Abstract
Background
Female hearts following ischemia/reperfusion (I/R) injury demonstrate improved functional recovery compared to male, which suggests a protective role for estrogen. Acute post-ischemic treatment with 17-β-estradiol (E2) attenuates myocardial dysfunction. However, it is unknown by which estrogen receptor (ER) E2 mediates this acute cardioprotection during I/R. Therefore, we hypothesize that post-ischemic infusion of the selective ER-α agonist (PPT) or the selective ER-β agonist (DPN) will improve myocardial function following I/R.
Methods
Isolated, perfused hearts (Langendorff) from adult male rats were subjected to 25-minute ischemia followed by 40-minute reperfusion. Hearts (n=4–6/group) were randomly infused with either perfusate, PPT or DPN at 1 nM, 10 nM, or 100 nM throughout reperfusion. After I/R, heart tissue was analyzed for TNF-α, IL-1β, VEGF and LDH.
Results
Post-ischemic treatment with 10 nM of PPT significantly improved myocardial function. Additionally, 10 or 100 nM of DPN significantly increased myocardial functional recovery following I/R, with maximum benefit at the 10 nM dose. A trend towards lower levels of LDH was noted in DPN and PPT treated groups following I/R. Neither PPT nor DPN affected myocardial production of TNF-α or IL-1β. However, higher levels of myocardial VEGF were noted in the PPT treated group compared to control.
Conclusions
Both ER-α and ER-β are involved in mediating E2-induced rapid cardioprotection following I/R. Advancing our understanding of both ER subtypes may be useful for the development of novel strategies that may benefit both males and females in response to myocardial ischemia.
Keywords: estrogen receptor, myocardial ischemia, cardiac function, sex hormones
Introduction
Gender disparities in cardiovascular disease suggest a protective role for estrogen in myocardial ischemia/infarction 1, 2. Estrogen has been reported to protect the myocardium from ischemia/reperfusion injury (I/R) in animal models through anti-apoptotic, anti-inflammatory signaling, as well as intracellular calcium regulatory mechanisms 1, 3. Following I/R injury, chronic estrogen administration has been shown to provide cardioprotection in in vitro and in vivo animal experiments 4. In addition, acute estrogen treatment before left anterior descending coronary artery ligation results in less cardiac myocyte necrosis and reduced infarct size 5. However, with respect to therapeutic potential in the treatment of acute myocardial infarction, post-insult administration of estrogen has more clinical appeal. Recently, our group demonstrated that post-ischemic infusion of 17β-estradiol (E2) throughout the 40 minute reperfusion period improved left ventricular (LV) function compared to control 6.
Estrogen exerts its effect on the target cell by diffusing through the cell membrane and binding to the receptors, estrogen receptor (ER)-α and/or ER-β 7–9. A selective ER-α agonist, 4,4′,4″-[4-propyl-(1H)-pyrazole-1,3,5-triyl]tris-phenol (PPT), has been shown to reduce infarct size after I/R in rabbit hearts 10. ICI 182,780 and ZM-182780, both potent ER antagonists, have also been found to abolish E2 11 or ER-α 10, 12 induced cardioprotection. In addition, our group has demonstrated that both ER-α and ER-β are involved in mediating cardiac protection following I/R injury by using ER-α knockout (KO) and ER-β KO mouse hearts 9, 13. However, it remains unknown by what mechanisms acute administration of E2 after ischemia protects the myocardium. Therefore, based on our previous findings, the purpose of this study was to determine which receptor (ER-α and/or ER-β) mediates acute cardiac protection by using selective ER agonists following I/R injury. We hypothesized that: 1) post-ischemic administration of the selective ER-α agonist (PPT) or the selective ER-β agonist 2,3-bis(4-hydroxyphenyl)-propionitrile (DPN) will improve left ventricular (LV) function; and 2) PPT- or DPN-induced cardioprotection will be associated with less myocardial damage (LDH release), reduced pro-inflammatory mediator production and increased VEGF production.
Materials and Methods
Animals
Normal male (250–300g, 8–10 weeks) Sprague-Dawley rats (Harlan, Indianapolis, IN) were fed a standard diet and acclimated in a quiet quarantine room for two weeks before the experiments. The animal protocol was reviewed and approved by the Indiana Animal Care and Use Committee of Indiana University. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” (NIH publication No. 85-23, revised 1996).
