Abstract
Intraperitoneal (IP) chemotherapy prolongs survival of ovarian cancer patients, but its utility is limited by treatment-related complications and inadequate drug penetration in larger tumors. Previous IP therapy used the paclitaxel/Cremophor formulation designed for intravenous use. The present report describes the development of paclitaxel-loaded microparticles designed for IP treatment (referred to as tumor penetrating microparticles or TPM). Evaluation of TPM was performed using IP metastatic, human ovarian SKOV3 xenograft tumor models in mice. TPM were retained in the peritoneal cavity and adhered to tumor surface. TPM consisted of two biocompatible and biodegradable polymeric components with different drug release rates; one component released the drug load rapidly to induce tumor priming while the second component provided sustained drug release. Tumor priming, by expanding interstitial space, promoted transport and penetration of particulates in tumors. These combined features resulted in the following advantages over paclitaxel/Cremophor: greater tumor targeting (16-times higher and more sustained concentration in omental tumors), lower toxicity to intestinal crypts and less body weight loss, greater therapeutic efficacy (longer survival and higher cure rate), and greater convenience (less frequent dosing). TPM may overcome the toxicities and compliance-related problems that have limited the utility of IP therapy.
Recommended section: Chemotherapy, Antibiotics, Gene Therapy
Introduction
A majority of ovarian cancer patients present with stage III or IV disease, accompanied by local metastasis. The standard of treatment is surgical debulking, followed by intravenous chemotherapy with platinum and taxane analogs. Concomitant intraperitoneal (IP) chemotherapy has been under development for several decades. Multiple studies have demonstrated significant targeting advantage for IP chemotherapy in patients, with peritoneal cavity-to-systemic blood ratios of drug exposure (measured as area-under-concentration-time curves or AUC) ranging from 12 for cisplatin to 1,000 for paclitaxel (Zimm et al., 1987; Markman et al., 1992; Kerr et al., 1993}. Adding IP chemotherapy to intravenous chemotherapy produces significantly longer progression-free and overall survival (Gadducci et al., 2000; Markman et al., 2001; Armstrong et al., 2006a; Goldberg, 2006); the most recent NCI Cooperative Group trial (GOG 172) in stage III patients with <1 cm tumors showed a 16-month longer overall survival. However, toxicities and other issues have prevented concomitant intravenous plus IP therapy to become a standard of care (Markman and Walker, 2006; Ozols et al., 2006).
Toxicities of IP therapy are generally related to procedures for administration and the drugs administered (Markman et al., 1992; Elias and Sideris, 2003; Wenzel et al., 2007). The use of IP catheter is associated with higher risk of infection and fever, and occasionally physical damages to peritoneal tissues (e.g., perforation). While hematologic toxicity is a major toxicity for drugs rapidly absorbed into the systemic circulation (e.g., cisplatin, carboplatin, melphalan, etoposide), local toxicity is dose-limiting for drugs that are slowly absorbed (e.g., paclitaxel, mitoxantrone, doxorubicin) or drugs that induce chemical peritonitis (e.g., mitomycin, 5-fluorouracil, oxaliplatin) or ileus (e.g., docetaxel) (Alberts et al., 1988; Demicheli et al., 1985; Elias and Sideris, 2003; Howell et al., 1984; Markman et al., 1992; Monk et al., 1988; Morgan, Jr. et al., 2003; O'Dwyer et al., 1991; Zimm et al., 1987). The GOG 172 trial showed 3-times more patients on the IP plus intravenous arm did not complete the assigned 6-treatment cycle compared to the intravenous arm (58% vs 17%). For the former, 20% terminated early due to catheter-related complications (infection, blocked or leaky catheter, port access problems), 22% due to other toxicities (gastrointestinal toxicities including abdominal pain or stomach cramp, dehydration, renal/metabolic, catheter-unrelated infection) and 9% due to patient refusal (Armstrong et al., 2006a).
For IP chemotherapy, residual tumor size is the most significant prognostic indicator, with a better prognosis and longer survival interval in patients with smaller tumors (≤0.5 cm) compared to larger tumors (≥2 cm) (Topuz et al., 1998; Alberts et al., 1996; Markman et al., 1998). These findings led to the recommendation of using IP therapy in optimally surgically debulked, stage III patients with tumors of less than 1 cm (Armstrong et al., 2006a). The tumor size restriction is likely due to the limited drug penetration into larger tumors. This notion is supported by the observations that while cisplatin and carboplatin were about equally effective in ovarian cancer patients present with only positive margins (<0.5 cm), the analog that shows inferior penetration and 7-times lower drug levels in rodent tumors (i.e., carboplatin) also shows and inferior activity in patients with larger tumors (1–3 cm) (Los et al., 1990; Los et al., 1991; Markman et al., 1993).
During IP therapy, drug delivery to peritoneal tumors is from two sources. Recirculation of drug absorbed from the peritoneal cavity via the systemic circulation is a minor source due to the relatively low concentration in blood. The primary source is drug diffusion or convection through tumor interstitium. Our laboratory has developed the tumor priming technology that uses an apoptosis-inducing drug (paclitaxel or doxorubicin) to expand the interstitial space and thereby promote the interstitial transport of particulates (Zheng et al., 2001; Kuh et al., 1999; Jang et al., 2001b; Chen et al., 1998; Jang et al., 2001a). The finding that tumor priming is effective in vitro, in the absence of blood flow or vasculature, indicates expansion of interstitial space is a major mechanism for enhanced transport. Tumor priming can also reduce interstitial fluid pressure and thereby decompress tumor microvessels and enhance extravasation and convection-mediated transport (Griffon-Etienne et al., 1999). Tumor priming is tumor selective, due to the greater susceptibility of tumor cells to apoptosis compared to normal cells (Lu et al., 2007).
The present study extended our earlier findings to the development of 2-component, biocompatible, biodegradable, polymeric (poly(D,L-lactide-co-glycolide) or PLG), paclitaxel-loaded microparticles (referred to as tumor penetrating microparticles or TPM) for IP treatment. TPM was designed to address several major limitations of IP therapy, and, as shown in the results, has the following properties. (a) The size of particles was optimized to reduce clearance and to enable wide distribution in the cavity (i.e., minimal sedimentation). (b) The microparticles adhered to tumor surface. (c) TPM consisted of two types of paclitaxel-loaded microparticles with different drug release rates; i.e., a rapid release component to enable tumor priming (referred to as Priming TPM) and promote interstitial transport of remaining particles, and a slow release component to provide sustained drug levels (referred to as Sustaining TPM) and to reduce the need of frequent dosing or indwelling catheter. The fractionated drug release approach lowers the drug exposure toxicity to host tissues as compared to the bolus, all-at-once dose presentation as is the case for the intravenous paclitaxel formulation used in previous IP therapy (paclitaxel solubilized in Cremophor/ethanol, referred to as paclitaxel/Cremophor).
Methods
Chemicals and Reagents
Paclitaxel (Hande Tech, Houston, TX), cephalomannine (National Cancer Institute, Bethesda, MD), and 3"-3H-paclitaxel (specific activity, 10.6 Ci/mmol, National Cancer Institute or Moravek Biochemicals, Brea, CA) were found to be >99% pure by high performance liquid chromatographic (HPLC) analysis. Cefotaxime sodium was purchased from Hoechst-Roussel Inc. (Somerville, NJ), gentamicin from Solo Pak Laboratories (Franklin Park, IL), and all other cell culture supplies from Life Technologies (Grand Island, NY). PLG was purchased from Birmingham Polymers (Birmingham, AL), O.C.T. embedding matrix from Miles Inc. (Elkhard, IN), Cremophor EL and poly(vinyl alcohol) or PVA from Sigma Chemical (St. Louis, MO), and HPLC solvents from Fisher Scientific (Fair Lawn, NJ). All chemicals and reagents were used as received.
Preparation of Paclitaxel- or Fluorophore-loaded PLG Microparticles
TPM were loaded with paclitaxel and consisted of two components, i.e., Priming and Sustaining TPM, prepared with different compositions of PLG co-polymers. Priming TPM was to produce rapid drug release and tumor priming, and comprised of 50:50 L:G (inherent viscosity of 0.17 dl/g in hexafluoroisopropanol). Sustaining TPM was to provide sustained release of paclitaxel, and comprised of 75:25 L:G (inherent viscosity: 0.67 dl/g in hexafluoroisopropanol). TPM were prepared using solvent evaporation method as previously described (Tsai et al., 2007). Briefly, PLG and paclitaxel were co-dissolved in 5 ml of methylene chloride, and emulsified in 20 ml of 1% PVA aqueous solution by homogenization for 30 sec. The emulsion was mixed with 500 ml of 0.1% PVA, and stirred at 1000 rpm at room temperature and ambient pressure to evaporate the methylene chloride. The residual microparticles pellet was collected by centrifugation, washed three times with deionized water to remove residual PVA, lyophilized, and stored at 4°C. The size of microparticles was determined using scanning electron microscopy (SEM). At least 300 particles were measured. Number-based mean diameter (Dn) and volume-based mean diameter (Dv) were obtained. Polydispersity index (PDI) is a measurement of distribution, and was calculated as (D90% – D10%)/D50%, where D90%, D50% and D10% were the respective volume diameters at 90%, 50%, and 10% cumulative volumes. The respective Dn, Dv and PDI were 3.6 µm, 5.7 µm, and 0.78 for a representative batch of Priming TPM, and 3.8 µm, 5.2 µm, and 0.63 for a representative batch of Sustaining TPM. Priming TPM released paclitaxel rapidly (70% drug load in 24 hr), whereas Sustaining TPM released 1% daily. The paclitaxel loading was 4.1±0.5% (mean±SD of 3 batches of Priming TPM and 3 batches of Sustaining TPM).
