Abstract
Objectives
To longitudinally evaluate five cerebrospinal fluid (CSF) biomarkers in the transition from Mild Cognitive Impairment (MCI) to Alzheimer’s disease (AD)
Methods
A baseline and 2-year follow-up clinical and CSF study of 86 subjects, including 22 MCI patients that declined to AD (MCI-AD), 43 MCI that did not deteriorate (MCI-MCI) and 21 controls (NL-NL). All subjects were studied for total and phosphorylated tau (T-tau, P-tau231), amyloid beta (Aβ) Aβ42/Aβ40 ratio, isoprostane (IP) as well as P-tau231/Aβ42/40 and T-tau/Aβ42/40 ratios.
Results
At baseline and at follow-up MCI-AD showed higher levels P-tau231, T-tau, IP, P-tau231/Aβ42/40 and T-tau/Aβ42/40 ratios and lower Aβ42/Aβ40 than MCI-MCI or NL-NL. Baseline P-tau231 best predicted MCI-AD (80%, p<0.001) followed in accuracy by P-tau231/Aβ42/40 and T-tau/Aβ42/40 ratios (both 75%, p’s <0.001), T-tau (74%, p<0.001), Aβ42/Aβ40 (69%, p<0.01), and IP (68%, p<0.01). Only IP showed longitudinal effects (p<0.05).
Conclusions
P-tau231 is the strongest predictor of the decline from MCI to AD. IP levels uniquely show longitudinal progression effects. These results suggest the use of CSF biomarkers in secondary prevention trials.
Search terms: Alzheimer’s disease, Mild Cognitive Impairment, CSF biomarkers, Early detection, Longitudinal, Prediction
1. INTRODUCTION
The incidence of Alzheimer’s disease (AD) will more than double by the 21st century [21]. The socioeconomic consequences of such increases warrant a reliable and accurate method that will enable the early diagnosis of AD and facilitate prevention studies. Evaluation of biochemical changes in cerebrospinal fluid (CSF) is seen as a promising strategy for a diagnosis of incipient AD [5]. The most frequently used CSF biomarkers include total tau (T-tau) and hyperphosphorylated tau protein (P-tau), which are associated with neuronal damage and intracellular neurofibrillary tangles (NFT); amyloid beta 1-42 (Aβ42), a predominant component of brain parenchyma amyloid plaques; amyloid beta 1-40 (Aβ40) found mainly in vascular walls [39]; and isoprostane (IP), a marker of lipid peroxidation and inflammation [30, 35]. Recent studies using T-tau and Aβ42 show that future AD can be accurately predicted in the MCI stage, which is considered by many as a prodromal stage of AD [5, 32]. Other studies have shown that both IP and P-tau231 can also predict future AD [3, 9, 12]. However, no single study has compared the predictive accuracy or longitudinal performance of all these commonly used biomarkers in the prediction of decline from MCI to AD. The present study was designed to evaluate the univariate and incremental accuracy of the most commonly used CSF biochemical markers in predicting decline from MCI to AD, as well as to examine their longitudinal utility in characterizing clinical change.
2. MATERIAL AND METHODS
2.1 Clinical Studies
Subjects
Eighty-six community-dwelling subjects participated in this 2-year longitudinal study. Using a standardized longitudinal protocol, the clinical evaluation consisted of medical (history, physical, MRI, and laboratory with apolipoprotein ε (APOE) genotyping) neurological and psychiatric evaluations. All subjects signed IRB approved informed consent. The three study groups were as follows: MCI patients who declined to AD (MCI-AD: n=22, age 71.3±7, 73% females; see Table 1); MCI patients that did not deteriorate over the 2-year study duration (MCI-MCI: n=43, age 72.1±8, 35% females); and a normal control group that remained normal over the 2-year study duration (NL-NL: n=21, age 68.7±11, 71% females). All consecutive MCI subjects were included as research subjects. A comparable number of healthy control subjects (NL-NL) balanced for age was selected from an ongoing 2-year longitudinal study (see inclusion and exclusion criteria below). The creation of the study groups was solely based on the clinical diagnosis and was blinded to all CSF, MRI and APOE data. All subjects received longitudinal 2-timepoint clinical, MRI-imaging. All NL-NL and MCI-AD and 33 of the 43 MCI-MCI received follow-up lumbar puncture.
Table 1.
Patient Demographics
| NL-NL (n=21) | MCI-MCI (n=43) | MCI-AD (n=22) | |
|---|---|---|---|
| age at baseline | 68.7 ± 11 | 72.1 ± 8.3 | 71.3 ± 7.2 |
| observation period [years] | 2.1 ± 0.7 | 2.0 ± 0.7 | 2.1 ± 0.5 |
| APOE4 carriers [%] | 29 % | 39 % | 73 % a, b |
| females [%] | 71 % b | 35 % | 73 % b |
| education [years] | 15.0 ± 3.8 | 13.9 ± 3.7 a | 10.7 ± 3.7a, b |
| MMSE at baseline [points] | 29.7 ± 0.5 | 28.1 ± 1.7 a | 27.3 ± 2.1 a, b |
NL-NL: normal control group, MCI-MCI: non-declining MCI group, MCI-AD: declining MCI group.
value significantly different than in NL-NL group
value significantly different than MCI-MCI group.