Experimental Groups
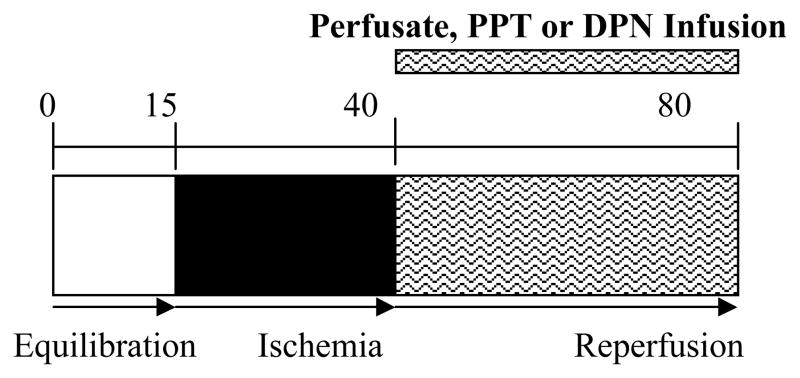
Rat hearts were randomly divided into 7 experimental groups and all hearts in each group were subjected to the same I/R protocol: 15 minute equilibration period (any drop of LVDP will be < 10% during this time), 25 minutes of global ischemia (37°C), and 40 minutes of reperfusion (figure 1). ER-α selective agonist PPT (1, 10, 100 nM/min, respectively, n=4–6/group), or ER-β selective agonist DPN (1, 10, 100 nM/min, respectively, n=4/group) was infused after ischemia and throughout the entire reperfusion period. Control hearts (n=6) were infused with Krebs-Henseleit (KH) solution. The proper agonist concentration was adjusted based on the coronary flow (CF) rate.
Figure 1.
Simplified schematic illustrates experimental protocol. Isolated rat hearts were subjected to 15 minutes of equilibration, followed by 25 minutes of warm ischemia (37°C) and then 40 minutes of reperfusion. During reperfusion period, either perfusate, PPT (1 nM, 10 nM, or 100 nM), or DPN (1 nM, 10 nM, or 100 nM) is infused.
Isolated Heart Preparation (Langendorff)
Rats were anesthetized (sodium pentobarbital, 60 mg/kg, intraperitoneal (ip) and heparinized (500 units, ip). Hearts where rapidly excised via median sternotomy and placed in 4°C KH solution. The aorta was cannulated and coronary arteries were retrograde perfused with oxygenated (95% O2 + 5% CO2), 37°C KH solution. Cardiac decompression was obtained by pulmonary arteriotomy. The left atrial appendage was excised and a water filled latex balloon was passed into the left ventricle. Pacing wires where affixed to the right atrium and left ventricle. The heart was paced at 350 BPM (6 Hz, 3 V, 2 ms). End diastolic pressure (EDP) was adjusted to a level between 6–10 mmHg and held constant throughout the entire experiment. Hearts unable to generate a left ventricular developed pressure (LVDP) >80 mmHg were excluded. Hearts were maintained at the same settings for 15 consecutive minutes of equilibration before proceeding to ischemia. A 3-way stopcock located above the aortic cannula was utilized to induce global ischemia. At the point of reperfusion, retrograde flow was slowly re-established (75 mmHg) and maintained for 40 minutes. Coronary flow rate was measured at regular intervals. Data (LVDP, EDP, HR, +dP/dt, and −dP/dt) were continuously recorded using a PowerLab 8 preamplifier/digitizer (AD Instruments Inc., Milford, MA) and a Mac mini computer (Apple Computer Inc., Cupertino, Ca). Immediately at the end of reperfusion, hearts were snap frozen with liquid nitrogen and stored at −70°C.
Lactate Dehydrogenase Activity (LDH)
Hearts (control n=6, 10 nM DPN n=4, 10 nM PPT n=6) were homogenized as previously described by Wang et al. 9. LDH assay was performed in supernatants and coronary effluents according to manufacturer instructions, in duplicate, using a commercially available Cytotoxicity Detection Kit (Roche Applied Science, Indianapolis, IN).
ELISA
Supernatants collected from the myocardial homogenate were analyzed for IL-1β, TNF-α, and VEGF concentration using commercially available ELISA kits (R & D Systems, Inc., Minneapolis, MN) (5). Cardiac tissue IL-1β, TNF-α and VEGF ELISAs were performed according to the manufacturer instructions. All standards and samples were measured in duplicate.
Presentation of Data and Statistical Analysis
All data are reported as mean ± SEM. Data were analyzed by 2-way ANOVA with post-hoc Bonferroni or a two-tailed Student’s t-test. A probability value of <0.05 was considered statistically significant.
