Abstract
Protein prenylation is a common post-translational modification present in eukaryotic cells. Many key proteins involved in signal transduction pathways are prenylated and inhibition of prenylation can be useful as a therapeutic intervention. While significant progress has been made in understanding protein prenylation in vitro, we have been interested in studying this process in living cells, including the question of where prenylated molecules localize. Here, we describe the synthesis and live cell analysis of a series of fluorescently labeled multifunctional peptides, based on the C-terminus of the naturally prenylated protein CDC42. A synthetic route was developed that features a key Acm to Scm protecting group conversion. This strategy was compatible with acid-sensitive isoprenoid moieties, and allowed incorporation of an appropriate fluorophore as well as a cell-penetrating sequence (penetratin). These peptides are able to enter cells through different mechanisms, depending on the presence or absence of the penetratin vehicle and the nature of the prenyl group attached. Interestingly, prenylated peptides lacking penetratin are able to enter cells freely through an energy-independent process, and localize in a perinuclear fashion. This effect extends to a prenylated peptide that includes a full “CAAX box” sequence (specifically, CVLL). Hence, these peptides open the door for studies of protein prenylation in living cells, including enzymatic processing and intracellular peptide trafficking. Moreover, the synthetic strategy developed here should be useful for the assembly of other types of peptides that contain acid sensitive functionalities.
Introduction
Protein prenylation is a post-translational modification that consists of the addition of 15 (farnesyl) or 20 (geranylgeranyl) carbon isoprenoid units to specific cysteine residues positioned near the C-terminus of a protein.1-3 Based on labeling experiments with tritiated mevalonic acid, the biosynthetic precursor to farnesyl diphosphate and other isoprenoids, it has been estimated that 2% of the mammalian proteome is prenylated.4 Moreover, the types of proteins that contain this modification include all members of the Ras superfamily of proteins and the gamma subunits of heterotrimeric G-proteins. Thus, essentially all signal transduction pathways involve prenylated proteins. The frequent occurrence of this modification, coupled with its crucial role in regulating cellular processes has generated intense interest in the enzymology and function of protein prenylation. The “CAAX box” (CVLL sequence in this paper) is the recognition sequence for prenylation. The mature isoprenylated protein arises from a three-step process that consists of prenylation, followed by proteolysis of the “AAX” sequence, and methyl esterification of the new C-terminal cysteine. Due to the participation of mutant forms of Ras and related proteins in cancer, there has been intense interest in developing inhibitors of the enzymes involved in the prenylation process. The most successful work to date has targeted farnesyltransferase, resulting in the development of several drug candidates that are in Phase 3 clinical trials.5-7 The proteolytic and methylating enzymes also show promise as possible drug targets. Progress has also been made in understanding the function of protein prenylation. In some cases, isoprenylation serves to direct membrane association,8 while in other situations, the prenyl group is involved in mediating protein-protein interactions.9
Despite the important developments in prenylation research noted above, much remains unclear, particularly concerning the biological function of this modification. For example, some proteins show different patterns of localization depending, on their prenylation state and their C-terminal amino acid sequences.10 Changes in prenylation state have also been shown to alter a protein's biological activity.11 One approach that can be used to investigate such issues is to prepare and study synthetic peptides derived from larger proteins. Such peptides allow the functions of particular segments of the protein to be studied in isolation; they also permit the introduction of synthetic moieties including non-natural residues, isoprenoid analogues, and spectroscopic probes that can facilitate biochemical analysis. To date, several studies have examined the binding of prenylated peptides to artificial lipid bilayers12, 13 and to microsomal membranes14 obtained via subcellular fractionation, but localization of prenylated peptides in living cells has yet to be probed. The purpose of the present studies is to complement such earlier in vitro work by developing methodology to prepare prenylated peptides that can be introduced into living cells and to examine their distribution therein.
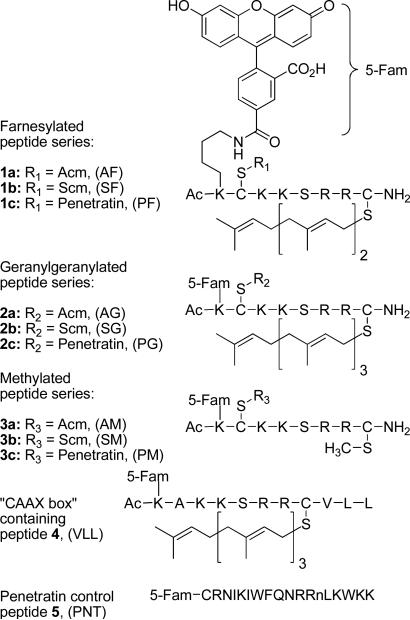
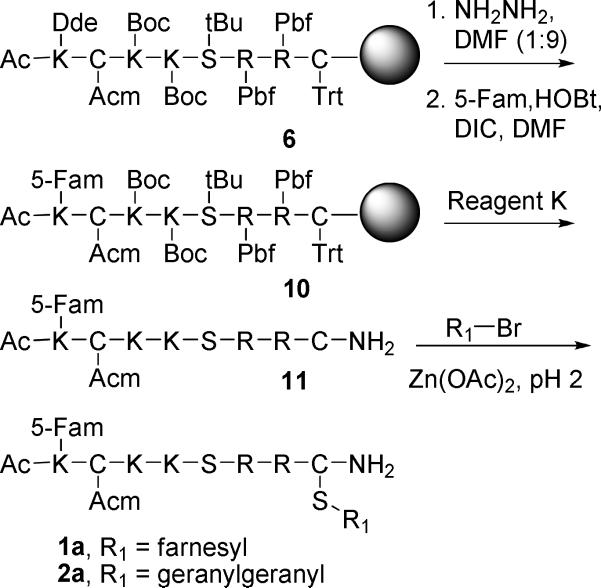
Due to the key role of the extensively characterized Rho protein CDC42 in regulating cytoskeletal assembly, we chose to study peptides based on its C-terminal sequence. To facilitate introduction of these peptides into living cells, we elected to conjugate them to the cell penetrating peptide (CPP) sequence known as penetratin, via a disulfide linkage that would subsequently be cleaved in the reducing environment of the targeted cells. However, because the resulting peptides contain two cysteine residues (one for penetratin attachment and the other as the site of prenylation), it was necessary to devise an orthogonal protecting group strategy that would enable the two thiols to be differentiated. Here we describe the synthesis of a series of peptides (Figure 1) based on the C-terminus of CDC42 that are functionalized with varying isoprenoids, along with a fluorophore and a penetratin sequence (1c, 2c and 3c). The peptides were synthesized as C-terminal amides to provide enhanced stability while retaining the neutral charge state of the C-terminal methyl ester present in naturally prenylated peptides. A second series of fluorescently labeled prenylated peptides lacking the penetratin group was also prepared (1a, 2a and 3a), along with a peptide containing a complete CVLL “CAAX box” sequence (4). Furthermore, a fluorescently labeled version of penetratin (5) was synthesized as a control.
Figure 1.
Multifunctional prenylated peptides for analysis in living cells.
Flow cytometry and confocal microscopy of living cells in conjunction with in vitro fluorescence measurement were then used to probe the entry and localization of these peptides. Interestingly, the presence of a farnesyl or geranylgeranyl group caused these peptides to target to the perinuclear region within the cell, in a similar fashion to what has been observed with the parent protein CDC42.15, 16 In the course of these experiments, it was observed that the prenylated peptides lacking the penetratin group could also enter the cell and localize in a perinuclear fashion; this includes a peptide containing a complete “CAAX box”. However, in those latter cases, the mechanism of entry appears to be different than for those peptides functionalized with penetratin. In summary, the methodology reported here has allowed us to prepare peptides containing natural and non-natural modifications that can be introduced into living cells. Moreover, these peptides should provide a means for studying the enzymatic processing, including proteolysis and methylation, of prenylated species in living cells.
Experimental Procedures
Materials and Methods
HeLa cells were the generous gift of Dr. Audrey Minden (Department of Chemical Biology, Rutgers University). Petri dishes (35 mm) fitted with microwells (14 mm) and a No. 1.5 coverglass were from MatTek Corporation. Wheat germ agglutinin Alexa Fluor 594 conjugate, ProLong(R) Gold antifade reagent, Hoechst 34850, and DMEM (Dulbecco's Modified Eagle Medium) were from Invitrogen. Fetal bovine serum was from Intergen Company. C18 Sep-Pak® cartridges were purchased from Waters. Vydac 12-Well plates were from Corning Inc. Vydac 218TP54 and 218TP1010 columns were used for analytical and preparative RP-HPLC, respectively. All analytical and preparative RP-HPLC solvents, water and CH3CN, contained 0.10% TFA; and retention times (tR) are based on linear gradients (unless otherwise noted) starting from 100% H2O to 40% H2O:60% CH3CN over 60 min. All solvents were of HPLC grade. DIEA and TFA were of Sequalog/peptide synthesis grade from Fisher. Fmoc-Cys(Me)-OH was from Bachem and Fmoc-Lys(Dde)-OH was from Nova Biochem. PAL-PEG-PS was from Applied Biostems. Preloaded CLEAR-Acid resins were from Peptides International. All other reagents were from Sigma Aldrich. In the following procedures, unless determined by UV spectroscopy, actual peptide content has not been taken into account. All procedures involving fluorescent derivatives were protected from light as much as possible in order to avoid bleaching the fluorophore.
