Abstract
B cells generated in the bone marrow of adult mice enter the periphery as transitional B cells and subsequently differentiate into one of two phenotypically and functionally distinct subsets, marginal zone (MZ) or follicular (Fo) B cells. Recent reports indicate, however, that in response to environmental cues, such as lymphopenia, mature Fo B cells can change to display phenotypic markers characteristic of MZ B cells. Previously, we found that splenic B cells transferred to SCID mice responded to polyoma virus (PyV) infection with T cell-independent (TI) IgM and IgG secretion, reducing the viral load and protecting mice from the lethal effect of the infection. The contribution of MZ and Fo B cell subsets to this antiviral TI-2 response, however, has not been addressed. In this study, we show that both sort-purified MZ and Fo B cells generate protective TI Ab responses to PyV infection when transferred into SCID mice. Moreover, the transferred Fo B cells in the spleens of the PyV-infected SCID mice change phenotype, with many of them displaying MZ B cell characteristics. These findings demonstrate the plasticity of the B cell subsets in virus-infected hosts and show for the first time that B cells derived exclusively from Fo B cells can effectively function in antiviral TI-2 responses.
B cells develop in adult mice from hematopoietic precursors into immature B cells in the bone marrow. These cells then migrate to the spleen and further differentiate into one of two mature B cell subsets, marginal zone (MZ)5 or follicular (Fo) B cells (1). The exact nature of signals and pathways determining the decision to become Fo or MZ B cell are not well understood. BCR signaling was shown to play a major role in selection into one subset vs the other and several reports suggest that B cells with stronger BCR signaling become Fo B cells, but there are also studies with the opposite conclusion (2–4). Other factors, for example, notch 2-Delta interactions are also thought to have a large effect (5). The importance of this decision is far-reaching, as the two B cell subsets have distinct phenotypes, functions, and anatomical locations. Fo B cells are characterized by high CD23 (FcεRII) and low CD21/CD35 (complement receptor CR2) expression, have a relatively short half-life of 4–5 mo (6), recirculate throughout the body, are present in the spleen, lymph nodes, and other lymphoid tissues, and represent a large fraction of mature peripheral B cells. In contrast, MZ B cells are CD23lowCD21/CD35high, have longer half-lives than Fo B cells, do not recirculate, are localized to the marginal sinuses of the spleen, and represent only a small fraction (~5%) of the splenic B cells. MZ B cells also have a restricted BCR repertoire (7, 8). Consistent with all of these observations, there are major differences between MZ and Fo B cells in gene expression patterns, which were documented recently (9).
MZ and Fo B cells are also thought to play distinct roles in the generation of T cell-independent (TI) and T cell-dependent (TD) Ab responses. Located at the marginal sinuses MZ B cells act as first responders to infection and produce robust TI responses to blood-borne pathogens (8), but studies using 4-hydroxy-3-nitro-phenyl acetyl (NP) coupled to chicken γ-globulin as a model Ag suggest that MZ B cells may also participate in some TD Ab responses (10). Fo B cells are the main producers of Abs after immunization with protein Ags. These Ab responses are TD and involve germinal center formation. It takes several days to develop these TD responses and rapid TI Ab responses to pathogens are usually not derived from Fo B cells (11, 12).
Although there are profound differences between MZ and Fo B cells, recent reports noted that mature Fo B cells can develop into MZ-like cells when transferred into lymphopenic environments, such as that occurring in RAG knockout (KO) mice (13, 14). The Fo B cell-derived MZ-like cells were CD21highCD23low and were localized to the splenic MZ (14). It is unclear what environmental cues in lymphopenic animals trigger these changes in phenotype and how Fo B-derived MZ-like cells would function in TI B cell responses against pathogens, particularly against infectious viruses.
We have demonstrated previously that PyV infection in mice induces a potent TI IgM and IgG response (15). The TI Ab responses to polyoma virus (PyV) could be induced in TCR βxδ KO mice and also in SCID mice reconstituted with B cells (16, 17). In adoptive transfer experiments, splenic B cells transferred into SCID mice responded to PyV infection with the secretion of TI IgM and IgG, and these Ab responses reduced the viral load and protected mice from the lethal effect of the infection (15). Since B cells with the Xid mutation did not secrete Abs in response to PyV infection after they were transferred into SCID mice, the TI response to PyV was defined as a TI-type 2 response (18). The contribution of MZ and Fo B cell subsets to this antiviral TI-type 2 response, however, has not been addressed. In this study, we demonstrate that adoptive transfer of either sort-purified MZ B cells or Fo B cells into SCID mice results in virus-specific TI IgM and IgG responses and enables the reconstituted mice to survive after PyV infection. Most of the transferred Fo B cells, however, do not retain their original CD21lowCD23high B cell phenotype, but instead adopt a CD21highCD23low MZ-like phenotype or some become CD21−CD23−. These findings demonstrate the plasticity of the B cell subsets in a virus-infected host and show for the first time that B cells derived exclusively from Fo B cells can effectively function in antiviral TI-type 2 responses.
