Abstract
Purpose
Expression of cellular adhesion molecules is altered in bullous keratopathy. The hypothesis that epithelial alterations in bullous keratopathy compromise the surface of the cornea and its glycocalyx was tested.
Methods
Studies were performed on eight cases each of pseudophakic bullous keratopathy and healthy corneas. The number of epithelial cell layers was determined with a stereological method of point counting. The minimum distance between points was established by estimates of cell size with variable pressure scanning electron microscopy performed in backscatter mode. The mean number of cell layers with mucin expression was identified by immunohistochemistry with mouse monoclonal antibodies for MUC1 and MUC16. Data were analyzed by Student's t-test if values showed a normal distribution or, alternatively, by the Wilcoxon rank-sum test.
Results
Mean numbers of wing cell and superficial cell layers were lower in bullous keratopathy specimens (1.6 vs. 2.0; P < 0.0001) than in controls (1.1 vs. 1.8; P < 0.000001). The number of exfoliated cell layers evident in sections was increased in the bullous keratopathy specimens compared with controls (0.36 vs. 0.03; P < 0.0001). The number of cell layers decorated with antibodies to MUC16 was lower in bullous keratopathy specimens than in controls (0.5 vs. 1.2; P < 0.025). The reduction of layers expressing MUC1 in bullous keratopathy was not statistically significant.
Conclusions
Pseudophakic bullous keratopathy manifests an abnormal corneal ocular surface in which superficial cell layers are exfoliated, leaving breaches in the protective MUC16 glycocalyx. The results provide a morphologic correlate for the surface epithelial abnormalities noted clinically in these patients.
Pseudophakic and aphakic bullous keratopathy are major indications for penetrating keratoplasty worldwide and account for more than one third of all corneal transplantations in some studies.1–5 Previous histopathologic studies of bullous keratopathy have understandably focused on the endothelial cell loss that constitutes the underlying pathogenic basis for this disorder. However, patients with bullous keratopathy are known to have painful surface epithelial defects, subepithelial bullae, and punctate staining. Bullous keratopathy may be accompanied clinically by superficial epithelial keratopathy.6 Eagle et al.7 noted that bullous keratopathy may manifest findings similar to map dot finger print dystrophy. Abnormalities include complete epithelial denudement, subepithelial bullae, intraepithelial duplication of basement membrane material, intraepithelial microcysts with degenerative material, and degenerative pannus. As expected, endothelial cell numbers are decreased.7 These features and stromal thickening with a degenerative lipid keratopathy have been reported.8 Absent in these descriptions is a quantitative analysis of the epithelial cell layers. In recent years, numerous antibodies to cellular adhesion and membrane proteins have become available. Alterations in the expression of cellular adhesion molecules have been demonstrated in bullous keratopathy, including elevated tenascin C integrins, elevated B6 integrins, and elevated expression of β-catenin throughout the epithelium.9,10 Some of these changes are putatively compensatory to augment interepithelial cell adhesion that has been compromised by bullous keratopathy.11 Some authors have noted increased apoptosis of corneal epithelium in Fuchs' dystrophy although the increase (P = 0.07) for bullous keratopathy was not statistically significant.12,13 Cell cycling inhibitors modulated by P53, P21, and P27 are reduced.14 These data suggest that surface epithelium may be compromised in bullous keratopathy by abnormal cellular and basement membrane adhesion and may be prone to increased cellular shedding. The superficial epithelium of the cornea is especially important because it expresses the ocular mucins MUC16, MUC1, and MUC4, which lubricate and protect the cornea.15–17 MUC16 is the largest corneal epithelial transmembrane mucin (22,000 amino acids) and displays extensive O-glycosylation.18 In human corneas, MUC16 is confined to the superficial apical cells and one subjacent layer.17 MUC16, but not MUC1, is important to prevent the permeability of Rose Bengal dye in islands of human cultured corneal epithelial cells.19,20 The pattern of expression of MUC4 is different from that of MUC1 and MUC16. MUC4 is expressed mainly at the corneal limbus in several layers of epithelium of the rat but perhaps only minimally in the central corneal epithelium of humans.16,21 Some ocular mucins have been shown to be altered in atopic keratoconjunctivitis, corneal ulcer, and dry eye disease.22,23 Given the clinically apparent abnormal surface characteristics and altered expression of adhesion molecules in bullous keratopathy, the hypothesis that exfoliation and cell drop out of the superficial epithelium affect the ocular surface glycocalyx was explored.
