. Sci Total Environ. 2004;328:185–193. doi: 10.1016/j.scitotenv.2004.01.030. [DOI] [PubMed] [Google Scholar]
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al revisited. J Parasitol. 1997;83:575–583. [PubMed] [Google Scholar]
- Campbell TW. Clinical pathology. In: Mader DR, editor. Reptile medicine and surgery. Pennsylvania: W.B. Saunders Co; 1996. pp. 248–257. [Google Scholar]
- Cartledge CA, Gartrell B, Jones SM. Adrenal and white cell count responses to chronic stress in gestating and postpartum females of the viviparous skink Egernia whitii (Scincidae) Comp Biochem Physiol A. 2005;141:100–107. doi: 10.1016/j.cbpb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Cyr NE, Earle K, Tam C, Romero LM. The effect of chronic psychological stress on corticosterone, plasma metabolites, and immune responsiveness in European starlings. Gen Comp Endocrinol. 2007;154:59–66. doi: 10.1016/j.ygcen.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Nat Acad Sci USA. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap KD, Schall JJ. Hormonal alterations and reproductive inhibition in male fence lizards (Sceloporus occidentalis) infected with the malarial parasite plasmodium-mexicanum. Physiol Zool. 1995;68:608–621. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF, Blust R, Bervoets L. Great and blue tits as indicators of heavy metal contamination in terrestrial ecosystems. Ecotoxicol Environ Safety. 1999;44:81–85. doi: 10.1006/eesa.1999.1828. [DOI] [PubMed] [Google Scholar]
- Fearn S, Robinson B, Sambono J, Shine R. Pythons in the pergola: The ecology of 'nuisance' carpet pythons (Morelia spilota) from suburban habitats in south-eastern Queensland. Wildlife Res. 2001;28:573–579. [Google Scholar]
- Fernandez-Juricic E. Can human disturbance promote nestedness? A case study with breeding birds in urban habitat fragments. Oecologia. 2002;131:269–278. doi: 10.1007/s00442-002-0883-y. [DOI] [PubMed] [Google Scholar]
- Ficetola GF, Sacchi R, Scali S, Gentilli A, De Bernardi F, Galeotti P. Vertebrates respond differently to human disturbance: Implications for the use of a focal species approach. Acta Oecologica-Intern J Ecol. 2007;31:109–118. [Google Scholar]
- Fokidis HB, Greiner EC, Deviche P. Interspecific variation in avian blood parasites and haematology associated with urbanization in a desert habitat. J Avian Bio. (In press) [Google Scholar]
- Fokidis HB, Orchinik M, Deviche P. Patterns of total and free corticosterone secretion in birds associated with urbanization in a desert city. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2008.12.005. (In review) [DOI] [PubMed] [Google Scholar]
- French SS, Matt KS, Moore MC. The effects of stress on wound healing in male tree lizards (Urosaurus ornatus) Gen Comp Endocrinol. 2006;145:128–132. doi: 10.1016/j.ygcen.2005.08.005. [DOI] [PubMed] [Google Scholar]
- French SS, DeNardo DF, Moore MC. Trade-offs between the reproductive and immune systems: Facultative responses to resources or obligate responses to reproduction? Am Nat. 2007a;170:79–89. doi: 10.1086/518569. [DOI] [PubMed] [Google Scholar]
- French SS, Johnston GIH, Moore MC. Immune activity suppresses reproduction in food-limited female tree lizards Urosaurus ornatus. Funct Ecol. 2007b;21:1115–1122. [Google Scholar]
- French SS, McLemore R, Vernon B, Johnston GIH, Moore MC. Corticosterone modulation of reproductive and immune systems trade-offs in female tree lizards: Long-term corticosterone manipulations via injectable gelling material. J Exp Biol. 2007c;210:2859–2865. doi: 10.1242/jeb.005348. [DOI] [PubMed] [Google Scholar]
- French SS, Moore MC. Immune function varies with reproductive stage and context in female and male tree lizards, Urosaurus ornatus. Gen Comp Endocrinol. 2008;155:148–156. doi: 10.1016/j.ygcen.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ, Erritzoe J, Moller AP. Feeding innovations and parasitism in birds. Biol J Linn Soc. 2007;90:441–455. [Google Scholar]
- Gering E, Atkinson CT. A rapid method for counting nucleated erythrocytes on stained blood smears by digital image analysis. J Parasitol. 2004;90:879–881. doi: 10.1645/GE-222R. [DOI] [PubMed] [Google Scholar]
- Germaine SS, Wakeling BF. Lizard species distributions and habitatoccupation along an urban gradient in Tucson, Arizona, USA. Biol Cons. 2001;97:229–237. [Google Scholar]