Fluorophore-labeled PLG microparticles (4 and 30 µm, without paclitaxel) were prepared with 50:50 L:G PLG copolymers. The smaller microparticles were prepared as described above, with the exception that paclitaxel was replaced by rhodamine or acridine orange, which respectively showed red and yellow fluorescence under UV light. The larger microparticles were prepared by emulsifying the polymer solution in 20 ml of 1% PVA for 1 min using magnetic stirring instead of homogenization; the respective Dn, Dv and PDI were 28.1 µm, 34.5 µm, and 0.65.
Metastatic IP Ovarian Tumor Models
Human ovarian SKOV3 xenograft tumors were maintained in female athymic BALB/c Nu/Nu mice (Charles River/NCI Laboratories; Wilmington, MD). Animals were cared for in accordance with institutional guidelines. SKOV3 tumor cells (ATCC, Manassas, VA) were maintained in McCoy’s media containing 9% fetal bovine serum, 2 mM L-glutamine, 90 µg/ml gentamicin, and 90 µg/ml cefotaxime sodium at 37°C in a humidified atmosphere of 5% CO2 in air. A metastatic subline was established by serial re-implantation of cells collected from peritoneal washings of mice given IP injections of the parent cells. Injection of the metastatic cells (2×107) into the peritoneal cavity yielded established tumors in 100% animals (n>50). In late stage (e.g., 6 weeks after injection of tumor cells), some mice showed tumors invading the parenchyma of visceral organs such as liver and kidney. Protein concentration in peritoneal fluid increased from 3% in normal mice to about 6% in tumor-bearing mice at 2 weeks. The volume of peritoneal fluid increased 7–10 folds after 4 weeks, and contained aggregates of tumor cells (mostly 5–10 cells). Tumor dissemination and disease progression of the metastatic IP SKOV3 model showed similarity to the following observations in late-stage ovarian cancer patients: (a) tumors appearing in bowel serosa, perihepatic and perisplenic ligaments, diaphragm, mesentery, and omentum, (b) high protein concentrations in peritoneal fluid (about 4% in late stage disease), due to leakage of serum proteins and/or presence of ascites in the peritoneal cavity, and (c) presence of tumor cell aggregates of similar size in ascites fluid (Tauchi et al., 1996).
Dose Preparation and Administration
Paclitaxel/Cremophor was prepared by dissolving paclitaxel in Cremophor:ethanol (1:1) and diluted with normal saline immediately before injection. TPM were suspended in either phosphate-buffered saline (PBS, pH=7.4) or physiological saline with 0.01% Tween 80 and injected IP into animals using 25 G needles. Anesthesia was attained using inhalation Isofluorane® (diluted to 15% in light mineral oil, Abbott Laboratories, North Chicao, IL), and euthanizaed using Isofluorane® overdose.
Microparticles Distribution in Peritoneal Cavity
Distribution of PLG microparticles within peritoneal cavity was evaluated in tumor-free or tumor-bearing animals. In the latter case, treatments were administered at 6 weeks after tumor implantation. Mice were given IP injection of 10 mg/ml of rhoadmine-loaded PLG microparticles dispersed in PBS. The two control groups received either rhodamine dissolved in PBS, or a combination of rhodamine in PBS plus drug-free blank microparticles. Mice were euthanized 24 hr later. The peritoneal cavity was exposed and examined under UV light at 254 nm.
Effects of Tumor Priming on Particle Penetration in Tumors
Spatial distribution of particles in tumors was studied using fluorescence-labeled latex beads (2 µm diameter, respective excitation and emission wavelengths of 580 and 605 nm; Molecular Probes, Eugene, OR). Mice (n=3 per data point) received either IP injection of Priming TPM (40 mg/kg paclitaxel equivalent), drug-free blank particles, or paclitaxel/Cremophor (40 mg/kg), followed by an IP injection of latex beads (2% w/v suspension, diluted 10-fold with physiologic saline, 0.5 ml per 25 g mouse) at 48, 144 and 216 hr. Another 24 hr later, mice were anesthetized and tumors located on the omentum were harvested, rinsed, blot-dried, embedded in the O.C.T matrix (Miles Inc., Elkhard, IN), and flash-frozen in the liquid nitrogen. The procedures were completed in less than 5 min. Tumors were cut into 20 µm sections using a cryotome at −30°C. The frozen sections were thaw-mounted onto glass slides and stored at 4°C.
Penetration of fluorescent latex beads in tumors was quantified as follows. Frozen sections were examined at low magnification (25×) to select the regions with the highest fluorescence signals or hot spots, which were photographed at 100× magnification. The regions containing connective or adipose tissues were avoided. We analyzed 3 fields per section, for a total of 10–12 sections per tumor, and 3 to 6 tumors per treatment group. The dispersion and total uptake of latex beads in tumors were quantified using Optimas® image analysis software (Mediacybernetics, Silver Spring, MD). Threshold luminescence intensity was determined by averaging the luminescence intensity (expressed in grey value, range 0∼255 units) of at least 5 images in a tumor section devoid of latex beads. For bead-containing tumor sections, pixels with luminescence intensity exceeding the threshold value were identified as the bead-occupied pixels. Dispersion of beads was quantified as (number of bead-occupied pixel) normalized by (total number of pixels of tumor-occupied region per 100× field). Accumulation of beads was measured as total luminescence intensity, i.e., multiplication product of (mean luminescence intensity of bead-occupied pixels) and (total number of bead-occupied pixels), normalized by (total number of pixels of tumor-occupied region per 100× field). Normalization was necessary to ascertain that the measurement was not confounded by unavoidable variations in the fraction of tumor-occupied area per field, and correctly reflected the number of beads in tumors.
Effects of TPM on Disposition of Paclitaxel in Tumors in vivo
Tumor-bearing mice were given IP treatments and euthanized at predetermined times. Tumor nodules (3–6 mm diameter) were excised, rinsed free of residual drug-containing peritoneal fluid using distilled water, and blot-dried. Drug levels in tumors were studied in two ways. In the first study, animals were given non-radiolabeled drug (10 mg/kg paclitaxel dose in Cremophor or Priming TPM) and the excised tumors were homogenized, and extracted and analyzed for total drug concentration using HPLC.
The second study used autoradiography to determine spatial drug distribution in tumors. Mice were treated with paclitaxel/Cremophor, Priming TPM, Sustaining TPM, or 2-component TPM (1:1 Priming: Sustaining). The total paclitaxel dose was 20 mg/kg for Cremophor and single component TPM groups and 40 mg/kg for the 2-component TPM group. All treatments consisted of a mixture of 3H-labeled and nonradiolabeled drug (1.6 mCi/20 mg paclitaxel). Tumors were flash-frozen and cut into 20 µm sections. In order to minimize potential data variation due to unavoidable differences in tumor size and shape, comparison of drug penetration used tumor sections obtained at equal depths, i.e., sequential sections obtained at each 200 µm depth. Autoradioluminographic images were captured by Quest Pharmaceutical Services, LLC (Newark, DE). Commercially available, pre-calibrated microscale tritium autoradiography standards (0.1 to 109.4 nCi/mg; Amersham Biosciences Corp., Piscataway, NJ) were placed onto glass slides holding tumor sections. Slides were placed against Fuji BAS-TR phosphor imaging plates (Stamford, CT) for 1 week at room temperature, and signals were scanned using Typhoon imaging system (Amersham Biosciences Corp., Piscataway, NJ). The limit of detection was 1–2 nCi/mg. Pixel gray-scale analysis was performed using Optimas® software. Drug concentration as a function of distance from tumor periphery was quantified by averaging the gray-scale intensities in four perpendicular directions spanning the length of the tumor; this procedure was to minimize the location-related variability within a tumor. Background radioactivity was determined by measuring signals in tumors obtained from control animals, which were typically <10% of the intensity in experimental groups. After correcting for background, drug concentrations were calculated using standard curves, and areas-under-concentration-depth-curves from periphery to 2 mm depth (AUC) were calculated using the trapezoid rule.
HPLC Analysis of Paclitaxel
Paclitaxel was extracted from aqueous samples with ethyl acetate using cephalomannine as the internal standard, and analyzed with column-switching HPLC assay, as previously described (Song and Au, 1995). For measuring paclitaxel levels in TPM, microparticles were dissolved in methylene chloride and analyzed without extraction. HPLC stationary phase consisted of a cleanup column (Nova-Pak C8, 4 µm particle, 3.9 mm × 75 mm; Waters, Milford, MA) and an analytical column (Bakerbond C18, 5 µm particle, 4.6 mm × 250 mm; Mallinckrodt Baker, Phillipsburg, NJ). Samples were injected into the cleanup column and eluted with a mobile phase consisting of 37.5% acetonitrile at a rate of 1.0 ml/min. The analytical mobile phase consisted of 49% acetonitrile and was passed through the analytical column at 1.2 ml/min. Paclitaxel was detected by UV absorbance at 229 nm; the lower limit of detection was 1 ng per injection.
Antitumor Activity
The in vivo therapeutic efficacy in tumor-bearing mice was measured as increase in overall survival time. Drug treatment was initiated on day 28, which equaled one-half of the median survival time of control group. Increase in life span (ILS) was calculated as [(median survival time of treatment group minus 28 days) divided by (median survival time of control group minus 28 days) × 100%] minus 100%. Mice received physiological saline, paclitaxel/Cremophor or TPM at equal mg and equi-toxic dose.
Post-mortem autopsy was performed to evaluate the cause of death. Typically, deaths that occurred within 10 days post-treatment were considered treatment-related deaths (e.g., >15% body weight loss, internal hemorrhage due to faulty injections). Deaths that occurred at later times and accompanied by presence of large tumor nodules (e.g., > 4 mm) and/or tumor infiltration into organs were considered deaths due to disease progression.