Inclusion Criteria
NL subjects were highly functioning individuals, had Clinical Dementia Rating (CDR) score of 0, Global Deterioration Scale (GDS) scores of 1 or 2 (differentiated by subjective reporting of age-related memory change) [38], MMSE score ≥ 28 and minimum of 8 years of education. The diagnosis of MCI was based on: progressive memory complaints corroborated by an informant, a CDR=0.5, GDS score = 3 [38] and clinically recognizable memory impairment without fulfilling either the DSM-IV [1] or NINCDS-ADRDA (8) criteria for dementia and AD. AD patients fulfilled the DSM-IV criteria for dementia and the NINCDS-ADRDA criteria for probable AD [28], and had GDS scores ≥ 4 [38].
Exclusion Criteria
Individuals with medical conditions or a history of significant conditions that may affect brain structure or function (e.g. stroke, other neurodegenerative diseases including fronto-temporal and Lewy body dementia, depression, uncontrolled hypertension or diabetes mellitus type 2) were excluded from the study. Additionally, all subjects with MRI-based evidence of lacunar or cortical infarctions as assessed with T1- and T2-weighted images at baseline were disqualified from participation in the study.
Study Protocol
All subjects underwent lumbar puncture within 3 months of their longitudinal clinical assessment periods as described above. At baseline and after an average period of 2 years (2.05±0.7 years; see table 1), a complete follow-up examination was conducted.
2.2 CSF study
After an overnight fast, at 11:00 A.M., a 25 gauge Sprott pencil point lumbar puncture needle was used to collect CSF. Samples were centrifuged, aliquoted to polypropylene tubes and stored at −80 °C. Assays were blinded to clinical data.
CSF T-tau levels were determined using a commercially available INNOTEST hTAU antigen kit (Innogenetics®, Gent, Belgium). The detection limit is 60 pg/ml for T-tau and the coefficient of variability are 5.5% (intra-assay) and 11.6% (inter-assay) [7].
A sandwich ELISA assay was used to detect tau phosphorylated at threonine 231 (P-tau231). In this assay, tau is captured with two backbone-directed antibodies, tau-1 and CP-27. The captured tau is then detected by CP9, which is specific for P-tau231 [25]. Detection limit for this assay is 9 pg/ml and the coefficients of variation range from 6.0 to 10.3% (intra-assay) and 11.6 to 14.4% (inter-assay).
The CSF amyloid β levels (Aβ40, Aβ42) were measured using a monoclonal antibody 6E10 (specific to an epitope present on Aβ-16) and to rabbit antisera to Aβ40 and Aβ42 respectively, in a double antibody sandwich ELISA [29]. The detection limit for Aβ40 and Aβ42 was 10 pg/ml while the reproducibility ranged from 8 to 14% (intra-assay) and 10 to 18% (inter-assay).
CSF levels of isoprostanes (8,12-iso-iPF2α-VI) were assayed by negative ion chemical ionization gas chromatography / mass spectrometry, after CSF samples were spiked with a fixed amount of internal standard (d4-8,12-iso-iPF2α-VI), extracted on a C18 cartridge column and purified by thin-layer chromatography [34]. The detection limit for this assay was 1 pg/ml. The coefficient of variation ranged from 4-7% (intra-assay) and 4.5-6.5% (inter-assay).
3. STATISTICAL ANALYSIS
For all analytes, the metric used was the CSF concentration. CSF levels of the analytes are presented in the text and the tables as mean value ± SD. Since prior studies and the current data demonstrate that Aβ40 alone is not a useful predictor of cognitive decline and that the Aβ42/Aβ40 ratio is superior to Aβ42 level in discriminating AD from NL or from other dementias [26], for predicting future MCI [18] or MCI transition to AD [19], the Aβ42/Aβ40 ratio was used for the analyses. Subjects with at least one APOE4 allele were classified as “APOE4 carriers”. Analysis of covariance with post-hoc Tukey tests was used to detect differences in univariate and combined CSF marker levels between diagnostic groups correcting for gender, education and APOE genotype. Differences in CSF marker levels between APOE4 carrier and non-carriers were corrected for gender and education. Non-normally distributed data, as determined by Shapiro-Wilk test, were examined using the Kruskal-Wallis nonparametric test after calculating residualized values using the previously mentioned covariates. The post hoc analysis for non-normally distributed data was performed with the Mann-Whitney U test with Bonferroni correction.
Logistic regression models were used to examine the CSF measures as predictors of cognitive decline to AD among the MCI subjects. The overall accuracy is the total proportion of correctly classified subjects across the diagnostic groups. Cut-off levels for sensitivity and specificity figures were derived from receiver operating characteristic (ROC) curves in two ways: 1) by maximizing their sum; and 2) by constraining sensitivity at 82% (in order to compare specificities across CSF analytes). The 82% sensitivity was chosen as the closest value on the ROC curve above 80%, consistent with the 80% threshold recommended for AD diagnostic tests by the Consensus Working Group [11]. CSF measures were tested for superiority with logistic regression prediction models by calculating step increase of the model after adding the other CSF measure to the equation. Correlations between CSF biomarkers were analyzed with partial correlations correcting for diagnostic group effects. P values < 0.05 were considered significant. All analyses were performed with SPSS 12.0 (SPSS, Chicago, IL 2004).
