Abstract
Hyperglycemia associated with diabetes mellitus results in the priming of neutrophils leading to oxidative stress that is, in part, responsible for diabetic complications. p47phox, a NADPH oxidase cytosolic subunit, is a key protein in the assembly of the NADPH oxidase leading to superoxide generation. Little is known about the priming mechanism of oxidative pathways in neutrophils of people with diabetes. In this study, the kinetics of p47phox activation was investigated by comparing neutrophils from diabetic and healthy subjects, and the mechanism of hyperglycemia-induced changes was studied by using neutrophil-like HL-60 cells as a model. In resting neutrophils from diabetic subjects, p47phox prematurely translocates to the cell membrane and preassembles with p22phox, a NADPH oxidase membrane subunit. This premature p47phox translocation and preassembly with p22phox were also observed in HL-60 cells cultured with high glucose (HG; 25 mM) and with the specific ligand for the receptor for advanced glycation end products (RAGE), S100B. Phosphorylation of ERK1/2, but not p38 MAPK, was the primary signaling pathway, as evidenced by PD98059 suppressing the translocation of p47phox in HL-60 cells incubated with HG and S100B. HL-60 cells cultured in HG and S100B exhibited a 1.8-fold increase in fMLP-induced superoxide generation compared with those cultured in normal glucose (5.5 mM). These data suggest that HG and increased AGE prime neutrophils and increase oxidative stress inducing the translocation of p47phox to the cell membrane and preassembly with p22phox by stimulating a RAGE-ERK1/2 pathway.
Keywords: inflammation, signal transduction, cell activation
INTRODUCTION
Diabetes mellitus is a common disease that affects more than 6.0% of the U.S. population [1] and is characterized by several major complications, such as cardiovascular disease, atherosclerosis, retinopathy, nephropathy, and neuropathy [2,3,4,5], which alter quality of life and reduce longevity. Several studies have reported that hyperglycemia and subsequent induced biochemical events are correlated with the development of diabetic complications, although the precise mechanism of action is still not clear [6, 7]. Recently, focus has been placed on the relationship between diabetes and inflammation, as all of the recognized complications of diabetes have a major inflammatory component. Periodontal disease, an oral inflammatory disease induced by biofilms on teeth, is more prevalent and more severe in patients with diabetes and has been accepted as a common diabetic complication [8]. Poor metabolic control of diabetes has often been related to the severity of periodontitis [9, 10].
One of the key elements in the development of diabetic complications is oxidative stress [11]. Previous studies in vascular endothelial cells and phagocytic leukocytes have linked generation of superoxide and other reactive oxygen species (ROS) and enhanced oxidative stress with hyperglycemia [9, 12,13,14]. Although multiple ROS sources are implicated in diabetes, a major source that is important in inflammatory lesions is the neutrophil NADPH oxidase [15]. Neutrophils represent the first line of cellular host defense against microorganisms, including periodontal pathogens. This function relies in part on the ability of these cells to generate large quantities of superoxide anion (O2–). The oxidative burst results from activation of NADPH oxidase, a multicomponent enzyme system that catalyzes the NADPH-dependent reduction of oxygen to the O2–. In resting cells, this multicomponent enzyme system is not activated (not assembled), and the components are divided between the membrane and the cytosol. The neutrophil NADPH oxidase is comprised of plasma membrane-bound subunits (gp91phox and p22phox) comprising flavoCytochrome b 558 and cytosolic subunits (p47phox, p67phox, p40phox, and Rac2). The activation of neutrophils by stimuli, such as fMLP or PMA, causes the phosphorylation of the cytosolic subunit, p47phox, and the translocation of the cytosolic components to the plasma membrane, where they interact with flavoCytochrome b 558 [16,17,18,19]. p47phox is a key protein in the assembly of the NADPH oxidase leading to superoxide generation, which is abolished completely in phagocytes from p47phox knockout mice compared with wild-type animals [20]. Transfection of THP-1 cells with p47phox small interfering RNA abrogates superoxide generation, demonstrating the pivotal role of p47phox in superoxide generation [21].
In neutrophils from healthy subjects, the activation of NADPH oxidase is regulated to avoid tissue and vascular damage. However, proinflammatory cytokines, such as TNF-α and GM-CSF, are known to modulate NADPH oxidase activity by “priming” neutrophils, which enhances bactericidal activity [22, 23]. In diabetes, it is thought that hyperglycemia is one of the priming factors for neutrophils [9]. However, the biochemical mechanisms involved in neutrophil priming in diabetes are not clear. Clinically, hyperglycemia is not limited to increased glucose concentrations, but it also encompasses increased advanced glycation end-product (AGE) concentrations and other changes [24]. Therefore, in this study, we focus on p47phox to characterize the superoxide generation by neutrophils from diabetic subjects as a possible pathway for priming.
AGE are produced by nonenzymatic glycation/oxidation of proteins/lipids that accumulate during natural aging, but in certain pathologies, such as diabetes, AGE are greatly increased [24,25,26]. Early glycation products, e.g., Schiff bases and Amadori products, are reversibly formed whenever plasma glucose levels are elevated. A small proportion of these glycation products undergoes further slow, irreversible chemical rearrangements to form AGE, which accumulate in the vasculature during hyperglycemia and when protein turnover is delayed [24]. Several receptors for AGE have been identified on vascular, renal, and other cells [27]. The well-studied cell surface receptor for AGE, termed RAGE, is a multiligand member of the Ig superfamily [25, 27, 28]. Ligands for RAGE include AGE, the S100/calgranulin family of proteins, amyloid β-peptide, amphoterin, and carboxymethyllysine adducts of protein [28,29,30]. Several short peptides, including S100B, which belongs to the S100/calgranulin family, signal through RAGE [29, 30]. Thus, S100B, as a defined ligand, is a valuable tool in the study of RAGE signaling [14, 31, 32]. Although AGE–RAGE interactions have been implicated in inflammatory responses [29, 30], the role of AGE in neutrophil priming in diabetes is not known. To investigate the activation of neutrophils by high glucose (HG) and AGE, we used the human promyelocytic cell line (HL-60) differentiated into neutrophil-like cells with DMSO stimulation [33]. Neutrophil-like HL-60 cells have been reported to be similar to human neutrophils in morphology, expression of receptors, superoxide generation, and chemotaxis [33,34,35,36,37]. Unlike primary neutrophils, which lose responsiveness to various agonists or proceed to apoptosis after relatively short culture periods (hours), these cells can be maintained for many days [38, 39].
In this study, we have analyzed the mechanism of neutrophil priming increased by chronic HG and increased RAGE ligand (S100B). We demonstrate that primary neutrophils from diabetic subjects are functionally primed with preassembly of NADPH oxidase components and that AGE priming leads to an increase in p47phox translocation to the cell membrane in neutrophil-like HL-60 cells. The results suggest that preassembly of p47phox leads to increased superoxide generation that may accelerate diabetic complications and may account, at least in part, for inflammatory complications.