Results
Post-ischemic infusion of ER-α agonist or ER-β agonist improved myocardial functional recovery
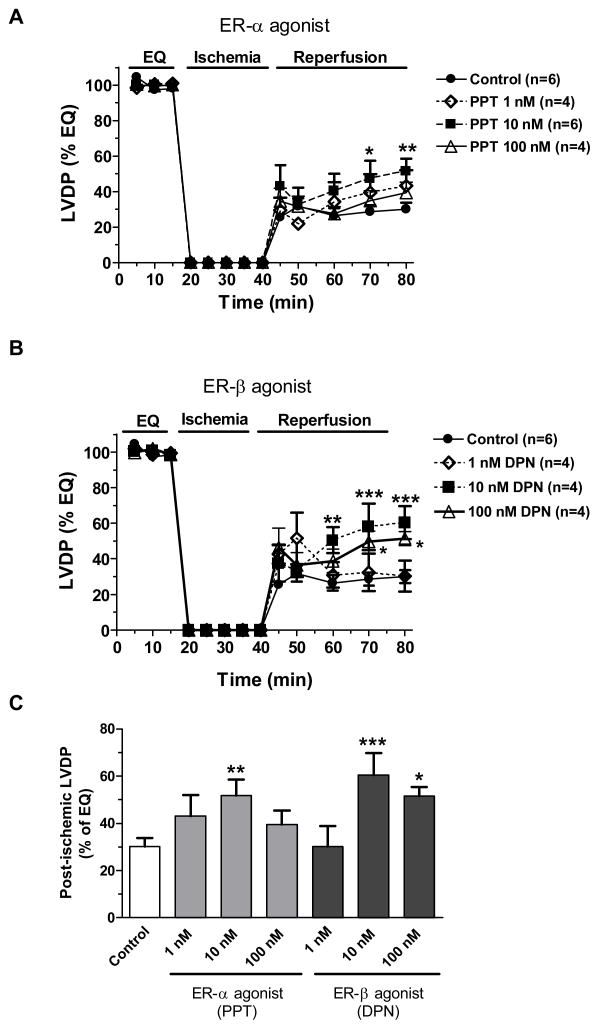
Ischemia and reperfusion injury resulted in markedly depressed myocardial function as demonstrated by a decrease in myocardial LVDP, +dP/dt and an elevation of EDP, −dP/dt (figure 2–5). Although a trend of increased myocardial LVDP recovery was noted in post-ischemic treatment with 1, 10 and 100 nM of the ER-α agonist (PPT), only 10 nM of PPT significantly improved myocardial function following I/R (figure 2A and 2C). In addition, both 10 and 100 nM doses of ER-β agonist (DPN) significantly restored myocardial LVDP after I/R, with the maximum effect at 10 nM concentration (figure 2B and 2C).
Figure 2.
Changes in LVDP (% of baseline) measured during equilibration, ischemia and reperfusion. (A) Post-ischemic infusion of PPT at concentration of 1 nM, 10 nM or 100 nM compared to control; (B) Post-ischemic administration of DPN at concentration of 1 nM, 10 nM or 100 nM compared to control; (C) LVDP recovery (% of baseline) at the end of reperfusion in all groups. Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. corresponding control.
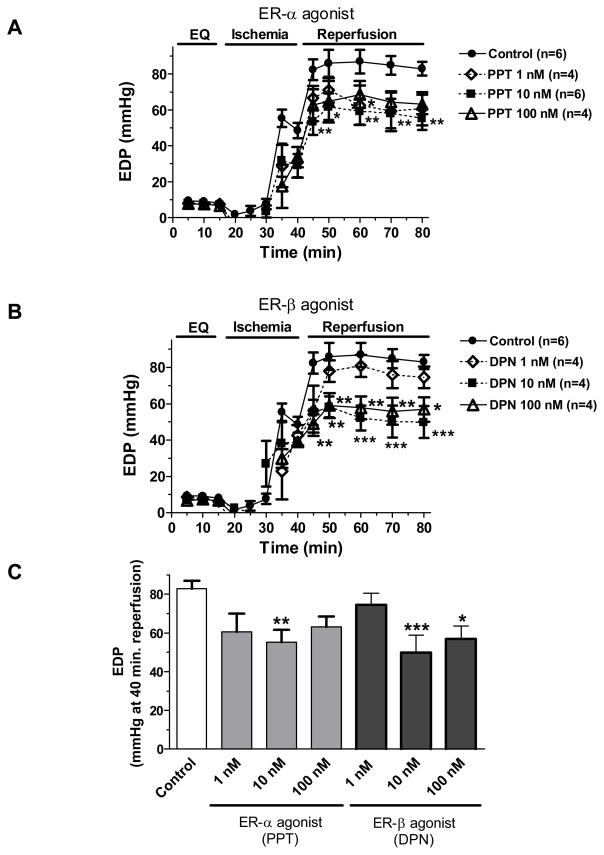
End diastolic pressure, at a constant preload during equilibration, indicated severity of myocardial injury by progressively increasing following ischemia and the beginning of reperfusion. The value of EDP was reduced after 15 minutes of reperfusion showing a recovery of myocardium from I/R injury. Similarly with LVDP, both PPT (10 nM) and DPN (10 and 100 nM) treatment after ischemia significantly improved recovery of EDP when compared to control (figure 3).