- Gillies C, Clout M. The prey of domestic cats (Felis catus) in two suburbs of Auckland City, New Zealand. J Zool. 2003;259:309–315. [Google Scholar]
- Giuliano WM, Lutz RS, Patino R. Physiological-responses of northern bobwhite (Colinus virginianus) to chronic water-deprivation. Physiol Zool. 1995;68:262–276. [Google Scholar]
- Godfrey RD, Fedynich AM, Pence DB. Quantification of hematozoa in blood smears. J Wildl Disease. 1987;23:558–565. doi: 10.7589/0090-3558-23.4.558. [DOI] [PubMed] [Google Scholar]
- Gross WB, Siegel PB. Effects of initial and 2nd periods of fasting on heterophil lymphocyte ratios and body-weight. Avian Disease. 1986;30:345–346. [PubMed] [Google Scholar]
- Hanley KA, Stamps JA. Does corticosterone mediate bidirectional interactions between social behaviour and blood parasites in the juvenile black iguana, Ctenosaura similis? Anim. Behav. 2002;63:311–322. [Google Scholar]
- Harmon BG. Avian heterophils in inflammation and disease resistance. Poult Sci. 1998;77:972–977. doi: 10.1093/ps/77.7.972. [DOI] [PubMed] [Google Scholar]
- Hostetler M. Scale, birds, and human decisions: A potential for integrative research in urban ecosystems. Landscape Urban Plann. 1999;45:15–19. [Google Scholar]
- Hostetler M, Knowles-Yanez K. Land use, scale, and bird distributions in the phoenix metropolitan area. Landscape Urban Plann. 2003;62:55–68. [Google Scholar]
- Jessop TS, Hamann M, Limpus CJ. Body condition and physiological changes in male green turtles during breeding. Marine Ecol-Progress. 2004;276:281–288. [Google Scholar]
- Jokimaki J, Kaisanlahti-Jokimaki ML, Sorace A, Fernandez-Juricic E, Rodriguez Prieto I, Jimenez MD. Evaluation of the "safe nesting zone"hypothesis across an urban gradient: A multi-scale study. Ecography. 2005;28:59–70. [Google Scholar]
- Koenig J, Shine R, Shea G. The dangers of life in the city: Patterns of activity, injury and mortality in suburban lizards (Tiliqua scincoides) J Herpetol. 2002;36:62–68. [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol. 2006;148:132–149. doi: 10.1016/j.ygcen.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Marquardt WC, Demaree RS, Grieve RB. Parasitology and vector biology. San Diego: Academic Press; 2000. [Google Scholar]
- McLean MA, Angilletta MJ, Williams KS. If you can't stand the heat, stay out of the city: Thermal reaction norms of chitinolytic fungi in an urban heat island. J Therm Biol. 2005;30:384–391. [Google Scholar]
- Moore IT, Jessop TS. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav. 2003;43:39–47. doi: 10.1016/s0018-506x(02)00038-7. [DOI] [PubMed] [Google Scholar]
- Moore IT, Mason RT. Behavioral and hormonal responses to corticosterone in the male red-sided garter snake, Thamnophis sirtalis parietalis. Physiol Behav. 2001;72:669–674. doi: 10.1016/s0031-9384(01)00413-9. [DOI] [PubMed] [Google Scholar]
- Moore MC. Elevated testosterone levels during nonbreeding-season territoriality in a fall-breeding lizard, Sceloporus jarrovi. J Comp Physiol A. 1986;158:159–163. doi: 10.1007/BF01338559. [DOI] [PubMed] [Google Scholar]
- Moore MC, Hews DK, Knapp R. Hormonal control and evolution of alternative male phenotypes: Generalizations of models for sexual differentiation. Am Zool. 1998;38:133–151. [Google Scholar]
- Moore MC, Thompson CW, Marler CA. Reciprocal changes in corticosterone and testosterone levels following acute and chronic handling stress in the tree lizard, Urosaurus ornatus. Gen Comp Endocrinol. 1991;81:217–226. doi: 10.1016/0016-6480(91)90006-r. [DOI] [PubMed] [Google Scholar]
- Morici LA, Elsey RM, Lance VA. Effects of long- term corticosterone implants on growth and immune function in juvenile alligators, Alligator mississippiensis. J Exp Zool. 1997;279:156–162. [PubMed] [Google Scholar]
- Munoz FJ, Galvan A, Lerma M, De la Fuente M. Seasonal changes in peripheral blood leukocyte functions of the turtle Mauremys caspica and their relationship with corticosterone, 17-beta-estradiol and testosterone serum levels. Vet. Immunol. Immunopathol. 2000;77:27–42. doi: 10.1016/s0165-2427(00)00228-2. [DOI] [PubMed] [Google Scholar]
- Parris KM, Hazell DL. Biotic effects of climate change in urban environments: The case of the grey-headed flying-fox (Pteropus poliocephalus) in Melbourne, Australia. Biol Cons. 2005;124:267–276. [Google Scholar]