MATERIALS AND METHODS
Reagents
Mouse anti-p47phox mAb, rabbit anti-p22phox, and Rac2 polyclonal antibodies and goat anti-p67phox polyclonal antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit antiphospho-p44/42 MAPK (ERK1/2) polyclonal antibody, rabbit antiphospho-p38 MAPK polyclonal antibody, and HRP-conjugated goat anti-mouse or rabbit IgG were purchased from Cell Signaling Technology (Danvers, MA, USA). The human promyelocytic leukemia cell line HL-60 was obtained from American Type Culture Collection (Manassas, VA, USA). Alexa Fluor 488-conjugated goat anti-mouse IgG, Prolong Gold antifade kit, endotoxin, glucose-free RPMI 1640, and heat-inactivated FBS were purchased from Invitrogen (Carlsbad, CA, USA). S100B, cytochrome C, PD98059 (a selective ERK1/2 inhibitor), and SB203580 (a p38 MAPK inhibitor) were purchased from Calbiochem (La Jolla, CA, USA). Polyvinylidene difluoride (PVDF) membrane was purchased from Millipore (Bedford, MA, USA). Super Signal West Pico chemiluminescent substrate (ECL) was purchased from Pierce (Rockford, IL, USA). Protein assay kit was purchased from Bio-Rad (Hercules, CA, USA). All other materials were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Selection of subjects
For the study of human neutrophils from people with diabetes, 22 diabetic subjects and 22 systemically healthy individuals were recruited at the dental unit of the General Clinical Research Center of Boston University Medical Center (Boston, MA, USA). Institutional Review Board approval and signed, informed consent from individuals were obtained prior to the study. People with diabetes (type 2) were selected according to the criteria of the American Diabetes Association [40]. Demographic and clinical data for the diabetic subjects, including age, gender, race, smoking status, duration of diabetes, HbA1c levels, fasting plasma glucose levels, total cholesterol levels, incidence of diabetic complications, treatment conditions, and periodontal status, were recorded (Table 1). Control subjects were matched as closely as possible based on age, gender, race, smoking status, and periodontal status.
TABLE 1.
Demographic Data of Healthy Subjects and Type 2 Diabetic Subjects (mean ± sem)
| Healthy subjects | Diabetic subjects | ||
|---|---|---|---|
| n | 22 | 22 | |
| Age | 43.5 ± 2.5 | 53.2 ± 1.6 | |
| Gender | Male | 13 | 13 |
| Female | 9 | 9 | |
| Race | White | 15 | 10 |
| African-American | 5 | 8 | |
| Hispanic, Asian | 2 | 4 | |
| Smoking status | Nonsmoker | 21 | 9 |
| Former-smoker | 0 | 5 | |
| Smoker | 1 | 8 | |
| Duration of diabetes (year) | 8.1 ± 1.3 | ||
| Diabetic complications | Cardiovascular disease (%) | 54.5 | |
| Retinopathy (%) | 12.5 | ||
| Periodontal condition | Healthy | 21 | 2 |
| Gingivitis, mild periodontitis | 1 | 20 | |
| Moderate, severe periodontitis | 0 | 0 | |
| Diabetic treatment | Insulin (%) | 36.4 | |
| Fasting plasma glucose (mg/dl) | 193.3.5 ± 16.3 | ||
| HbA1c (%) | 7.8 ± 0.4 | ||
| Total cholesterol (mg/dl) | 162.4 ± 11.6 |
Isolation of human neutrophils
Peripheral venous blood was collected into vacutainer tubes containing 10 U/ml heparin. Neutrophils were isolated using a discontinuous gradient system as previously reported [41]. Briefly, 3 ml MonoPoly 1119 and 2 ml Histopaque 1077 were layered in 15-ml polystyrene culture tubes. Peripheral blood (4.5 ml) was layered on the separating medium, and the tubes were centrifuged at 1000 g for 15 min. The neutrophil-enriched layers were collected, and contaminating erythrocytes were lysed with a hypotonic NH4Cl buffer [155 mM NH4Cl, 10 mM KHCO3, 120 mM EDTA (pH 7.4)]. The isolated cells were washed twice with PBS. Cell preparations were routinely 99% neutrophils with ≥95% viability, as determined by trypan blue exclusion.
Cell culture
HL-60 cells (1×106 cells/ml) were differentiated into neutrophil-like cells in endotoxin-free RPMI 1640 containing 5.5 mM glucose, 10% heat-inactivated FBS, and 1.25% DMSO at 37°C in a 5% CO2 atmosphere for 6 days as reported previously [33, 42] with minor modifications. After differentiation for 6 days, neutrophil-like HL-60 cells (1×106 cells/ml) were cultured for 24 h in 5.5 mM [normal glucose (NG)] or 25 mM glucose (HG), with or without the RAGE-specific ligand S100B (5 μg/ml). The nonphysiologic sugar, 19.5 mM mannitol (MN), was used as a control for studying the impact of osmotic pressure exerted by glucose and added with NG. Cell viability, as determined by trypan blue exclusion, was ≥95%. For inhibition studies, PD98059 (25 μM) or SB203580 (10 μM) was added to the cells after differentiation in each condition and incubated for 24 h.
Cell fractionation
Neutrophils or neutrophil-like HL-60 cells (10×106 cells each) were suspended in PBS. Cells were lysed rapidly by the addition of ice-cold extraction buffer [20 mM Tris-HCl (pH 7.4), 10 mM HEPES, 25 mM NaCl, 2 mM EDTA, 10 mM EGTA, 1% (w/w) protease inhibitor cocktail]. The subfractionation of cells was then conducted at 4°C using a method described previously [43] with minor modifications. The cells were ultrasonicated five times at 30 W for 5 s. An aliquot of sonicate was saved as the total fraction. The remaining sonicate was centrifuged at 1000 g for 5 min at 4°C to remove cell debris and nuclei. The collected supernatant was centrifuged at 15,000 g for 30 min at 4°C, and the supernatant was collected as the soluble fraction (cytosolic-rich fraction). The pellet was resuspended in the extraction buffer with 0.5% Triton X-100 and 1 mM PMSF by ultrasonication five times at 30 W for 5 s and incubated for 20 min at 4°C. The resulting suspension was used as the particulate fraction (membrane-rich fraction). Protein concentration was determined with Bio-Rad protein assay using BSA as the standard. We also confirmed the cell fractionation (membrane-rich or cytosol-rich fractions) by immunoblot using the plasma membrane-specific protein Na+-K+ ATPase antibody (data not shown).