Figure 3.
Changes in EDP (mmHg) throughout equilibration and ischemia/reperfusion in (A) post-ischemic treatment with PPT (1 nM, 10 nM or 100 nM) compared to control; (B) post-ischemic infusion of DPN (1 nM, 10 nM or 100 nM) compared to control; (C) comparison of EDP at the 40-minute reperfusion in all groups. Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. corresponding control.
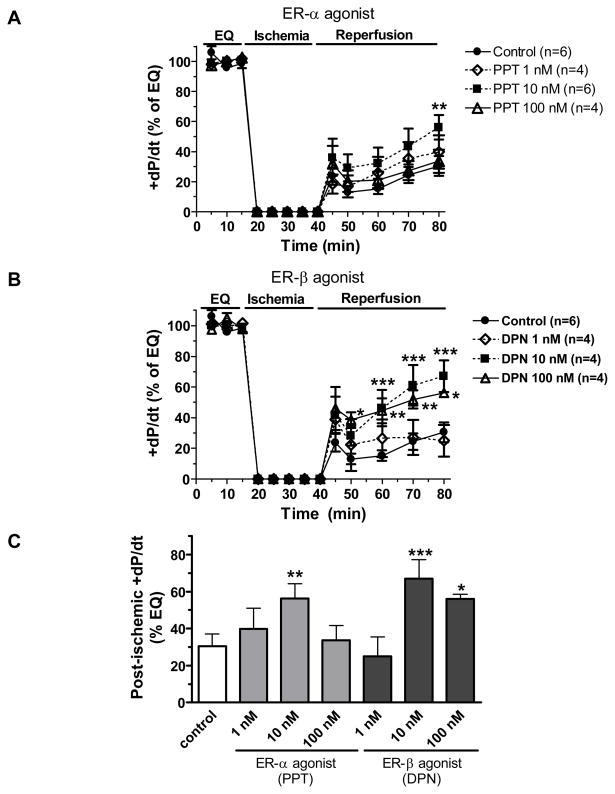
Other indices of myocardial function, +dP/dt (a measure of myocardial contractility) and −dP/dt (a measure of myocardial compliance), were also measured in this study. Not surprisingly, post-ischemic infusion of PPT and DPN significantly protected myocardial +dP/dt and −dP/dt during I/R injury. The maximum beneficial effects of PPT and DPN were induced at the 10 nM dose (figure 4 and 5).
Figure 4.
Changes in the maximum positive value of the first derivative of pressure (+dP/dt) throughout equilibration and I/R (% of baseline). (A) Post-ischemic infusion of PPT (1 nM, 10 nM or 100 nM) compared with control; (B) Post-ischemic treatment with DPN (1 nM, 10 nM or 100 nM) compared to control; (C) Comparison of +dP/dt at the end of reperfusion in all groups. Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. corresponding control.