4. RESULTS
Demographics of the study participants are presented in table 1. There were no significant differences between the three study groups with respect to age (F(2,83)=2.8, n.s.) or observation interval (F(2,83)=0.6, n.s., see table 1). The MCI-AD group had more APOE4 carriers (χ2(1)=7.2; p<0.01) and less education (F(2,83)=8.1, p<0.01) than both NL-NL and MCI-MCI groups (see table 1). The percentage of females was higher in both MCI-AD and NL-NL than in MCI-MCI (χ2(1)=7.6; p<0.001). Consequently, APOE genotype, gender and education were used as covariates in between-group analyses.
4.1 Study group discrimination effects
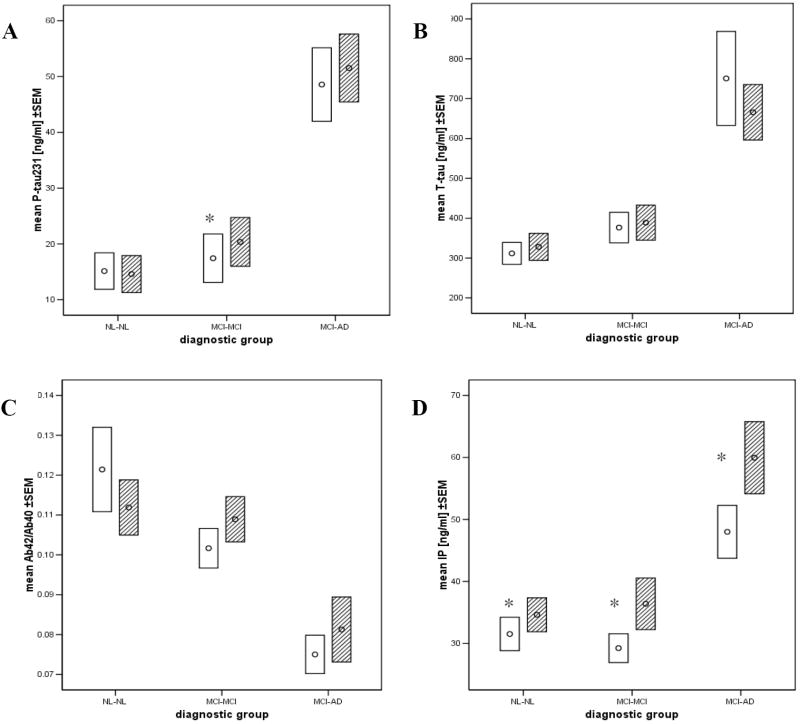
The baseline CSF analyte data by diagnostic group are presented in Table 2. After correcting for gender, education and APOE genotype, MCI-AD subjects presented with higher levels of CSF T-tau, P-tau231, IP, T-tau/Aβ42/40 and P-tau231/Aβ42/40 ratios as well as a lower Aβ42/Aβ40 ratio than either MCI-MCI or NL-NL (p’s<0.01). MCI-MCI did not show differences as compared to NL-NL (p>0.05). CSF measures are shown in Figure 1.
Table 2.
Cross-sectional and longitudinal CSF biomarker levels ± SD by diagnostic outcome group.
| NL-NL | MCI-MCI | MCI-AD | overall group difference corrected (ANCOVA) | overall group difference uncorrected (ANOVA) | |
|---|---|---|---|---|---|
| T-tau level [pg/ml]: | |||||
| baseline | 311±126 | 382±210 | 750±553 a, b | p<0.001 | p<0.001 |
| follow-up | 328±156 | 389±253 | 667±327a, b | p<0.001 | p<0.001 |
| annual rate of change | +8.51±38 | +14.64±81 | -38.7±144 | p = n.s | p = n.s. |
| P-tau231 [pg/ml]: | |||||
| baseline | 15.1±15.0 | 17.9±22.3 | 48.56±30.93a, b | p<0.001 | p<0.001 |
| follow-up | 14.6±15.2 | 20.4±25.1 * | 51.53±28.6a, b | p<0.001 | p<0.001 |
| annual rate of change | -0.57±4.6 | +1.50±1.9 | +1.50±3.3 | p = n.s. | p = n.s. |
| IP [pg/ml]: | |||||
| baseline | 31.5±12.3 | 30.6±16.3 | 48.00±20.0a, b | p<0.001 | p<0.001 |
| follow-up | 34.6±12.5 * | 36.4±24.0 * | 60.0±27.3 a, b * | p<0.001 | p<0.001 |
| annual rate of change | +1.28±5.1 | +4.4±9.1 a | +5.9±5.4 a, b | p<0.05 | p<0.05 |
| Aβ42 / Aβ40 ratio: | |||||
| baseline | 1.21 10-1 ± 0.5 10-1 | 1.01 10-1 ± 0.3 10-1 | 0.75 10-1 ± 0.2 10-1 a, b | p<0.01 | p<0.001 |
| follow-up | 1.12 10-1± 0.3 10-1 | 1.09 10-1 ± 0.3 10-1 | 0.81 10-1 ± 0.4 10-1 a,b | p<0.01 | p<0.01 |
| annual rate of change | -0.54 10-2 ± 2.8 10-2 | 0.38 10-2 ± 1.5 10-2 | 0.53 10-2 ± 2.5 10-2 | p = n.s. | p = n.s. |
| T-tau / Aβ42/40 ratio: | |||||
| baseline | 3274±2423 | 4687±4415 | 11932±11154 a,b | p<0.001 | p<0.001 |
| follow-up | 3794±3996 | 4403±4687 | 9621±5278 a,b | p<0.05 | p<0.01 |
| annual rate of change | +283±1632 | -202±1801 | -1148±3566 | p = n.s. | p = n.s. |
| P-tau231 / Aβ42/40 ratio: | |||||
| baseline | 177±226 | 267±440 | 782±589 a,b | p<0.001 | p<0.001 |
| follow-up | 174±239 | 263±417 | 778±466 a,b | p<0.001 | p<0.001 |
| annual rate of change | -5.83±136 | -19.22±144 | -2.64±119 | p = n.s. | p = n.s. |
P values from ANCOVA are given are after Bonferroni correction.