Materials and Methods
Mice and infections
All mice used were on the C57BL/6 (B6) background. SCID mice were obtained from The Jackson Laboratory and maintained in our breeding colony at the University of Massachusetts Medical School. Age- and gender-matched B6 and Ly5.1-congenic B6 mice were purchased from The Jackson Laboratory. To reconstitute SCID mice, B cells purified from B6 spleen cell populations were transferred in PBS by i.v. injection. Mice were inoculated i.p. with 2 × 106 PFU of PyV (strain A2). The mice were used between 8 and 12 wk of age; all procedures were done according to protocols approved by the University of Massachusetts Medical School Animal Care and Use Committee.
VP1-specific ELISA
VP1-specific ELISAs were done as previously described (19). Briefly, purified recombinant VP1 protein (0.1 µg/ml) was used to coat wells of 96-well plates (Falcon). Bound Ab was detected using biotin-conjugated goat Abs specific for either mouse IgM or IgG (Vector Laboratories) and streptavidin-conjugated HRP (Vector Laboratories). The plates were developed using 3,3′,5,5′-tetramethylbenzidine (Sigma-Aldrich) and were read at 450 nm using a microplate reader and Softmax software (Molecular Devices).
Isolation of MZ and Fo B cell subsets
Purified MZ and Fo B cells were obtained using a two-step process. First a MACS negative selection B cell isolation kit (Miltenyi Biotec) was used according to the manufacturer’s instructions, followed by fluorescence-activated cell sorting. Cells were treated with anti-CD16/32 (Fc block; clone 2.4G2; BD Pharmingen) and then stained with the following Abs: FITC-anti-mouse CD21 (clone 7G6; BD PharMingen), PE-anti-mouse CD23 (clone B3B4; BD Pharmingen), and allophycocyanin anti-mouse CD45R/B220 (clone RA3-6B2; BD Pharmingen), and AA4.1 (C1qRp; eBioscience) specific for the early B cell lineage Ag CD93. Live lymphocytes were gated based on forward scatter and side scatter profiles. In some experiments, a LIVE/DEAD fixable violet dead cell kit (Invitrogen) was also used to confirm the viability of the cells. Samples were sorted to ~95% purity at the University of Massachusetts Medical Center Flow Cytometry Core facility using DakoCytomation MoFlo and Summit softwares.
Results
Both Fo B cells and MZ B cells generate Ab responses to PyV infection when transferred into SCID mice
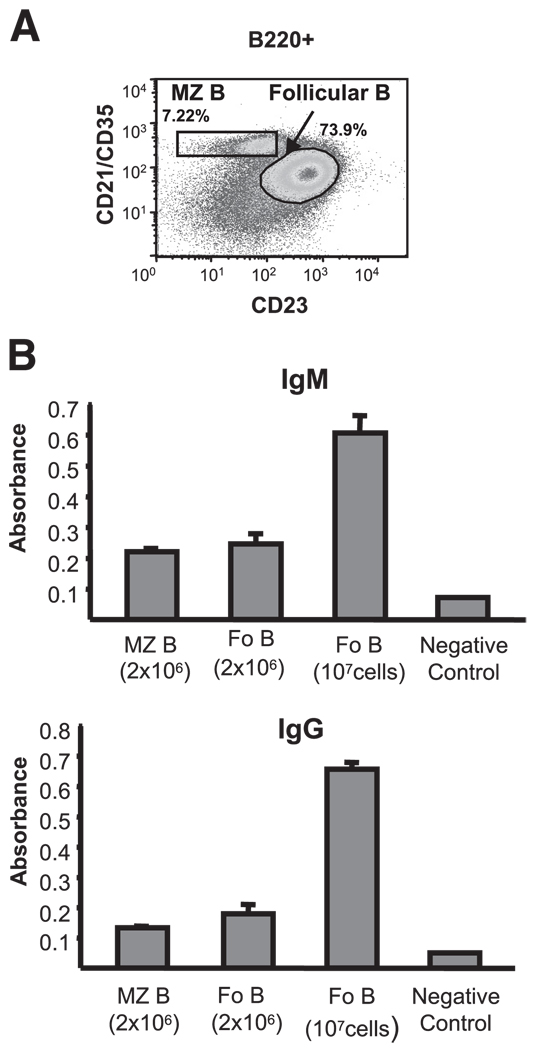
PyV infection induces antiviral IgG and IgM responses in T cell-deficient TCR βxδ KO mice and also in SCID mice reconstituted with T cell-depleted splenocytes or with purified B cells (15, 16). To determine the contribution of MZ and Fo B cells to the TI Ab response to PyV, we reconstituted SCID mice with either FACS-sorted splenic B220+CD21highCD23low MZ B cells or B220+ CD21lowCD23high Fo B cells (Fig. 1A), and examined serum IgM and IgG titers specific for the PyV major capsid protein VP1 in these mice at various time points after infection. PyV-infected SCID mice reconstituted with 2 × 106 MZ B cells generated both VP1-specific IgM and IgG (Fig. 1B), consistent with other studies showing a role for MZ B cells in the generation of TI-2 responses (11). Surprisingly, however, SCID mice reconstituted with 2 × 106 Fo B cells also generated VP1-specific IgM and IgG responses of similar magnitude. Fo B cells are present in the spleen of B6 mice at higher numbers than MZ B cells (Fig. 1A). Therefore, we also tested the response of SCID mice reconstituted with greater numbers (107) of Fo B cells. Indeed, these mice generated IgM and IgG responses much greater than that of SCID mice reconstituted with either 2 × 106 MZ or Fo B cells. Thus, both MZ and Fo B cells can contribute to the TI-2 Ab response to PyV in reconstituted SCID mice and Fo B cells, because of their greater numbers in mice, may be major contributors to TI responses to PyV.