Abbreviations
Ac, Acetyl; Acm, acetamidomethyl; BOC, t-butyloxycarbonyl; BOP, (benzotriazol-1-yloxy)tris-(dimethylamino)phosphonium hexafluorophosphate; t-Bu, tert-butyl; CLEAR, cross-linked ethoxylate acrylate resin; CPP, cell-penetrating peptide; DIC, N,N’-diisopropylcarbodiimide; Dde, 1-(4,4-dimethyl-2,6-dioxocyclohexylidene)ethyl; DIEA, diisopropylethylamine; DIC, diisopropylcarbodiimide; DMEM, Dulbecco's Modified Eagle Medium, DMF, N,N-dimethylformamide; DTT, dithiothreitol; ESI-MS, electrospray ionization mass spectrometry; 5-Fam, 5-carboxyfluorescein; Far, farnesyl; FITC, fluorescein isothiocyanate; Fmoc, 9-fluorenylmethyloxycarbonyl; Ger, geranylgeranyl; HOBt, 1-hydroxybenzotriazole; Me, methyl; nL, norleucine; OAc, acetate; PAL, peptide amide linker; Pbf, 2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl; PEG-PS, polyethylene glycolpolystyrene graft copolymer; PMSF, phenylmethanesulfonyl fluoride; Pnt, penetratin; RP-HPLC, reversed-phase high pressure liquid chromatography; Scm, S-carbomethoxysulfenyl; SPPS, solid-phase peptide synthesis; TFA, trifluoroacetic acid; Trt, trityl.
Peptide Synthesis
Linear peptides were synthesized by Fmoc-based SPPS on either Applied Biosystems 433A or Pioneer automated peptide synthesizers according to manufacturer protocols. Peptides were cleaved with freshly prepared Reagent K17 (TFA-phenol-thioanisole-water-ethanedithiol 82.5:5:5:5:2.5) for 2 h, precipitated with Et2O, and centrifuged to form a pellet which was washed with Et2O. Crude peptides were purified by RP-HPLC.
General Procedure for Cysteine Alkylation: Prenylation of AcK(5-Fam)C(Acm)KKSRRC-NH2 (11) to produce 1a and 2a
Peptide 11 (1.0 eq.) and Zn(OAc)2·2H2O (5.0 eq.) were dissolved in DMF/1-butanol/0.10% aqueous TFA (2:1:1, v/v/v) (1.0 mL solvent per 2.0 mg peptide).18 Either farnesyl bromide or geranylgeranyl bromide (4.0 eq.) was then added. The reaction was monitored by RP-HPLC, and once judged complete (typically 2 h), was diluted with 10 volumes of 0.10% aqueous TFA, filtered, and purified by RP-HPLC.
AcK(5-Fam)C(Acm)KKSRRC(Far)-NH2 (1a)
Reaction scale: 0.014 mmol, yield: 13 mg (54%), purity by RP-HPLC: 95%, tR = 45 min, deconvoluted ESI-MS: calculated 1681.8, found 1681.8.
AcK(5-Fam)C(Acm)KKSRRC(Ger)-NH2 (2a)
Reaction scale: 0.010 mmol, yield: 10.5 mg (62%), purity by RP-HPLC: 94%, tR = 58 min, deconvoluted ESI-MS: calculated 1973.1, found 1973.0.
AcK(5-Fam)C(Acm)KKSRRC(Me)-NH2 (3a)
Peptide 3a was synthesized by the above procedure, except Fmoc-Cys(Me)-OH was substituted for Fmoc-Cys(Trt)-OH during chain assembly on PAL-PEG-PS. Reaction scale: 0.025 mmol, yield: 19.9 mg (54%), purity by RP-HPLC: 99%, tR = 25 min, deconvoluted ESI-MS: calculated 1491.7, found 1491.9.
AcK(5-Fam)AKKSRRC(Ger)VLL (4)
The geranylgeranyl group was introduced onto the appropriate peptide precursor as it was for 2a. Reaction scale: 0.012 mmol, yield: 12.7 mg (53%), purity by RP-HPLC: 98%, tR = 50 min, deconvoluted ESI-MS: calculated 1749.9, found 1750.3.
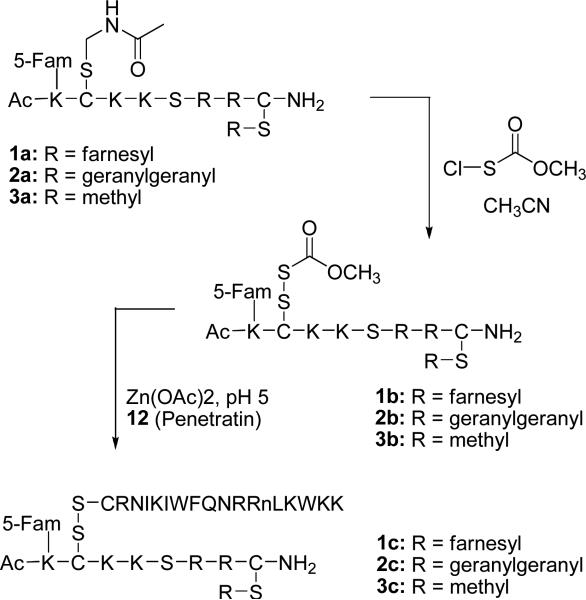
General Procedure for Conversion of Peptides with Cys(Acm) Side-chain Protection (1a, 2a and 3a) to Peptides with Cys(Scm) (1b, 2b and 3b)
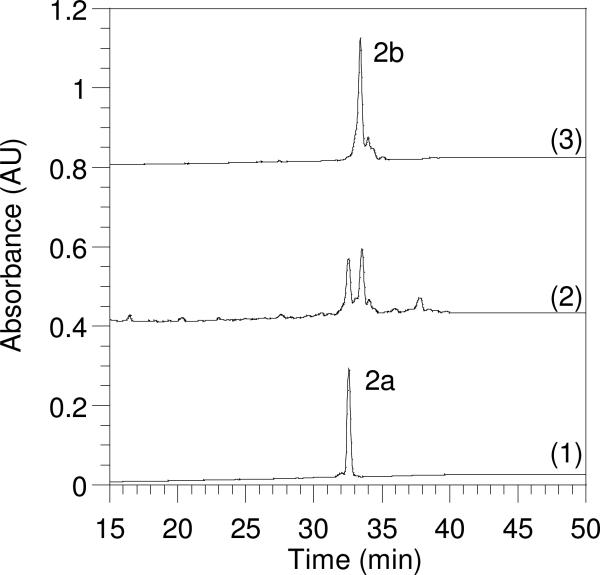
CH3O(CO)SCl (5.0 μL) was added to CH3CN (0.20 mL) to give a 0.27 M stock solution. The Cys(Acm)-containing peptide was dissolved in DMF/CH3CN (1:1, v/v) (1.0 mL solvent per 1.0 mg of peptide) and the peptide concentration was determined by UV spectroscopy of the 5-Fam chromophore (ε = 79,000, 492 nm, pH 9.0). The reaction and stock solution were cooled in an ice bath and 1.0 eq. of CH3O(CO)SCl stock solution was added to the reaction. After 1 h, the reaction was analyzed by RP-HPLC. The percent conversion of Cys(Acm) to Cys(Scm) was calculated by comparing the peak areas of the product and starting peptide peaks (Figure 2). Enough additional stock CH3O(CO)SCl was then added to complete the reaction, based on the percent conversion observed after addition of the first equivalent of CH3O(CO)SCl. The RP-HPLC monitoring and analysis was repeated after another 1 h of reaction time. If any precipitate of the product was observed, enough DMF was added to render the solution homogeneous, so as to ensure an accurate HPLC analysis of % conversion [Cys(Scm) is more hydrophobic than Cys(Acm), and hence less soluble]. When the reaction was judged complete, it was diluted with 10 volumes of 0.10% aqueous TFA, filtered, and purified by RP-HPLC. The pooled fractions of product from purification were used directly in the next step without concentration or lyophilization.
Figure 2.
Reversed-phase HPLC analysis monitoring the progress of Acm to Scm conversion in a geranylgeranylated C-terminal CDC42 peptide. Gradient: 20−60% CH3CN over 60 min. Chromatogram (1): Peptide 2a (tR = 32.5 min) before the addition of CH3O(CO)SCl. Chromatogram (2): A mixture of Acm and Scm protected peptides 2a and 2b 1 h after CH3O(CO)SCl was added. Chromatogram (3): Complete conversion of Acm-protected peptide 2a to Scm-modified peptide 2b (tR = 33.5 min).
AcK(5-Fam)C(Scm)KKSRRC(Far)-NH2 (1b)
Purity by RP-HPLC: 82%, tR = 46 min, deconvoluted ESI-MS: calculated 1700.8, found 1701.0.
AcK(5-Fam)C(Scm)KKSRRC(Ger)-NH2 (2b)
Purity by RP-HPLC: 69%, tR = 51 min, deconvoluted ESI-MS: calculated 1768.8, found 1768.6.
AcK(5-Fam)C(Scm)KKSRRC(Me)-NH2 (3b)
The crude product was isolated and used in the synthesis of 3c without analytical characterization, tR = 27 min.
AcK(5-Fam)C(Scm)KKSRRC(Scm)-NH2
In early experiments to convert Cys(Acm) to Cys(Scm) in the Cys(Far)- and Cys(Ger)-containing peptides, excess CH3O(CO)SCl was used. A major early-eluting contaminant peak (tR = 30 min), observed on RP-HPLC, was isolated and identified as the bis(Scm) derivative, in which the Cys(prenyl) group had been cleaved by the excess CH3O(CO)SCl to yield Cys(Scm). This bis(Scm) derivative was completely stable in 0.10% aqueous TFA solutions for at least several weeks. Treatment of peptides containing prenylated cysteine residues with CH3O(CO)SCl to yield the corresponding Cys(Scm) derivative, followed by treatment with DTT or 2-mercaptoethanol, may prove useful as a mild method for removal of prenyl groups from cysteine. Deconvoluted ESI-MS: calculated 1586.6, found 1586.6.