Western blotting
Western blotting was performed as described previously [44, 45]. Neutrophils or HL-60 cells (5×106 cells) were lysed rapidly (total cell lysates) by adding 40 μl 6× SDS sample buffer to 200 μl of the reaction mixture and then boiling the samples for 5 min. The final composition of SDS sample buffer after mixing was 2% (w/v) SDS, 58.3 mM Tris–HC1 (pH 6.8), 6% (v/v) glycerol, 5% (v/v) 2-ME, 0.002% (w/v) bromophenol blue, 1% (v/v) protease inhibitor cocktail, and 1 mM PMSF. Aliquots of these samples were separated by SDS-PAGE (10 μg/lane) on 10% or 13% (v/v) polyacrylamide slab gels. The separated proteins were transferred immediately, electrophoretically to PVDF membranes using Tris-borate buffer [25 mM Tris, 192 mM glycine, and 20% (v/v) methanol, pH 8.4]. Proteins were transferred at 100 V for 1 h at 4°C. Membranes were blocked for 1 h at room temperature with 5% skim milk in TBS (pH 7.6). The blocking buffer was removed, and the membranes were incubated with the appropriate primary antibodies (1:500 dilution for p47phox, p67phox, Rac2, and p22phox; 1:1000 dilution for phospho-p44/42 MAPK and phospho-p38 MAPK) overnight at 4°C in 20 mM Tris–HCl (pH 7.6) containing 250 mM NaCl, 0.1% (v/v) Tween 20, 1% (w/v) BSA, and 0.002% (w/v) NaN3. The membranes were subsequently washed three times (10 min/wash) with TBST [20 mM Tris–HC1 (pH 7.6) containing 150 mM NaCl and 0.1% (v/v) Tween 20] and then incubated with the secondary antibodies (goat anti-mouse or rabbit IgG-HRP conjugate; 1:4000 dilution) in TBST for 1 h at room temperature. Membranes were washed three times in TBST. The activity of HRP was visualized by incubating the membranes for 5 min at room temperature in an ECL detection system (Pierce), followed by autoradiography. At the end of these experiments, the immunodetection system and the bound antibody were removed from the blot by incubating the membrane with reprobing buffer [62.5 mM Tris-HCl (pH 6.7), 2% SDS, 100 mM 2-ME] for 30 min at 56°C, followed by three washes in TBST. The blots were then stained with an antiactin antibody (1:2000 dilution) to confirm that equal amounts of protein were present in each lane of the gel. The band density was measured using an imaging densitometer (Bio-Rad) and normalized to the actin band.
Immunoprecipitation
To investigate p47phox and p22phox interactions in plasma membranes from neutrophils or neutrophil-like HL-60 cells at rest (unstimulated), the cells (25×106 cells) were first fractionated to membrane or cytosol-rich fractions as described above. Membrane-rich fractions were suspended in 500 μl immunoprecipitation buffer (50 mM HEPES, pH 7.5, 5 mM MgCl2, 1 mM CaCl2, 1% protease inhibitor cocktail, and 1 mM PMSF). After adjusting the volume, the p47phox antibody (1:50 dilution) was added to the samples and incubated overnight at 4°C. Fifty percent slurry of protein A/G Sepharose beads (Santa Cruz Biotechnology; 20 μl) was added to the reaction mixture, which was then incubated at 4°C. The resulting beads containing the bound immune complexes were washed three times with immunoprecipitation buffer (500 μl/wash), volume was adjusted to 100 μl, and 20 μl 6× SDS sample buffer was added and boiled for 5 min. The proteins were separated by SDS-PAGE (20 μg/lane) on 13% (v/v) polyacrylamide slab gels and monitored by Western blotting as described above.
Superoxide measurement
Superoxide release was monitored spectrophotometrically at 37°C by measuring superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome C at 550 nm [46]. Assays were carried out in 96-well microtiter plates with flat-bottomed wells (Linbro type, Flow Laboratories, McLean, VA, USA). Control wells contained all of the components of the assay mixture plus SOD (20 U/ml) to assess ferricytochrome c reduction by agents other than O2–. Neutrophils or neutrophil-like HL-60 cells (0.5×105 cells/well) were suspended in PBS (200 μl/well), stimulated by the addition of fMLP, and the absorbance (OD) at 550 nm recorded in a Vmax kinetic microplate reader (Molecular Devices, Sunnyvale, CA, USA). Superoxide generation was monitored as a linear rate with respect to time and cell number and is expressed as nmole O2–/106 cells/min.
Confocal microscopy
HL-60 cells (2×105 cells/200 μl) cultured under each condition were fixed with 200 μl ice-cold 4% paraformaldehyde/PBS (final concentration, 2%) for 20 min at room temperature in the dark. After fixation, cells were attached to glass slides using a Cytospin centrifuge (Shandon, UK) and washed three times for 5 min in PBS. The cells were permeabilized with 0.5% Triton-X/PBS for 5 min and blocked with the Avidin/biotin blocking kit (Biogenex, San Ramon, CA, USA). The cells were incubated with anti-p47phox mAb (1:100) diluted in 2% FBS/PBS overnight at 4°C. Following this incubation, the cells were washed four times for 5 min in PBS and incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:250) diluted in 2% FBS/PBS for 1 h at room temperature in the dark. After washing five times with PBS, cells were mounted using the Prolong Gold antifade kit following the manufacturer’s instruction. Stained cells were examined with a ×63/1.4 numerical aperture objective under a Zeiss LSM510 confocal microscope (Carl Zeiss, Heidelberg, Germany), and the images were imported into an ISM5 image browser (Carl Zeiss) for analysis.
Statistical analysis
Experimental results are presented as the mean ± sem. Unpaired two-tailed Student’s t-test was used for analysis of relative density and fold induction. ANOVA was used for analysis of superoxide assay with Bonferroni correction for multiple comparisons. P values less than 0.05 were considered to be statistically significant.
RESULTS
Enhanced superoxide generation in neutrophils from diabetic subjects
To characterize the primed phenotype of neutrophils at the functional level, we first confirmed that superoxide generation is enhanced in neutrophils from diabetic subjects upon fMLP stimulation (Fig. 1A). Neutrophils from diabetic subjects produced ∼1.6 times more superoxide than neutrophils from healthy subjects (P=0.031; n=13 subjects each group).
Figure 1.
Neutrophil function and p47phox translocation in diabetic subjects. (A) Superoxide generation in neutrophils from diabetic [diabetes mellitus (DM)] subjects is enhanced upon fMLP stimulation. Isolated neutrophils from diabetic and control subjects were stimulated with fMLP (1 μM) or PBS for 5 min. Superoxide release was measured as the SOD-inhibitable reduction of cytochrome c and expressed as the percent of control [Cont.; fMLP stimulation=100% (0.84±0.11 nmole O2–/106 cells/min); *, P<0.05; n=13 subjects in each group]. (B) Membrane-associated proteins and whole cell lysates were extracted from unstimulated neutrophils from diabetic and healthy subjects and were analyzed by Western blotting with anti-p47phox, p67phox, Rac2, and p22phox antibodies as described in Materials and Methods. Western blots reveal that there is no difference in the total expression of p47phox in unstimulated neutrophils from diabetic and healthy subjects. p47phox is translocated to the cell membrane-rich fraction in neutrophils from diabetic subjects (P=0.041). Expression of p67phox, Rac2, and p22phox is not significantly different between healthy and diabetic subjects. The same membrane was stripped and reprobed with antiactin antibody. The levels of actin were evaluated as a loading control. The relative density of p47phox, p67phox, Rac2, and p22phox bands was quantified by densitometry, and the data were normalized to actin. Results are expressed as mean ± sem of 16 subjects in each group. IB, Immunoblot.