FIGURE 1.
MZ and Fo B cells transferred into SCID mice generate TI Ab responses to PyV. A, MZ (B220+CD21highCD23low) and Fo (B220+ CD21lowCD23high) B cells were sorted from B6 splenocytes by FACS; the isolated populations are shown. B, VP1-specific ELISA of serum IgM and IgG of SCID mice reconstituted with 2 × 106 MZ B cells (n = 5), 2 × 106 Fo B cells (n = 5), or 1 × 107 Fo B cells (n = 9) and infected with PyV. The sera for IgM was taken on day 14 and for IgG on day 21 after infection and tested at 1/100 dilutions. Sera of uninfected B6 mice were used as negative controls. Mean absorbance at 450 nm with SEM are shown. Data are representative of three experiments.
Transferred Fo B cells or MZ B cells prevent acute PyV-induced mortality in SCID mice
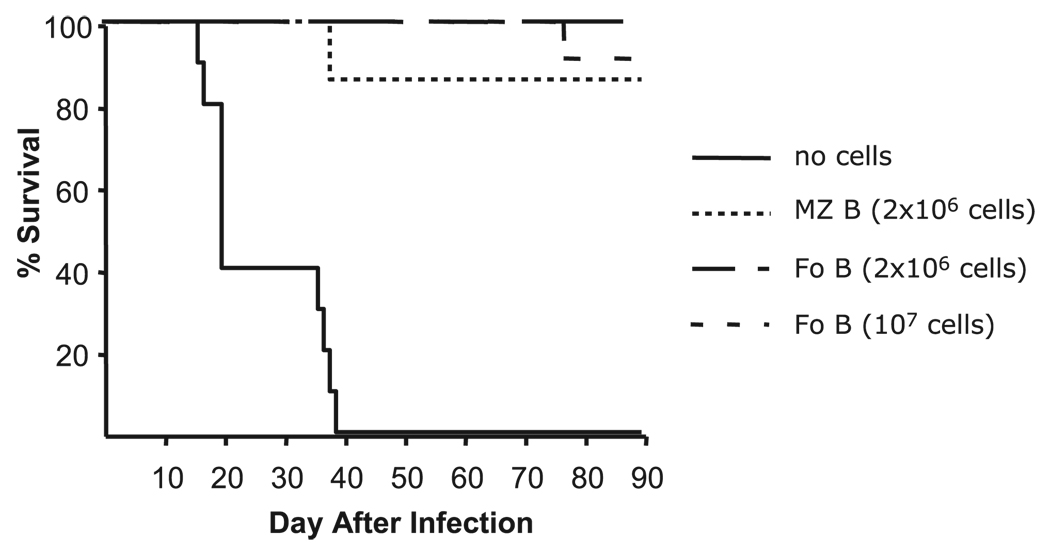
Following infection, PyV replicates to high levels in SCID mice and the mice succumb to an acute myeloproliferative disease (20). However, SCID mice reconstituted with splenocytes from TCRβxδ KO mice or Thy1-depleted splenocytes from B6 mice do not develop an acute myeloproliferative disease and survive for several months after infection (15). Moreover, i.v. transfer of IgG Abs purified from PyV- infected T cell KO mice also protects SCID mice from the lethal effects of PyV infection, indicating that TI Ab responses to PyV are sufficient to control the viral infection to the degree that allows for survival (15). To examine the protective capacity of the TI responses of MZ and Fo B cells to PyV, we monitored the survival of PyV-infected SCID mice reconstituted with either MZ or Fo B cells. Within 40 days of infection, all untreated SCID mice succumbed to PyV. However, reconstitution with either MZ or Fo B cells protected SCID mice from death for at least 90 days (Fig. 2), suggesting that the responses of MZ and Fo B cells to PyV have comparable biological consequences.