General Procedure for Conjugation of AcK(5-Fam)C(Scm)KKSRRC(prenyl)-NH2 with Penetratin to Provide Mixed Disulfide Peptides (1c, 2c and 3c)
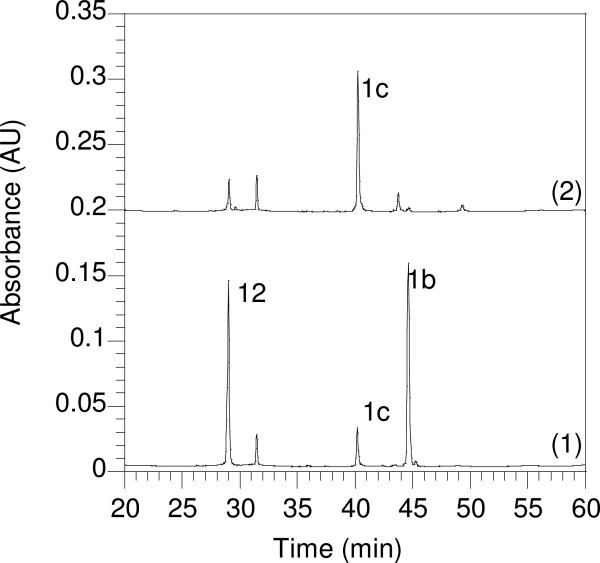
The concentrations of the Cys(Scm)-containing peptides were determined by UV spectroscopy of the 5-Fam chromophore (ε = 79,000, 492 nm, pH 9.0). Zn(OAc)2·2H2O (5.8 mg) was dissolved in 1.0 M sodium acetate (1.5 mL, pH 5.4) to give a 17 mM Zn2+ stock solution. Penetratin (12) was dissolved in 0.10% aqueous TFA (2.5 mg/mL, approximately 0.70 mM), and the exact concentration was determined by assaying the free thiol content with Ellman's reagent. Penetratin (1.0 eq.) was added from the stock solution to the pooled Cys(Scm)-containing fractions, the reaction mixture was cooled in an ice bath, and Zn(OAc)2 (5.0 eq.) from the stock solution was added. Next, enough sodium acetate (1.0 M, pH 5.4) was added to bring the overall acetate concentration to about 0.067 M, and the pH of the reaction mixture was adjusted to pH 5.0−5.2 with 0.10 N NaOH. After 15 min, the reaction was analyzed by RP-HPLC, and additional penetratin from the stock was added as necessary to drive the reaction to completion. When the reaction was judged complete by RP-HPLC analysis (Figure 3), the pH was adjusted to the range of 2−3 by addition of neat TFA, the mixture was filtered, and purified by RP-HPLC. It is important to note that since this is a bimolecular reaction, the rate is highly dependent on concentration. For more dilute peptide solutions, one may have to wait longer than 15 min to analyze the extent of reaction. UV spectroscopic analysis underestimated the concentration of AcK(5-Fam)C(Scm)KKSRRC(prenyl)-NH2 relative to penetratin. This is possibly due to the instability of the former peptide at pH 9.0.
Figure 3.
Reversed-phase HPLC analysis monitoring the progress of penetratin conjugation to a Scm-protected farnesylated C-terminal CDC42 peptide. Gradient: 0−60% CH3CN over 60 min. Chromatogram (1): Penetratin (12) (tR = 29.0 min) and peptide 1b (tR = 45 min) 24 h after the addition of Zn(OAc)2, pH 2. Chromatogram (2): Formation of penetratin-modified peptide 1c (tR = 40 min) 24 h after adjusting to pH 5.2.
AcK(5-Fam)C(Pnt)KKSRRC(Far)-NH2 (1c)
Yield: 1.9 mg over 2 steps from 5.2 mg 1a (16%), Purity by RP-HPLC: 95%, tR = 40 min, deconvoluted ESI-MS: calculated 3925.1, found 3925.0.
AcK(5-Fam)C(Pnt)KKSRRC(Ger)-NH2 (2c)
Yield: 1.3 mg over 2 steps from 8.9 mg 1b (6%), Purity by RP-HPLC: 99%, tR = 48 min, deconvoluted ESI-MS: calculated 3993.2, found 3993.2.
AcK(5-Fam)C(Pnt)KKSRRC(Me)-NH2 (3c)
Yield: 4.5 mg over 2 steps from 5.0 mg 1c (36%), Purity by RP-HPLC: 97%, tR = 31 min, deconvoluted ESI-MS: calculated 3734.9, found 3734.4.
AcK(5-Fam)C(Acm)KKSRRC-NH2 (11)
The linear sequence was assembled on either PAL-PEG-PS or Rink-amide resin. DIC and HOBt were used for coupling to avoid racemization of cysteine.19 The N-terminal lysine side-chain was orthogonally protected with Dde. Acetic acid was coupled in the last instrument cycle to acetylate the N-terminus. The Dde protecting group was removed selectively by treating protected peptide-resin (0.05 mmol) in a solid-phase reaction vessel with 5.0 mL of anhydrous NH2NH2/DMF (1:9, v/v), for 15 min, followed by washing with DMF (5 × 5.0 mL).20 5-Fam (38 mg, 0.10 mmol), DIC (16 mL, 0.10 mmol) and HOBt (13.5 mg, 0,10 mmol) were dissolved in DMF (2.0 mL) and then added to the peptide resin along with DMF washes (2 × 0.5 mL). The coupling reaction was allowed to tumble overnight, and then the peptide-resin was washed with DMF (3 × 5.0 mL) and CH2Cl2 (3 × 5.0 mL), and dried in vacuo. The peptide was cleaved with Reagent K17 (TFA-phenol-thioanisole-water-ethanedithiol 82.5:5:5:5:2.5) for 2 h, precipitated with Et2O, and centrifuged to form a pellet, which was washed with Et2O. The crude peptide was purified by RP-HPLC. Yield: 18−30 mg (24−40%), purity by RP-HPLC: > 95%, tR = 26 min, deconvoluted ESI-MS: calculated 1477.6, found 1477.4.
5-Fam-CRNIKIWFQNRRnLKWKK (5)
The linear peptide was synthesized as for compound 11 except acetic acid was not coupled in the last instrument cycle to acetylate the N-terminus. 5-Fam was coupled to the N-terminus of side-chain protected peptide-resin, using the same procedure described for fluorophore coupling used on compound 11. Purity by RP-HPLC: 97%, tR = 37 min, Deconvoluted ESI-MS: calculated 2674.4, found 2674.4.
AcK(5-Fam)AKKSRRCVLL (precursor to 4)
The linear peptide sequence was synthesized using CLEAR-Acid resin,21 and the 5-Fam fluorophore was coupled to the N-terminal lysine side-chain of otherwise fully protected peptide resin as described for compound 11. Purity by RP-HPLC: 99%, tR = 34 min, deconvoluted ESI-MS: calculated 1700.9, found 1700.8.
CRNIKIWFQNRRnLKWKK (penetratin) (12)
Purity by RP-HPLC: 99%, tR = 29 min, Deconvoluted ESI-MS: calculated 2316.3, found 2316.0.
Geranylgeranyl Bromide22
Geranylgeraniol (0.10 g, 0.69 mmol, 1.0 eq.) was dissolved in CH2Cl2 (4.2 mL). Next, polymer-supported PPh3 (0.45 g, 1.438 mmol, 2.0 eq.) was added, and the solution was gently shaken. After the addition, the heterogeneous solution turned a light brown color. After 30 min, CBr4 (0.27 g, 0.83 mmol, 1.2 eq.) was dissolved in 0.5 mL CH2Cl2 and added to the mixture via syringe. This solution was stirred for 6 h, after which the polymer-supported reagent was removed by filtration and the resulting solution was concentrated to yield 0.11 g of geranylgeranyl bromide (89% yield). This crude product was stored at −20° C. Just prior to use in the alkylation reaction, the calculated amount of geranylgeranyl bromide (4.0 eq. over peptide resin) was purified by solid phase extraction on a C18 Sep-Pak® cartridge. The cartridge was first equilibrated with 5% CH3CN in water that contained 0.10% TFA. The geranylgeranyl bromide was dissolved in 0.50 mL of DMF and applied to the column. The column was then washed with 10 mL of equilibration solvent and 10 mL of 50% CH3CN in water that contained 0.10% TFA. The geranylgeranyl bromide was then eluted from the column directly into the reaction vessel with 5.0 mL DMF. If this solid phase extraction procedure is not done, a large amount of disulfide impurity, rather than the desired geranylgeranyl alkylation product, is formed in the reaction. 1H NMR (300 MHz, CDCl3) δ = 1.62 (s, 6H), 1.70 (s, 3H), 1.77 (s, 3H), 1.79 (s, 3H), 2.00−2.16 (m, 12H), 4.04 (d, 1H, J=7.2 Hz), 5.11 (m, 3H) 5.54 (t, 1H, J=7.2 Hz). 13C NMR-DEPT (CDCl3) δ 16.0, 16.1, 16.2, 17.7, 25.8 (primary), 26.2, 26.7, 26.8, 29.8, 39.6, 39.7, 39.8, (secondary), 120.6, 123.5, 124.2, 124.4 (tertiary), 131.4, 135.1, 136.2, 143.7 (quaternary). HR-FAB-MS calculated for C20H33Br [M + NH4]+ 370.21, found 370.21.
Determination of Peptide Concentrations for Cell Studies
Stock peptide solutions were diluted using 20 mM Tris buffer, pH 9.0, and DMSO so that the final concentration of DMSO was no greater than 0.10% by volume. UV spectroscopy of the 5-Fam chromophore was used to determine the concentrations of filtered peptide stock solutions (ε492 = 79,000, 492 nm, 20 mM Tris·HCl, pH 9.0).23
Cell culture
HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum at 37 °C with 5.0% CO2. For all experiments, 2.6 × 104 cells/cm2 were seeded in culture dishes and grown for 24 h to approximately 50% confluency.