value significantly different than in NL-NL group,
value significantly different than in MCI-MCI group,
biomarker level significantly changed between baseline and follow-up.
Figure 1.
Mean values ± standard error of the mean (SEM) of: (A) P-tau231, (B) T-tau, (C) IP and (D) Aβ42/Aβ40 in the diagnostic outcome groups.
Legend: Baseline (open) and follow-up (dashed lines).
* - follow-up values significantly different than baseline (p<0.01).
Similar to the baseline results, after correcting for confounding variables the MCI-AD patients at follow-up presented with higher values of CSF T-tau, P-tau231, IP, T-tau/Aβ42/40 and P-tau231/Aβ42/40 ratios and lower Aβ42/Aβ40 ratio as compared with either MCI-MCI or NL-NL (p’s<0.01; see table 2). Again, there were no significant differences between MCI-MCI and NL-NL.
4.2 AD prediction effects
MMSE score at baseline did not predict MCI to AD decline χ2(1)= 2.7, p>0.05). Table 3 presents the sensitivity, specificity and overall accuracy for the baseline AD-prediction model calculated using CSF cut-off levels that maximized the combined sensitivity and specificity (part A) and by constraining sensitivity at 82 % (part B). Because there were no significant interactions between confounding variables and CSF biomarker levels, sensitivity and specificity figures are presented for uncorrected models (in order to provide cut-off levels). All the significant uncorrected predictions were confirmed in analyses correcting for demographic and APOE differences (p’s <0.01). The logistic regression and maximized ROC analysis showed that among univariate markers, both P-tau231 and T-tau demonstrated comparable overall accuracy in predicting decline (83%, χ2(1)=17.0 and 18.2 respectively, p<0.001), followed by IP (74%, χ2(1)=12.2, p<0.01). Among combined markers, the overall prediction accuracies of the P-tau231/Aβ42/40 (82%, χ2(1)=13.3, p<0.001) and T-tau/Aβ42/40 (80%, χ2(1)=15.9, p<0.001) provided superior results to Aβ42/Aβ40 (69%, χ2(1)=11.0, p<0.01), as determined by step wise logistic regression, but were not significantly different form T-tau and P-tau231 levels alone (p>0.05 for the step increase).
Table 3.
Sensitivity, specificity and overall diagnostic accuracy of the CSF analytes in the baseline prediction of MCI-AD (n=22) vs. MCI-MCI (n=43).
| Part A: Maximized sensitivity and specificity. | |||
|---|---|---|---|
| sensitivity | specificity | overall accuracy | |
| P-tau231 level: | |||
| cut-off level: 38.6 [pg/ml] | 73 % | 88 % | 83 % |
| T-tau level: | |||
| cut-off level: 605.5 [pg/ml] | 68 % | 91 % | 83 % |
| P-tau231 / Aβ42/40 ratio: | |||
| cut-off level: 445.9 | 73 % | 86 % | 82 % |
| T-tau / Aβ42/40 ratio: | |||
| cut-off level: 6127 | 77 % | 81 % | 80 % |
| IP level: | |||
| cut-off level: 47.5 [pg/m l] | 55 % | 91 % | 74 % |
| Aβ42/Aβ40 ratio: | |||
| cut-off level: 0.099 | 86 % | 60 % | 69 % |
| IP + Aβ42/Aβ40 ratio: | |||
| cut-off level: n/a | 64 % | 88 % | 80 % |
| Part B: Specificity and overall accuracy with sensitivity constrained at 82 %. | |||
| sensitivity | specificity | overall accuracy | |
| P-tau231 level: | |||
| cut-off level: 21.84 [pg/ml] | 80 % | 80 % | |
| T-tau / Aβ42/40 ratio: | |||
| cut-off level: 4970 | 74 % | 75 % | |
| P-tau231 / Aβ42/40 ratio: | |||
| cut-off level: 231.5 | 72 % | 75 % | |
| T-tau level: | |||
| cut-off level: 414.5 [pg/ml] | 82 % | 70 % | 74 % |
| Aβ42/Aβ40 ratio: | |||
| cut-off level: 0.093 | 63 % | 69 % | |
| IP level: | |||
| cut-off level: 30.5 [pg/ml] | 61 % | 68 % | |
| IP + Aβ42/Aβ40 ratio: | |||
| cut-off level: n/a | 58 % | 66 % | |
(the overall accuracy is the total proportion of correctly classified subjects)
By constraining sensitivity to 82%, baseline CSF levels of P-tau231 demonstrated the highest specificity (80%) and highest overall accuracy (80%) in discriminating MCI-AD from MCI-MCI, followed by T-tau/Aβ42/40 ratio (74% and 75%, respectively), P-tau231/Aβ42/40 ratio (72% and 75%), T-tau alone (70% and 74%), Aβ42/Aβ40 (63% and 69%) and IP (61% and 68%).