An earlier phase I trial of IP paclitaxel-loaded p(DAPG-EOP) particles (average size, 53 µm) revealed extensive, diffuse adhesions in a patient (Armstrong et al., 2006b). Hence, we evaluated whether TPM caused adhesion using the same definition in an earlier animal study, i.e., an abnormal connection between intra-abdominal contents that could not be disrupted by gentle separation is adhesion and microparticles attached to an intra-abdominal surface but not causing apposition of two surfaces are not adhesion (Kohane et al., 2006).
Gastrointestinal Toxicity
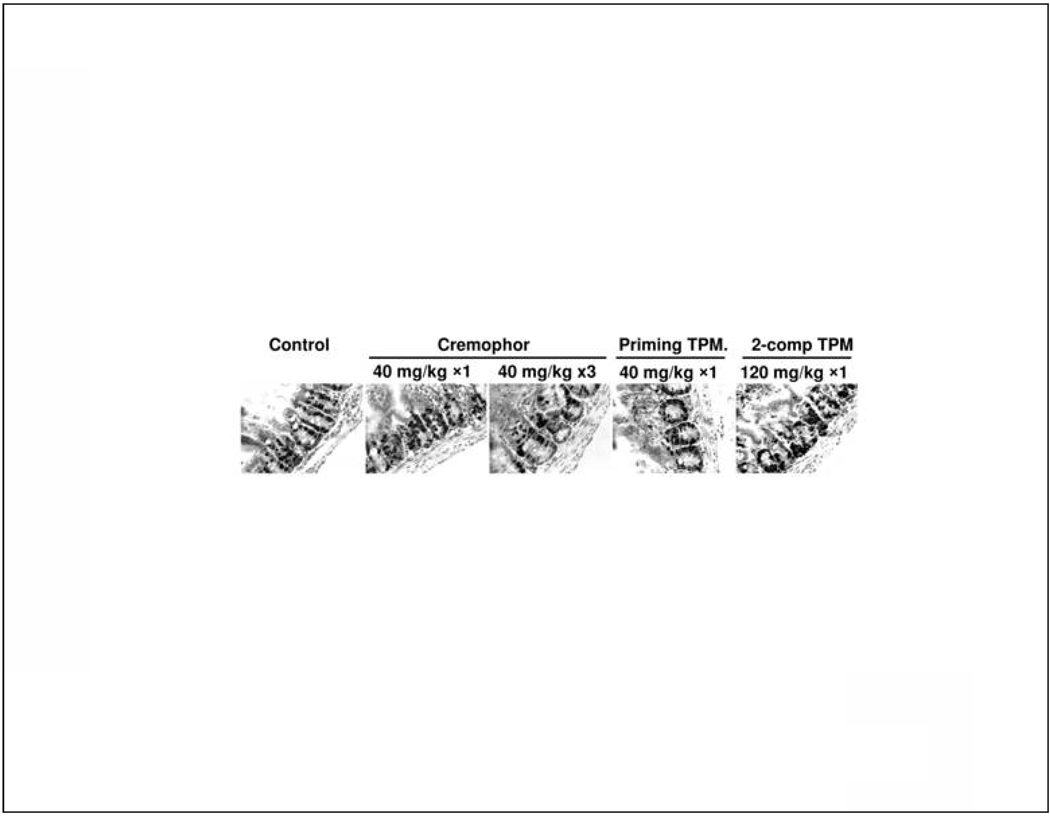
Gastrointestinal toxicity of IP paclitaxel was monitored by changes in body weight and labeling index of intestinal crypts. Tumor-free mice were given IP injections of paclitaxel/Cremophor (single dose of 40 mg/kg/day, 3 doses of 40 mg/kg on 3 consecutive days), Priming TPM at 40 mg/kg (single dose), or 2-component TPM at 120 mg/kg (1:2 Priming:Sustaining, single dose). Control group received physiological saline. Mice in single dose groups were euthanized at 24 hr post-treatment and mice in multiple dose and 2-component TPM groups were euthanized at 120 hr after the initial treatment. At 1 hr before euthanization, a DNA precursor bromodeoxyuridine (BrdU, 100 mg/kg) was injected intravenously. A segment of small intestine (jejunum) were excised, flushed with physiological saline, embedded in paraffin, and processed for immunostaining by BrdU using previously described methods (Gan et al., 1996).
Statistical Analysis
Survival data were analyzed with the log rank test between different treatment groups and Kaplan-Meier plots. Analysis used SAS software (Cary, NC). For the analysis of particle penetration data and intestinal crypts BrdU labeling data, comparisons between two groups used unpaired Student's t-test and comparisons between three groups used one-way ANOVA with post-hoc Tukey's test. Two-sided p values of less than 5% were considered statistically significant.
Results
Effects of Particle Size on Intraperitoneal Distribution and Tumor Localization
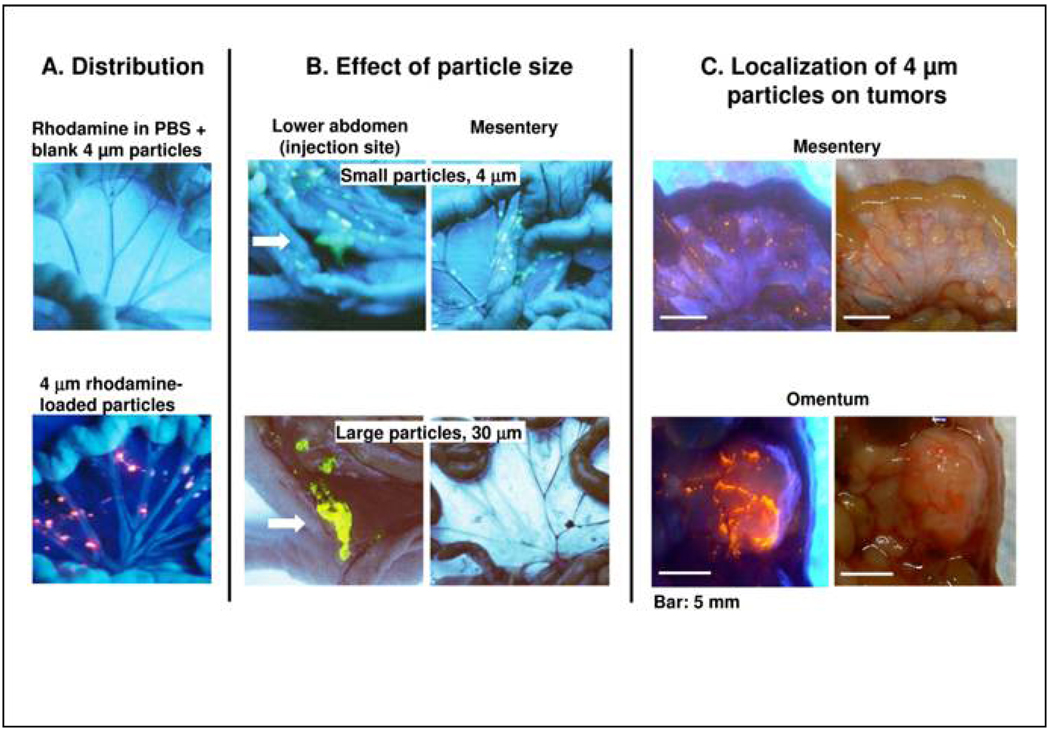
We first studied the distribution and retention of microparticles in peritoneal cavity, in tumor-free mice. Mice were treated with free rhodamine (dissolved in PBS), free rhodamine plus unlabeled microparticles (4 µm), or rhodamine-labeled microparticles (4 µm). In the first two groups, the fluorescence was evenly distributed throughout the abdominal cavity at early time points (e.g., 15 min) but rapidly declined to a level not distinguishable from background auto-fluorescence at 24 hr; the results for the second group are shown in Figure 1A (top panel). In contrast, mice treated with rhodamine-labeled microparticles showed clusters of strong fluorescence signals localized in the folds of gastrointestinal tract and other tissues at 15 min and remained detectable on the surface of diaphragm, omentum and mesentery at 24 hr (Figure 1A, bottom panel).
Figure 1. Intra-abdominal distribution of polymeric microparticles.
(A) Distribution. Tumor-free mice were given IP injections of rhodamine dissolved in vehicle (0.01% Tween 80 in PBS) plus blank microparticles (top panel), or rhodamine-labeled microparticles (bottom panel). Rhodamine appears red under UV light. (B) Effect of particle size. Tumor-free mice were given IP injections of acridine orange-labeled microparticles with average diameters of 4 or 30 µm. Acridine orange appears yellow under UV light. The smaller particles were dispersed throughout the cavity and on mesenteric membrane and omentum that are common sites of local metastases of ovarian tumors. The larger particles were localized in lower abdomen and were absent on mesenteric membrane and omentum. Arrow indicated injection site. (C) Localization of 4 µm particles on tumors. Mice were implanted with IP human ovarian SKOV3 xenograft tumors. After tumors were established (day 42), a mouse was given an IP dose of rhodamine-labeled microparticles. Three days later, the animal was anesthetized and the abdominal cavity exposed. Photographs were taken in the region of omentum and mesentery under UV light (left panel) and room light (right panel). Note the large tumor on omentum (∼13 mm longest diameter) and multiple small tumors on mesenteric membrane (1–3 mm longest diameter). Red color under UV light indicated localization of rhodamine-labeled particles on tumor surface.
We next studied the effect of particle size on distribution (4 and 30 µm, labeled with acridine orange), also in tumor-free mice. The smaller particles were widely dispersed throughout the cavity including omentum, mesentery, diaphragm and lower abdomen, whereas the larger particles were primarily localized in lower abdomen near the injection site (Figure 1B).