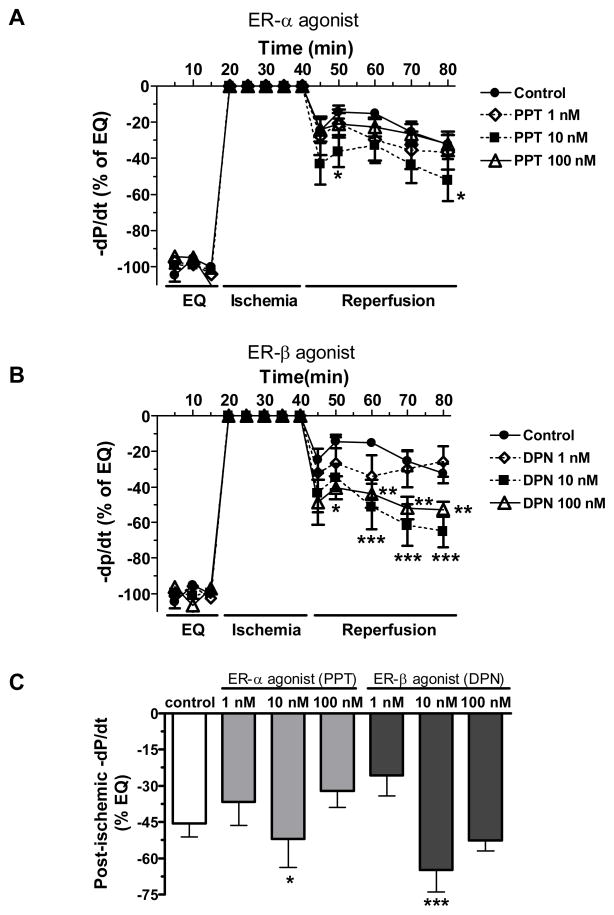
Figure 5.
Changes in the maximum negative value of the first derivative of pressure (−dP/dt) throughout equilibration and I/R (% of baseline). (A) Post-ischemic administration of PPT (1 nM, 10 nM or 100 nM) compared with control; (B) Post-ischemic infusion of DPN (1 nM, 10 nM or 100 nM) compared to control; (C) comparison of −dP/dt at the end of reperfusion in all groups. Results are mean ± SEM, *p<0.05, **p<0.01, ***p<0.001 vs. corresponding control.
Effects of ER-α agonist or ER-β agonist on myocardial damage following I/R
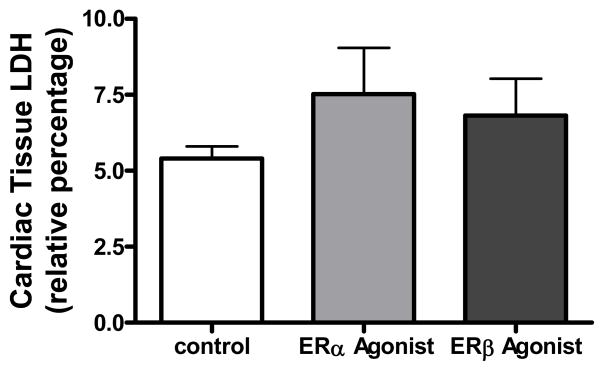
Lactate dehydrogenase (LDH) was measured in this study to demonstrate cardiac damage. Elevated LDH release usually indicates the damage of cell membrane integrity and is one of the most widely used indicators for cell viability and tissue injury. Theoretically, worse myocardial damage from I/R injury will result in higher levels of LDH in the coronary effluent and the less LDH will remain in cardiac tissue. Due to the under-detectable levels of LDH in coronary effluent, we measured the value of LDH in cardiac tissue. A trend of higher levels of LDH was observed in PPT (10 nM) and DPN (10 nM) -treated myocardium after I/R compared to the control group (figure 6). This suggests that post-ischemic treatment with 10 nM PPT or DPN might reduce LDH release into coronary effluent. In other words, infusion of ER-α agonist or ER-β agonist possibly decreased cardiac damage following I/R injury.
Figure 6.
Relative level of cardiac tissue LDH in Control (n=6), PPT 10 nM (n=6), and DPN 10 nM (n=4) after I/R injury.