In both stepwise logistic regression analyses, prediction accuracies of P-tau231 and T-tau were significantly better than that of IP (the step increase χ2(1)= 5.7 and; χ2(1)= 5.4, respectively, p’s <0.001) and of Aβ42/Aβ40 (the step χ2(1)= 4.4,and χ2(1)= 3.9, respectively, p’s <0.01).
Logistic regressions examining the incremental value of the individual baseline biomarkers to predict AD showed that the only significant accuracy increment was found after adding Aβ42/Aβ40 to IP. This increased the 74% accuracy χ2(1)=4.5, p=0.03) of the IP level, to a combined model with 80% accuracy χ2(2=16.7, p<0.01). The absence of other incremental effects was likely due to the significant correlations between the biomarkers.
Analysis of the correlations between CSF biomarkers at baseline showed that after setting diagnostic group as a covariate, most CSF biomarkers were significantly correlated with each other with (r’s ranging from 0.28 to 0.94, p’s<0.01; see Table 4). The strongest relationships were between IP and both P-tau231 and T-tau (r’s~ 0.70). IP was not associated with the Aβ42/Aβ40 ratio.
Table 4.
Correlations between CSF analytes after controlling for diagnostic group.
| P-tau231 | T-tau | Aβ42/40 ratio | IP | IP annual change | |
|---|---|---|---|---|---|
| P-tau231 | .79 p<0.001 |
-.47 p<0.001 |
.70 p<0.001 |
.44 p<0.001 |
|
| T-tau | -.28 p<0.01 |
.69 p<0.001 |
.38 p<0.001 |
||
| Aβ42/40 ratio | -.19 p = n.s. |
-.30 p<0.01 |
|||
| IP | .35 p<0.01 |
(top row shows Pearson’s r value and bottom row the statistical significance level.)
4.3 Longitudinal effects
Analyses of the longitudinal changes for the univariate and combined CSF markers showed that only the annualized rate of CSF IP level change (pg/ml/year) was different between the three diagnostic groups (χ2(2)=8.1; p<0.05). The rate of IP increase was higher in MCI-AD subjects (5.9±5.4 pg/ml/year) than in either MCI-MCI (4.4±9.0 pg/ml/year) or NL-NL (1.3±5.2 pg/ml/year). The IP rate of change was also higher in MCI-MCI as compared with NL-NL (p’s<0.05).
Interestingly, baseline levels of each of the CSF biomarkers predicted the longitudinal change in IP levels in both the MCI-MCI and MCI-AD groups (p’s <0.05), but not in the NL-NL group (p>0.05; see Table 4). The significant correlations ranged from r =.36 for T-tau in the MCI-MCI group to r = .72 for T-tau in the MCI-AD group. The longitudinal IP level change did not significantly correlate with the longitudinal changes in the other CSF measures (p’s>0.05).
4.4 APOE effects
In Analyses of Covariance examining APOE4 carrier status, diagnostic group and their interaction, the results showed significant genotype effects only for Aβ42/Aβ40. The diagnostic effects were the same as presented above and there was no interaction between APOE carrier status and diagnostic group. APOE4 carriers showed lower levels of Aβ42/Aβ40 as compared with non-carriers at both baseline (F(1,77)=12.7, p<0.01) and follow-up (F(1,69)=10.4, p<0.01) but not longitudinally (see Figure 2). Exploratory analyses showed that the difference in Aβ42/Aβ40 levels between carriers and non-carriers reached statistical significance in the MCI-MCI group at both time points (baseline: F(1,36)=16.6, p<0.01: follow-up: F(1,28)=5.2, p<0.05) and in the MCI-AD group at follow-up (F(1,17)=8.7, p<0.01).
Figure 2.
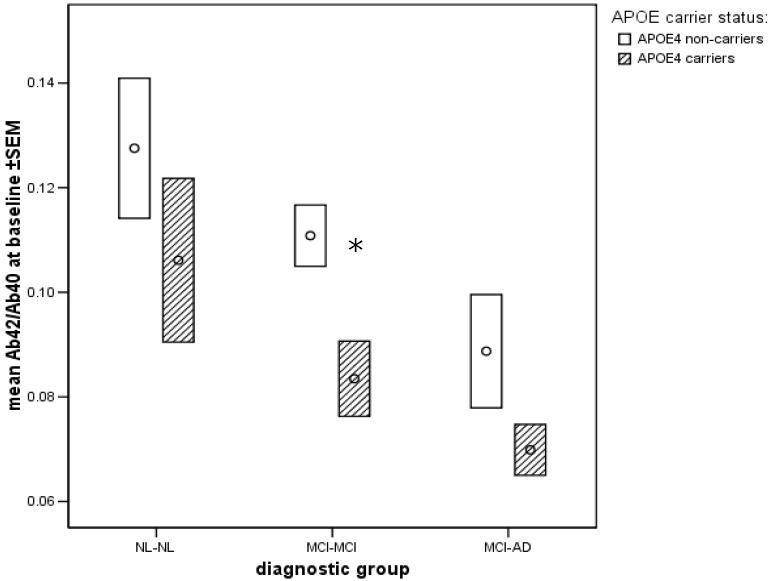
Mean ±SEM baseline values for the Aβ42/Aβ40 ratio in E4 carriers and non-carriers by diagnostic group.