The above data confirm our earlier finding that drug carriers significantly affect drug disposition within the peritoneal cavity (Tsai et al., 2007), and indicate microparticles as a useful tool to promote drug retention and distribution during IP therapy. The 4 µm microparticles were selected for subsequent studies in tumor-bearing mice. The results showed these particles (rhodamine-labeled) were localized on the surface of tumor nodules (Figure 1C) and visibly absent on the surface of peritoneum and other IP organs (not shown), indicating preferential adherence of microparticles to tumor surface.
Effects of Tumor Priming Treatments on Particle Penetration
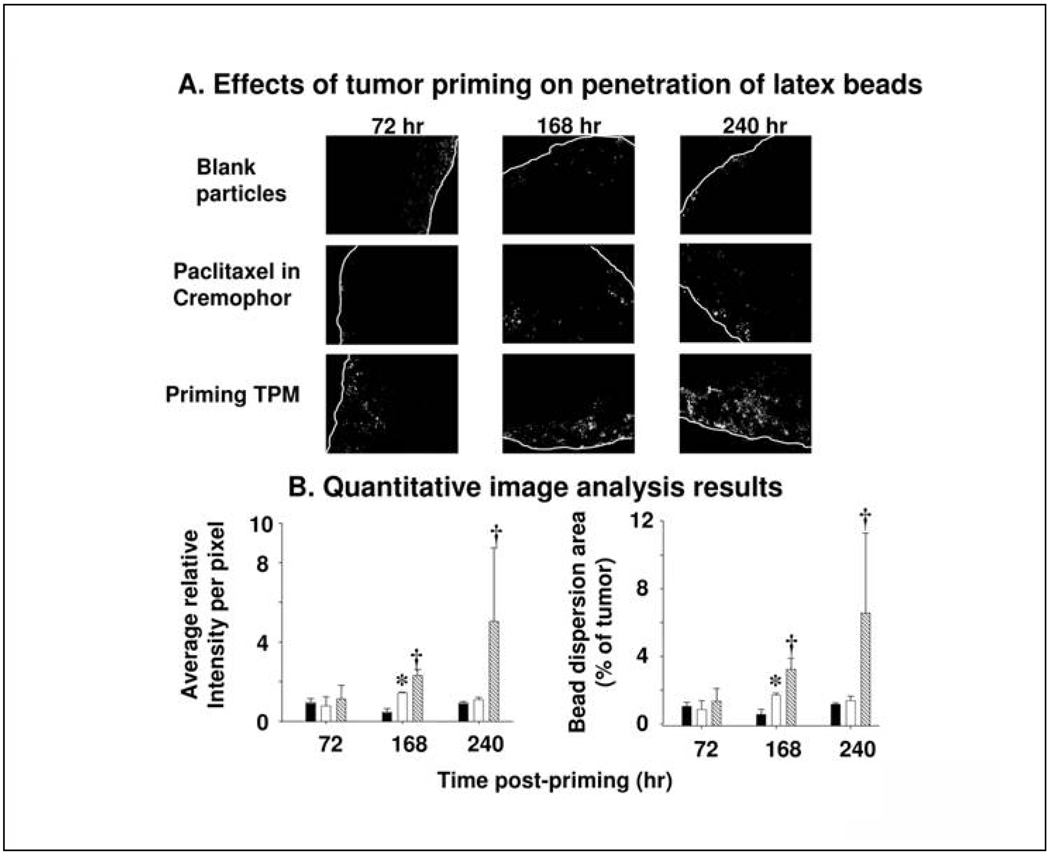
This study compared the efficiency of Priming TPM and paclitaxel/Cremophor for tumor priming, using micron-sized, drug-free fluorescent latex beads as the penetrant. The control group treated with IP blank particles showed restricted dispersion of latex beads on tumor periphery, whereas both tumor priming groups showed deeper penetration, wider dispersion, and greater total uptake of latex beads (Figure 2A). Among the two priming groups, results of quantitative image analysis showed significantly greater uptake as well as enhanced dispersion of latex beads in Priming TPM group compared to paclitaxel/Cremophor, especially at the later time point of 240 hr (Figure 2B), indicating greater and more sustained tumor priming for TPM.
Figure 2. Tumor priming promoted penetration of latex beads in IP SKOV-3 tumor nodules.
Mice bearing IP SKOV3 tumors were given an IP injection of a tumor priming treatment with either paclitaxel/Cremophor or Priming TPM (40 mg/kg), followed by an IP injection of fluorescent latex beads (2 µm diameter) given 48, 144 and 216 hr later. The dose of latex beads was 40 mg/kg (2% solid, 10 fold dilution in normal saline, 0.5 ml per 25 gram mice). Control group received blank, drug-free microparticles (i.e. no tumor priming pretreatment). (A) Representative tumor sections showing amount and dispersion of latex beads in tumors. Beads showed red fluorescence (shown as white dots in the black-and-white pictures). White lines indicate the outer perimeter of tumor nodules. 100× magnification. (B) Quantitative image analysis results. The amounts of latex beads in tumors are expressed as (total fluorescence intensity) normalized by (tumor area); a higher value indicates a greater amount. The bead dispersion results are expressed as percentages of tumor occupied by beads, a higher value indicates a greater dispersion. Solid bars: blank particles. Open bars, paclitaxel/Cremophor. Hatched bars: TPM. Error bars show 95% confidence intervals *: Elevated bead amounts or dispersion in paclitaxel/Cremophor group compared to blank particle group at corresponding time points (p<0.05, Student's t-test). †: Elevated bead amounts or dispersion in TPM group compared to paclitaxel/Cremophor or blank particle groups (p<0.05, ANOVA with post-hoc Tukey's test).
In vivo Tumor Targeting by TPM
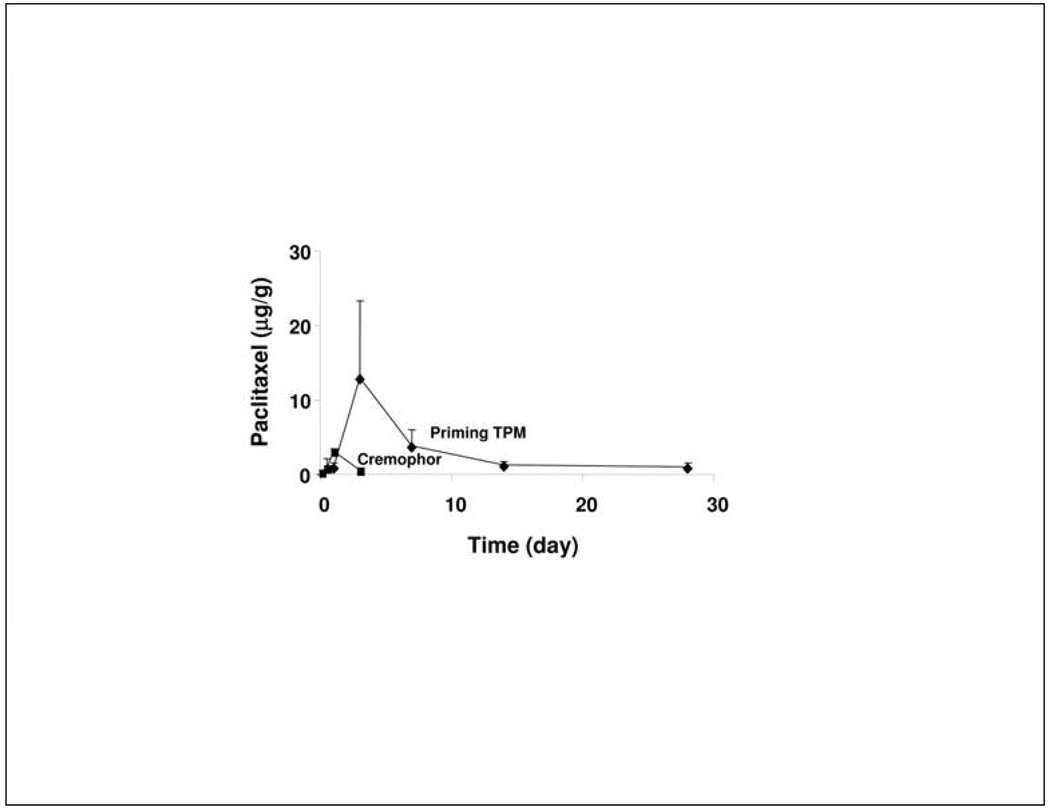
This study compared the tumor targeting of IP paclitaxel/Cremophor and Priming TPM at equal mg doses. Because drug penetration is dependent on tumor size (e.g., easier penetration in smaller tumors) and tumor location (e.g., greater drug exposure for tumors in contact with peritoneal fluid), the comparison was limited to tumors located on the omentum and of comparable sizes (3 to 6 mm diameter). HPLC analysis of total paclitaxel concentrations showed different kinetics for the two treatments (Figure 3). The paclitaxel/Cremophor treatment resulted in concentrations (Cmax) that peaked at an earlier time (24 hr) and declined more rapidly (below the detection limit of 0.5 µg/g at 72 hr), whereas TPM yielded slower uptake (Cmax at 72 hr) and slower decline (remained detectable for at least 28 days). TPM also yielded 4-fold higher Cmax and 16-fold higher area under concentration-time curve (AUC).
Figure 3. Tumor targeting advantage of TPM: HPLC results.
Mice bearing IP SKOV3 tumors were given IP injections of either paclitaxel/Cremophor or TPM (10 mg/kg). At predetermined times, tumors located on omentum were removed and analyzed for paclitaxel concentrations using HPLC. Compared to paclitaxel/Cremophor, TPM yielded 4-fold higher maximum concentration (13 vs 3.2 µg/g) and 16-fold higher AUC (82 vs 5 µg-day/g).