FIGURE 2.
MZ and Fo B cells protect SCID mice from death following PyV infection. SCID mice were reconstituted with 2 × 106 MZ B cells, 2 × 106 Fo cells, 1 × 107 Fo B cells, or no cells at all, and 1 day later infected with 2 × 106 PFU of PyV i.p. The mice were observed for 90 days.
Splenic B cells change phenotype following transfer into SCID mice and PyV infection
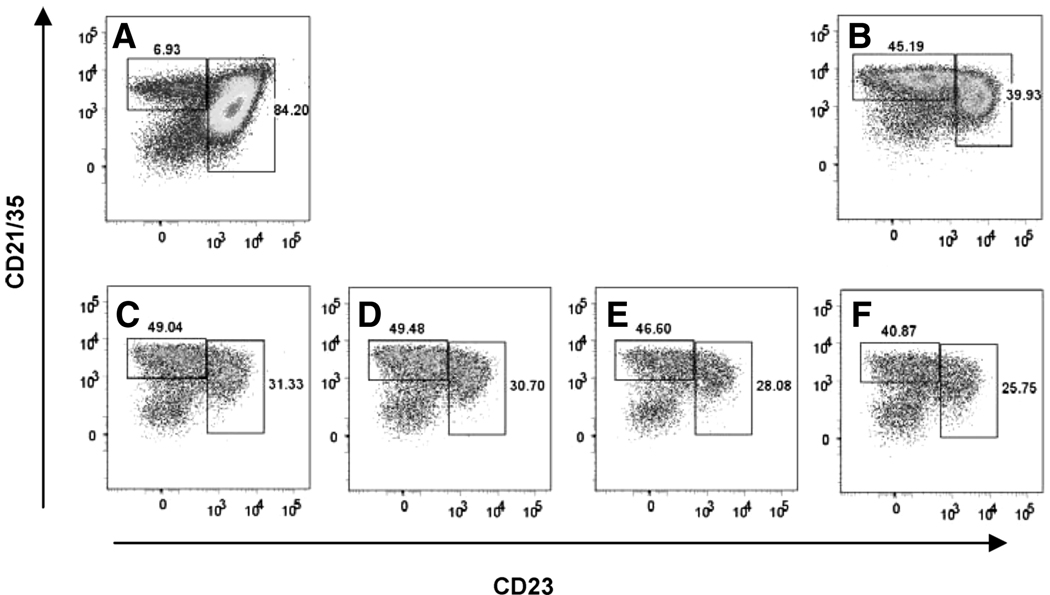
MZ and Fo B cells are thought to be derived from immature B cells via divergent differentiation pathways. It has been recently reported, however, that sorted Fo B cells acquired characteristics of MZ B cells when they were transferred into RAG KO hosts (14). Because this phenotypic change did not occur after Fo B cells were transferred into immune-competent B6 mice, it was assumed to be associated with homeostatic B cell proliferation in the “empty” RAG−/− recipients (14). We questioned whether major changes would occur in the B cell subsets after adoptive transfer of splenic B cells into SCID mice and subsequent PyV infection. To test for this possibility, we reconstituted SCID mice with 107 B cells isolated from spleens of B6 mice and infected the mice with PyV. Nearly 80% of the transferred B cells were CD21lowCD23high Fo B cells and ~6% were CD21highCD23low MZ B cells (Fig. 3A). Twenty-two days after infection, both MZ and Fo B cells could be identified in the spleens of the SCID mice. Notably, however, at this time point, B cells with the MZ phenotype outnumbered those with a Fo phenotype in the spleen of reconstituted SCID mice (Fig. 3, C–F). B cells in mice that received cell transfer but were left uninfected also underwent similar phenotypic changes (Fig. 3B). The increase in the MZ:Fo B cell ratio from 1:13 to 1.6:1 could be due to homing of Fo B cells to other tissues, reduced survival rate of Fo B cells, or MZ cells outcompeting the other subset. Alternatively, Fo B cells may have changed their surface markers following transfer into the SCID mice.
FIGURE 3.
Changes in the MZ and Fo B cell subsets following transfer to SCID mice and PyV infection. Phenotype of B cells in the spleens of naive B6 mice (A) and SCID mice reconstituted with B cells isolated from spleens of B6 mice (107cells/mouse) and left uninfected (B) or infected for 22 days with PyV (C–F). The FACS plots were gated on CD19+B220+ lymphoid cells. The percentage of CD21highCD23low and CD21low CD23highB cells are shown in each sample.