Confocal Microscopy
HeLa cells were seeded in 35 mm glass bottom microwell dishes. Approximately 24 h after plating cells were washed twice with serum-free DMEM, and incubated for 1 h at 37 °C and 5.0% CO2 in serum-free DMEM containing 1.0 μM peptide. Hoechst 34580 was added to 1 μg/ml during the final 20 minutes of incubation, and wheat germ agglutinin Alexa Fluor 594 conjugate was added to 5 μg/ml during the final 10 minutes of incubation. For ATP depletion, cells were washed twice with glucose-free, serum-free DMEM and then incubated for 2 h at 37 °C and 5.0% CO2 in glucose-free, serum-free DMEM supplemented with 12 μM rotenone and 15 mM 2-deoxyglucose24. Then peptides, Hoechst 34850 and wheat germ agglutinin AlexaFluor 594 conjugate were added and cells were incubated as described above. For peptide incubation at 4°C, cells were washed twice with chilled serum-free DMEM supplemented with 25 mM HEPES (pH 7.3) and incubated with peptide, Hoechst 34850 and wheat germ agglutinin Alexa Fluor 594 conjugate as described above at 4°C. After 1 h total incubation, all cells were washed twice with PBS. Cells were then either imaged live in serum-free DMEM, or cells were fixed for 10 min at room temperature in 3.7% formaldehyde in PBS. Coverslips were mounted with ProLong Gold Antifade Reagent on 1 mm glass microscope slides. Cells were imaged using an Olympus FluoView 1000 confocal microscope with a 60X objective with a N.A. of 1.42. The 5-Fam fluorophore was imaged using fluorescein settings, Hoechst 34850 was imaged using DAPI settings, and wheat germ agglutinin Alexa Fluor 594 conjugate was imaged using Alexa Fluor 594 settings. All fluorescence and brightfield images were acquired simultaneously.
Quantitation of Cellular Uptake of Fluorescent Peptides Using Cell Lysates
Peptide stock solutions in Tris (20 mM, pH 9.0) were prepared so that when the peptide (237 μL) was diluted into serum free DMEM (263 μL), the final concentrations of peptides would be 0.10, 0.30, 1.0, or 3.0 μM. The dilution kept the amount of Tris compared to DMEM constant, regardless of final peptide concentration. Dilution was required because of the differential solubility among peptides. HeLa cells (2.6 × 104 cells/cm2) were seeded in 12 well plates and grown for 20 h to approximately 50% confluency. Next, the media was removed and DMEM containing diluted peptide (500 μL) was added to each well. Cells were treated with each peptide in triplicate and were incubated for 1 h at 37 °C in 5.0% CO2. At this time, the peptide-containing media was removed, and the wells were washed with PBS (1.0 mL, 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.3). Next, the cells were lysed with RIPA buffer (100 μL, 50 mM Tris·HCl, pH 7.4, 1.0% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1.0 mM EDTA) and scraped from the wells. The lysates were placed in 1.6 mL microcentrifuge tubes and centrifuged (1.0 min, 10,000 g). The fluorescence of the supernatant (490 nm excitation, 520 nm emission, 5 nm slit widths) was measured to quantitate the amount of internalized peptide. This measurement was then normalized against the amount of protein in the sample using the Bradford assay (BioRad) to measure the protein concentration. Each experiment was performed in triplicate and the results are expressed as the mean relative fluorescence ± standard deviation.
Quantitation of Cellular Uptake of Fluorescent Peptides Using Flow Cytometry
HeLa cells were seeded in 6-well plates and treated with peptides as described for microscopy. After treatment for 1, 2, 3, or 4 h, cells were washed twice with PBS, trypsinized for 5 minutes, and resuspended to a total volume of 2 mL with DMEM/10% FBS. Cells were centrifuged for 5 minutes at 100 × g and resuspended in PBS to a final volume of 2 mL. A total of 10,000 events for each sample were analyzed using a BD FACSCalibur (BD Biosciences). Each experiment was performed in triplicate and the results are expressed as the geometric mean fluorescence ± standard deviation.
Results
Synthesis of Cysteine-Alkylated CDC42 C-terminal Peptides
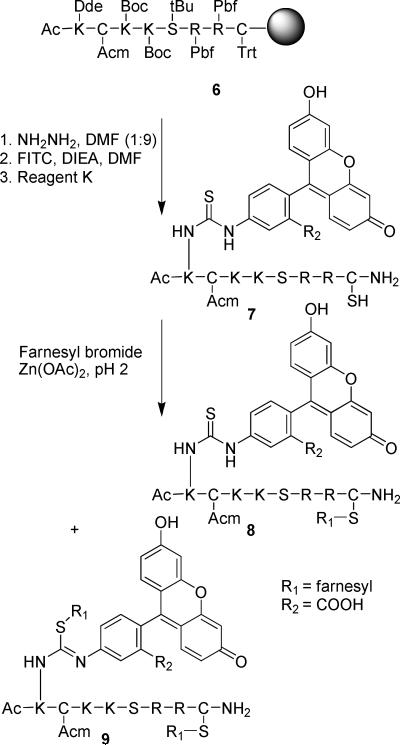
To prepare the desired multifunctional prenylated peptides for live cell analysis our initial strategy was to synthesize farnesylated peptide 8 by alkylation of peptide 7 derived after manipulation and cleavage of the peptide-resin 6. Fluorescein isothiocyanate (FITC) was chosen as the fluorescence reporter because of its reasonable cost, high fluorescence, and ready reactivity with primary amines via the isothiocyanate group. Thus, after selective on-resin removal of the Dde protecting group from the N-terminal Ac-Lys with anhydrous hydrazine (Scheme 1), FITC (3 eq.), in the presence of DIEA (6 eq.), was coupled onto the lysine side-chain (1 eq.) to install a thiourea-linked fluorophore. Final deprotection with Reagent K gave an orange peptide (7), in which one cysteine remains protected with the Acm group, while the free thiol of the second cysteine side-chain is available for alkylation. Interestingly, alkylation of the deprotected thiol in this peptide with farnesyl bromide in the presence of Zn(OAc)2 always resulted in mixtures of mono- (8) and dialkylated products (9) as determined by ESI-MS, even when only one equivalent of farnesyl bromide was added slowly to the peptide. Since it has been reported previously that Zn2+-catalyzed thiol alkylation under acidic conditions does not result in sulfonium ion formation18 (dialkylation of a single Cys), it was concluded that a second site in the peptide was undergoing alkylation at a rate that was competitive with alkylation of the free thiol of the C-terminal cysteine. While the most likely second site is the thiourea moiety linking fluorescein and the peptide (as suggested by peptide 9), we were unable to conclusively confirm the position of the second alkylation site by MS/MS sequence analysis due to complex fragmentation of the bis-alkylated peptide. To circumvent the dialkylation side reaction, FITC was replaced by 5-carboxyfluorescein (5-Fam) (Scheme 2) resulting in a fluorophore that was linked via an amide bond in lieu of a thiourea. As before, peptide-resin 6 was treated with anhydrous NH2NH2 to remove the orthogonal Dde protecting group, and this was followed by acylation of the resulting amine with 5-Fam using DIC and HOBt to produce peptide-resin 10. Cleavage from the resin and global deprotection afforded 11, that was subsequently alkylated with a prenyl bromide to yield 1a or 2a. In contrast to what was observed with peptide 7 where the fluorescein was linked via a thiourea, peptide 11 yielded only the monoalkylated products 1a or 2a, with no trace of dialkylated side products. Thus, peptides 1a and 2a were efficiently prepared for use in cellular experiments, as well as for subsequent conversion to 1c and 2c. The methylated analogue (3a) was prepared by direct incorporation of S-methyl cysteine (via Fmoc-Cys(Me)-OH) during SPPS, since the S-methyl derivative lacks the acid lability characteristic of prenylated peptides. The geranylgeranylated peptide containing a full length “CAAX box” (4) was prepared in a similar fashion to that described for 2a.
Scheme 1.
Initial strategy for synthesis of fluorescent prenylated peptides labeled via FITC modification. Although 8 elutes as a single peak in HPLC analysis, it is likely that it is an isomeric mixture of the structure shown and is the isomer containing the farnesyl group on the thiourea sulfur atom.
Scheme 2.
Revised strategy for the synthesis of fluorescent prenylated peptides labeled via 5-Fam incorporation.
Next, for the preparation of 1c-3c, it was necessary to unmask the Acm-protected cysteine present in 1a-3a so that penetratin could be linked via a disulfide bond. Multiple attempts using mercuric acetate,25 the standard method for Acm removal, to accomplish this transformation were unsuccessful. These results are likely due to the presence of the alkene functional groups in the isoprenoid moiety that might readily undergo oxymercuration. A revised strategy was chosen in which the Acm protecting group was transformed into a more active Scm derivative (Scheme 3).26 Conversion of Acm to Scm has the advantage of directly activating the thiol of cysteine to a species that can be used to form an unsymmetrical disulfide between the prenylated peptide and penetratin 12. Initial reactions on prenylated derivative 1a involved dissolving the peptide in MeOH/CH3CN mixtures and treating with CH3O(CO)SCl from a stock solution in MeOH. Although conversion of Acm to Scm was observed, the reaction was not reliably reproducible. From model studies on Boc-Cys(Acm)-OH, it was determined that the MeOH stock solutions of CH3O(CO)SCl rapidly lost activity (data not shown). In contrast, stock solutions of CH3O(CO)SCl in CH3CN are completely stable, and active for at least one day. Thus, to reproducibly effect the Cys(Acm) to Cys(Scm) conversion, alcohol-containing solvents must be avoided and the reaction should be performed in DMF/CH3CN solvent mixtures. It was also found that the use of excess CH3O(CO)SCl resulted in cleavage of thioether-linked isoprenoids to Cys(Scm). However, this side reaction was avoided by careful titration of the CH3O(CO)SCl reagent into the reaction, with monitoring by RP-HPLC. An analysis of a typical example of this Cys(Acm) to Cys(Scm) conversion is presented in Figure 2. Chromatogram 1 shows a pure sample of starting peptide 2a, chromatogram 2 shows a sample from the reaction at intermediate conversion and chromatogram 3 shows the reaction upon completion where the only significant species present is the Scm-protected peptide 2b. This approach was employed to prepare the Scm-protected peptides 1b-3b.
Scheme 3.
Strategy for linking penetratin to fluorescent prenylated peptides.