Effects of ER-α agonist or ER-β agonist on myocardial cytokine production
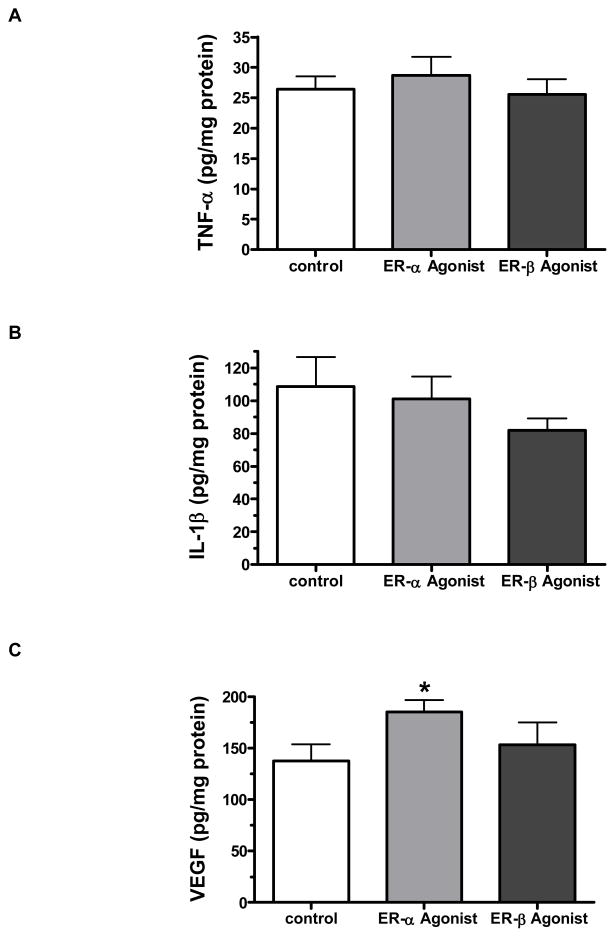
Treatment with 10 nM PPT or DPN appeared to reduce myocardial levels of IL-1β (figure 7A), whereas there was no change in TNF levels (figure 7C). IL-1β levels were 23% lower in the DPN group and 8% lower in the PPT group versus control. Interestingly, the PPT 10 nM group demonstrated significantly greater production of VEGF compared to control (185 ±11 pg/mg protein vs. 138±16 mg/mg protein), whereas DPN showed no effect (figure 7D).
Figure 7.
Myocardial production of TNF-α, IL-1 and VEGF after I/R injury. Neither PPT (ER-α agonist) nor DPN (ER-β agonist) post-ischemic administration significantly affected myocardial levels of TNF-α (A) and IL-1β (B). However, post-ischemic infusion of ER-α agonist – PPT, but not DPN throughout reperfusion significantly increased myocardial VEGF production (C). Results are mean ± SEM, *p<0.05 vs. control.
Discussion
Females demonstrate improved myocardial function following I/R injury when compared to males 3. Administration of E2 attenuates cardiac dysfunction, suggesting that E2 may be involved in mediating this cardioprotection in females 4. Additionally, post-injury treatment with E2, demonstrates protection of myocardial function against I/R and may have clinical potential in the treatment of acute myocardial infarction 6. In this study, we further determined by what mechanisms E2 conveys this acute cardiac protection following I/R. Herein, by utilizing specific agonists of ER-α or ER-β, we found that both ER-α and ER-β are involved in mediating E2-induced acute cardioprotection in response to I/R injury. Post-ischemic infusion of ER-α agonist-PPT at a concentration of 10 nM or ER-β agonist-DPN at a concentration of 10 nM or 100 nM reduces myocardial injury. In this study, post-injury treatment with either ER-α agonist or ER-β agonist improved myocardial systolic (LVDP) and diastolic function (EDP), as well as cardiac compliance (+/− dP/dt), suggesting that ER-α and ER-β may mediate E2-induced acute positive effects on myocardial function not only by increasing contractility, but by increasing the end diastolic fluid volume of the heart.
Estrogen has been shown to block voltage gated or receptor operated calcium channels and to promote coronary vasodilation, thereby resulting in improved cardiac perfusion, and overall protection of myocardial function 14. In addition, reactive oxygen species (ROS) are important mediators of myocardial dysfunction caused by I/R injury. E2 has been observed to decrease the production of oxygen radicals in I/R-injured myocardium 15. E2 demonstrates antioxidant effects by increasing I/R-reduced glutathione, a free radical scavenger in the heart 16. Therefore, it is possible that E2-reduced oxidative damage may mediate the protection of cardiac function in response to I/R injury.
Although the majority of effects of E2 are completed through genomic mechanism, the acute cardioprotective effects of E2 may occur via binding to membrane associated receptors. In fact, there is evidence that ER-α or ER-β is located in the membrane and mediates non-genomic events triggered by E2 17, 18. In addition, these receptors can be altered with post-translational modification such as phosphorylation, S-nitrosylation, and O-GlcNAcylation 1, thereby, initiating the effects of E2 through rapid, non-genomic pathways (e.g. MAPK, AKT, PI3K/AKT, PKA/PKC) 2. Estrogen-inhibited increase in ROS production has been shown to be mediated through ER-α 19. Conversely, ER-β is likely involved in regulating estrogen-increased NOS activation, NO production and E2–modulated intracellular calcium 20. Therefore, it is possible that an ER-α agonist or ER-β agonist may improve myocardial function through different mechanisms in this study. However, this requires further investigation to determine the detailed mechanisms by which ER-α or ER-β mediates cardiac protection following I/R injury.