Changes in phenotypes of Fo B cells following their transfer into SCID mice and PyV infection
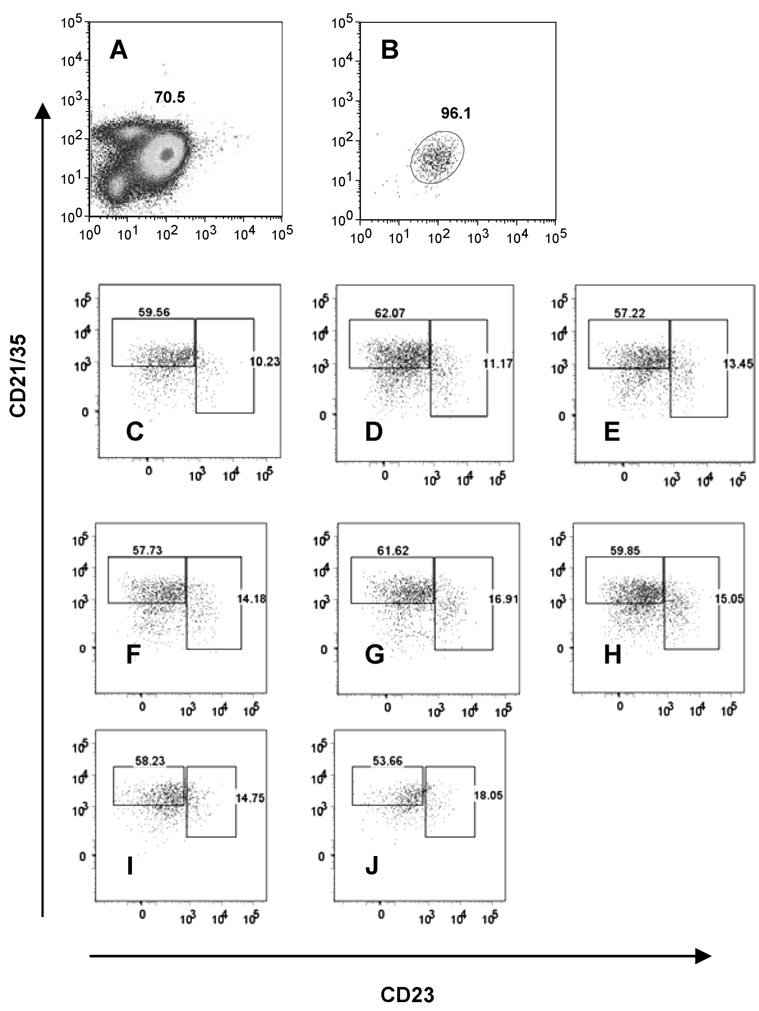
To determine whether Fo B cells undergo changes in their phenotype, we reconstituted SCID mice with sort-purified Fo B cells and examined the B cells in the spleens of these mice 17 days after cell transfer and PyV infection. Since the SCID mice used for these experiments expressed the Ly5.2 marker, we transferred Ly5.1-expressing B cells so that we could distinguish the donor and recipient cell populations. Although the B cells transferred into the SCID hosts were >96% pure CD21lowCD23high Fo B cells (Fig. 4B), we found that by day 17 after cell transfer and infection, only 10–13% of the cells in the spleens had retained this phenotype. Instead, the majority of the B cells displayed a CD21highCD23low MZ-like phenotype (~60%) and some were CD21−CD23− (Fig. 4, C–E). Uninfected SCID mice reconstituted with Fo B cells also had similar CD21highCD23low and CD21−CD23− B cell populations and relatively few (14–17%) CD21lowCD23high Fo B cells in their spleens 2 wk after cell transfer (Fig. 4, F–H), indicating that phenotypic changes of the transferred Fo B cells occurred regardless of the viral infection. Sorted CD21lowCD23high B cells contain a minor fraction (7–12%) of late transitional T2 B cells. These transitional B cells can be identified by staining with the Ab to the early B cell lineage marker AA4.1 (14). To exclude the possibility that the immature T2 cells were responsible for the appearance of MZ-like B cells and/or CD21−CD23− cells, we performed additional experiments with sort-purified AA4.1−CD21lowCD23high B cells. Adoptive transfer of these cells, which were all mature Fo B cells (>99.6% purity), yielded similar results to previous experiments. On day 14 after adoptive transfer and PyV infection, ~53% of spleen B cells had a CD21highCD23low MZ phenotype, whereas ~15% appeared as CD21lowCD23high Fo B cells (see Fig. 4, I and J), confirming our conclusion that the majority of Fo B cells changed their phenotypes in the SCID hosts.
FIGURE 4.