- Partecke J, Schwabl I, Gwinner E. Stress and the city: Urbanization and its effects on the stress physiology in European Blackbirds. Ecology. 2006;87:1945–1952. doi: 10.1890/0012-9658(2006)87[1945:satcua]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reed RN, Shine R. Lying in wait for extinction: Ecological correlates of conservation status among Australian elapid snakes. Cons Bio. 2002;16:451–446. [Google Scholar]
- Rich EL, Romero LM. Exposure to chronic stress downregulates corticosterone responses to acute stressors. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1628–R1636. doi: 10.1152/ajpregu.00484.2004. [DOI] [PubMed] [Google Scholar]
- Rodriguez AB, Marchena JM, Harvey S, Barriga C, Lea RW. Immobilization stress and daily variations of melatonin and corticosterone in the serum of ring dove (Streptopelia risoria) Biogenic Amines. 2001;16:185–197. [Google Scholar]
- Romero LM, Reed JM. Repeatability of baseline corticosterone concentrations. Gen Comp Endocrinol. 2008;156:27–33. doi: 10.1016/j.ygcen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Romero LM, Meister CJ, Cyr NE, Kenagy GJ, Wingfield JC. Seasonal glucocorticoid responses to capture in wild free-living mammals. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 2008;294:R614–R622. doi: 10.1152/ajpregu.00752.2007. [DOI] [PubMed] [Google Scholar]
- Romero LM, Remage-Healey L. Daily and seasonal variation in response to stress in captive starlings (Sturnus vulgaris): Corticosterone. Gen Comp Endocrinol. 2000;119:52–59. doi: 10.1006/gcen.2000.7491. [DOI] [PubMed] [Google Scholar]
- Saad AH, Elridi R. Endogenous corticosteroids mediate seasonal cyclic changes in immunity of lizards. Immunobiology. 1988;177:390–403. doi: 10.1016/S0171-2985(88)80007-X. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrin Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schall JJ, Smith TC. Detection of a malaria parasite (Plasmodium mexicanum) in ectoparasites (mites and ticks), and possible significance for transmission. J. Parasitol. 2006;92:413–415. doi: 10.1645/GE-688R.1. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Bowman R, Reynolds SJ. Corticosterone, nutrition, and timing of reproduction in Florida Scrub-Jays. Horm Behav. 2004;46:121–121. [Google Scholar]
- Shochat E, Warren PS, Faeth SH, McIntyre NE, Hope D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol Evol. 2006;21:186–191. doi: 10.1016/j.tree.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Sorace A. High density of bird and pest species in urban habitats and the role of predator abundance. Ornis Fenn. 2002;79:60–71. [Google Scholar]
- Thompson CW, Moore MC. Behavioral and hormonal correlates ofalternative reproductive strategies in a polygynous lizard - tests of the relative plasticity and challenge hypotheses. Horm Behav. 1992;26:568–585. doi: 10.1016/0018-506x(92)90023-o. [DOI] [PubMed] [Google Scholar]
- Vleck M. Reproductive season and water-stress in A sonoran desert bird – the Curve-Billed Thrasher. Am Zool. 1984;24:A5–A5. [Google Scholar]
- Vleck CM, Vertalino N, Vleck D, Bucher TL. Stress, corticosterone, and heterophil to lymphocyte ratios in free-living Adelie Penguins. Condor. 2000;102:392–400. [Google Scholar]
- Wada M, Shimizu T. Seasonal changes in adrenocortical responses to acute stress in polygynous male bush warblers (Cettia diphone) Gen Comp Endocrinol. 2004;135:193–200. doi: 10.1016/j.ygcen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Walberg J. White blood cell counting techniques in birds. Seminars Avian Exotic Pet Med. 2001;10:72–76. [Google Scholar]
- Wang G, Richardson MI, Moore IT, Soma KK, Li DM, Lei FM, Wingfield JC. Seasonal regulation of adrenocortical response to stress in two species of snowfinches on the Tibet plateau. Integrat Comp Bio. 2006;46:E151–E151. [Google Scholar]
- Wilson BS, Wingfield JC. Correlation between female reproductive condition and plasma-corticosterone in the lizard Uta stansburiana. Copeia. 1992:691–697. [Google Scholar]
- Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone-behavior interactions: The "emergency life history stage". Am Zool. 1998;38:191–206. [Google Scholar]
- Woodley SK, Moore MC. Plasma corticosterone response to an acute stressor varies according to reproductive condition in female tree lizards (Urosaurus ornatus) Gen Comp Endocrinol. 2002;128:143–148. doi: 10.1016/s0016-6480(02)00068-0. [DOI] [PubMed] [Google Scholar]
- Woods M, McDonald RA, Harris S. Predation of wildlife by domestic cats Felis catus in great britain. Mamm Rev. 2003;33:174–188. [Google Scholar]