TPM Enhanced Paclitaxel Penetration and Spatial Distribution in Tumors in vivo
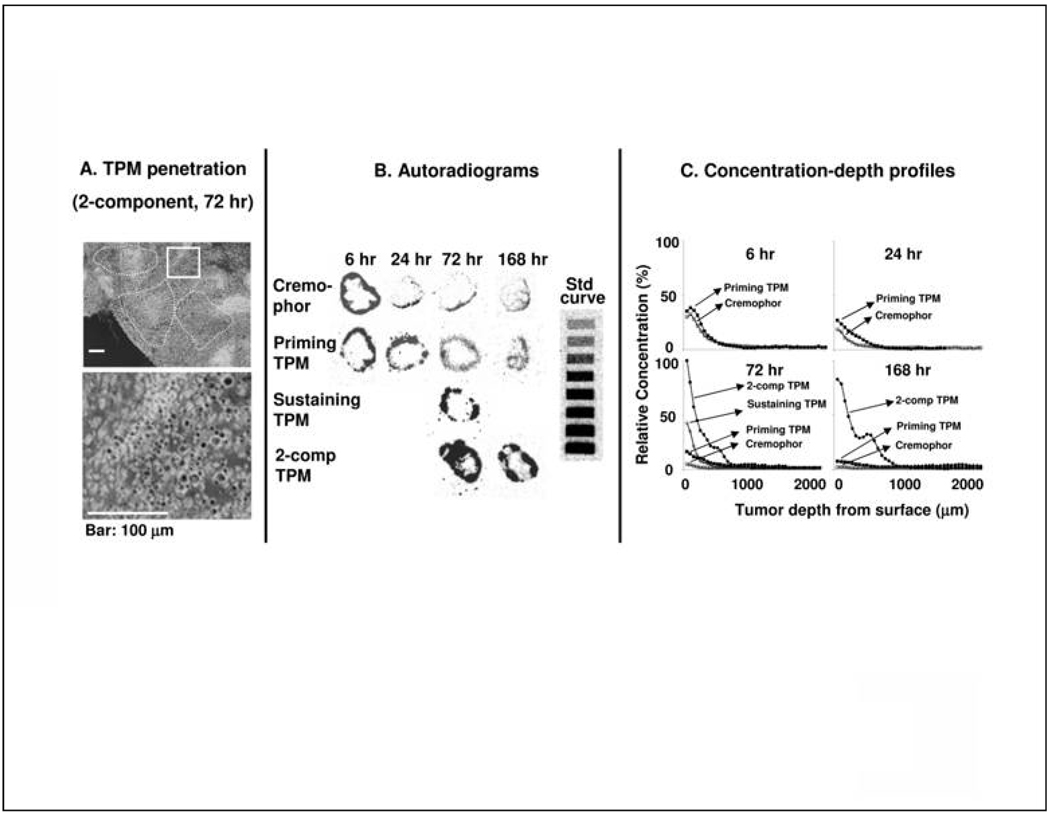
We tested the hypothesis that two-component TPM, due to the tumor priming property of Priming TPM and the sustained drug release from Sustaining TPM, improves the drug penetration and retention in tumors. Figure 4A shows the micrographs of sections of omental tumors excised from animals 72 hr after treatment; clusters of microparticles were observed in tumor interior, indicating TPM penetration into tumors.
Figure 4. Effect of tumor priming on spatial drug distribution in tumors: Autoradiographic results.
Mice bearing IP SKOV3 tumors were given IP injections of either paclitaxel/Cremophor or Priming TPM or Sustaining TPM (all at 20 mg/kg), or 2-component TPM 40 mg/kg, 1:1 Priming:Sustaining). (A) TPM penetration into tumor interior. An omental tumor was removed from a mouse at 72 hr after treatment with 2-component TPM, sectioned and stained with Hematoxylin and Eosin. The image was converted using Photoshop and TPM appeared as black dots. Top panel shows areas with clusters of TPM (circumscribed with dotted lines). Bottom panel shows the enlarged picture of the boxed area. (B) Autoradiograms of tumor sections (see Methods). (C) Concentration-depth profiles. Autoradiograms shown in B were processed to obtain measurements of total radioactivity using computer-assisted densitormetric analysis (see Methods). Radioactivity was expressed as paclitaxel-equivalents, with the highest level set at 100%.
We next used autoradiography to compare the spatial distribution of 3H-paclitaxel. The comparison was restricted to omental tumors of comparable sizes (as in the HPLC study) and to tumor mid-sections having the longest diameter. Applying these criteria across different treatment groups minimized differences due to unavoidable heterogeneities in tumor size and shape.
Figure 4B shows the autoradiograms in tumors removed from animals treated with paclitaxel/Cremophor, Priming TPM or Sustaining TPM (all at 20 mg/kg), or two-component TPM (40 mg/kg, 1:1 Priming:Sustaining). All four groups showed the highest concentration at tumor surface (Cmax,tissue), followed by a decline with increasing distance from the periphery. All three groups treated with different compositions of TPM showed higher concentrations and deeper penetration compared to the group treated with the Cremophor carrier. Figure 4C shows the concentration-depth profiles obtained from densitometric analysis of the autoradiograms; the data represented relative total concentrations and not the absolute concentrations because the actual weights of tissues on the tape sections could not be determined (Tsai et al., 2007). Due to the scarcity of the radiolabeled drug and the lower yield of Sustaining TPM, the 2-component and Sustaining TPM groups were studied only at the later time points (72 and 168 hr). Table 1 summarizes the results. With respect to changes in concentrations over time, paclitaxel/ Cremophor and TPM groups showed similar patterns, with the highest concentrations attained at the earliest time point of 6 hr followed by a decline with increasing time. With respect to rate of drug removal as shown by changes of Cmax,tissue with time, the decline was most rapid in paclitaxel/Cremophor group (14-fold decrease over 168 hr), followed by Priming TPM (5-fold decline over 168 hr) and 2-component TPM (<20% decline over 96 hr). With respect to total drug delivery, Priming TPM group showed significantly higher AUC compared topaclitaxel/Cremophor group, whereas 2-component TPM group showed dose-adjusted Cmax,tissue and AUC values that exceeded the individual values or the sums in the former two groups. The higher levels in the three TPM groups, relative to paclitaxel/Cremophor, could have resulted from the localization of TPM on tumor surface and interior.
Table 1. Drug penetration in peritoneal tumors.
Mice bearing IP SKOV3 tumors were given IP injections of either paclitaxel/Cremophor or Priming TPM or Sustaining TPM (all at 20 mg/kg), or 2-component TPM 40 mg/kg, 1:1 Priming:Sustaining); paclitaxel was radiolabeled. At predetermined times, tumors were excised, processed for autoradiography, and the radiographic signals were converted to drug concentrations, as described in Methods. Cmax,tissue is the maximum concentration found in the outer tumor perimeter in all treatment groups. Data are presented as relative concentrations, with the highest level set at 100%. AUCdepth represents area-under-concentration-depth curve. NA, not available. Data are mean ± SD (n=3 or 4 animals).
| 6 hr | 24 hr | 72 hr | 168 hr | |
|---|---|---|---|---|
| Cmax,tissue: % | ||||
| Paclitaxel/Cremophor | 32.4 ± 13.8 | 18.9 ± 10.5 | 4.8 ± 1.5 | 2.3 ± 1.1 |
| Priming TPM | 38.2 ± 14.6 | 31.2 ± 2.8 | 16.4 ± 10.6 | 7.7 ± 3.5 |
| Sustaining TPM | NA | NA | 42.9 ± 11.4 | NA |
| 2-Component TPM | NA | NA | 100.0 ± 33.2 | 82.7 ± 10.7 |
| AUCdepth: %*mm | ||||
| Paclitaxel/Cremophor | 11.5 ± 6.9 | 6.7 ± 1.2 | 2.8 ± 1.2 | 1.9 ± 0.8 |
| Priming TPM | 14.5 ± 7.0 | 9.6 ± 1.9 | 7.5 ± 3.6 | 5.2 ± 3.0 |
| Sustaining TPM | NA | NA | 9.5 ± 2.6 | NA |
| 2-Component TPM | NA | NA | 28.0 ± 17.0 | 31.9 ± 6.0 |
It is noted that the autoradiographic results differed from the HPLC results in that the former showed decreasing concentrations in tumors whereas the latter showed increasing paclitaxel concentrations, from 24 to 72 hr. As the two measurements used different parts of tumors (i.e., HPLC analysis used the whole tumor whereas autoradigraphy used only the widest part of a tumor), the different results could be due to unavoidable regional heterogeneities. For example, a greater drug accumulation in tumor periphery would result in a higher total concentration by HPLC. Moreover, autoradiography detected total radioactivity and did not distinguish the unchanged paclitaxel from its metabolites whereas HPLC measured only the unchanged drug.
Collectively, the above data indicate that Priming TPM and/or Sustaining TPM provided more favorable delivery of paclitaxel to tumors compared to the Cremophor carrier, and that Sustaining TPM produced sustained tumor priming and/or drug retention in tumors.
Comparison of Toxicity of TPM and Paclitaxel/Cremophor
Treatment-related toxicity was monitored as body weight loss and inhibition of intestinal crypt labeling index. The results are shown in Table 2 and Figure 5. For body weight loss, all tested treatments for paclitaxel/Cremophor (1×40 mg/kg, 4×10 mg/kg over 2 weeks, 8×15 mg/kg over 4 weeks) and TPM (40 mg/kg Priming TPM, 80 mg Sustaining TPM, 120 mg/kg 1:2 Priming:Sustaining ; all given as single dose) produced a maximum of less than 10% body weight loss in two days, followed by recovery to the baseline level in 3–7 days. In addition, no reduction of intestinal crypt labeling index was observed for a single dose of paclitaxel/Cremophor (1×40 mg/kg) or TPM (40 mg/kg Priming TPM or 120 mg 2-component TPM), compared to untreated control group. Reduction of intestinal crypt labeling index was observed at a more dose-dense schedule of paclitaxel/Cremophor (3 daily doses of 40 mg/kg for a total of 120 mg/kg). These results suggest that the intestinal toxicity of paclitaxel was determined to a greater extent by the dosing schedule than by the total dose. The lower toxicity for less dose-dense schedules supports the use of slow release TPM.