Phenotype of B cells in the spleens of mice reconstituted with FACS-sorted Fo B cells. CD21lowCD23high Fo B cells were sorted from spleens of B6 Ly5.1-congenic mice to ~96% purity and 107 cells were transferred into Ly5.2 SCID hosts i.v. Mice were infected the next day with 2 × 106 PFU of PyV i.p. or left uninfected. The FACS plots shown were gated on Ly5.1 (donor) CD19+B220+ cells. A, Unsorted spleen cells of B6 mice; B, sorted Fo B cells used for transfer; C–E, spleen cells of SCID mice 17 days after reconstitution and PyV infection; F–H, spleen cells of uninfected SCID mice 17 days after reconstitution. I and J, CD21lowCD23highAA4.1− Fo B cells were sorted from B6 Ly5.1 spleens (99.6% purity) and 7.5 × 106 cells were transferred into Ly5.2 SCID mice, then the mice were infected with 2 × 106 PFU of PyV i.p. on the next day. Spleen cells were analyzed by FACS on day 14 after infection. The FACS plots are gated on Ly5.1 CD19+B220+ cells. The data represent one of two similar independent experiments.
Discussion
TI Ab responses generated by splenic B cells of naive B6 mice transferred into SCID mice were previously shown to provide protection for the recipients against PyV infection, which is always lethal in SCID mice that receive no cell transfer (15). In this study, we questioned the role of spleen B cell subsets in these TI Ab responses, and we showed that SCID mice reconstituted with either sort-purified MZ B cells or Fo B cells survived PyV infection and that both MZ and Fo B cells have generated PyV major capsid protein VP1-specific IgM and IgG in the new host.
In a normal spleen, the ratio of Fo B cells to MZ B cells is 6–10:1. SCID mice reconstituted with equal numbers of Fo B cells or MZ B cells (2 × 106 cells/mouse each) responded to PyV with similar levels of IgM and IgG secretion. When we compared the responses of these B cell subsets taking into account their ratios in normal spleens, 5-fold higher numbers of Fo B cells (107 cells/mouse) produced significantly higher PyV-specific Ab levels than the MZ B cells (2 × 106 cells/mouse) (Fig. 1). These data suggest that not only MZ B cells, but also Fo B cells may make contribution to the TI humoral immune responses to PyV infection in this model.
The VP1-specific IgM and IgG levels detected in the mice reconstituted with MZ or Fo B cells were low, but still sufficient to provide protection against the lethal consequences of PyV infection. This finding is consistent with our previous results showing that SCID mice which were reconstituted with B cells with certain mutations (such as CD40 KO, IFN-γR KO) produced very small amounts of VP1-specific IgG, but still survived PyV infection much longer than mice that received no cells or Xid B cells which were completely incapable of responding to PyV with TI Ab secretion (17, 18). It is important to note that the ELISA used in our studies measure VP1-specific Abs, which accurately represent the antiviral Ab responses induced by PyV, as these responses are mostly directed against VP1, the major capsid protein. The other two structural proteins of PyV, VP2 and VP3, are only minor components of the capsid and not accessible on the surface of intact free virions (21).
TI responses to bacterial polysaccharides and other TI-2 Ags such as haptenated polymers (Ficoll or dextran) mostly derive from MZ B cells. MZ B cells are present only in the spleen in mice and splenectomized mice show low resistance to infections with encapsulated bacteria. Moreover, Pyk-2-deficient mice which fail to develop MZ B cells cannot respond to the TI-2 Ag NP-Ficoll (8, 22), and following sublethal irradiation of rats the reconstitution of MZ B cells in the spleen coincides with the appearance of Ab responses to TI-2 Ags (23). There are similar observations in humans: the appearance of MZ B cells in ontogeny at about 2 years of age marks the onset of efficient B cell responses to immunizations with TI-2 Ags (24). In contrast, Fo B cells are usually not implicated in responses directed against TI-2 Ags, but they represent the main B cell subset secreting Abs induced by TD protein Ags. On the other hand, the TI Ab responses induced by PyV differ from the “usual” TI responses induced by bacterial polysaccharides and conventional TI Ags in many respects. First, the PyV Ag is protein, not polysaccharide. Moreover, the responses are mostly isotype switched and the most abundant isotypes produced in response to PyV in T cell-deficient hosts are IgG2a and IgG2b, whereas TI responses to bacterial polysaccharides are mainly IgM and IgG3 responses (22). In addition to PyV, other viruses (e.g., vesicular stomatitis virus, rotaviruses) were also shown to induce isotype-switched TI Ab responses (25). Thus, it is of interest that we can demonstrate efficient protective Ab responses produced by either isolated MZ or Fo B cell subsets to PyV, which represents a novel group of viral TI Ags.