* - significantly different APOE4 carrier effect within MCI-MCI group (p<0.01).
5. DISCUSSION
This 2-year longitudinal study examined five commonly used CSF biomarkers for AD in a study of healthy controls, stable MCI and MCI patients who progressed to AD. There were three main findings. First, at baseline and at follow-up, all the CSF biomarkers separated declining MCI patients from stable MCI and normal controls. Second, all biomarkers were statistically significant predictors of the decline from MCI to AD with P-tau231 and T-tau the strongest univariate predictors. Third, only IP demonstrated longitudinal effects.
At baseline, all CSF measures accurately separated MCI subjects who later declined to AD from stable MCI subjects and from NL-NL. MCI-AD patients had higher CSF concentrations of P-tau231, T-tau, IP, P-tau231/Aβ42/40 and T-tau/Aβ42/40 ratios as well as lower Aβ42/Aβ40 measures as compared to either stable MCI or to normal elderly controls. This finding is in agreement with previous findings [2, 6, 9, 12, 22]. Our results confirm that before the onset of clinically overt AD, there are changes in the CSF biochemical composition that reflect AD pathology: neurofibrillary tangles, amyloid plaques [4, 36], and oxidative damage to neuronal cell membranes [30, 36].
Interestingly, IP was the only biomarker that showed longitudinal effects, such that IP levels increased over 2 years in association with MCI-AD conversion as compared to the same time interval for stable MCI and NL subjects. This finding is consistent with the results from smaller samples of MCI patients previously published by our group showing that longitudinal IP level provides diagnostic separation of MCI from healthy controls, and adds new evidence that longitudinal IP changes can be used to track the progression from MCI to AD [12, 13]. Since IP is a marker of membrane lipid peroxidation and inflammation, these data suggest that the increase of CSF IP levels in cognitively deteriorating patients reflects progressive neuronal oxidative stress and progression of neurodegenerative changes [35]. Although the cross-sectional baseline IP levels showed high correlation with P-tau231 and T-tau (r’s ~ .70), there was no correlation between longitudinal changes in these analytes. This finding suggests the unlikely summary that the processes of inflammation and neurodegeneration are not parallel. However, it remains of extreme interest to answer the question whether there is an order or staging effect, i.e. if oxidative stress precedes neurodegeneration or is merely a consequence of an already existing neurodegenerative process. The present 2 time point study of MCI patients is insufficient to answer this question. Possibly a study including normal subjects that experience longitudinal changes related to AD would reveal this sequence. It is also possible that other factors such as clearance of tau which is poorly understood and the dilution of a brain derived protein such as the tau molecule in the CSF has affected the sensitivity to measure brain progression effects [14]. As such, both additional groups and improved characterization of the physiology of tau are needed to understand the relationship to inflammation.
As recommended by the consensus report of the NIA Working Group on Biological Measures [11], an ideal diagnostic AD biomarker should have both sensitivity and specificity of at least 80% in separating AD from normal aging. We report that the prediction of decline with P-tau231 exceeds the recommended 80% threshold of sensitivity and specificity and, shows the highest specificity (80% recognition of non-declining MCI patients) among all biomarkers analyzed in the study. However, although our results show that only P-tau231 meets the criteria stated by the consensus group and provides highest sensitivity and specificity figures, its prediction accuracy was not statistically different from that of T-tau, T-tau/Aβ42/40 or P-tau231/Aβ42/40 ratios. However, larger samples may provide the statistical power to detect differences between these biomarkers.
The changes in P-tau231 are known to reflect neurofibrillary pathology [24] and clinical studies show that elevated levels confer diagnostic specificity for AD [10]. Although CSF P-tau231 and T-tau changes were better predictors of future cognitive decline than Aβ42/40 and IP, it remains to be established which biomarker is the first useful predictor of AD to be detected. A recent predictor CSF study by Fagan et al. suggested that CSF Aβ reductions occur in normal subjects prior to clinical decline and may precede tau elevations [16], but evidence for biomarker staging requires longitudinal data for a large number of clinical starting points and this is not yet available.
Although several studies show that the use of CSF T-tau/Aβ42 or Aβ42/Aβ40 ratios yield good AD diagnostic accuracy [2, 17, 23, 26, 31] and MCI-AD prediction effects [16, 20], it is usually not reported if the ratio statistically increments the prediction accuracy over the univariate measures. We observed a significant additive effect in our study only with the addition of Aβ42/Aβ40 to IP. Statistically, combinations of CSF measures will increase AD-prediction accuracy provided that the two markers are not highly correlated. Accordingly, our analysis shows a high correlation between most CSF biomarkers. Aβ42/Aβ40 and IP were weakly correlated and therefore have a greater potential to show incremental effects.
In the present study, only subjects with baseline diagnoses of NL or MCI were examined. The homogeneity of our study population was achieved by applying clearly defined baseline diagnostic criteria and excluding patients with cerebrovascular disease and other identifiable causes of poor cognitive performance. Therefore, we excluded subjects that in clinical settings have to be considered as part of the differential diagnosis of AD. It remains to be established to what extent our results apply to more heterogeneous patient samples. Future CSF biomarker studies are warranted to examine this issue.