Table 2. Comparison of antitumor activity and overall toxicity.
Mice were implanted with metastatic IP SKOV3 tumor cells. Treatment was initiated on day 28 after tumor implantation, which was about 50% of the median survival time (MST) of control group. Treatment consisted of physiological saline (Control), paclitaxel/Cremophor (single dose of 40 mg/kg or multiple, twice-weekly doses at 4 doses of 10 mg/kg or 8 doses at 15 mg/kg), Priming TPM (single dose of 40 mg/kg), Sustaining TPM (single dose of 80 mg/kg), or 2-component TPM (120 mg/kg total dose, 1:2 Priming:Sustaining). Increase in life span or ILS was calculated as [(median survival time of treatment group minus 28 days) divided by (median survival time of control group minus 28 days) × 100%] minus 100%. In some treatment groups (with long term cures), the upper limit of 95% confidence interval (CI) was not reached (experiments were terminated on day 163 or 174). Statistical significance for the differences between groups was analyzed using the log rank test.
| A. Treatment efficacy and toxicity | ||||||
|---|---|---|---|---|---|---|
| Group | n | Body Weight | Cure | MST (95% CI) | ILS |
|
| Change, % | % | days | Day | % | ||
| Control | 12 | −0.8 | 0 | 52 (48 – 58) | 24 | 0 |
| Paclitaxel/Cremophor (40 mg/kg) | 17 | −8 | 0 | 91 (85 – 93) | 63 | 163 |
| Paclitaxel/Cremophor (10 mg/kg × 4) | 8 | −3.5 | 0 | 72 (63 – 77) | 44 | 83 |
| Paclitaxel/Cremophor (15 mg/kg × 8) | 8 | −4.7 | 25 | 109 (49 – 174+) | 81 | 238 |
| Priming TPM (40 mg/kg) | 8 | −6.9 | 25 | 81 (76 – 163+) | 53 | 121 |
| Sustaining TPM (80 mg/kg) | 9 | −0.8 | 22 | 103 (101 – 107) | 75 | 213 |
| 2-Component TPM (120 mg/kg) | 9 | −7.2 | 33 | 117 (113 – 163+) | 89 | 271 |
| B. Statistical significance of between-group differences in ILS | |
|---|---|
| Group comparison | P value |
| Control vs each of six treatment groups | <0.003 |
| Single vs multiple divided doses of paclitaxel/Cremophor (40 mg/kg) | 0.30 |
| Priming TPM vs single dose of paclitaxel/Cremophor | 0.54 |
| Priming TPM vs multiple doses of paclitaxel/Cremophor (40 mg/kg) | 0.045 |
| Priming TPM vs 2-component TPM | 0.13 |
| 2-component TPM vs single dose of paclitaxel/Cremophor | <0.0001 |
| 2-component TPM vs multiple doses of paclitaxel/Cremophor (120 mg/kg) | 0.45 |
Figure 5. IP TPM produced less intestinal toxicity compared to IP paclitaxel/Cremophor.
Mice were given IP injections of paclitaxel/Cremophor at 40 mg/kg (single dose, or 3 daily doses over 3 consecutive days), Priming TPM at 40 mg/kg (singe dose), or 2-component TPM at 120 mg/kg (1:2 Priming:Sustaining single dose). Control group received physiological saline. Mice in the single dose groups were euthanized at 24 hr post-treatment and mice in the multiple dose group and 2-component TPM were euthanized at 120 hr after the initial treatment. Intestinal crypts were labeled by BrdU (brown color, shown as black dots in black-and-white figures). From left to right, the labeling index per crypt was 35.6±2.9%, 42.8±3.9%, 15.8±4.4%, 33.2±2.2% and 38.7±2.3% (mean±95% CI ,3 mice per group with at least 20 crypts counted). The group that received 3 doses of the Cremophor formulation had significantly lower labeling index compared to all other groups (p<0.05, ANOVA).
None of the 26 mice treated with a single dose of TPM (8 mice with 40 mg/kg Priming TPM, 9 with 80 mg/kg Sustaining TPM, and 9 with 120 mg/kg 2-component TPM) showed adhesion at the end of experiments (between day 42 to 135).
Treatment-induced lethality was observed only in the single dose paclitaxel/Cremophor group (2/15 or 13.3%); one animal died within 1 day and a second animal died 1 week later. The second animal showed significant body weight loss (26%). Neither death appeared to be due to tumor burden as the tumor size was several-times smaller compared to animals which died later from excessive tumor burden. A possible cause of death was accidental needle puncture of peritoneal organs.
In vivo Efficacy and Toxicity of TPM
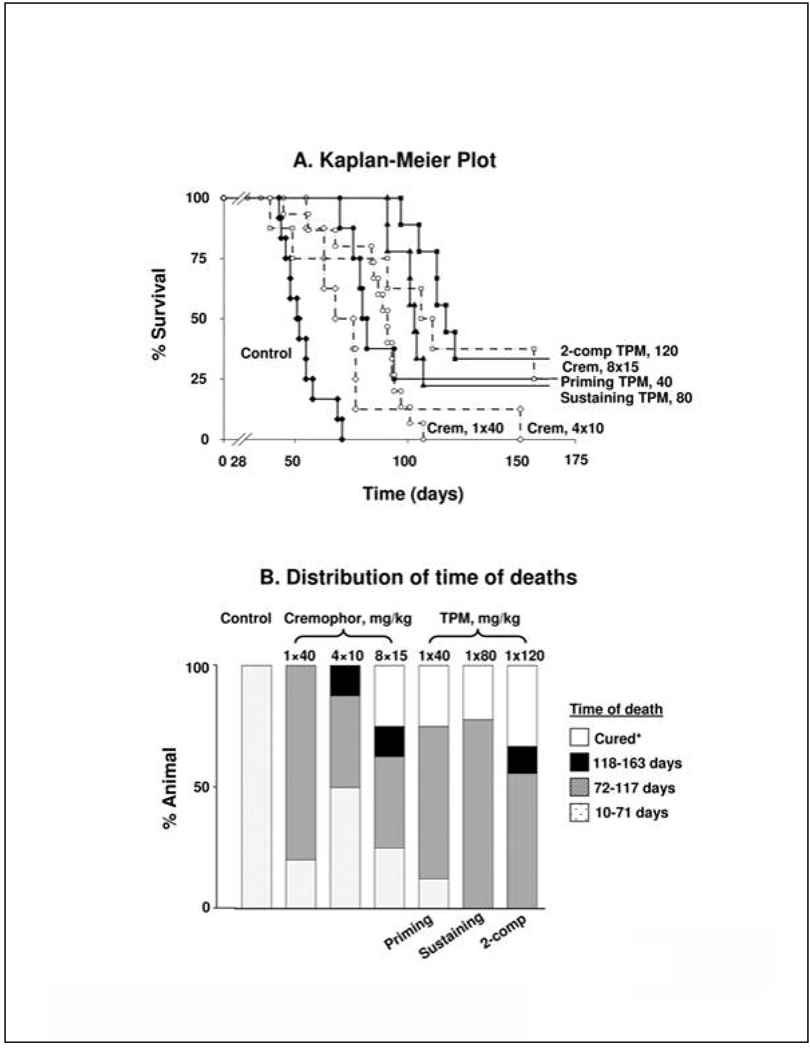
We compared the survival benefits of paclitaxel/Cremophor and TPM at equal mg and equi-toxic doses (six different treatments). Figure 6A shows the Kaplan-Meier plots and Table 2 summarizes the results. Without drug treatments, animals died rather quickly with all animals died within 71 days (referred to as early deaths). We measured treatment benefits in three ways, i.e., reducing early deaths, extending MST, and producing tumor-free cures. Tumor-free cures refer to animals that did not show tumor nodules in the peritoneal cavity on the last day of experiment (163–174 days after tumor implantation). Figure 6B compares the frequency of animal deaths at various time intervals (arbitrarily divided into 71 days for early deaths, followed by increments in 45 day intervals).
Figure 6. Antitumor activity of TPM.
(A). Kaplan-Meier plot; (B) Distribution of time of deaths. Mice were implanted with 20 × 106 SKOV3 cells IP on day 0. Twenty-eight days later, mice were treated with physiologic saline (control, n=12, solid diamonds, solid line), a single dose of 40 mg/kg paclitaxel/Cremophor (n=15, open circles, broken line), 4 doses of 10 mg/kg paclitaxel/Cremophor twice weekly (n=8, open diamonds, broken line), 8 doses of 15 mg/kg paclitaxel/Cremophor twice weekly (n=8, open squares, broken line), a single dose of Priming TPM (40 mg/kg paclitaxel, n=8, solid circles, solid line), a single dose of Sustaining TPM (80 mg/kg paclitaxel, n=9, solid triangles, solid line),or a single dose of 2-component TPM (120 mg/kg paclitaxel, 1:2 Priming:Sustaining, n=9, solid squares, solid line). Two animals in single dose paclitaxel/Cremophor died within 10 days after treatments and were censored. Animals remaining at the end of experiments (between 163–174 days) were euthanized; these include 2 mice in Priming TPM group, 2 in Sustaining TPM group, 3 in 2-component TPM group and 2 in 8×15 mg/kg paclitaxel/Cremophor group. None of these animals showed visible tumors in the peritoneal cavity and were considered long term cures. The survival times, increase in life span and statistical significance for between-group differences are shown in Table 2.