Materials and Methods
Corneal specimens were fixed in 10% formalin immediately after surgical removal. Corneal buttons specimens were carefully removed by the surgeon (RC) to avoid disturbing the central cornea epithelium. Control (normal) central buttons were taken from corneas obtained from enucleation or exenteration specimens, as previously described.24 Only specimens with grossly intact epithelia at the time of surgery were considered for this study. Mean ages of the control group and the group with disease were similar, 70.5, and 73.2 years, respectively. Specimens, both control and bullous keratopathy, were processed together in graded alcohols and xylene and were embedded in paraffin. To ensure uniformity of processing and staining, bullous keratopathy specimens and those from normal corneas were embedded and cut from the same paraffin block. Five-micron sections were stained for hematoxylin and eosin and for periodic acid Schiff (PAS). The study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of California, Los Angeles.
Variable Pressure Scanning Electron Microscopy
Scanning electron microscopy was performed to determine the minimum interval distance for point counting (size of the superficial cells) in bullous keratopathy specimens. Corneas, after fixation, were mounted on an aluminum platform stage of a variable pressure scanning electron microscope (Leo 1430; Carl Zeiss Inc., Oberkochen, Germany) equipped with a four-quadrant electron backscatter detector. Images were collected rapidly at 13 Pascal in variable pressure backscatter mode at 15 to 30 kV. No coating is necessary for specimens with this microscope.
Point Counting and Quantitative Assessment
Quantitative measurements for this study were confined to the central 1.2 mm of the corneal buttons because the number of cell layers varies at the far periphery of the cornea compared with the center.25 For an 8-mm button that is maximally offset in a 12-mm cornea, the distance between the sampled area and the closest peripheral edge is 3.4 mm. Superficial cells are thin and have indistinct lateral borders in histologic sections. Therefore, we chose a stereological method to count the number of cell layers at an interval that precluded counting any cell more than once.26 The number of cell layers was counted at random points where two lines on an ocular grid intersect (Fisher Scientific, Tustin, CA). Cell layers were counted in a line that extended perpendicularly to a tangent from the surface. Measurements were made at a total magnification 312.5×. Intersections of grid lines were counted at 100-μm intervals, which are greater than the diameter of the normal superficial cell of 30 to 45 μm.25,27 To determine whether a section of the central corneal buttons was representative, step sections were made through the entire central cornea at 200-μm intervals. Because results were consistent from section to section, a representative section from the central corneal buttons was used. For each cornea, 12 measurements were obtained for every parameter, and means of the values were taken. The specimens were coded with marking ink on the posterior surfaces to mask the diagnosis from the observer. Given that only cells observed in the section could be counted, the cells designated as exfoliated cells might have been partially exfoliated cells with connections to the surface in another plane of section. Therefore, for counting purposes, exfoliated cells were defined as partially or completely detached from the surface.
Immunohistochemistry
Immunohistochemistry was performed by the peroxidase-antiperoxidase technique, as previously described.28 Five-micron sections were deparaffinized in xylene, washed in graded alcohols, incubated for 15 minutes in Tris-buffered saline (pH 6.5), blocked with 1% bovine serum albumin, rinsed briefly, and incubated with mouse monoclonal antibodies MUC1 (clone DF3) and MUC16 (clone OC125) (DAKO Inc., Carpinteria, CA) diluted 1/4000 and 1:50, respectively (1 hour at room temperature). Secondary antibodies (goat anti–mouse) were obtained from DAKO. Omission of the primary antibody served as the negative control. Sections of human ovarian mucinous adenocarcinoma served as positive tissue controls. Slides were counterstained with hematoxylin. Because of drawbacks with the correlation of mucin expression and intensity of staining, measurements were limited to the number of cell layers with antigen reactivity.29
Statistical Analysis
Data were analyzed for differences in mean values between bullous keratopathy specimens and controls by the two tailed Student's t-test if values showed a normal distribution. Otherwise the Wilcoxon rank-sum test was used. Data from five to eight corneas—a minimum of 60 data points—were obtained for each group. Values represent mean ± SD. P < 0.05 was considered statistically significant.