In addition to decreasing oxidative damage, regulating intracellular calcium, and increasing myocardial NOS activation and NO production, estrogen may protect the myocardium from I/R injury by decreasing myocyte death 4, 21. Estrogen prevents the release of cytochrome c from myocardial mitochondria, and decreases its accumulation in the cytosol 21. Indeed, it is evident that ER-α and ER-β are localized in the mitochondrial membrane, and are responsible for reducing injury/stress-induced apoptosis 17. In addition, estrogen has been reported to suppress myocyte apoptosis via upregulation of the PI3K/Akt signaling pathway 22. In this study, post-ischemic infusion of either PPT (ER-α agonist) or DPN (ER-β agonist) demonstrates a trend toward reduction of myocardial necrosis as measured by cardiac tissue LDH, suggesting that ERα and ER-β likely reduce cardiac cell death and decrease myocardial damage, thereby leading to increased myocardial recovery following I/R.
Finally, the protective effects of E2 may also be mediated through reduced proinflammatory cytokine production. Estrogen has been shown to act as an immunomodulator of inflammation in chronic heart disease 23, 24. In addition, ER agonists have been demonstrated to have anti-inflammatory properties in some disease models 4. However, there is no significant difference in myocardial production of TNF-α and IL-1β between ER agonist treated group and untreated group. Further study is required to determine whether the decreased inflammation associated with E2 is mediated through rapid, non-genomic pathway during myocardial ischemia. Interestingly, in this study, we found that post-ischemic administration of ER-α agonist-PPT significantly increases myocardial VEGF production. Considering the protective effects of VEGF on the heart, it can be postulated that ER-α agonist may mediate cardiac protection through increased VEGF.
In this study, the Langendorff model was employed to test our hypothesis. This isolated heart perfusion system avoids the potential confounding effects of systemic actions as occurs when the heart is subjected to I/R in vivo. In addition, this model has direct clinical implications as global myocardial ischemia is routinely employed by cardiac surgeons during operation. However, this is an in vitro model which is not able to provide the evidence for the chronic effects of ER agonists on myocardial infarction. Therefore, further investigations using an in vivo myocardial infarction model are required for completely understanding the chronic actions of post-infarction treatment with ER agonists.
In summary, acute post-ischemic administration of ER-α agonist-PPT or ER-β agonist-DPN significantly attenuates myocardial dysfunction following I/R injury, suggesting that both ER-α and ER-β may mediate protection against myocardial I/R. Although a trend exists toward decreased myocardial damage and reduced pro-inflammatory cytokine production, as well as increased pro-survival cytokine (VEGF) has been noted in PPT or DPN treated group, further study is required to investigate the detailed mechanisms on the specific function of ER subtypes in the heart. Elucidation of knowledge regarding both ER subtypes will improve our understanding of gender and estrogen effects and may help in the development of novel strategies with benefit for both man and woman in response to acute myocardial injury.
Acknowledgments
This work was supported in part by NIH R01GM070628 (DRM), NIH R01HL085595 (DRM), NIH K99/R00 HL0876077 (MW).
Financial support: this work was supported in part by NIH R01GM070628 (DRM), NIH K99 HL0876077 (MW), and NIH R01HL085595 (DRM).
Footnotes
DISCLOSURES
None.
References
- 1.Murphy E, Steenbergen C. Gender-based differences in mechanisms of protection in myocardial ischemia-reperfusion injury. Cardiovasc Res. 2007;75(3):478–86. doi: 10.1016/j.cardiores.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Lahm T, Crisostomo PR, Markel TA, Wang M, Weil BR, Novotny NM, Meldrum DR. The effects of estrogen on pulmonary artery vasoreactivity and hypoxic pulmonary vasoconstriction: potential new clinical implications for an old hormone. Crit Care Med. 2008;36(7):2174–83. doi: 10.1097/CCM.0b013e31817d1a92. [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Baker L, Tsai BM, Meldrum KK, Meldrum DR. Sex differences in the myocardial inflammatory response to ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2005;288(2):E321–6. doi: 10.1152/ajpendo.00278.2004. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Tsai BM, Reiger KM, Brown JW, Meldrum DR. 17-beta-Estradiol decreases p38 MAPK-mediated myocardial inflammation and dysfunction following acute ischemia. J Mol Cell Cardiol. 2006;40(2):205–12. doi: 10.1016/j.yjmcc.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Node K, Kitakaze M, Kosaka H, Minamino T, Funaya H, Hori M. Amelioration of ischemia- and reperfusion-induced myocardial injury by 17beta-estradiol: role of nitric oxide and calcium-activated potassium channels. Circulation. 1997;96(6):1953–63. doi: 10.1161/01.cir.96.6.1953. [DOI] [PubMed] [Google Scholar]