Next, the preparation of the desired penetratin-linked prenylated peptides was undertaken, using the activated Scm-containing peptides described above. Initial attempts to form the desired unsymmetrical disulfides that link penetratin to the prenylated peptides involved dissolving a Cys(Scm)-containing peptide in phosphate buffer, pH 7.5, and then adding a solution of penetratin analogue 12 dissolved in 0.10% aqueous TFA. The solvents were carefully degassed, and the reaction was carried out under a N2 atmosphere. The only peptides that were identified under those conditions were unreacted 12 and its related symmetrical disulfide dimer, while the Cys(Scm)-containing peptide appeared to have undergone decomposition. The instability of Cys(Scm)-containing peptides in phosphate buffer was confirmed by dissolving prenylated peptide 1b in buffer and monitoring by RP-HPLC. Complete decomposition was observed within 15 min (data not shown). The decomposition in phosphate near neutral pH was surprising, since Cys(Scm)-containing peptides are stable in 0.10% aqueous TFA for several days, and acidic reaction mixtures containing them can be evaporated to dryness without ill effect. Thus, it appeared reasonable to explore acidic conditions for forming the desired disulfide. When Cys(Scm)-containing peptide 1b and penetratin analogue 12 were reacted in the presence of Zn2+ in 0.10% aqueous TFA, pH 2.0, a small amount of desired unsymmetrical disulfide 1c was observed. Unfortunately, after the initial burst of product formation, the reaction stopped and no additional 1c appeared, even after long reaction times. However, after the pH was raised into the range of 5.0−5.3 by titration with 0.10 N NaOH, the reaction rapidly proceeded to completion. No disulfide scrambling or air oxidation of cysteine was observed under these mildly acidic reaction conditions. An analysis of such a reaction is presented in Figure 3. Chromatogram 1 shows the reaction after 24 h at pH 2.0; a small burst of product initially forms and then the reaction stops. After the adjusting to pH 5.2, the reaction is essentially complete in 24 h (Figure 3, Chromatogram 2). This procedure was employed for the synthesis of three penetratin-linked peptides (1c-3c) containing S-farnesyl, S-geranylgeranyl and S-methyl modifications. With the synthesis of the desired prenylated peptide conjugates completed, we next proceeded to examine the distribution of these molecules in live cells.
Quantitative Uptake of Modified -KKSRRC-NH2 Peptides
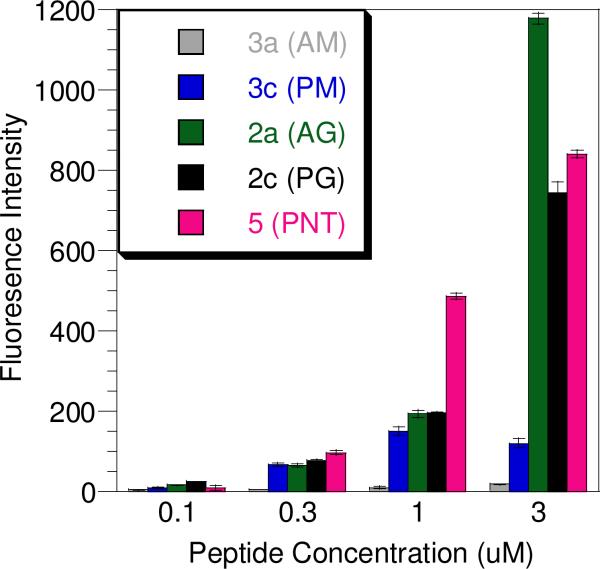
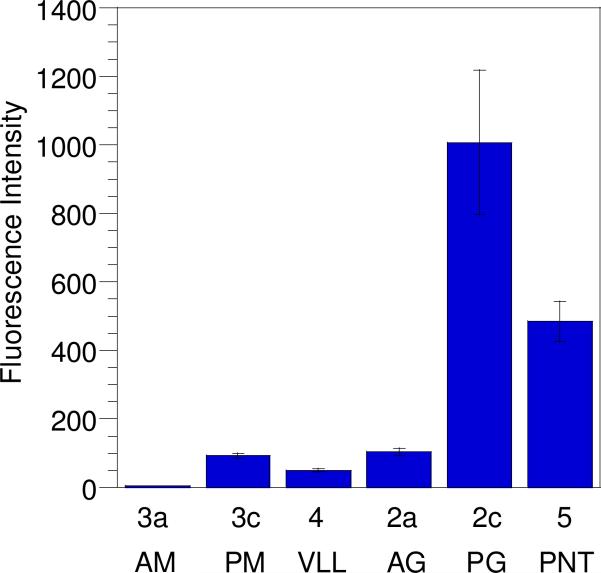
To evaluate the cellular uptake of the synthetic peptides prepared above, two methods were employed. Initially, this was accomplished by treating HeLa cells with each peptide over a range of concentrations, followed by washing, cell lysis, and quantitation of the internalized peptide via fluorescence spectroscopy. Using this method all peptides with the exception of 3a were able to enter cells at concentrations ranging from 0.1 to 3.0 μM. While the entry of the penetratin conjugates was expected, the uptake of the geranylgeranylated CDC42 peptide (2a) lacking any penetratin moiety was surprising. In order to ensure the observed uptake of prenylated peptides using this method was not merely due to peptide adsorption to plasma membranes, internalization was also measured using an alternative method where the cells were trypsinized prior to uptake analysis by flow cytometry. Trypsin digests and hence removes any peptide bound to the cell surface, thus allowing for the quantitation of only internalized peptide.27 Analysis of peptide concentrations in non-trypsinized cell samples (Figure 4) showed that incorporation of the geranylgeranylated CDC42 peptide without penetratin (2a) was comparable to that of the same peptide conjugated to penetratin (2c) at 0.3 μM and 1.0 μM; at 3.0 μM, the cellular uptake of 2a was actually higher than that of 2c. Such nonlinear changes in uptake at higher concentrations have been reported for other cationic cell-penetrating molecules.28 Analysis using trypsinized cell samples treated with 1.0 μM peptide showed 5-Fam labeled penetratin (5) entered cells 5-fold more than geranylgeranylated CDC42 peptide 2a (Figure 5A). Interestingly peptide 2c, which has both penetratin and geranylgeranyl moieties, entered cells 2-fold more than any of the other peptides examined suggesting that the effects of having an isoprenoid and a penetratin group may be additive. Thus, while these results indicate that some nonspecific surface adsorption occurs for the prenylated peptide 2a, that peptide is clearly capable of gaining entry into cells even through it lacks the penetratin substructure.
Figure 4.
Uptake of 5-Fam labeled peptides in HeLa cells evaluated by fluorescence spectroscopy. Cells were treated with peptide at different concentrations (0, 0.1, 0.3, 1.0, 3.0 μM) for 1 h, washed, and lysed in RIPA buffer. The fluorescence of the lysates was then normalized against the amount of protein in the sample. Each experiment was performed in triplicate and the results are expressed as the mean relative fluorescence ± standard deviation.
Figure 5.
Uptake of CPPs in HeLa cells at 37 °C determined by flow cytometry. After incubation with peptide (1.0 μM) for 1 h, the cells were trypsinized and washed to remove any surface-bound peptide. Each bar represents the geometric mean fluorescence of 10,000 cells counted by FACS analysis and each experiment was performed in triplicate and the results are expressed as the mean relative fluorescence ± standard deviation.
Cellular Internalization of Modified -KKSRRC-NH2 Peptides
To gain a better understanding of the mechanism of entry of these peptides into cells and their distribution, confocal microscopy experiments were performed. As noted above, studies were performed in parallel with a fluorescently labeled penetratin peptide lacking the CDC42 sequence (5) to provide a benchmark for comparison. Figure 6 shows several confocal microscope images (of fixed cells) obtained after treating cells with different peptides. In each case, HeLa cells were treated with a given peptide for 1 h followed by washing to remove unincorporated probe. Cells were treated with two additional fluorescent markers in order to visualize the nucleus (blue) and cell membrane (red), and then examined by confocal microscopy to observe the localization of the peptide (green).
Figure 6.
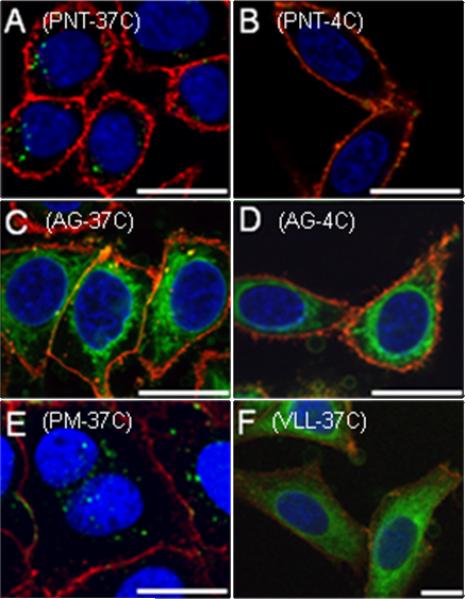
Confocal microscope images of fixed cells showing the uptake of CPPs in HeLa cells. After incubation with peptide (1.0 μM) for 1 h, the cells were washed, fixed, treated with ProLong Gold to limit fluorescence fading, and imaged. Panel A: 5-Fam labeled penetratin peptide 5 treated at 37 °C. Panel B: 5-Fam labeled penetratin peptide 5 treated at 4 °C. Panel C: Geranylgeranylated peptide 2a treated at 37 °C. Panel D: geranylgeranylated peptide 2a treated at 4 °C. Panel E: methylated penetratin linked peptide 3c treated at 37 °C. Panel F: “CAAX box” containing peptide 4 treated at 37 °C. Hoechst 34850 blue DNA staining dye was used to stain the nucleus blue, wheat germ agglutinin Alexa Fluor 488 was used to stain the plasma membrane red, and peptides were visualized as green. Orange staining is due to colocalization of green peptide with red plasma membrane dye. Bars in the lower corners of the images represent a distance of 20 microns.
Panel A in Figure 6 shows the results observed with HeLa cells treated with the fluorescently labeled penetratin control peptide (5). That peptide manifested a punctate pattern of green fluorescence within the cell that is characteristic of endocytic entry. Interestingly, treatment of cells with the farnesylated CDC42 peptide conjugated to penetratin (1c) produced a diffuse distribution of green fluorescence within the cells, accompanied by some punctate fluorescence (See supporting information, S1). In contrast, treatment of cells with the methylated CDC42 peptide conjugated to penetratin (3c) produced a punctate pattern of fluorescence exclusively (Figure 6, Panel E) similar to that of the simple penetratin peptide (5). These two results indicated that the presence of the isoprenoid moiety was playing a central role in controlling the observed localization of the internalized peptides. Finally, treatment of cells with the geranylgeranylated CDC42 peptide (2a) that retained the Acm protecting group, and hence was not conjugated to penetratin (Figure 6, Panel C), resulted in an intense diffuse distribution of green fluorescence within the cells localized to the perinuclear region. Similar, although less dramatic, results were observed with the Acm-protected farnesylated peptide (1a), but not with the corresponding methylated analogue (3a) (data not shown).