Results
Scanning Electron Microscopy
Scanning electron microscopy of the surface of a bullous keratopathy cornea determined the size of cells for point counting intervals. Numerous surface cells were apparently missing (Fig. 1). Sizes of the epithelial cells varied from 17 to 57 μm (mean, 31 μm).
FIGURE 1.
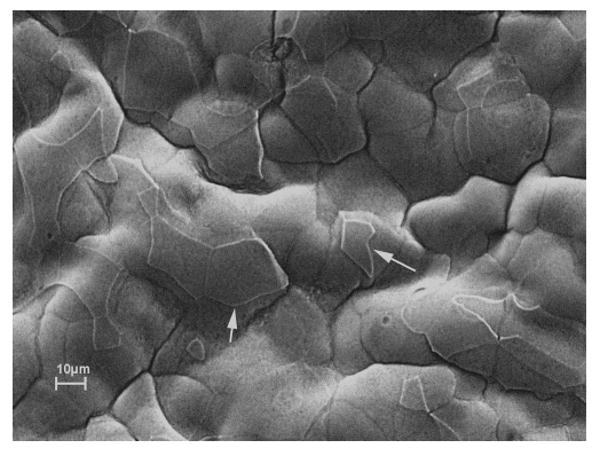
Scanning electron micrograph of bullous keratopathy specimen shows an irregular surface with numerous residual polygonal superficial epithelial cells of varying size and shapes after exfoliation (white arrows).
Analysis of Epithelial Cells in Bullous Keratopathy
A variety of epithelial manifestations in bullous keratopathy corneas were evident in this study. The thickness of the epithelium varied from a single layer to six layers. Some areas showed many exfoliated cells and other areas few. The epithelium appeared altered by basement membrane deposition and the presence of bullae.
Quantitative analysis showed a decrease in the mean of the total number of epithelial cell layers in bullous keratopathy specimens (3.9 ± 0.9 layers) compared with controls (4.9 ± 0.7 layers) (Fig. 2). In areas with only minimal pathologic changes, minor thinning of the epithelium often accompanied bullae (Fig. 3A). Less common were areas of marked thinning of the epithelium. Attenuation of Bowman layer was usually aligned with overlying bullae (Fig. 3B).
FIGURE 2.
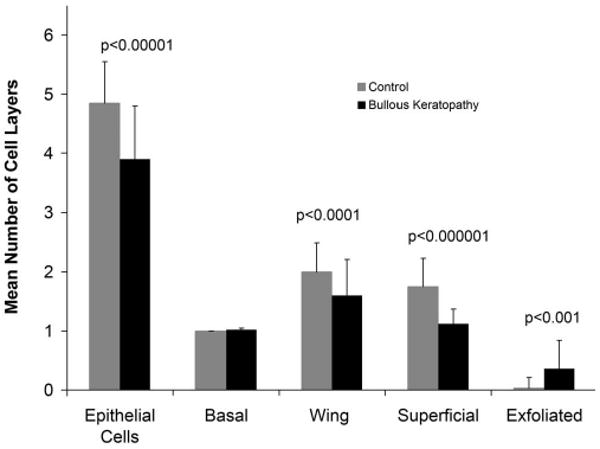
Mean numbers of cell layers in bullous keratopathy versus control specimens. A significant reduction is shown in the total epithelial cell layers and in wing cells and superficial cells in bullous keratopathy specimens. The number of exfoliated cells was significantly increased in bullous keratopathy compared with control specimens.
FIGURE 3.
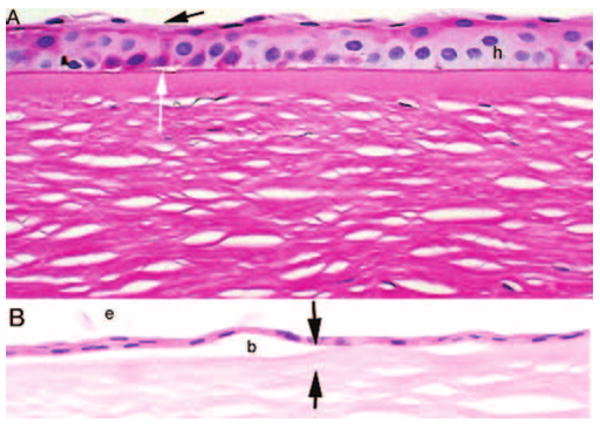
Photomicrographs of epithelial thinning in bullous keratopathy. (A) PAS stain shows thinning of the epithelium with loss of superficial cells (black arrow) and microbullae (white arrow). Hydropic change is evident within the epithelium (h). Many of the wing cell nuclei are faded. (B) Hematoxylin and eosin–stained sections show severe thinning of the epithelium with bullae (b) accompanied by attenuation of the Bowman layer (between black arrows). Exfoliated cells are evident (e). Original magnification, ×250.