Because the APOE4 genotype is a well known risk factor for AD [27], we examined its effects on the CSF biomarkers. We observed across the diagnostic groups that the Aβ42/40 ratio was lower in carriers as compared with non-carriers. Interestingly, we did not find any APOE genotype differences for the other CSF analytes. These data suggest a limited role for APOE genotype in the interpretation of CSF biomarkers. Our results are in agreement with Engelborghs et al. [15], but not with the CSF results reported by Prince et al. [37] who reported a link between APOE4 genotype and Aβ metabolism. Further study of these relationships is warranted.
A limitation of the current longitudinal dataset was the reliance on only one follow-up observation period. Based on other work, one would expect that about 12-15 % of amnestic MCI patients (and less for the non-amnestic) will progress within 1 year to AD [33]. While our data are consistent with this expectation, with a 2-years follow-up interval our results may underestimate the differences between stable and progressing MCI as our MCI-MCI subject group are likely to include MCI patients who will later develop dementia. In other words, the inclusion of future declining MCI subjects within the stable MCI group would have the conservative effect of reducing the statistical differences between groups. Consequently, expanding the observation period may increase the baseline prediction accuracies for the biomarkers. As an example, in a similarly designed study published by Hansson et al., using T-tau and Aβ42, an observation period greater than 4 years provided sensitivity and specificity values predicting MCI to AD decline as high as 95% and 83%, respectively,[20]. The fact that our sensitivity and specificity estimates for P-tau231 are about 80% suggests that reliable predictions can be made over a 2 year interval.
Although several AD-prediction studies are published, these reports do not include longitudinal CSF measurements [8]. Our paper presents longitudinal CSF data for five most common biomarkers, and tested the incremental potential of combining them in the prediction models. Our results identify P-tau231 as the better predictor of AD at the MCI stage and that IP may be useful for monitoring the course of decline to AD. We conclude that CSF biomarkers may facilitate the design of secondary prevention trials studies by enabling subject enrichment and monitoring clinical course. Future studies with larger, more naturalistic study populations with longer and more frequent follow-up intervals will further contribute to determining the most useful sets of biomarkers and relationships among the CSF biomarkers for AD.
Acknowledgments
Authors would like to thank Drs. Daniel Kerkman and John DeBernardis at Applied NeuroSolutions for help with P-tau231 assays and Dr. Elizabeth Javier at NYU for help with CSF collection and processing.
This project was funded by: P30 AG008051, R01 AG012101, R01 AG022374, R01 AG003051, M01 RR000096.
Footnotes
Financial disclosure Dr. Zinkowski owns stocks and stock options in Applied NeuroSolutions, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) 4. Washington, D.C.: American Psychiatric Association; 1994. [Google Scholar]
- 2.Andreasen N, Minthon L, Vanmechelen E, Vanderstichele H, Davidsson P, Winblad B, Blennow K. Cerebrospinal fluid tau and Abeta42 as predictors of development of Alzheimer’s disease in patients with mild cognitive impairment. Neurosci Lett. 1999;273(1):5–8. doi: 10.1016/s0304-3940(99)00617-5. [DOI] [PubMed] [Google Scholar]
- 3.Arai H, Ishiguro K, Ohno H, Moriyama M, Itoh N, Okamura N, Matsui T, Morikawa Y, Horikawa E, Kohno H, Sasaki H, Imahori K. CSF phosphorylated tau protein and mild cognitive impairment: a prospective study. Exp Neurol. 2000;166(1):201–3. doi: 10.1006/exnr.2000.7501. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 5.Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 6.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2(10):605–13. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 7.Blennow K, Wallin A, Agren H, Spenger C, Siegfried J, Vanmechelen E. Tau protein in cerebrospinal fluid: a biochemical marker for axonal degeneration in Alzheimer disease? Mol Chem Neuropathol. 1995;26(3):231–45. doi: 10.1007/BF02815140. [DOI] [PubMed] [Google Scholar]
- 8.Brys M, Mosconi L, De Santi S, Rich KE, de Leon MJ. CSF Biomarkers for Mild Cognitive Impairment. Aging Health. 2006;2(1):111–21. [Google Scholar]
- 9.Buerger K, Teipel SJ, Zinkowski R, Blennow K, Arai H, Engel R, Hofmann-Kiefer K, McCulloch C, Ptok U, Heun R, Andreasen N, DeBernardis J, Kerkman D, Moeller H, Davies P, Hampel H. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59(4):627–9. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 10.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59(8):1267–72. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 11.Consensus Working Group. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging. Neurobiology of Aging. 1998;19:109–16. [PubMed] [Google Scholar]
- 12.de Leon MJ, Mosconi L, Li J, De Santi S, Yao YTWH, Pirraglia E, Rich K, Javier E, Brys M, Sobanska L, Switalski R, Saint Louis LA, Pratico D. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. Journal of Neurology. 2007 doi: 10.1007/s00415-007-0610-z. in press. [DOI] [PubMed] [Google Scholar]