- 6.Terrell AM, Crisostomo PR, Markel TA, Wang M, Abarbanell AM, Herrmann JL, Meldrum DR. Postischemic infusion of 17-beta-estradiol protects myocardial function and viability. J Surg Res. 2008;146(2):218–24. doi: 10.1016/j.jss.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340(23):1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 8.Babiker FA, De Windt LJ, van Eickels M, Grohe C, Meyer R, Doevendans PA. Estrogenic hormone action in the heart: regulatory network and function. Cardiovasc Res. 2002;53(3):709–19. doi: 10.1016/s0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. 2006;290(6):H2204–9. doi: 10.1152/ajpheart.01219.2005. [DOI] [PubMed] [Google Scholar]
- 10.Booth EA, Obeid NR, Lucchesi BR. Activation of estrogen receptor-alpha protects the in vivo rabbit heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2005;289(5):H2039–47. doi: 10.1152/ajpheart.00479.2005. [DOI] [PubMed] [Google Scholar]
- 11.Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res. 2004;95(7):692–9. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- 12.Pelzer T, Jazbutyte V, Hu K, Segerer S, Nahrendorf M, Nordbeck P, Bonz AW, Muck J, Fritzemeier KH, Hegele-Hartung C, Ertl G, Neyses L. The estrogen receptor-alpha agonist 16alpha-LE2 inhibits cardiac hypertrophy and improves hemodynamic function in estrogen-deficient spontaneously hypertensive rats. Cardiovasc Res. 2005;67(4):604–12. doi: 10.1016/j.cardiores.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Crisostomo PR, Markel T, Wang Y, Lillemoe KD, Meldrum DR. Estrogen receptor beta mediates acute myocardial protection following ischemia. Surgery. 2008;144(2):233–8. doi: 10.1016/j.surg.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Moritz A, Radtke OA, Gust R, Glusa E, Pertz HH. Characterisation of the relaxant response to raloxifene in porcine coronary arteries. Eur J Pharmacol. 2006;545(2–3):153–60. doi: 10.1016/j.ejphar.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Pedram A, Razandi M, Levin ER. Estrogen prevents cardiomyocyte apoptosis through inhibition of reactive oxygen species and differential regulation of p38 kinase isoforms. J Biol Chem. 2006;281(10):6760–7. doi: 10.1074/jbc.M511024200. [DOI] [PubMed] [Google Scholar]
- 16.Urata Y, Ihara Y, Murata H, Goto S, Koji T, Yodoi J, Inoue S, Kondo T. 17Beta-estradiol protects against oxidative stress-induced cell death through the glutathione/glutaredoxin-dependent redox regulation of Akt in myocardiac H9c2 cells. J Biol Chem. 2006;281(19):13092–102. doi: 10.1074/jbc.M601984200. [DOI] [PubMed] [Google Scholar]
- 17.Stefano GB, Prevot V, Beauvillain JC, Cadet P, Fimiani C, Welters I, Fricchione GL, Breton C, Lassalle P, Salzet M, Bilfinger TV. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;101(13):1594–7. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- 18.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407(6803):538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 20.Parkash J, Felty Q, Roy D. Estrogen exerts a spatial and temporal influence on reactive oxygen species generation that precedes calcium uptake in high-capacity mitochondria: implications for rapid nongenomic signaling of cell growth. Biochemistry. 2006;45(9):2872–81. doi: 10.1021/bi051855x. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YC, Yu HP, Suzuki T, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Upregulation of mitochondrial respiratory complex IV by estrogen receptor-beta is critical for inhibiting mitochondrial apoptotic signaling and restoring cardiac functions following trauma-hemorrhage. J Mol Cell Cardiol. 2006;41(3):511–21. doi: 10.1016/j.yjmcc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88(10):1020–7. doi: 10.1161/hh1001.090858. [DOI] [PubMed] [Google Scholar]
- 23.Asai K, Hiki N, Mimura Y, Ogawa T, Unou K, Kaminishi M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock. 2001;16(5):340–3. doi: 10.1097/00024382-200116050-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ozveri ES, Bozkurt A, Haklar G, Cetinel S, Arbak S, Yegen C, Yegen BC. Estrogens ameliorate remote organ inflammation induced by burn injury in rats. Inflamm Res. 2001;50(12):585–91. doi: 10.1007/PL00000238. [DOI] [PubMed] [Google Scholar]