The superficial epithelium often showed numerous partially exfoliated cells that formed festooned microblebs (Fig. 4A). A reduction in superficial cells was evident in bullous keratopathy specimens (1.1 ± 0.3) compared with control specimens (1.8 ± 0.5). Fewer layers of wing cells were counted in bullous keratopathy (1.6 ± 0.6) than in the controls (2.0 ± 0.5) (Fig. 2). The mean number of basal cell layers in bullous keratopathy specimens (1.0 ± 0.0) was not different from that of control specimens in this study (1.0 ± 0.0) (Fig. 2), yet basal cell and wing cell layers showed distinct abnormalities (Fig. 4). Abnormal cells were still counted if identifiable.
FIGURE 4.
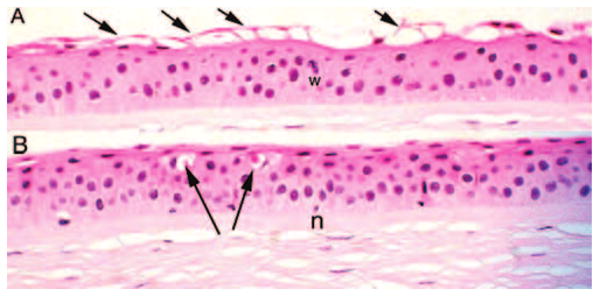
(A) Photomicrographs of bullous keratopathy show numerous exfoliated superficial cells (arrows) producing the appearance of multiple small surface blebs. A wing cell nucleus appears fragmented (w). (B) In some areas, wing cell dropout (arrows) is accompanied by a relatively intact superficial cell layer. Effete nuclei (n) are seen within the basal cell layer (original magnification, ×250; hematoxylin and eosin).
Mucin Localization in Epithelium
PAS stained superficial cells and some wing cells in control and bullous keratopathy specimens (Fig. 5A), However, staining was obfuscated by extensive intraepithelial deposition of basement membrane (Fig. 5B). When fulminant, the basement membrane material could even account for most of the overall thickness of the epithelial layer (Fig. 5C).
FIGURE 5.
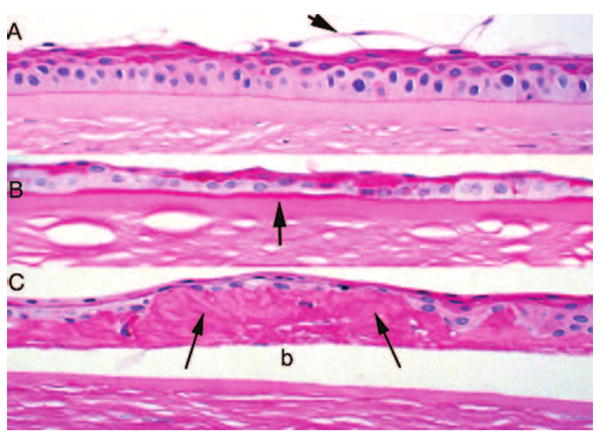
PAS stains of bullous keratopathy (original magnification, ×250). (A) Poorly stained superficial cells are partially exfoliated (arrow) with underlying wing cells that stain. (B) Basement membrane thickening is evident beneath the epithelium (arrow), and the thinned epithelium shows variable staining of various layers with PAS. (C) Marked intraepithelial deposition of basement membrane material (arrows) lies beneath and around the detached epithelium (b).
Although the mean number of cell layers staining with PAS was slightly less in the epithelium of bullous keratopathy (2.0 ± 1.0) than in control (2.6 ± 0.9) specimens, the difference was not significant (Fig. 6). Immunohistochemistry was performed to search with more specificity for ocular surface mucin. Mean numbers of cell layers expressing MUC16 were decreased significantly in bullous keratopathy (0.5 ± 0.6) compared with control (1.2 ± 0.6) specimens. For MUC1 the decrement from control specimens was not significant (Fig. 6).