Translocation of p47phox to the cell membrane in unstimulated neutrophils from diabetic subjects
To investigate alterations in superoxide generation pathways by neutrophils in diabetes, unstimulated neutrophils from diabetic and healthy subjects were isolated. Subcellular localization of p47phox, a key component of the NADPH oxidase, was monitored by Western blotting. In whole cell lysates at rest, there was no difference in the total expression of p47phox in neutrophils from diabetic and healthy subjects (Fig. 1B). However, there was more p47phox in the cell membrane-rich fraction of diabetic subjects than in the cell membrane-rich fraction of healthy subjects (P=0.041; n=16 subjects each group), demonstrating the unstimulated p47phox translocation in neutrophils from diabetic subjects. To test the specificity of p47phox translocation to the cell membrane in neutrophils from diabetic subjects, we have further analyzed lysates of neutrophils for other cytosolic components of the NADPH oxidase complex (p67phox and Rac2) and a membrane-bound component (p22phox). Figure 1B shows that in neutrophils from diabetic subjects, p67phox, Rac2, and p22phox expression at whole cell or membrane levels was not different from cells from healthy subjects, supporting the specificity of p47phox translocation.
Superoxide generation in neutrophil-like HL-60 cells
Neutrophil-like HL-60 cells were cultured in glucose, with and without the RAGE-specific ligand S100B for 24 h, and superoxide generation was measured (Fig. 2). When HL-60 cells were cultured in NG with S100B, fMLP-induced superoxide generation was higher than that of cells cultured with NG alone (P=0.024). When HL-60 cells were cultured in HG with S100B, superoxide levels were 1.8-fold greater than those in cells cultured in NG (P=0.028). To determine if the HG induction was glucose-specific or alternatively, the result of osmotic changes, HL-60 cells were cultured in NG with MN (5.5 mM glucose+19.5 mM MN). There was a trend for higher superoxide generation from the cells cultured in HG or MN compared with that of the cells cultured in NG alone, but the difference between these two groups was not significant (NG vs. HG: P=0.063; NG vs. MN: P=0.055).
Figure 2.
fMLP-induced superoxide generation in neutrophil-like HL-60 cells, which were cultured in NG or HG, with or without the RAGE-specific ligand S100B for 24 h. Cells were stimulated with fMLP (100 nM) or PBS for 5 min, and superoxide generation was quantified as the SOD-inhibitable reduction of ferricytochrome C as described in Materials and Methods. Neutrophil-like HL-60 cells cultured in NG with S100B or in HG with S100B exhibited greater fMLP-induced superoxide generation than the cells cultured in NG alone. Results are expressed as mean ± sem of at least six different experiments (*, P<0.05, compared with the cells cultured in NG).
p47phox translocation to the cell membrane in neutrophil-like HL-60 cells
After differentiation, neutrophil-like HL-60 cells were incubated in NG or HG, with or without S100B for 24 h. Cells were collected, and subcellular localization of p47phox was monitored by Western blotting (Fig. 3). Western blot data showed that HG + S100B did not alter the total expression of p47phox in HL-60 cells, which is similar to the result in human neutrophils (Fig. 3A). Examining subcellular fractions revealed that in unstimulated HL-60 cells cultured in HG, there was only a minor increase of p47phox in the membrane-rich fraction compared with the cells cultured in NG alone (P=0.12; Fig. 3B). In HL-60 cells cultured in NG with S100B, p47phox translocation to the membrane-rich fraction was significant when compared with cells cultured in NG alone (P=0.042). When cells were cultured in HG with S100B and compared with cells cultured in NG and HG alone, greater levels of p47phox translocated to the membrane-rich fraction (P=0.008; Fig. 3B). In response to MN, there was only a minor increase of p47phox in the membrane fraction compared with the cells cultured in NG, similar to that seen with HG, suggesting that the differences induced by HG are the result of osmotic pressure changes (Fig. 3B). To further confirm these findings, we have analyzed cells by confocal microscopy (Fig. 4). Confocal microscopic analyses clearly show that HG with S100B induced translocation of p47phox to the periphery of neutrophil-like HL-60 cells (Fig. , 4A4B4C4D4E).
Figure 3.
p47phox translocation to the cell membrane in neutrophil-like HL-60 cells. Membrane-associated proteins and whole cell lysates were extracted from unstimulated, neutrophil-like HL-60 cells cultured in NG or HG, with or without S100B for 24 h, and were analyzed by Western blotting with the anti-p47phox, p67phox, Rac2, or p22phox antibodies as described in Materials and Methods. (A and C) There is no difference of the expression of total p47phox, p67phox, Rac2, and p22phox in unstimulated, neutrophil-like HL-60 cells regardless of the culture condition. (B and C) p47phox translocated to the cell membrane-rich fraction in neutrophil-like HL-60 cells cultured in HG with S100B (HG+S, P=0.008, vs. NG, P=0.016, vs. NG with S100B (S; NG+S), P=0.009, vs. HG, P=0.016, vs. NG+MN) and NG with S100B (P=0.042 vs. NG), and p67phox, Rac2, and p22phox expression did not change. The same membrane was stripped and reprobed with antiactin antibody. The levels of actin were evaluated as a loading control. p47phox, p67phox, Rac2, and p22phox were quantified by densitometry, and the data were normalized to actin. Results are expressed as mean ± sem of at least three different experiments. *, P < 0.05.
Figure 4.
Confocal microscopic evaluation of p47phox translocation to the cell membrane in neutrophil-like HL-60 cells. Unstimulated, neutrophil-like HL-60 cells cultured in NG or HG, with or without S100B for 24 h, were fixed and attached to glass slides. The cells were incubated with anti-p47phox antibody and visualized using confocal microscopy. (A) NG, (B) NG with S100B; NG + S, (C) HG, (D) HG with S100B; HG + S, (E) NG + MN, (F) HG with S100B and PD98059 (PD); HG + S + PD. The results from three independent experiments are represented by the image. (Original scale bars: 5 μm.)
To study the specificity of p47phox as the targeted component of NADPH oxidase during hyperglycemia-induced neutrophil priming, we analyzed p67phox, Rac2, and p22phox expression in neutrophil-like HL-60 cells (Fig. 3). Neither whole cell nor membrane preparations from cells exposed to various conditions demonstrated any detectable changes in p67phox, Rac2, and p22phox expression, confirming the specificity of p47phox as the target for hyperglycemic events in primary cells (Fig. 1B) as well as in neutrophil-like HL-60 cells.