Recirculating mature B cells residing in the perisinusoidal areas of the bone marrow were recently shown to be involved in TI Ab responses to bacteria, but these Fo B cell-derived “perisinusoidal B cells” could secrete only IgM, as they did not express activation-induced cytidine deaminase which is required for isotype switching (26). In this study, we report that Fo B cell-derived cells respond to PyV infection with protective IgM and IgG secretion after their transfer into SCID hosts.
Adoptive transfer of B cells into SCID mice places the donor cells into a lymphopenic milieu, which may trigger changes in cell behavior and phenotype. In our experiments, transfer of splenic B cell populations containing a mixture of MZ and Fo B cells in a 1:10 ratio resulted in the recovery of predominantly MZ phenotype B cells 3 wk after the transfer and PyV infection. The major change in the ratios of MZ vs Fo B cells could result from several different mechanisms. The transferred MZ B cells could outcompete Fo B cells in the spleen by proliferation, Fo B cells could migrate and home to various locations other than the spleen (e.g., lymph nodes) after the cell transfer, or Fo B cells could change into MZ-like B cells after transfer into SCID mice. Because 3 wk after the transfer of sort-purified Fo B cells into SCID mice MZ phenotype B cells also outnumbered Fo B cells in the spleens, we think that most likely the transferred Fo B cells changed their phenotype in the new host. Supporting this conclusion are recent reports indicating that Fo B cells placed into a lymphopenic environment, such as that of SCID or RAG KO mice, eventually change their surface markers by down-regulating CD23 and up-regulating CD21 expression, appearing as MZ B cells (13, 14). These B cells localize to the MZ and generate IgM responses to classical TI Ags such as NP-Ficoll, thus exhibiting functional attributes of MZ B cells as well (13). Our report now shows that Fo B cell-derived B cells, most of them with newly acquired MZ phenotypes, respond to a PyV, a viral TI Ag with IgM and IgG secretion and can successfully control the otherwise lethal infection.
Several observations suggest that in a lymphopenic milieu, MZ B cells are preferentially generated and maintained. When the supply of new B cells from the bone marrow is scarce or missing, such as in mice with IL-7 or IL7Rα deficiency (27, 28) and in conditional Rag2 KO mice (29), the MZ B cell pool is maintained at the expense of other B cell subsets (30, 31). Gradual changes in the surface markers of the transferred Fo B cells in RAG KO mice shifting toward the MZ B cell phenotype are also consistent with this idea (14). Thus, it seems that differentiation of B cells into distinct B cell compartments is not a one-way terminal process; instead it is reversible. Studies showing an enlarged MZ B cell compartment in B cell-activating factor of the TNF family (BAFF) overexpressing mice suggest that BAFF may be a key factor regulating the size of the B cell subsets (32, 33).
Our findings suggest that Fo B cells give rise to MZ phenotype B cells in lymphopenic hosts even under the conditions of an ongoing virus infection. Furthermore, we show for the first time that the Fo B cell-derived cells are fully functional in TI antiviral IgG responses in vivo. These studies do not distinguish between two scenarios, whether Fo B cells first need to acquire MZ B cell-like phenotypes to respond to PyV in a TI manner or whether the phenotypic changes occur in some cells, but are not required for the responses. In either case, the transferred cells can rapidly provide protection against a life-threatening infection, demonstrating the remarkable functional and phenotypic plasticity of the B cell subsets.
Acknowledgments
We thank the UMMS Flow Cytometry Core facility and Bhavana Priyadharshini for help with the experiments and Dr. Rachel Gerstein for critical reading of this manuscript.
Footnotes
This work was supported by National Institute of Health Grants CA66644 and AI073651, Training Grant AI07272, and Institutional Diabetes Endocrinology Research Center Grant DK32520.