FIGURE 6.
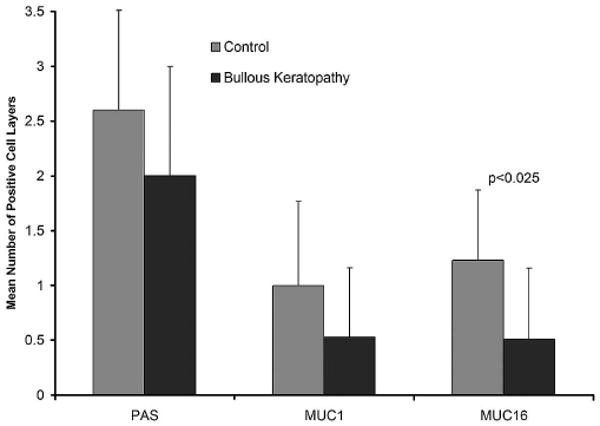
Identification of mucin-positive cell layers in bullous keratopathy compared with control specimens. PAS staining was insignificantly decreased in bullous keratopathy specimens. Immunohistochemical staining for MUC16, but not MUC1, showed a significant decrease in the number of cell layers that were positive.
Immunohistochemical staining for MUC16 was consistent in controls and was confined to one to two cell layers (Fig. 7). However, in bullous keratopathy specimens, areas of thinning showed apparent removal of superficial cells often with very little residual staining (Fig. 7B). The exfoliated cells generally showed some reactivity and left gaps in which the epithelium was bereft of MUC16 surface coverage (Figs. 7B, 7C).
FIGURE 7.
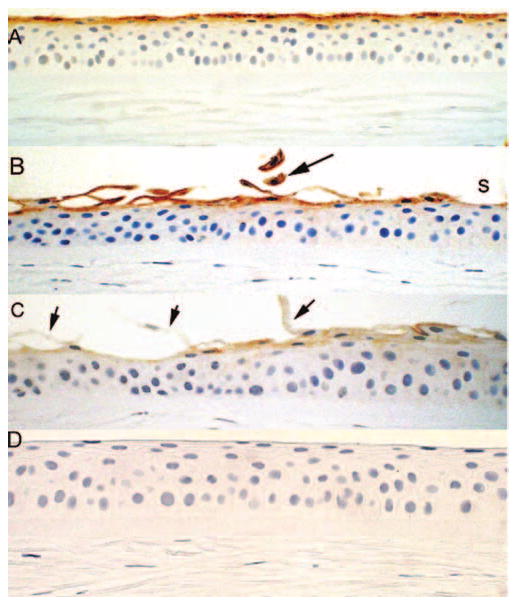
Immunohistochemistry for MUC16 (A) in control specimens shows one to two superficial cell layers staining consistently and (B, C) in bullous keratopathy specimen. (B) Exfoliated cells show intense MUC16 staining (black arrow). Some areas underlying shed cells shows faint staining (s). (C) In some cases exfoliated cells (black arrows) and the underlying epithelium show weak to absent reactivity to MUC16. (D) Negative control of normal cornea with deletion of the primary antibody.
Discussion
The previously reported major morphologic features of bullous keratopathy,7 including separation of epithelium from Bowman layer in large bullae, intraepithelial basement membrane deposition, stromal scarring, and reduced endothelial cells, were observed in this study. Patients with gross epithelial defects were not included because only epithelium was germane to this study. This selection bias would only underestimate the severity of more advanced disease.