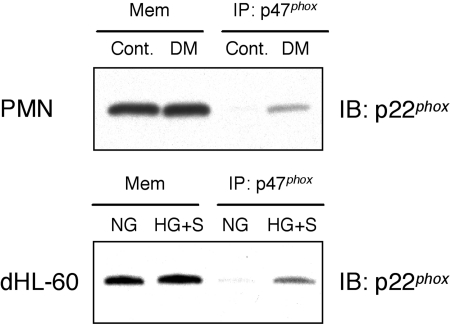
p47phox preassembly with p22phox on the cell membrane in neutrophils from diabetic subjects
Based on our observation of premature translocation of p47phox to the cell membrane-rich fraction in neutrophils, we next investigated the interaction between p47phox and p22phox in the membrane-rich fractions by coimmunoprecipitation. Membrane fractions from unstimulated neutrophils from diabetic or healthy subjects or neutrophil-like HL-60 cells cultured in NG or HG with S100B for 24 h were immunoprecipitated with p47phox antibody. Samples were then analyzed by Western blotting using anti-p22phox antibody as described in Materials and Methods. Western blotting data revealed that there was no difference in the expression of p22phox in membrane-rich fractions of unstimulated neutrophils from diabetic and healthy subjects similar to Figures 1Band 3. Samples immunoprecipitated with p47phox antibody revealed that p47phox coprecipitated with p22phox on the cell membrane in unstimulated neutrophils from diabetic subjects as well as unstimulated, neutrophil-like HL-60 cells cultured in HG with S100B (HG+S; Fig. 5).
Figure 5.
p47phox preassembled with p22phox in the cell membrane (Mem) fraction in unstimulated neutrophils from diabetic subjects or AGE-stimulated, neutrophil-like HL-60 cells. Unstimulated neutrophils from diabetic or healthy subjects and neutrophil-like HL-60 cells cultured in NG or HG with S100B for 24 h were fractionated to membrane and cytosolic fractions. The membrane-rich fraction samples were immunoprecipitated (IP) with p47phox mAb, and immunoprecipitated samples were analyzed by Western blotting using anti-p22phox antibody as described in Materials and Methods. Western blotting data reveal that there is no difference in the expression of p22phox in the membrane-rich fraction of unstimulated neutrophils from diabetic and healthy subjects. p47phox is preassembled with p22phox in the cell membrane-rich fraction in unstimulated neutrophils from diabetic subjects as well as unstimulated, neutrophil-like HL-60 cells cultured with AGE. Results are representative of at least three independent experiments. PMN, Polymorphonuclear neutrophil; dHL-60, differentiated neutrophil-like HL-60 cells.
Activation of RAGE induces the continuous phosphorylation of ERK1/2, but not p38 MAPK
Engagement of RAGE by AGE activates key signal transduction pathways, such as p21ras, ERK1/2, p38 MAPK, and NF-κB, in a variety of cells [28, 47, 48]. To determine the RAGE downstream signaling in neutrophils, HL-60 cells cultured in NG or HG were stimulated with S100B (5 μg/ml) for the indicated periods of time and monitored by Western blotting (Fig. 6).
Figure 6.
AGE induces the continuous phosphorylation of ERK1/2, but not p38 MAPK. Neutrophil-like HL-60 cells cultured in NG or HG were stimulated with S100B (5 μg/ml) for the indicated periods of time. Whole cell lysates were analyzed by Western blotting using antibodies to phospho-ERK1/2 and phospho-p38 MAPK as described in Materials and Methods. Stimulation with S100B for 5 min resulted in the rapid phosphorylation of ERK1/2 and p38 MAPK. The phosphorylation of ERK1/2 continued for 240 min, particularly ERK1. However, the phosphorylation of p38 MAPK was undetectable after 15 min. There was no detectable difference between NG and HG. The same membrane was stripped and reprobed with antiactin antibody. The levels of actin were evaluated as a loading control. Results are representative of three independent experiments.
Stimulation with S100B for 5 min resulted in the strong phosphorylation of ERK1/2 in cells cultured under NG or HG. This phosphorylation event continued for 240 min, particularly, ERK1. Stimulation with S100B for 5 min resulted in the phosphorylation of p38 MAPK. However, this phosphorylation event was undetectable after 15 min. There was no difference between NG and HG conditions, suggesting that RAGE activation leads to signal transduction regardless of the glucose availability in the extracellular milieu.
p47phox translocation to the cell membrane is mediated by ERK1/2
To investigate RAGE signaling upstream of ERK1/2 for translocation of p47phox to the cell membrane, HL-60 cells were cultured for 24 h, with or without PD98059, an inhibitor of MEK1/2 (the upstream activator of ERK1/2), and SB203580, a p38 MAPK inhibitor. The translocation of p47phox to the cell membrane in neutrophil-like HL-60 cells was studied by Western blotting (Fig. 7) and confocal microscopy (Fig. 4). PD98059 suppressed p47phox translocation to the cell membrane in HL-60 cells cultured in NG with S100B and cells cultured in HG with S100B (Fig. 7A). Confocal microscopic analysis confirmed these results (Fig. 4F). SB203580 did not inhibit p47phox translocation to the cell membrane-rich fraction of HL-60 cells cultured in NG with S100B and HG with S100B (Fig. 7B), suggesting that hyperglycemia-mediated p47phox translocation in neutrophils is ERK1/2-dependent. The inhibition of ERK1/2 was also specific for p47phox translocation, as evidenced by the lack of any detectable change in p22phox expression in membrane-rich fractions of cells treated with the same conditions (Fig. 7).
Figure 7.
PD98059 suppresses p47phox translocation. Membrane-associated proteins were extracted from unstimulated, neutrophil-like HL-60 cells cultured in NG or HG, with or without PD98059 (25 μM) or SB203580 (10 μM) for 24 h, and were analyzed by Western blotting with anti-p47phox and p22phox antibodies as described in Materials and Methods. PD98059 inhibited p47phox translocation to the cell membrane-rich fraction of HL-60 cells cultured in NG with S100B (NG+S) and completely inhibited p47phox translocation to the cell membrane-rich fraction of HL-60 cells cultured in HG with S100B (HG+S; Fig. 7A). SB203580 did not suppress p47phox translocation to the cell membrane (Fig. 7B). PD98059 and SB203580 did not alter p22phox expression (Fig. 7). The same membrane was stripped and reprobed with antiactin antibody, where actin was used as the loading control. Results are representative of three independent experiments.
DISCUSSION
In this paper, we report that the component of chronic hyperglycemia in diabetes responsible for priming neutrophils for exaggerated superoxide release is AGE or signaling through RAGE. The direct action of HG is minimal, but HG does augment the AGE-induced response. Enhanced superoxide generation from neutrophils in diabetes is induced by p47phox, a key component of the NADPH oxidase, translocation to the cell membrane, and preassembly with p22phox. These findings suggest that significant changes in the innate-immune response occur with chronic hyperglycemia, which may contribute to diabetic complications.