Abbreviations used in this paper: MZ, marginal zone; Fo, follicular; TI, T cell independent; TD, T cell dependent; KO, knockout; PyV, polyoma virus; BAFF, B cell-activating factor of the TNF family; NP, 4-hydroxy-3-nitrophenyl acetyl.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv. Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 2.Cariappa A, Tang M, Parng C, Nebelitskiy E, Carroll M, Georgopoulos K, Pillai S. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–615. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 3.Lopes-Carvalho T, Kearney JF. Development and selection of marginal zone B cells. Immunol. Rev. 2004;197:192–205. doi: 10.1111/j.0105-2896.2004.0112.x. [DOI] [PubMed] [Google Scholar]
- 4.Samardzic T, Marinkovic D, Danzer CP, Gerlach J, Nitschke L, Wirth T. Reduction of marginal zone B cells in CD22-deficient mice. Eur. J. Immunol. 2002;32:561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda K, Han H, Tani S, Tanigaki K, Tun T, Furukawa T, Taniguchi Y, Kurooka H, Hamada Y, Toyokuni S, Honjo T. Regulation of marginal zone B cell development by MINT, a suppressor of Notch/RBP-J signaling pathway. Immunity. 2003;18:301–312. doi: 10.1016/s1074-7613(03)00029-3. [DOI] [PubMed] [Google Scholar]
- 6.Forster I, Rajewsky K. The bulk of the peripheral B-cell pool in mice is stable and not rapidly renewed from the bone marrow. Proc. Natl. Acad. Sci. USA. 1990;87:4781–4784. doi: 10.1073/pnas.87.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J. Immunol. 2000;165:6156–6169. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 8.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 9.Kin NW, Crawford DM, Liu J, Behrens TW, Kearney JF. DNA microarray gene expression profile of marginal zone versus follicular B cells and idiotype positive marginal zone B cells before and after immunization with Streptococcus pneumoniae. J. Immunol. 2008;180:6663–6674. doi: 10.4049/jimmunol.180.10.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song H, Cerny J. Functional heterogeneity of marginal zone B cells revealed by their ability to generate both early antibody-forming cells and germinal centers with hypermutation and memory in response to a T-dependent antigen. J. Exp. Med. 2003;198:1923–1935. doi: 10.1084/jem.20031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 12.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Vinuesa CG, Sze DM, Cook MC, Toellner KM, Klaus GG, Ball J, MacLennan IC. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur. J. Immunol. 2003;33:297–305. doi: 10.1002/immu.200310003. [DOI] [PubMed] [Google Scholar]
- 14.Srivastava B, Quinn WJ, 3rd, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J. Exp. Med. 2005;202:1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szomolanyi-Tsuda E, Welsh RM. T cell-independent antibody-mediated clearance of polyoma virus in T cell-deficient mice. J. Exp. Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szomolanyi-Tsuda E, Le QP, Garcea RL, Welsh RM. T-cellindependent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J. Virol. 1998;72:6665–6670. doi: 10.1128/jvi.72.8.6665-6670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szomolanyi-Tsuda E, Brien JD, Dorgan JE, Welsh RM, Garcea RL. The role of CD40-CD154 interaction in antiviral T cell-independent IgG responses. J. Immunol. 2000;164:5877–5882. doi: 10.4049/jimmunol.164.11.5877. [DOI] [PubMed] [Google Scholar]
- 18.Szomolanyi-Tsuda E, Brien JD, Dorgan JE, Garcea RL, Woodland RT, Welsh RM. Antiviral T-cell-independent type 2 antibody responses induced in vivo in the absence of T and NK cells. Virology. 2001;280:160–168. doi: 10.1006/viro.2000.0766. [DOI] [PubMed] [Google Scholar]
- 19.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J. Immunol. 2007;178:5124–5131. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 20.Szomolanyi-Tsuda E, Dundon PL, Joris I, Shultz LD, Woda BA, Welsh RM. Acute, lethal, natural killer cell-resistant myeloproliferative disease induced by polyomavirus in severe combined immunodeficient mice. Am. J. Pathol. 1994;144:359–371. [PMC free article] [PubMed] [Google Scholar]
- 21.Cole CN, Conzen SD. Polyomaviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Martin MA, Lamb RA, Roizman B, Straus SE, editors. Fields Virology. Vol. 2. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2141–2174. [Google Scholar]
- 22.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr. Opin. Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 23.Lane PJ, Gray D, Oldfield S, MacLennan IC. Differences in the recruitment of virgin B cells into antibody responses to thymus-dependent and thymus-independent type-2 antigens. Eur. J. Immunol. 1986;16:1569–1575. doi: 10.1002/eji.1830161216. [DOI] [PubMed] [Google Scholar]
- 24.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infancy: possible contribution to the deficient infant immune response. J. Immunol. 1999;143:3200–3206. [PubMed] [Google Scholar]
- 25.Szomolanyi-Tsuda E, Welsh RM. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 1998;10:431–435. doi: 10.1016/s0952-7915(98)80117-9. [DOI] [PubMed] [Google Scholar]
- 26.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7−/− mice. J. Exp. Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erlandsson L, Licence S, Gaspal F, Bell S, Lane P, Corcoran AE, Martensson IL. Impaired B-1 and B-2 B cell development and atypical splenic B cell structures in IL-7 receptor-deficient mice. Eur. J. Immunol. 2004;34:3595–3603. doi: 10.1002/eji.200425217. [DOI] [PubMed] [Google Scholar]
- 29.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin F, Kearney JF. Marginal-zone B cells. Nat. Rev. Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 31.Srivastava B, Lindsley RC, Nikbakht N, Allman D. Models for peripheral B cell development and homeostasis. Semin. Immunol. 2005;17:175–182. doi: 10.1016/j.smim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]