The key finding of decreased number of superficial cell layers observed in the present study appears to be related to exfoliation from the surface. The number of exfoliated cell layers observed in section was approximately 10-fold greater in bullous keratopathy specimens (0.36 ± 0.48) than in controls (0.03 ± 0.18) (Fig. 2). These exfoliated cells often accompanied thinning of the epithelium, but the thinnest epithelia had few identifiable cells (Figs. 3B, 5C). It seems probable that the number of exfoliated cells was underestimated. Corneas with bullous keratopathy may be predisposed to cell loss with minor shear stresses. Indeed previous studies have shown that cellular adhesion molecules are altered in bullous keratopathy. Elevated expression of tenascin C integrins and of β-catenin are considered compensatory to aid cell adhesion because B4 integrins are reduced throughout the epithelium. Shear stress may increase the shedding of corneal epithelial cells in rabbits.30 Additional factors such as rubbing by the patient because of irritation, manipulation during penetrating keratoplasty, or even processing may add shear stress to the corneas. Exfoliated cells were only occasionally observed in control corneas taken from enucleation and exenteration specimens, eyes that were also surgically manipulated (Figs. 2, 7A). In addition data collection was confined to the central cornea, away from the surgical incision. The loss of epithelial cells in bullous keratopathy may also result from accelerated apoptosis or nonviability.31 The loss of wing cells in bullous keratopathy is not accounted for solely by external shear forces because superficial cells overlying the degenerated wing cells were frequently intact (Fig. 4B). The extensive loss of epithelium included basal cell and wing cell layers in areas of dramatic bullae (Fig. 3B). Apoptosis of all cell layers has been described in bullous keratopathy13 and may account for the significant decrease observed in wing cell layers (Figs. 2, 4B).
PAS is a common histochemical stain used in the analysis of pathologic corneas. The histologic reagent stains a wide variety of mucins. PAS is not specific for mucins and reacts with a variety of glycosylated moieties, including glycogen, basement membrane materials, and glycolipids. The nonspecificity of this stain is evident in the extensive basement membrane staining that may reside in the epithelium of corneas with bullous keratopathy (Fig. 5). These findings prompted immunohistochemical studies for the two major ocular surface mucins.
MUC1 stains minimally in the human superficial cornea epithelium,16 and its role does not seem as important for ocular surface protection; previous studies demonstrate that its presence does not exclude such dyes as Rose Bengal.19
MUC16, also known as CA 125, localizes to microplicae at the apical surfaces of the cornea epithelial cells and is linked to the actin cytoskeleton domain.32,33 The localization of MUC16 in our study conforms to this finding. MUC16, unlike other ocular mucins, lacks epidermal growth factor domains and is a likely candidate to serve mainly as a protectant of the ocular surface.32,34 MUC16 expression is diminished on the conjunctival epithelium of patients with non-Sjögren dry eye syndrome.17,35 MUC16 mRNA expression is upregulated in allergic keratoconjunctivitis, possibly a compensatory mechanism for a reduction in MUC5AC, produced by Goblet cells.36 Given the excessive epithelial shedding of superficial cells in bullous keratopathy, the variation of epithelial thickness and cellular distribution, the restricted expression pattern of MUC16 in the cornea, and the lack of correlation between transcript and mucin surface product,29 immunohistochemistry was preferable to RT-PCR for localization of MUC16 and quantification restricted to positive cell layers. Immunohistochemical localization permits direct visualization of gaps produced by exfoliated superficial cells in bullous keratopathy to correlate with MUC16 expression (Fig. 7). Cell shedding appears to be a dominant factor in bullous keratopathy in determining the ocular surface composition of MUC16. Previous immunoelectron microscopy in animals has suggested that apical cells that are poorly adherent to the cornea surface show less membrane mucin expression.33,37 Such a conclusion in bullous keratopathy is supported by Figures 5A and 7C, though masking of antigens with fixation and heavy glycosylation may lead to underestimation of mucin expression as measured by the intensity of staining in immunohistochemistry.29 However, in areas in which exfoliation is excessive, MUC16 antigen appears abundant in shedding cells (Fig. 7B). It is possible that premature shedding of cells in bullous keratopathy preempts the release of antigen by ectodomain shedding.38 The reduction of MUC16-positive cell layers beneath shed cells may indicate that compensatory upregulation of MUC16 expression in response to cell shedding could be insufficient for the increased superficial cell exfoliation. The implication from exfoliative-related breaches in the MUC16 glycocalyx is one of compromised barrier function with exposure and decreased wetting of the ocular surface.
A number of complex factors regulate mucin expression.29,38,39 The excessive shedding of superficial cells that express MUC16 characterizes the ocular surface disorder present in bullous keratopathy. Such a phenomenon is possible in other disorders in which the epithelium is chronically stressed by edema, inflammation, and increased apoptosis. In Sjögren's original histologic description of a cornea in dry eye, many findings of the epithelium were identical with those of bullous keratopathy.40 Bullous keratopathy presents a potential disease model in which the superficial epithelium and the glycocalyx protective coat of the ocular surface appear compromised.