Oxidants produced by NADPH oxidase are highly toxic, not only to infectious agents but also to neighboring host tissues, causing cell dysfunction, DNA damage, and apoptosis [49]. Neutrophil superoxide generation can be potentiated to a great extent by prior exposure to priming agents such as GM-CSF, TNF-α, IL-8, and LPS [22, 50,51,52]. Neutrophil priming is a process whereby the cells are preactivated such that a subsequent exposure to an inflammatory stimulus generates an enhanced response. Primed cells are important in the enhanced reactivity of neutrophils in various inflammatory diseases such as diabetes, rheumatoid arthritis, and periodontitis [9, 22, 53], and an understanding of the mechanisms involved in the priming response may point to sites of potential therapeutic intervention. However, the mechanism of priming in neutrophils from diabetic subjects is still not clear. In this report, we demonstrate for the first time that p47phox, one of the cytosolic components of the NADPH oxidase system and a key protein for superoxide generation, is translocated to the cell membrane-rich fraction in neutrophils from diabetic subjects (Fig. 1B). The hyper-responsive phenotype of neutrophils from diabetic subjects reported here suggests that the cells are functionally primed. In diabetes, one of the major risk factors that correlates with neutrophil priming is hyperglycemia [9]. We have previously reported that the hyper-responsive phenotype for superoxide generation in neutrophils [9] or mononuclear phagocytes [14] is correlated with the HbA1c level, which is an indicator of poor chronic glycemic control. Two major factors that may mediate priming are HG concentration and AGE.
Neutrophils play a key role in the host defense against invading microorganisms and have a major role in inflammatory pathology [54,55,56,57]. In response to a variety of agents, neutrophils release large quantities of O2– and other ROS in a phenomenon known as oxidative burst [57]. Neutrophil production of O2– is dependent on activation of the NADPH oxidase, a multicomponent enzyme system that catalyzes NADPH-dependent reduction of oxygen to O2– [18, 58, 59]. NAPDH oxidase is the primary source of superoxide generation in neutrophils. Components of the NADPH oxidase are dissociated in the resting state into cytoplasmic (p47phox, p67phox, p40phox, and Rac2) and membrane-bound components (gp91phox and p22phox). The activation of neutrophils by stimuli, such as fMLP or PMA, subsequently leads to the phosphorylation of p47phox and its translocation to the cell membrane. This is a key, early event, resulting in full assembly of the NADPH oxidase and consequent generation of superoxide [60]. In neutrophils from diabetic subjects, p47phox is membrane-associated, indicating premature assembly of the NADPH oxidase. Gyurko et al. [61] have shown that in bone marrow neutrophils from the Akita mouse, a hyperglycemic mouse model, p47phox is partially translocated to the cell membrane, and these cells demonstrate enhanced superoxide generation after fMLP stimulation. As reported here, primary neutrophils from diabetic subjects show increased translocation of p47phox from the cytosol to the cell membrane. The same result was observed in an HL-60 cell model of hyperglycemia, and the actions were attributed to stimulation of cells through RAGE with a mild osmotic stimulation induced by elevated glucose. The initiating protein was specifically p47phox but not p67phox and Rac2; stimulation of RAGE did not impact the expression of membrane-bound p22phox. In addition, several studies suggest that phosphorylation of p47phox induces conformational changes that initiate assembly of the active enzyme via interaction of the Src homology 3 (SH3) domains with the proline-rich region of p22phox. p47phox contains two SH3 domains, one of which interacts with a p47phox-polyproline sequence in the nonphosphorylated protein. This interaction switches to the p22phox-polyproline sequence when p47phox is phosphorylated [62, 63]. The findings from our work demonstrate the expression of p47phox and p22phox in the membrane fraction, showing that the translocation of p47phox to the cell membrane is the driving force in increased neutrophil superoxide generation (Fig. 1A). Meanwhile, the expression of p67phox, Rac2, and p22phox in the cell membrane fraction does not change, suggesting that hyperglycemia specifically induces the p47phox pretranslocation to the cell membrane.
To determine which component of hyperglycemia causes p47phox translocation to the membrane compartment in neutrophils from diabetic subjects, the focus was then placed on HG and AGE–RAGE interactions. Primary neutrophils are end-stage cells that cannot be cultured for long periods of time (>4 h) without losing responsiveness to various agonists or becoming apoptotic [38, 39]. Therefore, we have used DMSO-induced, differentiated HL-60 cells instead of primary neutrophils to determine the long-term (chronic) changes induced by HG concentrations and RAGE ligand in an attempt to mimic the diabetic conditions in vitro. Neutrophil-like HL-60 cells are widely used as a model in functional studies of neutrophil chemotaxis and superoxide generation [33,34,35,36,37]. We confirmed that the NADPH subunit expression and superoxide generation from neutrophil-like HL-60 cells were similar to primary neutrophils.
When neutrophil-like HL-60 cells were cultured with HG alone, there was only a minimal translocation of p47phox, which was similar to that observed when the cells were cultured in NG with MN. As hyperosmolarity may impact the cell response regardless of the level of glucose [14], we have used MN as an osmotic control in this study. The cells treated with MN showed similar response to those treated with HG (Figs. 2and 3). However, when the cells were cultured in NG with S100B, p47phox significantly translocated to the membrane fraction. Also, when the cells were cultured in HG with S100B, the translocation was further enhanced in the neutrophil-like HL-60 cells, and this result was similar to that observed in neutrophils obtained from diabetic subjects. HG and S100B also resulted in a similar pattern of superoxide generation in neutrophil-like HL-60 cells (Fig. 2). However, the HG and AGE do not change the total expression of p47phox in neutrophil-like HL-60 cells, which is also similar to the data obtained in human neutrophils from diabetic subjects. These observations suggest that AGE–RAGE interactions are the primary priming event for the translocation of p47phox to the cell membrane. The changes induced by HG or MN alone are minor and probably represent a mild osmotic effect; the translocation of p47phox is an important priming event, as this resulted in enhanced oxidative burst upon activation with fMLP.
To further characterize the signaling pathways induced by AGE–RAGE interactions, post-receptor, downstream signaling events were evaluated. RAGE, a member of the Ig superfamily of cell surface molecules, is the central signal-transduction receptor for AGE. Binding of RAGE by these ligands activates key signal transduction pathways, including p21ras, ERK1/2, p38 MAPK, and NF-κB in endothelial cells, monocytes, and vascular smooth muscle cells. Collison et al. [64] have previously demonstrated that RAGE is expressed on human neutrophils. The cascade of events leads to RAGE-mediated, enhanced expression of proinflammatory mediators as demonstrated by suppression of these modified adducts in the presence of blocking antibodies to RAGE, soluble RAGE, the extracellular ligand-binding domain of the receptor, or by transient transfection of cDNA encoding cytosolic, tail-deleted RAGE into RAGE-bearing cells [14, 28, 47, 48]. These studies provided critical evidence that ligand engagement and signal transduction through RAGE were importantly involved in mediating the effects of AGE [28]. Our data have revealed that the RAGE-specific ligand, S100B, induced phosphorylation of ERK1/2 and p38 MAPK in neutrophil-like HL-60 cells. However, the signaling was differential; for ERK1/2, phosphorylation was maintained for 240 min. The p38 MAPK phosphorylation was undetectable after 15 min (Fig. 6), suggesting that ERK1/2 is the major pathway. To investigate this possibility further, the pharmacological inhibitors PD98059 or SB203580 were used. PD98059 completely inhibited p47phox translocation to the cell membrane-rich fraction in Western blotting (Fig. 7A) and confocal microscopy experiments (Fig. 4F), whereas a p38 MAPK inhibitor did not (Fig. 7B). These data suggest that RAGE ligation by S100B in neutrophil-like HL-60 cells induces the translocation of p47phox to the cell membrane compartment, at least in part, via ERK1/2. Recently, Dang et al. [22] have reported a pivotal role of a specific serine-phosphorylated site (Ser345) on p47phox by convergent MAPKs in the NADPH oxidase priming of neutrophils using the proinflammatory cytokines TNF-α and GM-CSF. They also suggested that prephosphorylation of p47phox on Ser345 by ERK1/2 or p38 MAPK could facilitate conformational changes induced by a secondary phosphorylation step. Further experiments will be necessary to elucidate whether RAGE induces prephosphorylation of p47phox at Ser345.