The observation that prenylated peptides lacking a cell penetrating peptide fusion could efficiently enter cells, coupled with their non-punctate distribution, suggested that their mechanism of cellular uptake might differ from that of penetratin. To investigate that possibility, we repeated the experiments described above at lower temperature, since it has been well established that endocytic uptake is inhibited at 4°C. Treatment of HeLa cells with the fluorescently labeled penetratin control peptide (5) at 4°C showed no green fluorescence within the cells (Figure 6, Panel B). Instead, the green peptide remained on the surface of the cells, colocalizing with the red plasma membrane marker producing an orange color at the plasma membrane (See also supporting information, S1). In contrast, treatment of HeLa cells at 4°C with the geranylgeranylated CDC42 peptide (2a) lacking the penetratin moiety showed substantial uptake of the peptide (Figure 6, Panel D); essentially, the behavior of this peptide was identical at the two different temperatures, suggesting that it does not enter through an endocytic pathway via depletion of intracellular ATP. To confirm that conclusion, cellular uptake was also examined in the presence of rotenone and 10 mM 2-deoxyglucose, which are known to inhibit processes that are energy dependent including endocytosis.24 Treatment of HeLa cells with the fluorescently labeled penetratin control peptide (5) in the presence of rotenone and 10 mM 2-deoxyglucose resulted in no uptake, whereas significant uptake was observed with the geranylgeranylated peptides 2a and 4. (See supporting information, S2).
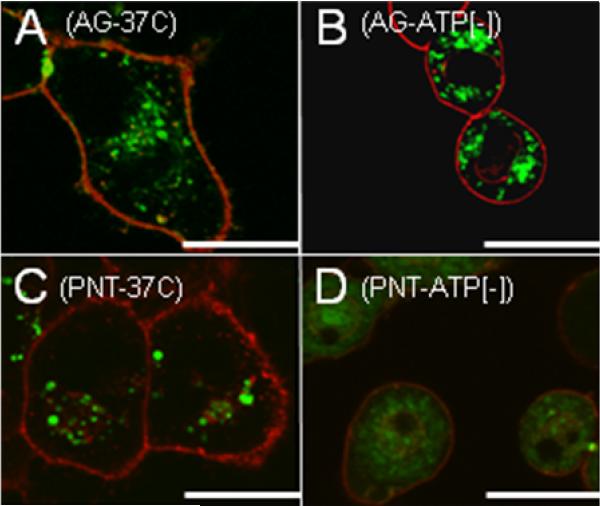
Recent reports have surfaced which indicate that fixing cell samples can in some cases render the cells more permeable to penetrating molecules.27 Since the microscopy experiments discussed above were completed on fixed cell samples, confocal microscopy imaging was also carried out on live HeLa cells treated with prenylated peptide 2a and penetratin linked peptide 5. Confocal images of live cells treated with penetratin peptide 5 at 37 °C showed perinuclear punctate fluorescence indicative of endocytotic uptake and looked identical to fixed samples treated under the same conditions (Compare Figure 6, Panel A to Figure 7, Panel C). Images of ATP-depleted cells that were treated with peptide 5 lacked punctate fluorescence, but showed a diffuse green staining throughout the cell that required an increase of laser power for visualization (Figure 7, Panel D). The intensity of green peptide signal was observed to be significantly less for ATP-depleted samples incubated with peptide 5. The decrease of internalized peptide 5 in ATP-depleted samples was later confirmed by FACS analysis (Figure 8). Together these observations confirm previous experiments by Letoha et al. who concluded that penetratin-linked peptides internalize mainly by an endocytotic mechanism, but can still gain entry to a lesser degree by direct transport.29 In contrast, prenylated peptide 2a did not show differential localization hinging on whether the treated cells were ATP-depleted (Figure 7, Panels A and B). The images observed of live cells treated with peptide 2a are very similar to those obtained of cells that were fixed after treatment with peptide (Compare Figure 6, Panel C to Figure 7, Panel A). The only observed difference between live and fixed images of cells treated with prenylated peptides is an increase in green signal intensity for fixed samples. This corresponds to previous results by Richard et al. that showed fixing cells treated with CPPs can lead to an increase in cell permeability.27 Even though fixing may increase the amount of peptide that internalizes, it does not change the phenotypic localization of peptide. Thus, quantitation of CPP internalization is more accurately assessed from live cell samples using flow cytometry rather than using signal intensities from confocal images.
Figure 7.
Live cell images showing the uptake of CPPs in HeLa cells. After incubation with peptide (1.0 μM) for 1 h, the cells were washed, and imaged without fixation. Panel A: Geranylgeranylated peptide 2a treated at 37 °C. Panel B: Geranylgeranylated peptide 2a treated to ATP-depleted cells. Panel C: 5-Fam labeled penetratin peptide 5 treated at 37 °C. Panel D: 5-Fam labeled penetratin peptide 5 treated to ATP-depleted cells. Images in panels A-C were taken using the same laser parameters while the image in Panel D required higher laser power for visualization. The intensity of the red channel in Panel D was also reduced to avoid obscuring the small amount of green fluorescence. Wheat germ agglutinin Alexa Fluor 488 was used to stain the plasma membrane red, and peptides were visualized as green. Orange staining is due to colocalization of green peptide with red plasma membrane dye. Bars in the lower corners of the images represent a distance of 20 microns.
Figure 8.
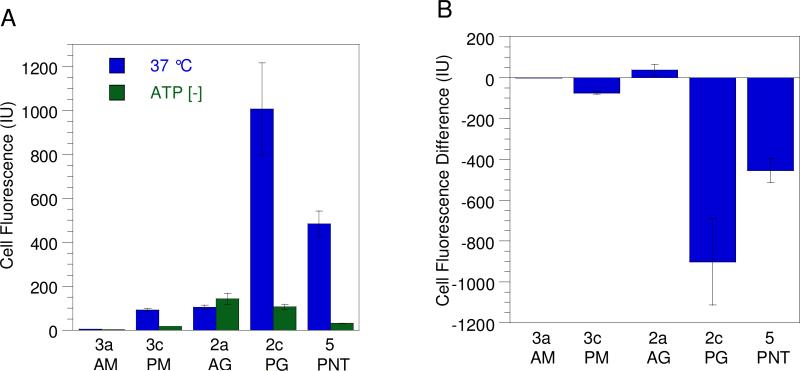
A) Uptake of CPPs in HeLa cells treated at 37 °C (blue) and under ATP-depleting conditions (green) obtained by flow cytometry. (B) The difference between peptide uptake under ATP-depleted conditions and at 37 °C. This is calculated by subtracting the geometric mean fluorescence of cells treated at 37 °C from the geometric mean fluorescence of cells treated under ATP-depleted conditions. After incubation with 1.0 μM peptide for 1 h, the cells were trypsinized and washed to remove any surface-bound peptide. Each bar represents the fluorescence of 10,000 cells counted by FACS analysis and each experiment was performed in triplicate and the results are expressed as the mean relative fluorescence ± standard deviation.
The mechanistic conclusions from the above confocal experiments were corroborated by results acquired from peptide uptake data obtained via flow cytometry and fluorescence measurements. Penetratin-linked peptides 5 and 3c showed significantly less cellular uptake in ATP-depleted cell samples compared to non-ATP-depleted controls, while geranylgeranylated peptide 2a showed similar levels of internalization regardless of whether the treated cells were ATP-depleted (Figure 8A). The changes in the amount of peptide internalization are more clearly illustrated in Figure 8B which plots the difference between uptake under ATP-depleted conditions and uptake under normal (37 °C) conditions; negative values in that plot indicate that internalization requires ATP. As expected, large decreases were observed for peptides 5 and 3c consistent with the established ATP-dependant mechanism for penetratin-mediated cell entry. In contrast peptide 2a shows no similar decrease. Since ATP-depletion affects only endocytotic uptake, the above results suggest that prenylated peptides enter cells through a mechanism distinct from the endocytotic pathway that has been proposed for penetratin-linked peptides. The residual uptake observed for penetratin linked peptides 5 and 3c can be attributed to a small amount of non-endosomal uptake that has been previously reported for penetratin linked molecules.29 Peptide 2c which has both a geranylgeranyl and a penetratin moiety showed ATP-depleted cell uptake similar to that of prenylated peptide 2a. Thus under ATP-depleting conditions that prevent endocytic uptake, the presence of a prenyl group within peptides 2a and 2c is clearly the dominant structural feature responsible for the cell penetrating capability of these molecules.