Acknowledgments
The authors thank Anne Coleman and Felice Wei for advice with statistical analysis.
Supported by United States Public Health Service Grants NIH EY11224 and NIH EY00331 and by The Edith and Lew Wasserman Endowed Professorship in Ophthalmology.
Footnotes
Disclosure: B.J. Glasgow, None; O.K. Gasymov, None; R.C. Casey, None
References
- 1.Cosar CB, Sridhar MS, Cohen EJ, et al. Indications for penetrating keratoplasty and associated procedures: 1996–2000. Cornea. 2002;21:148–151. doi: 10.1097/00003226-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Dobbins KR, Price FW, Jr, Whitson WE. Trends in the indications for penetrating keratoplasty in the Midwestern United States. Cornea. 2000;19:813–816. doi: 10.1097/00003226-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Chen WL, Hu FR, Wang IJ. Changing indications for penetrating keratoplasty in Taiwan from 1987 to 1999. Cornea. 2001;20:141–144. doi: 10.1097/00003226-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Liu E, Slomovic AR. Indications for penetrating keratoplasty in Canada: 1986–1995. Cornea. 1997;16:414–419. [PubMed] [Google Scholar]
- 5.Narayanan R, Gaster RN, Kenney MC. Pseudophakic corneal edema: a review of mechanisms and treatments. Cornea. 2006;25:993–1004. doi: 10.1097/01.ico.0000214225.98366.83. [DOI] [PubMed] [Google Scholar]
- 6.Taylor DM, Atlas BF, Romanchuk KG, Stern AL. Pseudophakic bullous keratopathy. Ophthalmology. 1983;90:19–24. doi: 10.1016/s0161-6420(83)34607-8. [DOI] [PubMed] [Google Scholar]
- 7.Eagle RC, Jr, Laibson PR, Arentsen JJ. Epithelial abnormalities in chronic corneal edema: a histopathological study. Trans Am Ophthalmol Soc. 1989;87:107–119. discussion 119-124. [PMC free article] [PubMed] [Google Scholar]
- 8.Yuen HK, Rassier CE, Jardeleza MS, et al. A morphologic study of Fuchs dystrophy and bullous keratopathy. Cornea. 2005;24:319–327. doi: 10.1097/01.ico.0000148288.53323.b2. [DOI] [PubMed] [Google Scholar]
- 9.Akhtar S, Bron AJ, Hawksworth NR, Bonshek RE, Meek KM. Ultrastructural morphology and expression of proteoglycans, betaig-h3, tenascin-C, fibrillin-1, and fibronectin in bullous keratopathy. Br J Ophthalmol. 2001;85:720–731. doi: 10.1136/bjo.85.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spirin KS, Ljubimov AV, Castellon R, et al. Analysis of gene expression in human bullous keratopathy corneas containing limiting amounts of RNA. Invest Ophthalmol Vis Sci. 1999;40:3108–3115. [PubMed] [Google Scholar]
- 11.Ljubimov AV, Saghizadeh M, Pytela R, Sheppard D, Kenney MC. Increased expression of tenascin-C-binding epithelial integrins in human bullous keratopathy corneas. J Histochem Cytochem. 2001;49:1341–1350. doi: 10.1177/002215540104901102. [DOI] [PubMed] [Google Scholar]
- 12.Li QJ, Ashraf MF, Shen DF, et al. The role of apoptosis in the pathogenesis of Fuchs endothelial dystrophy of the cornea. Arch Ophthalmol. 2001;119:1597–1604. doi: 10.1001/archopht.119.11.1597. [DOI] [PubMed] [Google Scholar]
- 13.Szentmary N, Szende B, Suveges I. Epithelial cell, keratocyte, and endothelial cell apoptosis in Fuchs' dystrophy and in pseudophakic bullous keratopathy. Eur J Ophthalmol. 2005;15:17–22. doi: 10.1177/112067210501500103. [DOI] [PubMed] [Google Scholar]
- 14.Szentmary N, Szende B, Suveges I. P53, CD95, cathepsin and survivin pathways in Fuchs' dystrophy and pseudophakic bullous keratopathy corneas. Histol Histopathol. 2008;23:911–916. doi: 10.14670/HH-23.911. [DOI] [PubMed] [Google Scholar]
- 15.Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–1827. [PubMed] [Google Scholar]
- 16.Pflugfelder SC, Liu Z, Monroy D, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. 2000;41:1316–1326. [PubMed] [Google Scholar]