In addition to the independent activation of p47phox in diabetes, it would be of further interest to explore the interaction of NADPH oxidase components with other membranous structures such as lipid rafts [65,66,67]. In a recent report, Shao et al. [66] have shown that membrane-bound NADPH oxidase subunits (gp91phox and p22phox) are present in the lipid raft compartment of neutrophils, and cytosolic NADPH oxidase components (p47phox, p67phox, and p40phox) are absent from, but recruited to, rafts upon FcγR activation. This finding, together with our demonstration that hyperglycemia activates the translocation of NADPH oxidase components in cytosol to the membrane of neutrophils, suggests that lipid rafts may play a role in diabetes-induced neutrophil priming. Another recent report by Guichard et al. [65] has shown that IL-8 induced the sequential preassembly and recruitment of the cytosolic NADPH oxidase components in lipid rafts, which have important regulatory functions in fMLP-induced oxidative burst in neutrophils. As diabetes is a disease of lipid metabolism as well as glucose metabolism, it will be of interest in future studies to evaluate the role of changes in lipid composition of lipid rafts in the activation and assembly of the oxidase. Thus, the association among lipid rafts, activation of NADPH oxidase, and neutrophil priming in the context of diabetes require further experimentation.
In conclusion, this study points to the importance and specificity of translocation of p47phox to the cell membrane and preassembly of the NADPH oxidase in priming of neutrophils in diabetes. Primed neutrophils likely play a role in accelerating diabetic complications. Complete elucidation of these pathways will provide targets for future therapeutic interventions.
Acknowledgments
This work was supported in part by U.S. Public Health Service grants DE-15566, DE-16933, and RR-00533. We thank Amanda Blackwood and A. Mi Kim for laboratory assistance and Juliana J. Kim for help preparing the manuscript. We also thank Drs. Tsugumichi Saito and Haiyan Gong for their technical suggestions in confocal microscopy.
References
- Zimmet P., Alberti K. G., Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- Ritz E., Orth S. R. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999;341:1127–1133. doi: 10.1056/NEJM199910073411506. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., Nakamura K., Matsui T., Takenaka K., Jinnouchi Y., Imaizumi T. Cardiovascular disease in diabetes. Mini Rev Med Chem. 2006;6:313–318. doi: 10.2174/138955706776073501. [DOI] [PubMed] [Google Scholar]
- Renard C., Van Obberghen E. Role of diabetes in atherosclerotic pathogenesis What have we learned from animal models? Diabetes Metab. 2006;32:15–29. doi: 10.1016/s1262-3636(07)70243-4. [DOI] [PubMed] [Google Scholar]
- Pop-Busui R., Sima A., Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22:257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- Cameron N. E., Eaton S. E., Cotter M. A., Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44:1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- Kahn B. B., Flier J. S. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soskolne W. A., Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001;6:91–98. doi: 10.1902/annals.2001.6.1.91. [DOI] [PubMed] [Google Scholar]
- Karima M., Kantarci A., Ohira T., Hasturk H., Jones V. L., Nam B. H., Malabanan A., Trackman P. C., Badwey J. A., Van Dyke T. E. Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol. 2005;78:862–870. doi: 10.1189/jlb.1004583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacopino A. M. Periodontitis and diabetes interrelationships: role of inflammation. Ann Periodontol. 2001;6:125–137. doi: 10.1902/annals.2001.6.1.125. [DOI] [PubMed] [Google Scholar]
- Rosen P., Nawroth P. P., King G., Moller W., Tritschler H. J., Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored by UNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17:189–212. doi: 10.1002/dmrr.196. [DOI] [PubMed] [Google Scholar]
- Bellin C., de Wiza D. H., Wiernsperger N. F., Rosen P. Generation of reactive oxygen species by endothelial and smooth muscle cells: influence of hyperglycemia and metformin. Horm Metab Res. 2006;38:732–739. doi: 10.1055/s-2006-955084. [DOI] [PubMed] [Google Scholar]
- Wautier J. L., Schmidt A. M. Protein glycation: a firm link to endothelial cell dysfunction. Circ Res. 2004;95:233–238. doi: 10.1161/01.RES.0000137876.28454.64. [DOI] [PubMed] [Google Scholar]
- Ding Y., Kantarci A., Hasturk H., Trackman P. C., Malabanan A., Van Dyke T. E. Activation of RAGE induces elevated O2– generation by mononuclear phagocytes in diabetes. J Leukoc Biol. 2007;81:520–527. doi: 10.1189/jlb.0406262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtz-Swirski R., Sela S., Herskovits A. T., Shasha S. M., Shapiro G., Nasser L., Kristal B. Involvement of peripheral polymorphonuclear leukocytes in oxidative stress and inflammation in type 2 diabetic patients. Diabetes Care. 2001;24:104–110. doi: 10.2337/diacare.24.1.104. [DOI] [PubMed] [Google Scholar]
- Babior B. M. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Ago T., Nunoi H., Ito T., Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox) Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- Chanock S. J., el Benna J., Smith R. M., Babior B. M. The respiratory burst oxidase. J Biol Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- Park J. W., Babior B. M. The translocation of respiratory burst oxidase components from cytosol to plasma membrane is regulated by guanine nucleotides and diacylglycerol. J Biol Chem. 1992;267:19901–19906. [PubMed] [Google Scholar]
- Jackson S. H., Gallin J. I., Holland S. M. The p47phox mouse knock-out model of chronic granulomatous disease. J Exp Med. 1995;182:751–758. doi: 10.1084/jem.182.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siow Y. L., Au-Yeung K. K., Woo C. W., O K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cβ activation. Biochem J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang P. M., Stensballe A., Boussetta T., Raad H., Dewas C., Kroviarski Y., Hayem G., Jensen O. N., Gougerot-Pocidalo M. A., El-Benna J. A specific p47phox-serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J Clin Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeish K. R., Knall C., Ward R. A., Gerwins P., Coxon P. Y., Klein J. B., Johnson G. L. Activation of mitogen-activated protein kinase cascades during priming of human neutrophils by TNF-α and GM-CSF. J Leukoc Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Brett J., Yan S. D., Wautier J. L., Stern D. Cellular receptors for advanced glycation end products Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994;14:1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Huebschmann A. G., Regensteiner J. G., Vlassara H., Reusch J. E. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29:1420–1432. doi: 10.2337/dc05-2096. [DOI] [PubMed] [Google Scholar]
- Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev. 2001;17:436–443. doi: 10.1002/dmrr.233. [DOI] [PubMed] [Google Scholar]
- Kislinger T., Fu C., Huber B., Qu W., Taguchi A., Du Yan S., Hofmann M., Yan S. F., Pischetsrieder M., Stern D., Schmidt A. M. N(ε)-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J Biol Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Yan S. D., Yan S. F., Stern D. M. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M. A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M. F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A. M. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Reddy M. A., Li S. L., Sahar S., Kim Y. S., Xu Z. G., Lanting L., Natarajan R. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- Shanmugam N., Kim Y. S., Lanting L., Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–34844. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci USA. 1978;75:2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papac R. J., Brown A. E., Schwartz E. L., Sartorelli A. C. Differentiation of human promyelocytic leukemia cells in vitro by 6-thioguanine. Cancer Lett. 1980;10:33–38. doi: 10.1016/0304-3835(80)90062-2. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Fu J., Suzuki K., Sendo D., Nitto T., Sendo F., Araki Y. Expression of GPI-80, a β2-integrin-associated glycosylphosphatidylinositol-anchored protein, requires neutrophil differentiation with dimethyl sulfoxide in HL-60 cells. Exp Cell Res. 2003;286:199–208. doi: 10.1016/s0014-4827(03)00071-5. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Speier C., Borregaard N., Walsh C. E., Whitin J. C., Simons E. R. Development of the superoxide-generating system during differentiation of the HL-60 human promyelocytic leukemia cell line. J Biol Chem. 1984;259:3771–3776. [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P. G., Karnovsky M. L., Badwey J. A. Protein phosphorylation associated with synergistic stimulation of neutrophils. J Biol Chem. 1989;264:14935–14939. [PubMed] [Google Scholar]
- Cowburn A. S., Cadwallader K. A., Reed B. J., Farahi N., Chilvers E. R. Role of PI3-kinase-dependent Bad phosphorylation and altered transcription in cytokine-mediated neutrophil survival. Blood. 2002;100:2607–2616. doi: 10.1182/blood-2001-11-0122. [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl. 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Fujita T., Zawawi K. H., Kurihara H., Van Dyke T. E. CD38 cleavage in fMLP- and IL-8-induced chemotaxis is dependent on p38 MAP kinase but independent of p44/42 MAP kinase. Cell Signal. 2005;17:167–175. doi: 10.1016/j.cellsig.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Batista E. L., Jr, Warbington M., Badwey J. A., Van Dyke T. E. Differentiation of HL-60 cells to granulocytes involves regulation of select diacylglycerol kinases (DGKs) J Cell Biochem. 2005;94:774–793. doi: 10.1002/jcb.20356. [DOI] [PubMed] [Google Scholar]
- Kurihara H., Murayama Y., Warbington M. L., Champagne C. M., Van Dyke T. E. Calcium-dependent protein kinase C activity of neutrophils in localized juvenile periodontitis. Infect Immun. 1993;61:3137–3142. doi: 10.1128/iai.61.8.3137-3142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira T., Bannenberg G., Arita M., Takahashi M., Ge Q., Van Dyke T. E., Stahl G. L., Serhan C. N., Badwey J. A. A stable aspirin-triggered lipoxin A4 analog blocks phosphorylation of leukocyte-specific protein 1 in human neutrophils. J Immunol. 2004;173:2091–2098. doi: 10.4049/jimmunol.173.3.2091. [DOI] [PubMed] [Google Scholar]
- Omori K., Naruishi K., Nishimura F., Yamada-Naruishi H., Takashiba S. High glucose enhances interleukin-6-induced vascular endothelial growth factor 165 expression via activation of gp130-mediated p44/42 MAPK-CCAAT/enhancer binding protein signaling in gingival fibroblasts. J Biol Chem. 2004;279:6643–6649. doi: 10.1074/jbc.M311688200. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978;61:1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M., et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Martindale J. L., Holbrook N. J. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Fukushima T., Fialkow L., Waddell T. K. Intracellular signaling in neutrophil priming and activation. Semin Cell Biol. 1995;6:345–356. doi: 10.1016/s1043-4682(05)80005-4. [DOI] [PubMed] [Google Scholar]
- Hallett M. B., Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- DeLeo F. R., Renee J., McCormick S., Nakamura M., Apicella M., Weiss J. P., Nauseef W. M. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci A., Oyaizu K., Van Dyke T. E. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: findings from localized aggressive periodontitis. J Periodontol. 2003;74:66–75. doi: 10.1902/jop.2003.74.1.66. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- Segal A. W. How neutrophils kill microbes. Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- Quinn M. T., Gauss K. A. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol. 2004;76:760–781. doi: 10.1189/jlb.0404216. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Ohira T., Badwey J. A. Regulation of the NADPH-oxidase complex of phagocytic leukocytes Recent insights from structural biology, molecular genetics, and microscopy. Histochem Cell Biol. 2004;122:293–304. doi: 10.1007/s00418-004-0672-2. [DOI] [PubMed] [Google Scholar]
- Gyurko R., Siqueira C. C., Caldon N., Gao L., Kantarci A., Van Dyke T. E. Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice. J Immunol. 2006;177:7250–7256. doi: 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. D., Helgerson S. L., Davis A. R., Nelson L. K., Quinn M. T. Analysis of activation-induced conformational changes in p47phox using tryptophan fluorescence spectroscopy. J Biol Chem. 1997;272:29502–29510. doi: 10.1074/jbc.272.47.29502. [DOI] [PubMed] [Google Scholar]
- Groemping Y., Lapouge K., Smerdon S. J., Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- Collison K. S., Parhar R. S., Saleh S. S., Meyer B. F., Kwaasi A. A., Hammami M. M., Schmidt A. M., Stern D. M., Al-Mohanna F. A. RAGE-mediated neutrophil dysfunction is evoked by advanced glycation end products (AGEs) J Leukoc Biol. 2002;71:433–444. [PubMed] [Google Scholar]
- Guichard C., Pedruzzi E., Dewas C., Fay M., Pouzet C., Bens M., Vandewalle A., Ogier-Denis E., Gougerot-Pocidalo M. A., Elbim C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J Biol Chem. 2005;280:37021–37032. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- Shao D., Segal A. W., Dekker L. V. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- Kannan K. B., Barlos D., Hauser C. J. Free cholesterol alters lipid raft structure and function regulating neutrophil Ca2+ entry and respiratory burst: correlations with calcium channel raft trafficking. J Immunol. 2007;178:5253–5261. doi: 10.4049/jimmunol.178.8.5253. [DOI] [PubMed] [Google Scholar]