Compared to untreated controls, treatments with either paclitaxel/Cremophor or TPM significantly reduced the fraction of early deaths and extended MST. In general, increasing the total paclitaxel-equivalent dose and/or treatment frequency delayed disease-related deaths. At the equivalent total dose of 120 mg/kg, a single dose of 2-component TPM was equally effective as 8 doses of paclitaxel/Cremophor, and both treatments were significantly more efficacious compared to all other treatments. The qualitative and quantitative differences among Cremophor and TPM formulations are as follows. First, 9 of 33 (27.3%) mice in three paclitaxel/Cremophor groups showed early deaths within 71 days, whereas only one of 26 mice (3.8%, death on day 70) in the three TPM groups showed early deaths. For paclitaxel/Cremophor, no long term, tumor-free survivors were observed for the two groups receiving 40 mg/kg (either single dose or 4 divided doses over 2 weeks) whereas the group receiving 120 mg/kg in 8 divided doses (twice weekly for 4 weeks) showed 25% cures. In contrast, all three TPM groups, irrespective of the dose or drug release rate (40 mg/kg Priming TPM, 80 mg/kg Sustaining TPM, 120 mg/kg 2-component TPM) yielded about 22–33% tumor-free cures.
We next tested the hypothesis that a single dose of TPM was as efficacious as multiple doses of paclitaxel/Cremophor. At the equal mg dose of 40 mg/kg, a single dose of Priming TPM had comparable activity as a single dose of paclitaxel/Cremophor. Furthermore, a single dose of Priming TPM, but not the single dose of paclitaxel/Cremophor, yielded significantly longer survival time compared to the twice weekly schedule of paclitaxel/Cremophor (4×10 mg/kg). At the equal mg dose of 120 mg/kg, a single dose of 2-component TPM (120 mg/kg, 1:2 Priming: Sustaining) had comparable activity as 8 doses of paclitaxel/Cremophor (8×15 mg/kg twice weekly over 4 weeks).
Discussion
The present study provided several findings that may be applied to improving IP therapy. First, tumor priming using paclitaxel/Cremophor or TPM promoted the transport of micron-sized particles in tumor nodules, and, hence, may improve the efficacy of IP treatment of bulky disease. Second, the properties of TPM were selected such that they were localized on the surface of tumor nodules and penetrated tumor interior. This, together with the two-component feature providing gated and sustained drug release, resulted in significantly greater tumor targeting advantage. TPM formulation was developed based on the desired pharmacodynamics for successful IP therapy; the two-component feature is unique compared to other commercially available formulations of paclitaxel. For example, compared to paclitaxel/Cremophor, TPM yielded higher and more sustained paclitaxel concentrations (16-times higher CxT), lower host toxicity (less body weight loss and lower toxicity to intestinal crypts), and greater therapeutic efficacy (longer survival), at equal mg or equi-toxic doses. We propose that the lower toxicity of TPM is likely a result of the fractionated dose presentation compared to the bolus injection of the entire dose all-at-once as in the case for paclitaxel/Cremophor. Another important benefit of TPM is its apparent ability to eliminate the need of frequent dosing, as a single dose of TPM (40 or 120 mg/kg) was equally or more effective compared to multiple divided doses of paclitaxel/Cremophor (4× 10 or 8×15 mg/kg). Taken together, these findings support our contention that TPM, specifically tailored to the unique properties of peritoneal cavity and IP tumors, represent a potentially useful strategy for IP therapy of ovarian cancer.
We previously reported the effects of carriers on the peritoneal clearance of paclitaxel (Tsai et al., 2007). A major clearance mechanism for drug-containing carriers that are not readily transported through the peritoneum is drainage through the lymphatics. The smaller, nano-size Cremophor and polymeric formulations are readily cleared through the lymphatics, whereas TPM, which has a diameter (4 µm) approaches or exceeds that of subdiaphragmic lymphatic openings (∼3 µm), is better retained in the peritoneal cavity. The present study further showed that the size of TPM affected its distribution in the peritoneal cavity; smaller TPM (4 µm) was widely distributed in the peritoneal cavity and adhered to tumor surface whereas larger TPM (e.g., 30 µm) localized in the lower abdomen.
Several factors affect the pharmacokinetic-pharmacodynamic relationship of IP therapy. First, as shown by other investigators (see Introduction) and confirmed in the present study, penetration of most drugs (without using TPM) into tumors is limited to the periphery. TPM adhered to tumor surface, penetrated and resided in tumor interior. The intra-tumoral heterogeneity in drug penetration and distribution makes it difficult to extrapolate efficacy based on tumor pharmacokinetics, in vitro drug release rate or dosing intensity and dosing rate. In addition, IP metastatic tumors often comprise of (a) loosely attached tumor cells that typically grow rapidly and (b) tumors embedded or attached to intraperitoneal structures such as mesenteric membrane or omentum that typically grow slowly (unpublished observation). As it is likely that rapidly growing tumors result in early deaths and slowly growing tumors result in deaths at later times, successful treatments against rapidly growing cells will be more effective in reducing early deaths whereas successful treatments against slowly growing tumors will be effective in producing long term cures. This hypothesis is supported by several findings in the present study. First, administration of 40 mg/kg paclitaxel/Cremophor all-at-once in a single dose would be more likely to produce greater effects against the rapidly growing cells compared to administration in four divided doses over two weeks; this is consistent with the finding that the single dose schedule was more effective in reducing the early deaths. Second, long term cures, suggestive of eradication of the slowly growing tumors embedded in intraperitoneal tissues, were possible only for extended treatments (i.e., 4 weeks for paclitaxel/Cremophor or in the form of sustained release formulations such as TPM). Third, TPM (Priming TPM, 40 mg/kg), which yielded greater and more sustained drug concentrations in slowly growing omental tumors compared to paclitaxel/Cremophor (1×40 mg/kg), did not reduce early deaths but produced long term cures whereas paclitaxel/Cremophor did not produce cures. Additional studies to evaluate the 3-way relationship between drug release/dosing rate, tumor growth rate and treatment efficacy may improve the utility of IP therapy.
Locoregional administration of PLG copolymer is generally well tolerated in humans, as intramuscular administration of PLG microparticles elicited mild tissue response followed by complete recovery (Visscher et al., 1985; Shive and Anderson, 1997). On the other hand, one of 13 patients in the phase I trial of IP paclitaxel-loaded particles made with a different polymer (i.e., poly(D,L-lactide-co-ethyl phosphate)) and with a relatively large size (53 µm) showed extensive, diffuse adhesions completely obliterating the peritoneal space in the lower abdomen and pelvis (Armstrong et al., 2006b). A recent animal study evaluated whether IP injection of microparticles (5–250 µm diameter) made of PLG with different molecular weights (7–90 kDa) caused adhesion of abdominal tissues. The results show higher frequency of adhesion for high molecular weight PLG microparticles (e.g., 90 kDa) (Kohane et al., 2006). The present study showed that TPM comprised of low molecular weight polymers (∼8 and 40 kDa) and having a relatively small size (4 µm) did not cause adhesion in all of the 26 mice examined. Another possibility is that paclitaxel suppresses adhesion, as reported previously (Jackson et al., 2002). Whether TPM produces adhesion in humans needs to be investigated.
Another potentially interesting finding is the selective tumor-adhering property of TPM. We speculate this may be a result of interaction between PLG and tumor surface. Other carriers such as activated carbon particles also showed selective adherence to surface of IP Yoshida sarcoma (Hagiwara et al., 1990). As tumor adherence may provide a tumor-selective delivery platform, further investigations on polymer-tumor interactions are warranted.
In summary, two-component TPM was designed to address the key challenges in IP treatment of ovarian cancer. The present study demonstrated the several significant advantages of two-component TPM over the commercially available paclitaxel/Cremophor. These advantages may help to eliminate the need of indwelling catheter, minimize the local toxicity and improve the compliance of patients and medical staff. The use of multi-components with different drug release rates presents an additional theoretical advantage in that the combination of rapid and slow drug presentation enables the control of tumor cells with different growth rates. Finally, the good safety records of paclitaxel and PLG copolymers in humans support the clinical evaluation of 2-component TPM.
Acknowledgments
The authors would like to thank Eric Solon and Alfred Lordi at Quest Pharmaceutical Services for graciously providing phosphor imaging services for autoradiography.