- 17.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 18.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 19.Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the Rose Bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864–1871. doi: 10.1167/iovs.07-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–1692. [PubMed] [Google Scholar]
- 22.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 23.Dogru M, Asano-Kato N, Tanaka M, et al. Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers. Curr Eye Res. 2005;30:897–908. doi: 10.1080/02713680500196715. [DOI] [PubMed] [Google Scholar]
- 24.Gasymov OK, Abduragimov AR, Prasher P, Yusifov TN, Glasgow BJ. Tear lipocalin: evidence for a scavenging function to remove lipids from the human corneal surface. Invest Ophthalmol Vis Sci. 2005;46:3589–3596. doi: 10.1167/iovs.05-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan MJ, Alvarado JA, Weddell JE. Histology of the Human Eye: An Atlas and Textbook. Philadelphia: Saunders; 1971. p. xiii.p. 687. [Google Scholar]
- 26.Weibel ER. Stereological Methods. New York: Academic Press; 1979. pp. 101–161. [Google Scholar]
- 27.Blumcke S, Morgenroth K., Jr The stereo ultrastructure of the external and internal surface of the cornea. J Ultrastruct Res. 1967;18:502–518. doi: 10.1016/s0022-5320(67)80200-4. [DOI] [PubMed] [Google Scholar]
- 28.Glasgow BJ. Tissue expression of lipocalins in human lacrimal and von Ebner's glands: colocalization with lysozyme. Graefes Arch Clin Exp Ophthalmol. 1995;233:513–522. doi: 10.1007/BF00183433. [DOI] [PubMed] [Google Scholar]
- 29.Theodoropoulos G, Carraway KL. Molecular signaling in the regulation of mucins. J Cell Biochem. 2007;102:1103–1116. doi: 10.1002/jcb.21539. [DOI] [PubMed] [Google Scholar]
- 30.Ren H, Wilson G. The effect of a shear force on the cell shedding rate of the corneal epithelium. Acta Ophthalmol Scand. 1997;75:383–387. doi: 10.1111/j.1600-0420.1997.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 31.Ren H, Wilson G. Apoptosis in the corneal epithelium. Invest Ophthalmol Vis Sci. 1996;37:1017–1025. [PubMed] [Google Scholar]
- 32.Blalock TD, Spurr-Michaud SJ, Tisdale AS, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–4518. doi: 10.1167/iovs.07-0430. [DOI] [PubMed] [Google Scholar]
- 33.Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–388. doi: 10.1016/s0014-4835(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 34.Singh AP, Chauhan SC, Bafna S, et al. Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate. 2006;66:421–429. doi: 10.1002/pros.20372. [DOI] [PubMed] [Google Scholar]
- 35.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 36.Dogru M, Matsumoto Y, Okada N, et al. Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy. 2008;63:1324–1334. doi: 10.1111/j.1398-9995.2008.01781.x. [DOI] [PubMed] [Google Scholar]
- 37.Gipson IK, Yankauckas M, Spurr-Michaud SJ, Tisdale AS, Rinehart W. Characteristics of a glycoprotein in the ocular surface glycocalyx. Invest Ophthalmol Vis Sci. 1992;33:218–227. [PubMed] [Google Scholar]
- 38.Dartt DA. Control of mucin production by ocular surface epithelial cells. Exp Eye Res. 2004;78:173–185. doi: 10.1016/j.exer.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Paulsen F, Jager K, Worlitzsch D, et al. Regulation of MUC16 by inflammatory mediators in ocular surface epithelial cell lines. Ann Anat. 2008;190:59–70. doi: 10.1016/j.aanat.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 40.Sjogren H. Zur kenntnis der keratoconjunctivitis sicca. Acta Ophthalmol (Copenh) 1933;(suppl 11):151. doi: 10.1111/j.1755-3768.1971.tb08678.x. [DOI] [PubMed] [Google Scholar]