Kinetics of Peptide Uptake
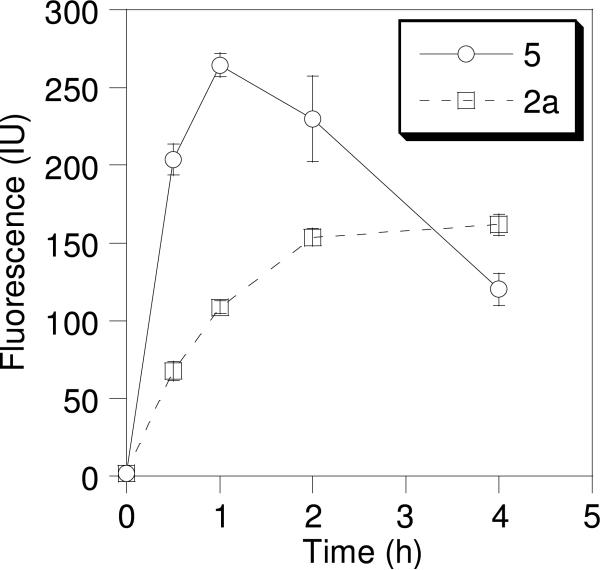
Since the results described above suggested that the prenylated peptides reported here internalize through a nonendosomal pathway, in contrast to penetratin, we decided to examine the rate of internalization for two representative peptides. Accordingly, we analyzed the kinetics of internalization for a prenylated peptide, geranylgeranylated peptide 2a and for the Fam-labeled penetratin peptide 5 (Figure 9). In those experiments, HeLa cells were incubated with peptide (1.0 μM) for varying amounts of time, trypsinized, and the resulting cells subjected to FACS analysis to quantify the amount of internalized peptide. 5-Fam-labeled penetratin (5) reached its maximum internalization level after 1 h of incubation and was observed to decrease with longer incubation periods. Internalization of geranylgeranylated peptide 2a was at 70% of its maximum level after 1 h and leveled off after 2 h. After 4 h the internalized level of geranylgeranylated peptide 2a exceeded that of 5-Fam-linked penetratin (5); this may reflect the great stability of 2a compared to 5. Since peptide 2a possesses both a blocked N-terminus (due to the 5-Fam) and a modified cysteine at its C-terminus, it is probably less susceptible to degradation by exoproteases; resistance to carboxypeptidase has previously been demonstrated for other prenylated peptides.1, 30 Since internalized levels of 5-Fam-linked penetratin begin to decrease and levels of geranylgeranylated peptide 2a are near their maximum after 1 h, this length of incubation represents a good compromise for comparing the internalization of the various peptides reported here.
Figure 9.
Time dependant uptake of CPPs. Cells were treated with 1 μM of geranylgeranylated peptide 2a (□) or 5-Fam-linked penetratin peptide 5 (○). After incubation for different amounts of time the cells were trypsinized and washed to remove any surface-bound peptide. Each time point represents the fluorescence of 10,000 cells counted by FACS analysis and each experiment was performed in triplicate and the results are expressed as the mean relative fluorescence ± standard deviation. Data points are connected to indicate the trend in each case.
Internalization of a Prenylated Peptide Including an Intact “CAAX Box”
Given the interesting result that prenylated CDC42-derived peptides lacking a penetratin moiety could freely enter cells, we wanted to determine whether related peptides that contain an intact C-terminal “CAAX box” could also be introduced into living cells. To study this, a CDC42-derived peptide that included the natural C-terminal sequence CVLL was prepared and geranylgeranylated; this peptide lacked the second cysteine present in the other peptides and was hence considerably easier to synthesize. Standard SPPS assembly, followed by on-resin labeling with 5-Fam, cleavage, and alkylation of the resulting free thiol in the presence of Zn(OAc)2 using geranylgeranyl bromide, gave peptide 4. Treatment of HeLa cells with peptide 4 at 1.0 μM, followed by confocal microscopy, showed that the peptide was able to enter the cells and that it localized in a fashion similar to what was observed with the geranylgeranylated peptide lacking the intact “CAAX box” (2a) (Figure 6, compare Panels F and C). Similar experiments performed at 4°C and under ATP-depleted conditions showed that the peptide was still able to enter the cells, suggesting a non-endocytic mechanism for cellular uptake (See supporting information, S1 and S2). Interestingly the distribution of 4 appears more diffuse than that of 2a. This probably reflects the more polar nature of 4 compared to 2a since the C-terminus of 4 is anionic (carboxylate) whereas that of 2a is neutral (amide). The greater polarity of 4 would then result in less dramatic partitioning between the cytosol and internal membranes compared with 2a.
Discussion
Previous studies have shown how prenylated peptides localize in natural 14 and artificial membranes.12, 13 Such studies showed what types of membranes bind prenylated peptides, but did not allow examination of the subcellular localization of prenylated peptides in live cells, or their processing therein. The goal of the work reported here was to develop synthetic methods for the preparation of multifunctional prenylated peptides, and to demonstrate that they could be introduced into living cells and visualized by fluorescence techniques.
The peptide sequence used in this work, -KKSRRC-NH2, is based on the C-terminal hexapeptide from fully processed CDC42, a small GTP binding protein from the Ras superfamily that is involved in regulation of cytoskeletal assembly. This peptide, when prenylated, is known to bind to the effector protein RhoGDI.31 Thus, this peptide sequence was chosen to facilitate studies of prenylation and its role(s) in biological systems.
The syntheses of the desired multifunctional peptides 1a-3a and 1c-3c contained several design challenges. Once the linear peptide sequence was synthesized, it was necessary to achieve three subsequent modifications. First, the C-terminal cysteine needed to be prenylated, and second, a fluorophore needed to be attached so that the peptide could be visualized. Third, it was thought that a CPP sequence would be needed in order to traverse the cell membrane. In order to accommodate these modifications, two amino acids were added to the N-terminus, and a protection scheme that included three dimensions of orthogonality was chosen. Since the all-trans double bonds of the prenyl groups are not stable to the acidic final cleavage conditions used in Fmoc-based SPPS, these moieties were attached to the peptide in solution subsequent to cleavage from the resin.32 Also, to avoid scrambling the disulfide linkage amongst the two cysteines, penetratin was added last in the synthetic sequence. To differentiate among the two cysteines, orthogonal protection for the cysteine closest to the N-terminus was accomplished with an Acm group,25 and for the C-terminal cysteine a Trt group was employed.
The selective introduction of a fluorophore onto the N-terminal lysine side-chain is best accomplished on-resin while the peptide is still fully protected, through the use of orthogonal Dde protection.20 Initially, a FITC fluorophore was coupled through a thiourea linkage; however this linkage was subject to alkylation during the subsequent prenylation reaction (Scheme 1). Thus, the fluorescent labeling reagent, FITC, was replaced by 5-Fam which resulted in a less problematic amide linkage to the Lys side-chain. Following installation of the fluorophore and subsequent cleavage from the resin, the thiol group on the C-terminal cysteine was prenylated with Zn2+ catalysis using the elegant procedure developed by Naider and coworkers;32 these conditions have been shown to not disturb the all-trans alkene stereochemistry present in prenyl groups, and they allow the Acm protected cysteine to remain masked.
With the peptide containing the prenyl group selectively installed on the desired cysteine, attention was next focused on linking the penetratin moiety to the second cysteine. Initially, it was envisioned that the Acm group would be deprotected to produce a free thiol that could then be reacted with an activated form of penetratin to produce the desired disulfide-linked peptides. However, efforts to remove the Acm group with Hg2+ were unsuccessful, probably due to additional reactions with the alkenes present in the isoprenoid moiety. To circumvent this problem, the Acm element was converted to the more reactive Scm group via treatment with CH3O(CO)SCl, following the pioneering work of Kamber.33 Investigations by Hiskey and coworkers26 and Kemp et al.34 suggested that thioethers exhibit a range of reactivity with CH3O(CO)SCl, and our own experiments showed that excess sulfenyl chloride reagent converts a prenylated cysteine moiety to Cys(Scm). The key finding here is that titration of peptides containing both Cys(Acm) and a prenylated Cys with CH3O(CO)SCl and carefully monitoring by HPLC leads to clean conversion to the desired Scm-modified molecules. This methodology for selective Acm removal in the presence of allylic thioethers may be generalized to peptides that contain multiple cysteines that must be differentiated, including prenylated and palmitylated sequences. A further important advantage of this strategy is that it results in the production of an activated disulfide that can then be used for direct reaction with penetratin; no separate activation of the latter peptide is required. This feature enabled the preparation of molecules in which the cell penetrating group (penetratin) is conjugated to the target peptide by a cleavable disulfide linker. Internally, cells house a reductive environment which is known to cleave disulfide bonds linking penetratin to its cargo.35 In contrast to simple fusion proteins (e.g. penetratin fused to the N-terminus of the CDC42 peptide) this approach allows for the study of the effects of the peptide independently from the effects of penetratin. Even though penetratin is internalized, it has been shown to be nontoxic at concentrations well above those employed here.36
Once the peptides were synthesized, they were tested for uptake into cells. Initially it was thought that a cell-penetrating peptide sequence would be needed for cellular uptake of these CDC42 C-terminal prenylated peptides. Interestingly, all prenylated peptides were able to gain entry into the cells, regardless of whether or not penetratin was attached. Imperiali et al. have shown that polybasic sequences followed by a large hydrophobic group exhibit cell-penetrating properties.37 The prenylated peptides used in the present study have the sequence (KKSRRC-NH2) with multiple positively charged lysine and arginine residues and a hydrophobic prenyl chain. Wender et al. have thoroughly studied how changing the hydrophobicity and altering the number of Arg residues in a polyarginine-based CPP modulates its cell penetrating properties.38 In those studies, peptides with less than six Arg or Lys residues showed little cellular internalization. Interestingly the peptide studied in the work reported here has only four charged residues but still exhibits large levels of internalization due to the presence of a nonpolar isoprenoid; consistent with that requirement, it should be noted that peptide 3a which lacked prenyl or penetratin modifications was unable to cross cellular membranes. These results demonstrate the importance of prenyl modification of CDC42 C-terminal peptides for transversing cellular membranes. Moreover, the molecules reported here manifest an additional feature that makes them particularly interesting; peptide isoprenylation appears to result in specific subcellular localization to membrane bound organelles around the nucleus. While earlier work by Imperiali and coworkers demonstrated that cationic peptides functionalized with hydrophobic groups can gain entry into cells, those peptides manifested a uniform distribution within the cells. In contrast, the prenylated peptides described here show specific localization to the perinuclear region of the cell. That localization may be due to the physical properties of the peptides, their association with specific proteins within the cell or some combination of both; experiments to address this question are in progress.