Grant support: National Cancer Institute, National Institutes of Health, Department of Human Health and Services, R37CA49816 (J.L-S. Au), R43CA103133 (Z. Lu) and R44CA103133 (Z. Lu)
List of abbreviations
- AUC
area under concentration-time/depth curves
- BrdU
bromodeoxyuridine
- Cmax
maximum concentration
- Cmax,tissue
highest tissue concentration
- HPLC
high performance liquid chromatography
- ILS
increase in life span
- IP
intraperitoneal
- PBS
phosphate-buffered saline
- PLG
poly(D,L-lactide-co-glycolide)
Footnotes
J.L.-S. Au and M.G. Wientjes have personal financial and ownership interests in the technology discussed in the paper
References
- Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- Alberts DS, Surwit EA, Peng YM, McCloskey T, Rivest R, Graham V, McDonald L, Roe D. Phase I clinical and pharmacokinetic study of mitoxantrone given to patients by intraperitoneal administration. Cancer Res. 1988;48:5874–5877. [PubMed] [Google Scholar]
- Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N.Engl.J Med. 2006a;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- Armstrong DK, Fleming GF, Markman M, Bailey HH. A phase I trial of intraperitoneal sustained-release paclitaxel microspheres (Paclimer(R)) in recurrent ovarian cancer: A Gynecologic Oncology Group study. Gynecol.Oncol. 2006b;103:391–396. doi: 10.1016/j.ygyno.2006.02.029. [DOI] [PubMed] [Google Scholar]
- Chen CT, Au JL, Wientjes MG. Pharmacodynamics of doxorubicin in human prostate tumors. Clin.Cancer Res. 1998;4:277–282. [PubMed] [Google Scholar]
- Demicheli R, Bonciarelli G, Jirillo A, Foroni R, Petrosino L, Targa L, Garusi G. Pharmacologic data and technical feasibility of intraperitoneal doxorubicin administration. Tumori. 1985;71:63–68. doi: 10.1177/030089168507100112. [DOI] [PubMed] [Google Scholar]
- Elias DM, Sideris L. Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg.Oncol.Clin.N.Am. 2003;12:755–769. doi: 10.1016/s1055-3207(03)00051-6. xiv. [DOI] [PubMed] [Google Scholar]
- Gadducci A, Carnino F, Chiara S, Brunetti I, Tanganelli L, Romanini A, Bruzzone M, Conte PF. Intraperitoneal versus intravenous cisplatin in combination with intravenous cyclophosphamide and epidoxorubicin in optimally cytoreduced advanced epithelial ovarian cancer: a randomized trial of the Gruppo Oncologico Nord-Ovest. Gynecol Oncol. 2000;76:157–162. doi: 10.1006/gyno.1999.5677. [DOI] [PubMed] [Google Scholar]
- Gan Y, Wientjes MG, Schuller DE, Au JLS. Pharmacodynamics of taxol in human head and neck tumors. Cancer Res. 1996;56:2086–2093. [PubMed] [Google Scholar]
- Goldberg KE. Phase III Trial Shows Benefit for Old Drug, Device, for Ovarian Cancer; Will Practice Change? The Cancer Letter. 2006;32(2):1–4. [Google Scholar]
- Griffon-Etienne G, Boucher Y, Brekken C, Suit Hd, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776–3782. [PubMed] [Google Scholar]
- Hagiwara A, Takahashi T, Iwamoto A, Yoneyama C, Itoh M, Sasabe T. Affinity of intraperitoneally injected activated carbon particles adsorbing mitomycin C to tumor surface of Yoshida sarcoma. Anticancer Drug Des. 1990;5:359–369. [PubMed] [Google Scholar]
- Howell SB, Pfeifle CE, Olshen RA. Intraperitoneal chemotherapy with melphalan. Ann.Intern.Med. 1984;101:14–18. doi: 10.7326/0003-4819-101-1-14. [DOI] [PubMed] [Google Scholar]
- Jackson JK, Skinner KC, Burgess L, Sun T, Hunter WL, Burt HM. Paclitaxel-loaded crosslinked hyaluronic acid films for the prevention of postsurgical adhesions. Pharm.Res. 2002;19:411–417. doi: 10.1023/a:1015175108183. [DOI] [PubMed] [Google Scholar]
- Jang SH, Wientjes MG, Au JL. Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs. 2001a;19:113–123. doi: 10.1023/a:1010662413174. [DOI] [PubMed] [Google Scholar]
- Jang SH, Wientjes MG, Au JL. Enhancement of paclitaxel delivery to solid tumors by apoptosis-inducing pretreatment: effect of treatment schedule. J.Pharmacol.Exp.Ther. 2001b;296:1035–1042. [PubMed] [Google Scholar]
- Kerr DJ, Los G. Pharmacokinetic principles of locoregional chemotherapy. Cancer Surv. 1993;17:105–122. [PubMed] [Google Scholar]
- Kohane DS, Tse JY, Yeo Y, Padera R, Shubina M, Langer R. Biodegradable polymeric microspheres and nanospheres for drug delivery in the peritoneum. J Biomed.Mater.Res.A. 2006;77:351–361. doi: 10.1002/jbm.a.30654. [DOI] [PubMed] [Google Scholar]
- Kuh HJ, Jang SH, Wientjes MG, Weaver JR, Au JL. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther. 1999;290:871–880. [PubMed] [Google Scholar]
- Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG. Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol. 1990;25:389–394. doi: 10.1007/BF00686048. [DOI] [PubMed] [Google Scholar]
- Los G, Verdegaal EM, Mutsaers PH, McVie JG. Penetration of carboplatin and cisplatin into rat peritoneal tumor nodules after intraperitoneal chemotherapy. Cancer Chemother Pharmacol. 1991;28:159–165. doi: 10.1007/BF00685503. [DOI] [PubMed] [Google Scholar]
- Lu D, Wientjes MG, Lu Z, Au JL. Tumor priming enhances delivery and efficacy of nanomedicines. J Pharmacol.Exp.Ther. 2007;322:80–88. doi: 10.1124/jpet.107.121632. [DOI] [PubMed] [Google Scholar]
- Markman M, Brady MF, Spirtos NM, Hanjani P, Rubin SC. Phase II trial of intraperitoneal paclitaxel in carcinoma of the ovary, tube, and peritoneum: a Gynecologic Oncology Group Study. J Clin Oncol. 1998;16:2620–2624. doi: 10.1200/JCO.1998.16.8.2620. [DOI] [PubMed] [Google Scholar]
- Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- Markman M, Reichman B, Hakes T, Rubin S, Lewis JL, Jr, Jones W, Barakat R, Curtin J, Almadrones L, Hoskins W. Evidence supporting the superiority of intraperitoneal cisplatin compared to intraperitoneal carboplatin for salvage therapy of small-volume residual ovarian cancer. Gynecol.Oncol. 1993;50:100–104. doi: 10.1006/gyno.1993.1171. [DOI] [PubMed] [Google Scholar]
- Markman M, Rowinsky E, Hakes T, Reichman B, Jones W, Lewis JL, Jr, Rubin S, Curtin J, Barakat R, Phillips M. Phase I trial of intraperitoneal taxol: a Gynecoloic Oncology Group study. J Clin Oncol. 1992;10:1485–1491. doi: 10.1200/JCO.1992.10.9.1485. [DOI] [PubMed] [Google Scholar]
- Markman M, Walker JL. Intraperitoneal chemotherapy of ovarian cancer: a review, with a focus on practical aspects of treatment. J.Clin.Oncol. 2006;24:988–994. doi: 10.1200/JCO.2005.05.2456. [DOI] [PubMed] [Google Scholar]
- Monk BJ, Surwit EA, Alberts DS, Graham V. Intraperitoneal mitomycin C in the treatment of peritoneal carcinomatosis following second-look surgery. Semin.Oncol. 1988;15:27–31. [PubMed] [Google Scholar]
- Morgan RJ, Jr, Doroshow JH, Synold T, Lim D, Shibata S, Margolin K, Schwarz R, Leong L, Somlo G, Twardowski P, Yen Y, Chow W, Lin P, Paz B, Chu D, Frankel P, Stalter S. Phase I trial of intraperitoneal docetaxel in the treatment of advanced malignancies primarily confined to the peritoneal cavity: dose-limiting toxicity and pharmacokinetics. Clin.Cancer Res. 2003;9:5896–5901. [PubMed] [Google Scholar]
- O'Dwyer PJ, LaCreta FP, Daugherty JP, Hogan M, Rosenblum NG, O'Dwyer JL, Comis RL. Phase I pharmacokinetic study of intraperitoneal etoposide. Cancer Res. 1991;51:2041–2046. [PubMed] [Google Scholar]
- Ozols RF, Bookman MA, Young RC. Intraperitoneal chemotherapy for ovarian cancer. N.Engl.J.Med. 2006;354:1641–1643. doi: 10.1056/NEJMc060264. [DOI] [PubMed] [Google Scholar]
- Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv.Drug Deliv.Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Song D, Au JL. Isocratic high-performance liquid chromatographic assay of taxol in biological fluids and tissues using automated column switching. J Chromatogr.B Biomed.Appl. 1995;663:337–344. doi: 10.1016/0378-4347(94)00456-f. [DOI] [PubMed] [Google Scholar]
- Tauchi PS, Caraway N, Truong LD, Kaplan AL, Ramzy I. Serous surface carcinoma of the peritoneum: useful role of cytology in differential diagnosis and follow-up. Acta Cytol. 1996;40:429–436. doi: 10.1159/000333894. [DOI] [PubMed] [Google Scholar]
- Topuz E, Saip P, Aydmer A, Salihoglu Y, Berkman S, Bengisu E. Intraperitoneal cisplatin-mitoxantrone and intravenous ifosfamide combination as first-line treatment of ovarian cancer. Eur J Gynaecol.Oncol. 1998;19:265–270. [PubMed] [Google Scholar]
- Tsai M, Lu Z, Wang J, Yeh TK, Wientjes MG, Au JL. Effects of carrier on disposition and antitumor activity of intraperitoneal Paclitaxel. Pharm.Res. 2007;24:1691–1701. doi: 10.1007/s11095-007-9298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher GE, Robison RL, Maulding HV, Fong JW, Pearson JE, Argentieri GJ. Biodegradation of and tissue reaction to 50:50 poly(DL-lactide-co-glycolide) microcapsules. J Biomed.Mater.Res. 1985;19:349–365. doi: 10.1002/jbm.820190315. [DOI] [PubMed] [Google Scholar]
- Wenzel LB, Huang HQ, Armstrong DK, Walker JL, Cella D. Health-related quality of life during and after intraperitoneal versus intravenous chemotherapy for optimally debulked ovarian cancer: a Gynecologic Oncology Group Study. J Clin.Oncol. 2007;25:437–443. doi: 10.1200/JCO.2006.07.3494. [DOI] [PubMed] [Google Scholar]
- Zheng JH, Chen CT, Au JL, Wientjes MG. Time-and concentration-dependent penetration of doxorubicin in prostate tumors. AAPS PharmSci. 2001;3:E15. doi: 10.1208/ps030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimm S, Cleary SM, Lucas WE, Weiss RJ, Markman M, Andrews PA, Schiefer MA, Kim S, Horton C, Howell SB. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res. 1987;47:1712–1716. [PubMed] [Google Scholar]