- 13.de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, Clark CM, Tarshish C, Li Y, Lair L, Javier E, Rich K, Lesbre P, Mosconi L, Reisberg B, Sadowski M, DeBernadis JF, Kerkman DJ, Hampel H, Wahlund LO, Davies P. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27(3):394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 14.de Leon MJ, Segal S, Tarshish CY, DeSanti S, Zinkowski R, Mehta PD, Convit A, Caraos C, Rusinek H, Tsui W, Saint Louis LA, DeBernardis J, Kerkman D, Qadri F, Gary A, Lesbre P, Wisniewski T, Poirier J, Davies P. Longitudinal cerebrospinal fluid tau load increases in mild cognitive impairment. Neurosci Lett. 2002;333(3):183–6. doi: 10.1016/s0304-3940(02)01038-8. [DOI] [PubMed] [Google Scholar]
- 15.Engelborghs S, Sleegers K, Cras P, Brouwers N, Serneels S, De LE, Martin JJ, Vanmechelen E, Van BC, De Deyn PP. No association of CSF biomarkers with APOE4, plaque and tangle burden in definite Alzheimer’s disease. Brain. 2007 doi: 10.1093/brain/awm136. [DOI] [PubMed] [Google Scholar]
- 16.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal Fluid tau/beta-Amyloid42 Ratio as a Prediction of Cognitive Decline in Nondemented Older Adults. Arch Neurol. 2007 doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 17.Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998;55(7):937–45. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 18.Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma abeta42/abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64(3):354–62. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 19.Hansson O, Zetterberg H, Buchhave P, Andreasson U, Londos E, Minthon L, Blennow K. Prediction of Alzheimer’s disease using the CSF Abeta42/Abeta40 ratio in patients with mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23(5):316–20. doi: 10.1159/000100926. [DOI] [PubMed] [Google Scholar]
- 20.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5(3):228–34. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 21.Hebert LE, Beckett LA, Scherr PA, Evans DA. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord. 2001;15(4):169–73. doi: 10.1097/00002093-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Herukka SK, Helisalmi S, Hallikainen M, Tervo S, Soininen H, Pirttila T. CSF Abeta42, Tau and phosphorylated Tau, APOE epsilon4 allele and MCI type in progressive MCI. Neurobiol Aging. 2007;28(4):507–14. doi: 10.1016/j.neurobiolaging.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Hulstaert F, Blennow K, Ivanoiu A, Schoonderwaldt HC, Riemenschneider M, De Deyn PP, Bancher C, Cras P, Wiltfang J, Mehta PD, Iqbal K, Pottel H, Vanmechelen E, Vanderstichele H. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555–62. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 24.Hyman BT, Van Hoesen GW, Wolozin BL, Davies P, Kromer LJ, Damasio AR. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 1988;23(4):371–9. doi: 10.1002/ana.410230410. [DOI] [PubMed] [Google Scholar]
- 25.Kohnken R, Buerger K, Zinkowski R, Miller C, Kerkman D, DeBernardis J, Shen J, Moller HJ, Davies P, Hampel H. Detection of tau phosphorylated at threonine 231 in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 2000;287(3):187–90. doi: 10.1016/s0304-3940(00)01178-2. [DOI] [PubMed] [Google Scholar]
- 26.Lewczuk P, Esselmann H, Otto M, Maler JM, Henkel AW, Henkel MK, Eikenberg O, Antz C, Krause WR, Reulbach U. Neurochemical diagnosis of Alzheimer’s dementia by CSF A[beta]42, A[beta]42/A[beta]40 ratio and total tau. Neurobiology of Aging. 2004;25(3):273–81. doi: 10.1016/S0197-4580(03)00086-1. [DOI] [PubMed] [Google Scholar]
- 27.Mayeux R, Saunders AM, Shea S, Mirra S, Evans D, Roses AD, Hyman BT, Crain B, Tang MX, Phelps CH. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. New England Journal of Medicine. 1998;338:506–11. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57(1):100–5. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- 30.Montine TJ, Markesbery WR, Morrow JD, Roberts LJ. Cerebrospinal fluid F2-isoprostane levels are increased in Alzheimer’s disease. Ann Neurol. 1998;44(3):410–3. doi: 10.1002/ana.410440322. [DOI] [PubMed] [Google Scholar]
- 31.Motter R, Vigo-Pelfrey C, Kholodenko D, Barbour R, Johnson-Wood K, Galasko D, Chang L, Miller B, Clark C, Green R. Reduction of beta-amyloid peptide42 in the cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1995;38(4):643–8. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 32.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 33.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 34.Pratico D, Clark CM, Lee VM, Trojanowski JQ, Rokach J, FitzGerald GA. Increased 8,12-iso-iPF2alpha-VI in Alzheimer’s disease: correlation of a noninvasive index of lipid peroxidation with disease severity. Ann Neurol. 2000;48(5):809–12. [PubMed] [Google Scholar]
- 35.Pratico D, Rokach J, Lawson J, FitzGerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem Phys Lipids. 2004;128(12):165–71. doi: 10.1016/j.chemphyslip.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 36.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45(3):358–68. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.Prince JA, Zetterberg H, Andreasen N, Marcusson J, Blennow K. APOE epsilon4 allele is associated with reduced cerebrospinal fluid levels of Abeta42. Neurology. 2004;62(11):2116–8. doi: 10.1212/01.wnl.0000128088.08695.05. [DOI] [PubMed] [Google Scholar]
- 38.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–9. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki N, Iwatsubo T, Odaka A, Ishibashi Y, Kitada C, Ihara Y. High tissue content of soluble beta 1-40 is linked to cerebral amyloid angiopathy. Am J Pathol. 1994;145(2):452–60. [PMC free article] [PubMed] [Google Scholar]