It is also important to point out apparent differences in the internalization mechanisms for the peptides described here. Penetratin-linked peptides have been shown to enter cells mainly though an energy dependent-pathway.39 Other CPPs have been shown to enter cells through both energy-dependent and -independent pathways. The data reported here shows that while prenylated peptides conjugated to penetratin may enter cells through multiple pathways, the peptides lacking the penetratin moiety clearly enter via an energy-independent process. This is particularly significant since it allows prenylated peptides to be introduced directly into cells, and avoids the potential problem of having them become trapped within the vesicular transport system. Beyond their intended use in studies of protein prenylation, the peptides described here may serve as cell penetrating peptides in their own right. Compared to polyarginine sequences, penetratin and Tat, the molecules reported here are significantly smaller; in fact, they are more similar in size to the cholesterol-based synthetic receptors created by Petersen and coworkers.40-42
In summary, we describe here the synthesis of a series of multifunctional peptides based on the C-terminus of CDC42. The molecules include a fluorophore for cellular visualization, a cell penetration moiety to facilitate cellular entry, and a prenyl group. These peptides readily enter cells and are nontoxic at physiologically relevant concentrations. The ability of these peptides to be readily internalized, coupled with their lack of toxicity, makes them ideal to probe the relationship between prenyl group structure and localization in living cells. Since a prenylated peptide incorporating an intact “CAAX box” is also internalized, this molecule makes it possible to follow the enzymatic processing of prenylated peptides in living cells. Given the fact that the enzymes involved in prenylation and subsequent processing events are important targets for the development of therapeutic agents for cancer treatment, the synthetic probes reported here should provide significant utility in the drug development process. Moreover, the methodology reported here is not limited to the specific peptides described herein. Given the large number of prenylated proteins and their crucial role in signal transduction pathways, the synthetic strategy described here should enable the preparation of peptides with a diverse range of non-natural isoprenoid structures and a variety of different sequences.43-46 Finally, it should be noted that prenylated peptides are not the only type of post-translational modification that can be prepared using the strategy reported here. The synthesis of cell-penetrating forms of glycosylated and phosphorylated peptides also present similar challenges. Introduction of disulfide-linked penetratin and other cell penetrating moieties via the mild methods reported here should prove to be useful for the preparation of those and many other types of modified peptides.
Supplementary Material
Acknowledgement
The authors thank Dr. Marion Navratil for helpful discussions and Mr. Jerry Sedgewick and Mr. John Oja of the University of Minnesota Biomedical Image Processing Lab for technical assistance regarding cell imaging. We would like to acknowledge the assistance of the Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598. This work was supported by the National Institutes of Health (Grant No GM058842, CA104609 and T32GM008347).
Footnotes
Supporting Information Available: S1: Multichannel fluorescent images available for peptides 1c, 2a, 3c, 4, 5 treated at 37 °C and 4°C. S2: Representative images of HeLa cells treated with rotenone and 2-deoxyglucose before addition of penetratin (5) or geranylgeranyled peptide (4). This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Schafer WR, Rine J. Annu. Rev. Genet. 1992;26:209–37. doi: 10.1146/annurev.ge.26.120192.001233. [DOI] [PubMed] [Google Scholar]
- 2.Zhang FL, Casey PJ. Annu. Rev. Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 3.Chow M, Der CJ, Buss JE. Curr Opin Cell Biol. 1992;4:629–36. doi: 10.1016/0955-0674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 4.Epstein WW, Lever D, Leining LM, Bruenger E, Rilling HC. Proc. Natl. Acad. Sci. 1991;88:9668–70. doi: 10.1073/pnas.88.21.9668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sebti SM, Hamilton AD. Oncogene. 2000;19:6584–6593. doi: 10.1038/sj.onc.1204146. [DOI] [PubMed] [Google Scholar]
- 6.Sousa SF, Fernandes PA, Ramos MJ. Curr. Med. Chem. 2008;15:1478–1492. doi: 10.2174/092986708784638825. [DOI] [PubMed] [Google Scholar]
- 7.Zhu K, Hamilton AD, Sebti SM. Curr. Opin. Invest. Drugs. 2003;4:1428–1435. [PubMed] [Google Scholar]
- 8.Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Proc. Natl. Acad. Sci. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall CJ. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 10.Michaelson D, Silletti J, Murphy G, D'Eustachio P, Rush M, Philips MR. J. Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts MJ, Troutman JM, Chehade KAH, Cha HC, Kao JPY, Huang X, Zhan C-G, Peterson YK, Subramanian T, Kamalakkannan S, Andres DA, Spielmann HP. Biochemistry. 2006;45:15862–15872. doi: 10.1021/bi061704+. [DOI] [PubMed] [Google Scholar]
- 12.Ghomashchi F, Zhang X, Liu L, Gelb MH. Biochemistry. 1995;34:11910–8. doi: 10.1021/bi00037a032. [DOI] [PubMed] [Google Scholar]
- 13.Silvius JR, l'Heureux F. Biochemistry. 1994;33:3014–22. doi: 10.1021/bi00176a034. [DOI] [PubMed] [Google Scholar]
- 14.Thissen JA, Casey PJ. J. Biol. Chem. 1993;268:13780–3. [PubMed] [Google Scholar]
- 15.Osmani N, Vitale N, Borg J-P, Etienne-Manneville S. Curr. Biol. 2006;16:2395–2405. doi: 10.1016/j.cub.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Nalbant P, Hodgson L, Kraynov V, Toutchkine A, Hahn KM. Science. 2004;305:1615–1619. doi: 10.1126/science.1100367. [DOI] [PubMed] [Google Scholar]
- 17.King DS, Fields CG, Fields GB. Int. J. Pept. Protein Res. 1990;36:255–266. doi: 10.1111/j.1399-3011.1990.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 18.Xue CB, Becker JM, Naider F. Tetrahedron Lett. 1992;33:1435–8. [Google Scholar]
- 19.Han Y, Albericio F, Barany G. J. Org. Chem. 1997;62:4307–4312. doi: 10.1021/jo9622744. [DOI] [PubMed] [Google Scholar]
- 20.Bycroft BW, Chan WC, R. CS, Hone ND. J. Chem. Soc., Chem. Commun. 1993:778–779. [Google Scholar]
- 21.Kempe M, Barany G. J. Am. Chem. Soc. 1996;118:7083–7093. [Google Scholar]
- 22.Kale TA. Ph.D. University of Minnesota; Minneapolis: 2001. [Google Scholar]
- 23.Haugland RP. The Handbook: A Guide to Fluorescent Probes and Labeling Technologies. 10th ed. Invitrogen; Carlsbad, CA: 2005. p. 65. [Google Scholar]
- 24.Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, Zon L, Sherman MY. Mol. Cell Biol. 1999;19:2547–55. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veber DF, Milkowski JD, Varga SL, Denkewalter RG, Hirschmann R. J. Am. Chem. Soc. 1972;94:5456–61. doi: 10.1021/ja00770a600. [DOI] [PubMed] [Google Scholar]
- 26.Hiskey RG, Muthukumaraswamy N, Vunnam RR. J. Org. Chem. 1975;40:950–3. doi: 10.1021/jo00895a600. [DOI] [PubMed] [Google Scholar]
- 27.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Leblue B. J. Biol. Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 28.Duchardt F, Fotin-Mleczek M, Schwarz H, Fischer R, Brock R. Traffic. 2007;8:848–66. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 29.Letoha T, Gaal S, Somlai C, Czajlik A, Perczel A, Penke B. J. Mol. Recognit. 2003;16:272–279. doi: 10.1002/jmr.637. [DOI] [PubMed] [Google Scholar]
- 30.Edelstein RL, Weller VA, Distefano MD, Tung JS. J. Org. Chem. 1998;63:5298–5299. [Google Scholar]
- 31.Hoffman GR, Nassar N, Cerione RA. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 32.Naider FR, Becker JM. Biopolymers. 1997;43:3–14. doi: 10.1002/(SICI)1097-0282(1997)43:1<3::AID-BIP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 33.Kamber B. Helv. Chim. Acta. 1973;56:1370–81. doi: 10.1002/hlca.19730560420. [DOI] [PubMed] [Google Scholar]
- 34.Kemp DS, Carey RI. J. Org. Chem. 1989;54:3640–6. [Google Scholar]
- 35.Saito G, Swanson JA, Lee K-D. Adv. Drug Deliv. Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 36.Saar K. In: The Handbook of Cell-Penetrating Peptides. 2nd ed. Langel U, editor. CRC Press; Boca Raton, FL: 2007. pp. 553–565. [Google Scholar]
- 37.Carrigan CN, Imperiali B. Anal. Biochem. 2005;341:290–298. doi: 10.1016/j.ab.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. Proc. Natl. Acad. Sci. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abes S, Richard J-P, Thierry A, Clair P, Lebleu B. In: The Handbook of Cell-Penetrating Peptides. 2nd ed. Langel U, editor. CRC Press; Boca Raton, FA: 2007. pp. 29–42. [Google Scholar]
- 40.Boonyarattanakalin S, Hu J, Dykstra-Rummel SA, August A, Peterson BR. J. Am. Chem. Soc. 2007;129:268–269. doi: 10.1021/ja067674f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boonyarattanakalin S, Martin SE, Dykstra SA, Peterson BR. J. Am. Chem. Soc. 2004;126:16379–16386. doi: 10.1021/ja046663o. [DOI] [PubMed] [Google Scholar]
- 42.Martin SE, Peterson BR. Bioconjug. Chem. 2003;14:67–74. doi: 10.1021/bc025601p. [DOI] [PubMed] [Google Scholar]
- 43.Duckworth BP, Chen Y, Wollack JW, Sham Y, Mueller JD, Taton TA, Distefano MD. Angew. Chem. Int. Ed. 2007;46:8819–8822. doi: 10.1002/anie.200701942. [DOI] [PubMed] [Google Scholar]
- 44.Krzysiak AJ, Rawat DS, Scott SA, Pais JE, Handley M, Harrison ML, Fierke CA, Gibbs RA. ACS Chem. Biol. 2007;2:385–389. doi: 10.1021/cb700062b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawe AL, Becker JM, Jiang Y, Naider F, Eummer JT, Mu YQ, Gibbs RA. Biochemistry. 1997;36:12036–12044. doi: 10.1021/bi9709755. [DOI] [PubMed] [Google Scholar]
- 46.Kale TA, Raab C, Yu N, Dean DC, Distefano MD. J. Am. Chem. Soc. 2001;123:4373–4381. doi: 10.1021/ja0012016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.